Abstract
Background
There is increasing evidence that chronic immune activation predisposes to non-Hodgkin’s lymphoma (NHL). Whether this association exists among women representative of the current HIV epidemic in the U.S. who are at high risk of HIV-associated NHL (AIDS-NHL), remains to be determined.
Methods
We conducted a nested case-control study within the Women’s Interagency HIV Study with longitudinally collected risk factor data and sera. Cases were HIV-infected women with stored sera collected at three time-windows 3–5 years, 1–3 years, and 0–1 year prior to AIDS-NHL diagnosis (n=22). Three to six HIV-infected controls, without AIDS-NHL, were matched to each case on age, race, CD4+ T cell count, and study follow-up time (n=78). Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between one unit increase in log-transformed biomarker levels and AIDS-NHL were computed using random effect multivariate logistic regression models.
Results
Elevated levels of sCD27 (OR=7.21, 95% CI=2.62–19.88), sCD30 (OR=2.64, 95% CI=1.24–5.64), and CXCL13 (OR=2.56, 95% CI=1.32–4.96) were associated with subsequent diagnosis of AIDS-NHL overall. Elevated sCD23 was associated with a 2-to 4-fold increased risk of AIDS-NHL in certain subgroups, while elevated IL6 was associated with a 2-fold increased risk in the 0–1 year time-window, only.
Conclusion
These findings support the hypothesis that chronic B cell activation contributes to the development of AIDS-NHL in women.
Impact
sCD23, sCD27, sCD30, and CXCL13 may serve as biomarkers for AIDS-NHL.
Keywords: lymphoma, B cell, cytokines, AIDS, immune activation
Introduction
AIDS-associated B cell non-Hodgkin lymphoma (AIDS-NHL) is the most common malignancy among people with HIV-infection in some regions where highly active antiretroviral therapy (HAART) is readily available, including the United States, Europe, and Australia (1). Prolonged infection with HIV causes immune dysfunction including chronic immune suppression and B cell hyperactivation. The depletion of CD4+ T cells contributes to the development of AIDS-NHL, particularly primary central nervous system lymphoma (PCNSL), through the loss of immunoregulatory control over Epstein-Barr virus (EBV) -infected B cells (2). Some of the more common subtypes of non-PCNSL (or systemic) AIDS-NHL, including diffuse large B cell lymphoma (DLBCL), occur in the setting of chronic B cell activation (3) which may be an indirect response to gut microbial translocation or other factors (4). The downstream effects of chronic B cell activation, with ongoing engagement of the B cell receptor complex, on lymphomagenesis are numerous, and include the accumulation of oncogene mutations and translocations resulting from aberrant expression and gene targeting of the DNA-mutating enzyme, activation-induced cytidine deaminase (AID) (5, 6).
Several recent studies have reported significant associations between elevated levels of B cell activation biomarkers and subsequent risk of AIDS-NHL, including numerous cytokines, chemokines, soluble receptors, kappa and lambda free light chains, and AID (7–12). These studies have been conducted in populations predominantly composed of men who were not treated with HAART prior to the diagnosis of AIDS-NHL. HAART results in suppression of HIV replication and partial restoration of immune competence, although markers of inflammation remain elevated. Thus, although several immune biomarkers have been found to be reduced following HAART initiation, these markers have not normalized (13). It has also been suggested that HAART exposure modifies the relationship between biomarkers of B cell activation and AIDS-NHL risk (9).
In order to examine the relationship between markers of B cell activation and subsequent development of AIDS-NHL, we examined the longitudinal circulating levels of B cell activation and immune stimulatory molecules (soluble CD23 [sCD23], sCD27, sCD30, CXCL13/BCA1, interleukin-6 [IL6], and complement-reactive protein [CRP]) in relation to AIDS-NHL risk in a cohort of HIV infected women, about 40% of whom had been treated with HAART prior to the development of AIDS-NHL.
Materials and Methods
Study Design and Population
A nested case-control study was performed within an ongoing prospective study of HIV infection among women, the Women’s Interagency HIV Study (WIHS) (14, 15). The WIHS includes 3,768 adult women from five metropolitan areas (the San Francisco Bay Area, Los Angeles, Chicago, Washington D.C., and New York City) enrolled during two recruitment periods (1994–1995 and 2001–2002). WIHS participants are seen semiannually for an in-person interview, physical exam, and specimen collection. Baseline and follow-up interviews elicited a wide range of detailed information, including demographic data, medications taken, and clinical events. Self-reported antiretroviral use was ascertained at each visit, with the aid of photo-medication cards, and was summarized according to Department of Health and Human Services/Kaiser Panel to define HAART usage (16). Venous blood samples were drawn at each 6-month study visit and processed; sera were stored in a central repository. HIV plasma load and CD4+ T cell counts were measured at each 6-month study visit.
Case and Control Definitions
AIDS-NHL cases were ascertained through 6-monthly self-reports and confirmed by pathology records and state cancer registries. Cases included all HIV-infected WIHS participants with AIDS-NHL diagnosed after their baseline visit and prior to 2006 who had at least one available pre-AIDS-NHL diagnosis serum specimen (n=22). The median follow-up time for cases was 3.0 years. Based on classification of ICD codes and InterLymph recommendation for epidemiologic research (17), 17 cases were systemic and five were central nervous system lymphomas (Table 1). Of the systemic cases, 11 were diffuse large B cell lymphomas, two were B-cell precursor lymphoblastic leukemia/lymphoma, one was Burkitt lymphoma, and three B cell lymphoid neoplasms, not otherwise specified. Up to three serum samples were obtained for each case, collected 2.7 to 4.7 years pre-NHL (median = 4.0 years, n=10), 1.0 to 2.9 years pre-NHL (median = 1.9 years, n=14), and 0.1 to 0.9 years pre-NHL (median = 0.5 years, n=19). At least three, and up to six (if available), HIV-infected WIHS participants without a diagnosis of AIDS-NHL were individually matched to each case on 1) time since first HIV positive study visit (± 1.0 year), 2) age (± 2.5 years), 3) CD4+ T cell count at first HIV positive study visit (± 50 cells/mm3), and 4) race/ethnicity. Reference date for controls was a date matched on HIV+ follow-up time to the case of each matched set at time of AIDS-NHL diagnosis. Specimens for controls were obtained from three time-windows with a median of 3.9, 2.1, and 0.4 years prior to reference date.
Table 1.
Select characteristics of AIDS-NHL cases and HIV-infected controls from the Women’s Interagency HIV Study
| AIDS-NHL cases (n=22)
|
HIV-infected controls (n=78)
|
p-value | |||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Baseline characteristics | |||||
| WIHS site | |||||
| New York | 9 | (40) | 24 | (31) | |
| DC | 1 | (5) | 9 | (12) | |
| California | 11 | (50) | 28 | (35) | |
| Chicago | 1 | (5) | 17 | (22) | 0.16 |
| Race/ethnicity | |||||
| African-American, non-Hispanic | 11 | (50) | 47 | (60) | |
| White, non-Hispanic | 8 | (36) | 20 | (26) | |
| Latina/Hispanic | 2 | (9) | 11 | (14) | |
| Other | 1 | (5) | 0 | (0) | 0.18 |
| Education | |||||
| Less than high school | 7 | (32) | 28 | (36) | |
| High school graduate | 6 | (27) | 23 | (30) | |
| More than high school | 9 | (41) | 26 | (34) | 0.82 |
| HCV positivea | |||||
| No | 18 | (82) | 58 | (75) | |
| Yes | 4 | (18) | 19 | (25) | 0.53 |
| Characteristics at AIDS-NHL diagnosis or reference date | |||||
| Reference year | |||||
| 1995–2001 | 16 | (73) | 53 | (68) | |
| 2002–2008 | 6 | (27) | 25 | (32) | 0.67 |
| Age (years) | |||||
| <30 | 4 | (18) | 8 | (10) | |
| 30–39 | 10 | (45) | 41 | (53) | |
| 40–49 | 7 | (32) | 24 | (31) | |
| ≥50 | 1 | (5) | 5 | (6) | 0.76 |
| Cigarette smokingb | |||||
| Never | 5 | (24) | 26 | (34) | |
| Current | 8 | (38) | 38 | (50) | |
| Former | 8 | (38 | 12 | (16 | 0.08 |
| CD4+ T cells/mm3 c | |||||
| <200 | 13 | (72) | 28 | (41) | |
| 200–400 | 3 | (17) | 12 | (18) | |
| >400 | 2 | (11) | 28 | (41) | 0.04 |
| HIV RNA copiesd | |||||
| <4000 | 2 | (11) | 25 | (36) | |
| 4000–20,000 | 4 | (21) | 15 | (22) | |
| >20000 | 13 | (68) | 29 | (42) | 0.07 |
| Prior AIDS diagnosis | |||||
| No | 9 | (41) | 34 | (44) | |
| Yes | 13 | (59) | 44 | (56) | 0.82 |
| Prior HAART exposuree | |||||
| No | 13 | (59) | 41 | (53) | |
| Yes | 9 | (41) | 37 | (47) | 0.66 |
| Tumor subtypef | |||||
| Systemic | 17 | (77) | |||
| Diffuse large B cell lymphoma | 11 | ||||
| Precursor lymphoblastic leukemia/lymphoma, B cell | 2 | ||||
| Burkitt lymphoma | 1 | ||||
| B cell lymphoid neoplasm, NOS | 3 | ||||
| Central Nervous System | 5 | (23) | |||
RNA positive or antibody positive at baseline study visit
Missing values for three participants
Missing values for 14 participants
Missing values for 12 participants
“No” indicates unexposed at all three serum sampling time points “Yes” indicates exposed at least one time point
Classified according to the InterLymph recommendation for epidemiologic research (17).
Serum Biomarker Determination
Immune biomarkers were measured in archived sera by enzyme-linked immunosorbent assays (ELISA). Assays were carried out according to the manufacturer’s protocol for sCD23 and sCD30 (Bender MedSystems USA, San Bruno, California), sCD27 (PeliKine-compact ELISA kit, CLB/Sanquin, Netherlands, with 1:20 dilutions), CXCL13 (R&D Systems, Minneapolis, MN), IL6 (Biosource/Invitrogen, Carlsbad, California with color development time extended to 40 minutes to ensure consistent low level detection), and CRP (high sensitivity protocol, Virgo CRP 150, Hemagen, Columbia, Maryland). All samples from each case and matched controls were tested together as a set on the same assay plate; laboratory personnel were blinded to the identity of the samples within each set. All immune markers were detectable in greater than 98% of samples tested, except for sCD23 (90.9%, Table 2). All of the immune markers had intra-assay CVs ≤7%. The inter-assay CVs were comparatively higher, particularly for IL6 and CRP, which also exhibited low concentrations in tested samples.
Table 2.
Descriptive and quality control data for immune markers
| lower limit of detection | % detectable (# undetectable/total) | Intra-assay CV% | Inter-assay CV% | |
|---|---|---|---|---|
| sCD23 | 13 units/mL | 90.9 (19/208) | 2.6% | 19.8% |
| sCD27 | 32 units/mL | 100 (0/208) | 2.6% | 4.4% |
| sCD30 | 6 units/mL | 100 (0/208) | 6.1% | 20.8% |
| CXCL13 | 7.8 pg/mL | 100 (0/208) | 4.1% | 15.7% |
| IL6 | 0.16 pg/mL | 98.6 (3/208) | 3.1% | 25.5% |
| CRP | 0.25 μg/mL | 98.1 (4/208) | 7.0% | 29.2% |
CV=coefficient of variation
Statistical Analysis
We calculated frequencies for select covariates and χ2 p-values to test for differences between cases and controls. Samples with biomarker levels below the detection limit were set equal to half the value of the lower detection limit. Unmatched comparisons of mean natural log transformed (loge) biomarker levels at each time-window for cases compared to controls were performed using t-tests, and the means were reported as back-transformed geometric means. Geometric means were also calculated for categories of select covariates among HIV-infected control women. One-way ANOVAs were used to compare means across categories for each covariate, and a p-values ≤0.05 was considered evidence for statistically significant variation. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between loge immune biomarker levels and AIDS-NHL were computed using random effects multivariate logistic regression with the GLIMMIX procedure in SAS (18). The ORs represent risk of AIDS-NHL associated with one unit increase in loge biomarker levels. The matching by design between each case/control pair and correlation between samples from the same individual in the models including measurements from all available time-windows combined were incorporated into the models by adding a random effect term, with the following equation: logit P(D=1|X)= β0+ β1Biomarker + β2–7Covariates + ρiMatch, where logit P(D=1|X) is the log odds of being a case versus control, and all effects are fixed effects except for ρiMatch which is a random effect variable (mean zero, constant variance) that has the same value for all visits belonging to the ith matched case-control pair (19). Covariates were selected a priori if they were plausibly related to AIDS-NHL risk, HIV disease progression, and/or immune biomarker levels. Covariates included in the final multivariate models were education status and HCV positivity (RNA or antibody) at baseline, absolute CD4+ T cell counts, HIV RNA levels, and HAART exposure ascertained at the study visit which the biomarkers were measured, and cigarette smoking status (never, former, or current) at the study visit closest to and preceding AIDS-NHL diagnosis date in the cases and reference date in the controls. Multiple imputation was used to estimate missing covariate data in the multivariate models with the MIANALYZE procedure in SAS (20).
Results
Cases and controls were similarly distributed by race, reference year and age (reflective of the matching criteria), and education (Table 1). The large percentage of black, non-Hispanic women in this case-control study is representative of the WIHS cohort at large, and of the HIV epidemic in women in the United States (15). More cases than controls were from New York and California, although this difference was not statistically significant. Less than one third of cases and controls were HCV positive at study entry. Most cases and controls were current or former smokers, although fewer cases were never smokers compared to controls (p=0.08). Cases had a lower CD4+ T cell count (p=0.04) and higher HIV RNA load (p=0.07) compared to controls. The majority of cases and controls had a prior AIDS diagnosis, while 41% of cases and 46% of controls had been exposed to HAART. A larger proportion of lymphomas were systemic tumors (n=17, 77%) compared to PCNSL tumors (n=5, 23%), and a majority of systemic tumors were of the DLBCL subtype.
Biomarkers were explored among the HIV-infected control women to understand factors related to biomarker levels in absence of NHL (Table 3). All of the biomarkers showed significant or borderline significant variation by categories of CD4+ T cell count or HIV RNA, except for sCD23. Women with the most progressive HIV disease, (i.e., CD4+ T cell count <200 or HIV RNA copies >20,000) had the highest mean biomarker levels; the only exception was sCD23, which had the highest mean level in women with CD4+ T cell >400. Additionally, there were significant variations across categories of WIHS site for IL6 (p=0.044), race for CXCL13 (p=0.018), age for sCD30 (p=0.016), and cigarette smoking for sCD27 (p=0.001) and IL6 (p=0.025). Furthermore, sCD27 was higher (p=0.022) and CRP lower (p=0.005) among HCV positive controls. Women with a reference year between 1995 and 2001 (early HAART period) had higher levels of sCD30 (p=0.008) and CXCL13 (p=0.039), and sCD30 was significantly higher among women unexposed to HAART (p=0.005)
Table 3.
Geometric means for immune markers at visit 0–1 year prior to reference date among HIV-infected control women by select characteristics
| sCD23 (units/mL) | sCD27 (units/mL) | sCD30 (units/mL) | CXCL13 (pg/mL) | IL6 (pg/mL) | CRP (μg/mL) | |
|---|---|---|---|---|---|---|
|
|
||||||
| Baseline characteristics | ||||||
| WIHS site | ||||||
| New York | 49 | 494 | 115 | 213 | 2.7 | 3.3 |
| DC | 56 | 361 | 103 | 220 | 1.5 | 1.5 |
| California | 39 | 342 | 76 | 153 | 1.2 | 2.0 |
| Chicago | 64 | 406 | 78 | 198 | 2.0 | 2.3 |
| p-value | 0.338 | 0.075 | 0.052 | 0.230 | 0.044 | 0.470 |
| Race/ethnicity | ||||||
| African-American, non- Hispanic | 47 | 409 | 92 | 211 | 2.0 | 2.3 |
| White, non-Hispanic | 52 | 422 | 92 | 173 | 1.4 | 2.7 |
| Latina/Hispanic | 54 | 339 | 76 | 113 | 1.3 | 2.0 |
| p-value | 0.877 | 0.557 | 0.698 | 0.018 | 0.372 | 0.872 |
| Education | ||||||
| Less than high school | 59 | 420 | 97 | 197 | 1.7 | 1.7 |
| High school graduate | 40 | 409 | 83 | 194 | 1.9 | 3.1 |
| More than high school | 49 | 381 | 90 | 175 | 1.7 | 2.6 |
| p-value | 0.342 | 0.785 | 0.670 | 0.760 | 0.894 | 0.263 |
| HCV positivea | ||||||
| No | 49 | 376 | 87 | 177 | 1.7 | 3.0 |
| Yes | 49 | 519 | 103 | 233 | 2.1 | 1.0 |
| p-value | 0.977 | 0.022 | 0.324 | 0.116 | 0.469 | 0.005 |
| Characteristics at AIDS-NHL diagnosis or reference date | ||||||
| Reference year | ||||||
| 1995–2001 | 51 | 425 | 103 | 210 | 2.0 | 2.1 |
| 2002–2008 | 46 | 366 | 71 | 154 | 1.5 | 2.8 |
| p-value | 0.633 | 0.224 | 0.008 | 0.039 | 0.216 | 0.404 |
| Age (years) | ||||||
| <30 | 35 | 397 | 130 | 241 | 1.8 | 1.9 |
| 30–39 | 55 | 399 | 94 | 196 | 1.9 | 3.1 |
| 40–49 | 47 | 433 | 90 | 177 | 1.5 | 1.6 |
| ≥50 | 31 | 322 | 44 | 141 | 1.7 | 1.8 |
| p-value | 0.427 | 0.683 | 0.016 | 0.502 | 0.896 | 0.318 |
| Cigarette smokingb | ||||||
| Never | 53 | 340 | 79 | 158 | 1.1 | 2.2 |
| Current | 51 | 504 | 105 | 208 | 2.2 | 2.1 |
| Former | 43 | 336 | 83 | 209 | 2.6 | 3.0 |
| p-value | 0.781 | 0.001 | 0.129 | 0.200 | 0.025 | 0.726 |
| CD4+ T cells/mm3 c | ||||||
| <200 | 44 | 450 | 123 | 243 | 2.6 | 3.0 |
| 200–400 | 45 | 427 | 95 | 189 | 1.1 | 1.1 |
| >400 | 52 | 354 | 65 | 142 | 1.5 | 2.4 |
| p-value | 0.731 | 0.171 | <0.001 | 0.002 | 0.019 | 0.065 |
| HIV RNA copiesd | ||||||
| <4000 | 45 | 299 | 55 | 128 | 1.2 | 2.2 |
| 4000–20,000 | 43 | 429 | 106 | 200 | 1.4 | 2.1 |
| >20000 | 56 | 504 | 126 | 253 | 2.8 | 2.6 |
| p-value | 0.544 | <0.001 | <0.001 | <0.001 | 0.005 | 0.837 |
| Prior AIDS diagnosis | ||||||
| No | 51 | 426 | 94 | 179 | 1.8 | 2.5 |
| Yes | 47 | 387 | 87 | 195 | 1.8 | 2.2 |
| p-value | 0.710 | 0.434 | 0.570 | 0.560 | 0.945 | 0.682 |
| Prior HAART exposureb | ||||||
| No | 50 | 447 | 110 | 207 | 2.2 | 2.0 |
| Yes | 48 | 366 | 75 | 172 | 1.5 | 2.8 |
| p-value | 0.836 | 0.091 | 0.005 | 0.203 | 0.094 | 0.284 |
RNA positive or antibody positive at baseline study visit
“No” indicates unexposed at all three serum sampling time points, “Yes” indicates exposed at least one time point
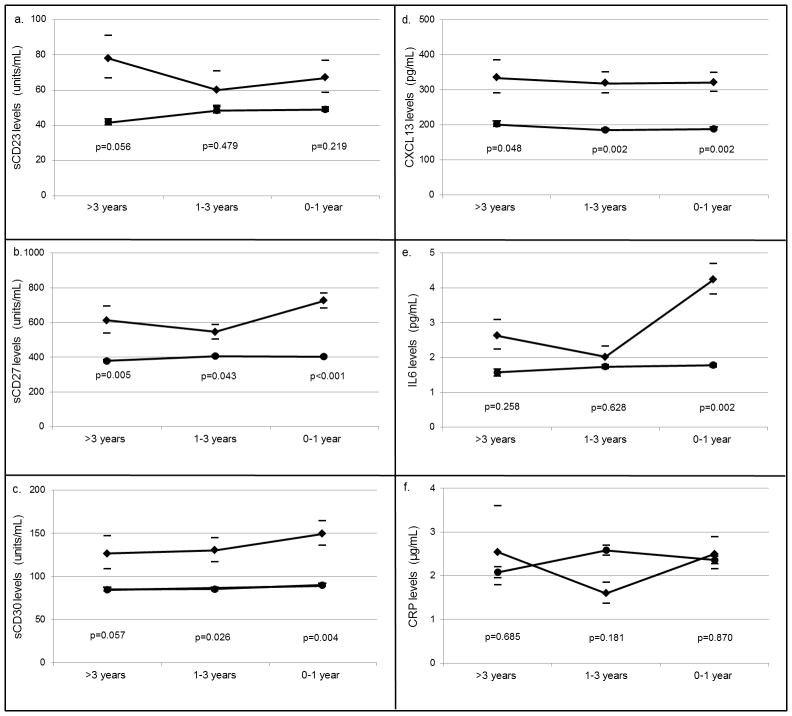
Next biomarker levels were compared between the case and control groups. In univariate analyses, mean levels of sCD27 were significantly higher in cases compared to controls at all three time-windows, while sCD30 and CXCL13 showed increased levels of borderline significance at >3 years, and clearly significant increased levels at the 1–3 and 0–1 years time windows (Figure 1). IL6 was elevated in cases at the 0–1 year time-window only. Levels of sCD23 and CRP were not significantly different between cases and controls at any time-window.
Figure 1. Mean serum biomarker levels in AIDS-NHL cases and matched HIV-infected controls.
Geometric means for cases (black diamonds) and controls (black circles) and 95% confidence intervals (bars) at three time-windows preceding an AIDS-NHL diagnosis in cases and reference date in controls for a. sCD23, b. sCD27, c. sCD30, d. CXCL13, e. IL6, and f. CRP. P-values represent the significance level for the difference in mean biomarker values between cases and matched controls.
In multivariate models including serum measurements from all available time-windows combined, sCD27 was strongly associated with AIDS-NHL risk across all subgroups (Table 4). Elevations of sCD23, sCD30 and CXCL13 were statistically associated with an increased risk of systemic AIDS-NHL and in those who were HAART unexposed. IL6 and CRP were generally not associated with AIDS-NHL risk. When the biomarker data were analyzed according to sampling time-window, there were no clear trends with respect to biomarker associations increasing or decreasing with time to NHL diagnosis (Table 5). However, sCD27 appeared to be more strongly associated with AIDS-NHL at the time-window closest to AIDS-NHL diagnosis date, as was IL-6, although the latter was not statistically significant, overall.
Table 4.
Association between immune markers and AIDS-NHL risk overall and in select subgroups
| All samples 22 Cases/78 Controls |
Systemic 17 Cases/62 Controls |
HAART Unexposedb 13 Cases/41 Controls |
HAART Exposed 9 Cases/37 Controls |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| ORa | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| sCD23 | 1.45 | (0.96–2.19) | 1.91 | (1.14–3.23) | 2.00 | (1.07–3.74) | 1.18 | (0.61–2.31) |
| sCD27 | 7.21 | (2.62–19.88) | 29.07 | (6.72–125) | 6.99 | (1.94–25.3) | 13.07 | (1.87–91.7) |
| sCD30 | 2.64 | (1.24–5.64) | 4.30 | (1.70–10.91) | 3.61 | (1.36–9.63) | 1.55 | (0.46–5.30) |
| CXCL13 | 2.56 | (1.32–4.96) | 5.52 | (2.29–13.32) | 2.42 | (1.05–5.58) | 2.15 | (0.67–6.91) |
| IL6 | 1.26 | (0.88–1.81) | 1.57 | (1.05–2.37) | 1.17 | (0.69–2.00) | 1.37 | (0.75–2.53) |
| CRP | 0.96 | (0.71–1.31) | 1.21 | (0.86–1.73) | 1.26 | (0.86–1.87) | 0.64 | (0.37–1.10) |
The ORs represent risk of AIDS-NHL associated with one unit increase in the biomarker on the natural log scale. ORs were adjusted for CD4+ T cell count, HIV load, and HAART exposure at each visit where the biomarker was measured, cigarette smoking, HCV infection, and education, in addition to the matching factors (time since first HIV positive study visit, age, CD4+ T cell count at first HIV positive study visit, and race/ethnicity)
HAART exposure at the time of the blood draw for the sample used in the immune marker assays
Table 5.
Association between immune markers and AIDS-NHL risk at three sampling time-windows
| 3–5 years 10 Cases/42 Controls |
1–3 years 14 Cases/54 Controls |
0–1 year 19 Cases/69 Controls |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| ORa | 95% CI | OR | 95% CI | OR | 95% CI | |
| sCD23 | 4.56 | (1.14–18.35) | 1.24 | (0.63–2.5) | 1.45 | (0.82–2.58) |
| sCD27 | 3.44 | (0.42–28.27) | 3.41 | (0.65–18.01) | 16.43 | (2.93–92.19) |
| sCD30 | 1.80 | (0.32–10.41) | 2.37 | (0.68–8.35) | 2.90 | (0.95–8.94) |
| CXCL13 | 1.25 | (0.38–4.18) | 4.40 | (1.25–15.61) | 2.70 | (0.91–8.08) |
| IL6 | 1.94 | (0.85–4.47) | 0.88 | (0.43–1.82) | 2.11 | (1.17–3.83) |
| CRP | 1.48 | (0.76–2.92) | 0.54 | (0.29–1.04) | 1.01 | (0.66–1.56) |
The ORs represent risk of AIDS-NHL associated with one unit increase in the biomarker on the natural log scale. ORs were adjusted for CD4+ T cell count, HIV load, and HAART exposure at each visit where the biomarker was measured, cigarette smoking, HCV infection, and education, in addition to the matching factors (time since first HIV positive study visit, age, CD4+ T cell count at first HIV positive study visit, and race/ethnicity)
Discussion
Elevated serum levels of sCD23, sCD27, sCD30, CXCL13, and IL6 were associated with an increased risk of AIDS-NHL overall or in specific subgroups in our study of racially- and ethnically-diverse HIV-infected women. Of particular interest were the strong and consistent associations observed for sCD27, sCD30, and CXCL13, which is consistent with previous studies. In the Multicenter AIDS Cohort Study (MACS), among the eight immune biomarkers examined, sCD27, sCD30, and CXCL13 exhibited the strongest (3 to 8 –fold) and most consistent (over three time-windows up to five years prior to an AIDS-NHL diagnosis) associations with increased risk of AIDS-NHL (7, 11). Additionally, among HIV uninfected study populations, sCD27, sCD30, and CXCL13 have been reported increased up to 13 years prior to B cell NHL; these associations were the most pronounced for the DLBCL subtype which is also the main subtype observed in the setting of HIV (12, 21). Replication of these biomarker associations in the HIV-infected women of the WIHS, in light of the major differences between this and previous study populations evaluated (in terms of gender, race, and other factors), adds support to the hypothesis that prolonged chronic B cell activation is important in the etiology of B cell NHL. Furthermore, we and others have observed that these immune marker associations are not limited to the time-window closest to NHL diagnosis date, and can be observed up to 13 years prior to NHL diagnosis, which suggests that these elevations in immune marker levels do not result from an already existing, though not yet clinically diagnosed, case of AIDS-NHL.
CD27 and CD30 are receptors for TNF-like immune stimulatory molecules. CD27 is expressed on the surface of B cells following activation, and is a marker for memory B cells (22). CD30 is expressed on activated T and B lymphocytes, and is considered a marker for cells producing Th2 cytokines that support B cell activation and differentiation into antibody-secreting plasma cells (23). Soluble forms of these receptors (sCD27 and sCD30), cleaved from the cell surface following cellular activation, are found in relatively low levels in the blood of healthy individuals, while high levels are associated with a number of viral infections and immune related disorders dominated by a Th2 immune response (24–27). CXCL13 is a chemokine produced by follicular dendritic and T helper cells, which plays a central role in homeostatic trafficking of antigen-naïve B cells into and within follicles of secondary lymphoid organs, an essential step in the development and structure of secondary lymphoid organs and the differentiation of B cells into antibody-producing plasma cells (28–30).
In previous studies, higher serum levels of sCD27 and CXCL13 were observed among HIV-infected men when compared to HIV-uninfected controls (27, 31), and elevated sCD30 levels predicted faster progression to AIDS in HIV-infected patients (32). Serum levels for sCD27, CXCL13, and sCD30 were significantly reduced in HIV-infected men following HAART exposure compared to their pre-HAART levels in a recent study, yet levels still remained higher when compared to HIV-uninfected controls (13). In our study, we also observed important associations between HIV disease progression and HAART exposure on biomarker levels among HIV-infected control women. After controlling for indicators of HIV disease progression and immune status (CD4+ T cell count, HIV RNA, and HAART exposure), sCD27, sCD30, and CXCL13 remained significantly elevated prior to AIDS-NHL.
Interestingly, similar to what was observed recently in the MACS, sCD23 was associated with an increased risk of AIDS-NHL at >3 years pre-diagnosis but not at visits closer to the time of diagnosis (11). Somewhat paradoxically, among HIV-positive controls without AIDS-NHL, slightly higher mean levels of sCD23 were seen in the most immunocompetent women (CD4+ T cells >400 cells/mm3) as well as in those with the highest HIV viral loads. CD23 is a receptor for IgE that is upregulated on activated B cells, and in its soluble form, has B cell stimulatory properties including enhancement of Ig class switch recombination. Prior observations that sCD23 levels are higher among HIV-uninfected versus HIV-infected individuals (33, 34), plus the observations noted above, allow for the possibility that there is a complex interaction between the competence of the immune system (number and/or function of CD4+ T helper cells), B cell activation due to high HIV viral load, and lymphomagenesis that cannot yet be teased apart. Furthermore, our observation that sCD23 was significantly elevated in the systemic subgroup but not overall (after inclusion of PCNSL cases, which are almost uniformly EBV positive) is consistent with this possibility, as well as with prior data demonstrating an association between sCD23 and EBV tumor negativity (35).
IL6 and CRP were not strongly associated with AIDS-NHL in our study. IL6 is a pluripotent cytokine produced by many different cell types whose major functions include driving acute and chronic inflammation, participation in B cell maturation and activation, and involvement in tumor initiation and growth. One previous study in the MACS found that elevated serum IL6 levels were associated with subsequent development of Burkitt lymphoma, but not DLBCL, which represents the majority cases in our study (33). However, a subsequent larger study in the MACS found that IL6 levels were associated with AIDS-NHL overall (11). CRP, which is produced by hepatocytes in response to IL6, participates in the clearance of necrotic and apoptotic cells and is considered a marker for the bioactivity of IL6. Our observation that the ORs for CRP were low in all subgroups is similar to what was observed in the MACS where ORs for CRP were significant, but low (11). The high inter-assay CVs for IL6 and CRP and the fact that these immune markers exhibited levels at the lower end of the detection curve in our samples may contribute to our inability to detect significant associations with AIDS-NHL risk, as may the relatively small number of AIDS-NHL cases in the current study.
In subgroup analyses, the ORs for all immune markers except CRP were significantly increased when the analysis was restricted to cases with systemic AIDS-NHL and their matched controls. Although we could not estimate the associations for the PCNL subgroup due to sparse data, our observations are consistent with the hypothesis that chronic B cell activation is a primary pathway for development of systemic AIDS-NHL. An alternative explanation may be that the contribution of B cell dysfunction to lymphomagenesis is obscured among people who have severely suppressed T cell immunity which is characteristic of PCNSL. Furthermore, the ORs in the HAART exposed subgroup appeared to be slightly attenuated for sCD23, sCD30, and CXCL13, and strengthened for sCD27, compared to the HAART unexposed. The variability in ORs may be due to the small sample size, or may also reflect an effect of HAART on immune marker levels (13). Additionally, there are a number of factors influencing HAART initiation and continuation, which may confound the association (36).
Among the study’s limitations, we did not have an adequate sample size to reliably assess immune marker associations in all desired subgroups. In addition, we could not address the role of EBV in our immune marker associations because we lacked data on EBV status of the tumors. The main strength of this study is the inclusion of HIV-infected women from a large prospective study, including detailed longitudinal data and stored serum specimens. This study population has several unique aspects compared to the populations investigated in previous AIDS-NHL biomarker studies. The WIHS participants were predominantly African-American or Latina, in comparison to the White, non-Hispanic male populations studied previously (8, 9, 11), and are representative of the HIV epidemic in women in the United States (15). Furthermore, the large percentage of women exposed to HAART allowed us to conduct stratified analyses by HAART exposure.
In the current era of HAART, HIV-infected individuals are living longer in the setting of chronic B cell activation, and will continue to be at increased risk of developing AIDS-NHL. We have added evidence to support previous reports that biomarkers of B cell activation are important predictors of AIDS-NHL in diverse populations including African and Latina women, and those who are receiving HAART.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (K07-CA-140360 to S.K. Hussain, an NCI supplement to U01-AI-035040 to O. Martínez-Maza, R01-CA-121195 to O. Martínez-Maza, R01-CA-168482 to O. Martínez-Maza). This work was carried out in the facilities of the UCLA AIDS Institute, which were supported, in part, by funds from the James B. Pendleton Charitable Trust and the McCarthy Family Foundation, and by NIH grant AI-028697: UCLA Center for AIDS Research (CFAR). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). We are sincerely thankful to the women who consented to be part of this study. We also thank Mr. Larry Magpantay for technical support in the performance of the immune assays, and Dr. Jeffrey Gornbein for statistical consultation.
Footnotes
Conflicts of Interest: None of the authors have a conflict of interest to report.
References
- 1.Grulich AE, Li YM, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination antiretroviral therapy. AIDS. 2001;15:629–33. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 2.Epeldegui M, Widney DP, Martinez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Curr Opin Oncol. 2006;18:444–8. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- 3.Vendrame E, Martínez-Maza O. Assessment of Pre-Diagnosis Biomarkers of Immune Activation and Inflammation: Insights on the Etiology of Lymphoma. Journal of Proteome Research. 2010;10:113–9. doi: 10.1021/pr100729z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks MA, Rabkin CS, Engels EA, Busch E, Kopp W, Rager H, et al. Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS. 2013;27(3):469–74. doi: 10.1097/QAD.0b013e32835c1333. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki I, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, et al. Constitutive expression of AID leads to tumorigenesis. Journal of Experimental Medicine. 2003;197:1173–81. doi: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komeno Y, Kitaura J, Watanabe-Okochi N, Kato N, Oki T, Nakahara F, et al. AID-induced T-lymphoma or B-leukemia/lymphoma in a mouse BMT model. Leukemia. 2010;24:1018–24. doi: 10.1038/leu.2010.40. [DOI] [PubMed] [Google Scholar]
- 7.Hussain SK, Zhu W, Chang S-C, Breen EC, Vendrame E, Magpantay L, et al. Serum Levels of the Chemokine CXCL13, Genetic Variation in CXCL13 and Its Receptor CXCR5, and HIV-Associated Non-Hodgkin B-Cell Lymphoma Risk. Cancer Epidemiology Biomarkers & Prevention. 2013;22:295–307. doi: 10.1158/1055-9965.EPI-12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabkin CS, Engels EA, Landgren O, Schuurman R, Camargo MC, Pfeiffer R, et al. Circulating cytokine levels, Epstein-Barr viremia, and risk of acquired immunodeficiency syndrome-related non-Hodgkin lymphoma. American Journal of Hematology. 2011;86:875–8. doi: 10.1002/ajh.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, et al. Circulating Serum Free Light Chains As Predictive Markers of AIDS-Related Lymphoma. J Clin Oncol. 2010;28:773–9. doi: 10.1200/JCO.2009.25.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martinez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS. 2007;21:2265–70. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- 11.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-Cell Stimulatory Cytokines and Markers of Immune Activation Are Elevated Several Years Prior to the Diagnosis of Systemic AIDS-Associated Non-Hodgkin B-Cell Lymphoma. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purdue MP, Lan Q, Martinez-Maza O, Oken MM, Hocking W, Huang WY, et al. A prospective study of serum soluble CD30 concentration and risk of non-Hodgkin lymphoma. Blood. 2009;114:2730–2. doi: 10.1182/blood-2009-04-217521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regidor DL, Detels R, Breen EC, Widney DP, Jacobson LP, Palella F, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25:303–14. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 15.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clinical and Diagnostic Laboratory Immunology. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DHHS/Henry J Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV infection. Nov 3, 2008. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. revision. [Google Scholar]
- 17.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim MR, Zeger SL. Generalized linear models with random effects; salamander mating revisited. Biometrics. 1992;48:631–44. [PubMed] [Google Scholar]
- 19.Kleinbaum DG, Klein M. Logistic Regression: A Self Learning Text. 2. New York: Springer; 2002. pp. 406–25. [Google Scholar]
- 20.Desai M, Kubo J, Esserman D, Terry MB. The Handling of Missing Data in Molecular Epidemiology Studies. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1571–9. doi: 10.1158/1055-9965.EPI-10-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Roos AJ, Mirick DK, Edlefsen KL, LaCroix AZ, Kopecky KJ, Madeleine MM, et al. Markers of B-Cell Activation in Relation to Risk of Non-Hodgkin Lymphoma. Cancer Research. 2012;72:4733–43. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agematsu K. Memory B cells and CD27. Histology and Histopathology. 2000;15:573–6. doi: 10.14670/HH-15.573. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson A. The role of CD30 in atopic disease. Allergy. 2001;56:593–603. doi: 10.1034/j.1398-9995.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 24.Caligariscappio F, Bertero MT, Converso M, Stacchini A, Vinante F, Romagnani S, et al. Circulating levels of soluble CD30, a marker of cells producing TH2-type cytokines, are increased in patients with systemic lupus-erythematosus and correlate with disease-activity. Clinical and Experimental Rheumatology. 1995;13:339–43. [PubMed] [Google Scholar]
- 25.Ihn H, Yazawa N, Kubo M, Yamane K, Sato S, Fujimoto M, et al. Circulating levels of soluble CD30 are increased in patients with localized scleroderma and correlated with serological and clinical features of the disease. Journal of Rheumatology. 2000;27:698–702. [PubMed] [Google Scholar]
- 26.Nolte MA, van Olffen RW, van Gisbergen K, van Lier RAW. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunological Reviews. 2009;229:216–31. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 27.Widney D, Gundapp G, Said JW, van der Meijden M, Bonavida B, Demidem A, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clinical Immunology. 1999;93:114–23. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 28.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. Journal of Experimental Medicine. 1998;187:655–60. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 30.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–9. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 31.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, et al. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. Journal of Interferon and Cytokine Research. 2005;25:702–6. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 32.Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–6. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Crabb Breen E, van der Meijden M, Cumberland W, Kishimoto T, Detels R, Marti, et al. The Development of AIDS-Associated Burkitt’s/Small Noncleaved Cell Lymphoma Is Preceded by Elevated Serum Levels of Interleukin 6. Clinical Immunology. 1999;92:293–9. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 34.Yawetz S, Cumberland W, van der Meyden M, Martinez-Maza O. Elevated serum levels of soluble CD23 (sCD23) precede the appearance ofacquired immunodeficiency syndrome--associated non-Hodgkin’s lymphoma. Blood. 1995;85:1843–9. [PubMed] [Google Scholar]
- 35.Schroeder JR, Saah AJ, Ambinder RF, Martinez-Maza O, Breen EC, Variakojis D, et al. Serum sCD23 level in patients with AIDS-related non-Hodgkin’s lymphoma is associated with absence of Epstein-Barr virus in tumor tissue. Clinical Immunology. 1999;93:239–44. doi: 10.1006/clim.1999.4793. [DOI] [PubMed] [Google Scholar]
- 36.Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P, et al. Medically Eligible Women Who Do Not Use HAART: The Importance of Abuse, Drug Use, and Race. American Journal of Public Health. 2004;94:1147–51. doi: 10.2105/ajph.94.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]