Abstract
Objective
To study agomelatine (S 20098), a potent agonist at melatonin receptors and antagonist at serotonin-2C (5-HT2C) receptors, in an animal model of depression, namely, the rodent forced swimming test (FST).
Methods
The effects of acute and repeated administration of S 20098 were compared with those of melatonin (4, 8, 16, 32, 64 mg/kg intraperitoneally [IP] for mice), imipramine (64 mg/kg orally for rats, 8 mg/kg IP for mice) and fluoxetine (16 mg/kg IP for mice). The influence of the pretreatments with 5-HT1A or 5-HT1B receptor agonists (8-OH-DPAT, anpirtoline) and 5-HT1A/1B, 5-HT2A/2C or 5-HT3 antagonists (pindolol, ritanserin, ondansetron) on the effects of S 20098 or melatonin were compared with imipramine and fluoxetine in mice.
Results
Acute or repeated (13 days) administration of S 20098 or imipramine in rats significantly decreased the duration of immobility during the FST at all doses. A dose-dependent effect was observed after the repeated treatment with S 20098. When given for 10 days to mice in the evening, S 20098 was active on the FST at doses of 4, 16 and 32 mg/kg, whereas the acute administration of S 20098 (in the morning and evening) was without any significant effect. Acute or repeated administration of S 20098 did not modify the locomotor activity of mice. The combination of S 20098 with the above-mentioned pretreatments enhanced the effects of S 20098, given alone, on the duration of immobility. By comparison, acute melatonin was inactive in the FST and only pretreatment with 8-OH-DPAT or pindolol revealed an anti-immobility effect. A pretreatment with 8-OH-DPAT also induced anti-immobility effects with imipramine, but not fluoxetine, whereas pindolol exerted additive effects with fluoxetine but not imipramine.
Conclusion
These results demonstrate the antidepressant-like activity of repeated administration of S 20098 in the FST. Moreover, the combination of 5-HT agonists and antagonists leads to more powerful effects with S 20098 than with melatonin, thereby emphasizing the contribution of 5-HT receptors to the antidepressant activity of S 20098. Compared with imipramine and fluoxetine, the 5-HT receptor subtypes that may be involved in the antidepressant-like activity of S 20098 are not similar. Indeed, when considering the binding properties of S 20098, the 5-HT2C receptor subtype appears to be the most attractive candidate. It is concluded that the antidepressant-like activity of S 20098 in this model most probably involves a combination of both its melatonin agonist and 5-HT2C receptor antagonist properties.
Medical subject headings: antidepressive agents; melatonin; mice; models, animal; serotonin; rats
Abstract
Objectif
Étudier l'activité d'agomélatine (S 20098), un puissant agoniste des récepteurs de la mélatonine et antagoniste des récepteurs de la sérotonine-2C (5-HT2C), dans un modèle animal de la dépression : le test de la nage forcée.
Méthodes
Les effets de l'administration aiguë et répétée d'agomélatine ont été comparés à ceux de l'administration de mélatonine (4, 8, 16, 32, 64 mg/kg par voie intrapéritonéale [i.p.] chez la souris), d'imipramine (64 mg/kg par voie orale [p.o.] chez le rat, 8 mg/kg i.p. chez la souris) et de fluoxétine (16 mg/kg i.p. chez la souris). L'influence de prétraitements aux agonistes des récepteurs 5-HT1A ou 5-HT1B (8-OH-DPAT, anpirtoline) et des antagonistes des récepteurs 5-HT1A/1B, 5-HT2A/2C ou 5-HT3 (pindolol, ritansérine, ondansétron) sur les effets de l'agomélatine ou de la mélatonine ont été comparés chez la souris avec les effets de l'imipramine et de la fluoxétine.
Résultats
L'administration aiguë ou répétée (13 jours) d'agomélatine ou d'imipramine chez le rat diminue significativement le temps d'immobilité au cours du test de la nage forcée à toutes les doses étudiées. Une relation dose/effet a été observée après administration répétée d'agomélatine. Chez la souris, l'agomélatine agit sur le test de la nage forcée aux doses de 4, 16, et 32 mg/kg après une administration répétée de 10 jours le soir, alors qu'une administration aiguë d'agomélatine (le matin et le soir) n'a pas eu d'effet significatif. Par ailleurs, l'administration aiguë ou répétée d'agomélatine ne modifie pas l'activité motrice chez la souris. L'association des traitements précités avec l'agomélatine potentialise les effets de celle-ci sur le temps d'immobilité, par rapport à l'administration d'agomélatine seule. À titre de comparaison, l'administration aiguë de mélatonine n'a pas eu d'effet sur le test de la nage forcée, et seul le prétraitement au 8-OH-DPAT ou au pindolol a produit un effet anti-immobilité. Le prétraitement au 8-OH-DPAT a aussi produit un effet anti- immobilité en association avec l'imipramine, mais pas avec la fluoxétine, tandis que le pindolol a potentialisé l'activité de la fluoxétine mais non celle de l'imipramine.
Conclusion
Ces résultats démontrent l'activité de nature antidépressive de l'administration répétée de S 20098 dans le test de la nage forcée. De plus, l'association d'agonistes et d'antagonistes des récepteurs 5-HT produit des effets plus puissants avec l'agomélatine qu'avec la mélatonine, soulignant ainsi la contribution de récepteurs sérotoninergiques à l'activité antidépressive de l'agomélatine. En comparaison avec l'imipramine et la fluoxétine, les sous-types du récepteur 5-HT susceptibles d'intervenir dans l'activité de nature antidépressive de l'agomélatine ne sont pas les mêmes. En fait, vu les propriétés de fixation de l'agomélatine, le candidat le plus intéressant semblerait être le récepteur de sous-type 5-HT2C. En conclusion, il est suggéré que l'activité de nature antidépressive de S 20098 dans ce modèle met fort probablement en cause une combinaison de ses propriétés agonistes mélatoninergiques et antagonistes des récepteurs 5-HT2C.
Introduction
Agomelatine (S 20098, N-[2-(7-methoxy-1-naphthyl)ethyl] acetamide) is a potent agonist at melatoninergic receptors.1,2,3 S 20098 shows a high affinity for cloned human melatonin receptor MT1 and MT2 subtypes (Ki = 6.15 х 10–11 mol/L and 2.68 х 10–10 mol/L, respectively). The affinity of S 20098 at melatonin receptors is comparable to that of melatonin (Ki = 8.52 х 10–11 mol/L and 2.63 х 10–10 mol/L, respectively) and, in line with this, S 20098 displaces iodomelatonin from its binding sites in the suprachiasmatic nucleus of the hypothalamus, that is, the brain region involved in the mechanism of the endogenous biological clock.4 S 20098 functions as an agonist in that it mimics the effects of melatonin on several systems.2,5,6,7,8,9,10 S 20098 also shows a high affinity (IC50 = 2.7 х 10–7 mol/L) for human cloned serotonin-2C (5-HT2C) receptors, and acts as an antagonist at this receptor subtype.11,12 Only very low affinities of S 20098 for a number of other types of receptors have been obtained.13
Preclinical studies have suggested that S 20098 may induce antidepressant-like effects in several animal models such as the learned helplessness test,14 chronic mild stress15 and in a transgenic mouse model of depression.16
In the present study, the antidepressant-like activity associated with the acute and repeated administration of a wide range of doses of S 20098 was assessed in another animal model of depression, namely, the forced swimming test (FST).17,18 In this model, rodents forced to swim in a situation from which they cannot escape rapidly become immobile, floating in an upright position and making only small movements to keep their heads above water. The FST is a validated test of antidepressant activity, because the immobility of animals can be reversed by many different classes of antidepressant.18,19 Moreover, antidepressant-like effects of melatonin have been reported in the FST.20,21,22 As S 20098 possesses chronobiotic properties, which can be shown in the chronic mild stress model of depression,15 the present experiments were carried out to see if there is a difference in the activity of S 20098 between morning and evening testing.
It has been recently demonstrated that pretreatment with the 5-HT1A receptor agonist 8-OH-DPAT or the 5-HT2A/2C receptor antagonist ritanserin enhances the anti-immobility effects of a normally subactive dose of several tricyclic antidepressants.23 On the other hand, pretreatment with a 5-HT1A autoreceptor antagonist (pindolol), 5-HT1B receptor agonist (anpirtoline) or 5-HT3 receptor antagonist (ondansetron) enhances the antidepressant-like activity of selective serotonin reuptake inhibitors (SSRIs)24 in the mouse FST. More recently, it has been demonstrated that the anti- immobility effects of subactive doses of the serotonin–noradrenaline reuptake inhibitor venlafaxine are enhanced by drugs acting at all these receptor subtypes.25 These results suggest that these compounds can be used to separate different classes of antidepressant drugs and that these receptor systems are implicated in the mechanism of action of the drugs tested. This experimental approach has been used here to investigate the receptors involved in the possible antidepressant-like activity of S 20098 in the FST, namely, the 5-HT1A, 5-HT1B, 5-HT2 and 5-HT3 receptor subtypes. The effects of S 20098 were compared with those of melatonin, imipramine as the most representative of tricyclic antidepressants and fluoxetine as representative of SSRIs.
Methods
Following a 5-day acclimatization period, male Swiss mice (n = 10/group, weight range 22–24 g, 4 weeks old; Elevage Janvier, Le Genest, France) or Wistar rats (n = 6/group, weight range 224–278 g; Elevage Janvier) were used. Animals were maintained in cages under light/dark 12/12 illumination (light on at 7 am and 8 am for mice and rats, respectively) at stable ambient temperature (mean 20°C, standard deviation 2°C) and relative humidity 50%, with free access to food and tap water until tested. All the experiments were carried out in accordance with international guidelines for animal experimentation.
S 20098 was administered to rats orally (PO) and mice intraperitoneally (IP) 1 hour and 30 minutes, respectively, before the FST. The effects of S 20098 were compared with those of melatonin, imipramine and fluoxetine according to the doses listed in Table 1.
Table 1
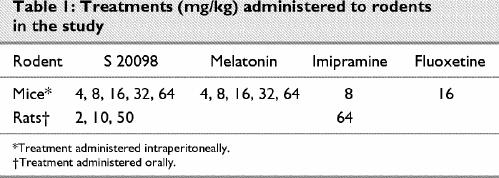
In mice, acute administrations of S 20098, melatonin, imipramine and fluoxetine were performed either in the morning (between 8 am and 1 pm) or in the evening (at 5 pm). The repeated treatment (10 days) with S 20098 was administered in the evening (at 5 pm).
In rats, acute and repeated (13 days) treatments were administered during the artificial lighting period.
S 20098 or melatonin (4, 16, 64 mg/kg) were injected as part of the acute treatment into the mice in the evening, either alone or in combination with 8-OH-DPAT (1 mg/kg), anpirtoline (1 mg/kg), pindolol (32 mg/kg), ritanserin (4 mg/kg) or ondansetron (0.01 μg/kg). The influence of these pretreatments on the effects of S 20098 or melatonin were compared with the tricyclic antidepressant imipramine (8 mg/kg IP) and the SSRI fluoxetine (16 mg/kg IP) alone or in combination with 8-OH-DPAT or pindolol. All drugs were dissolved in distilled water with the exception of S 20098 and melatonin, which were homogeneously suspended in a 1% solution of hydroxyethylcellulose, and pindolol and ritanserin, which were dissolved in a 1% aqueous solution of Tween 80. Drugs were administered in a constant volume of 0.5 mL/20 g body weight, and doses used refer to the salt form of the drug.
S 20098 or the vehicle was injected into mice 30 minutes before the measurement of the spontaneous locomotor activity.26 The animals were placed in activity monitors with photoelectric cells, and locomotor activity was recorded over a 10-minute testing period.
S 20098 was injected 1 hour before testing. The FST employed was essentially similar to that described elsewhere.17,18 Briefly, rodents were dropped individually into glass cylinders (mice: height 25 cm, diameter 10 cm; rats: height 35 cm, diameter 24 cm) containing 10–13.5 cm of water, maintained at 23°C–25°C, and left there for 5 minutes (rats) or 6 minutes (mice). In such a situation, from which they cannot escape, animals rapidly became immobile, that is, floating in an upright position and making only small movements to keep their heads above water. The duration of immobility was recorded for 5 minutes in rats and during the last 4 minutes of the 6-minute testing period in mice.
All results for locomotor activity and the FST were expressed as mean (and standard error of the mean). For the interaction studies with agonists and antagonists in the FST, the mean immobility time of the combined treatment group is also expressed as the percentage of the score obtained with the appropriate drug alone.
For each group, normality of distribution was first examined using the nonparametric Kolmogorov– Smirnov test. Data were then subjected to an analysis of variance (ANOVA) according to the homogeneity of variances. Only 1 ANOVA was performed for all the data obtained with the rat FST (acute and repeated administration). A nonparametric test was performed when the homogeneity of variances did not permit a direct ANOVA. Additional parametric post hoc tests were performed for comparison between independent groups, that is, drug per se and distilled water control, using the Dunnett t test, and for comparison between interaction groups using the a posteriori Sidak test.27 Results were considered significant at p < 0.05.
Results
Acute administration of S 20098 significantly decreased the duration of immobility in rats at all doses tested. The effect was not dose-dependent (Fig. 1). Imipramine (64 mg/kg PO) administered under the same conditions, clearly decreased the duration of immobility (F8,45 = 28.26, p ≤ 0.001).
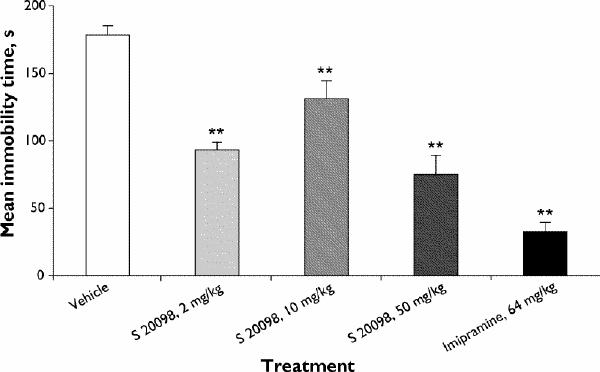
Fig. 1: Effects of acute administration of S 20098 or imipramine on the duration of immobility in the forced swimming test (FST) in the rat. Results are expressed as mean and standard error of the mean (SEM) (n = 6/group). Dunnett's t test, **p < 0.01.
On the other hand, in mice, acute administration of S 20098 did not induce any significant changes in the FST or locomotor activity at any dose or time (F5,54 = 9.65, p ≤ 0.06 and F5,54 = 1.12, p ≤ 0.36 for FST and locomotor activity, respectively, for the morning and F5,54 = 2.33, p = 0.06 and F5,54 = 1.48, p = 0.21 for FST and locomotor activity, respectively, for the evening).
In mice, acute administration of melatonin (4, 8, 16, 32, 64 mg/kg) was inactive in the FST for all the doses tested (F5,54 = 0.0, p ≤ 0.005).
Acute melatonin in the morning induced a slight change in locomotor activity from 4 mg/kg, and a sedative effect was constant and significant for the doses 8, 16, 32 mg/kg. A strong decrease in spontaneous locomotor activity was noted at the highest tested dose (64 mg/kg; F5,54 = 12.84, p ≤ 0.001).
The repeated administration of S 20098 moderately decreased the duration of immobility in rats in a dose-dependent manner. Imipramine (64 mg/kg) under the same experimental conditions markedly decreased the duration of immobility (Fig. 2; F8,45 = 28.26, p ≤ 0.001).
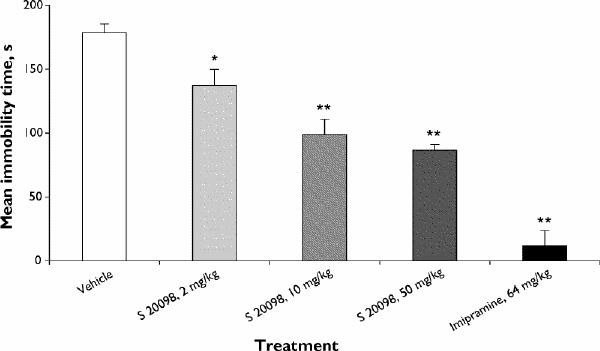
Fig. 2: Effects of repeated administration of S 20098 or imipramine on duration of rat immobility in the FST. Results are expressed as mean and SEM (n = 6/group). Dunnett's t test, *p < 0.05, **p < 0.01.
When given for 10 days to mice, S 20098 (administered in the evening) was active in the FST at 4, 16 and 32 mg/kg and induced a weak but statistically significant decrease in duration of immobility (Fig. 3; F5,54 = 5.55, p ≤ 0.005) but did not induce any statistically significant changes in locomotor activity (F5,54 = 1.92, p ≤ 0.11; data not shown).
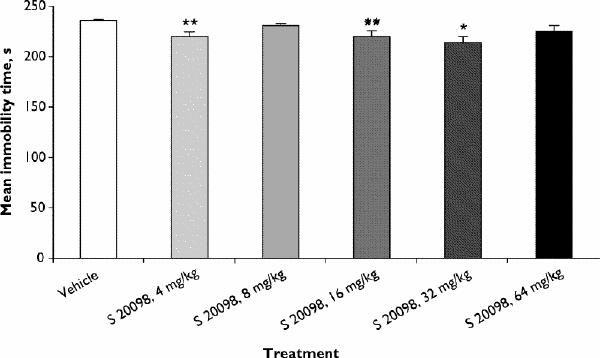
Fig. 3: Effects of repeated administration of S 20098 on duration of immobility in the FST in mice. Results are expressed as mean and SEM (n = 10/group). Dunnett's t test, *p < 0.05, **p < 0.01.
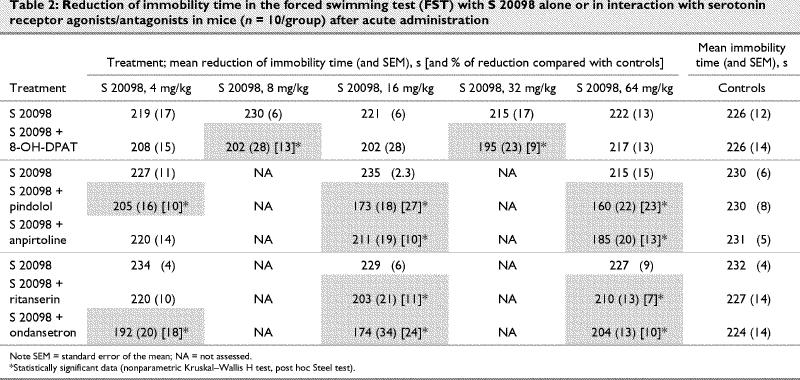
Under the same experimental conditions (acute administration in the morning), the combination of S 20098 with 5-HT agonist or antagonist pretreatments induced an anti-immobility effect in the mice FST (Table 2). Thus, pretreatment with 8-OH-DPAT (1 mg/kg) enhanced the anti-immobility effects of S 20098 (8 and 32 mg/kg, p < 0.05) versus the S 20098 group without pretreatment (Kruskal–Wallis test H11,108 = 29.45, p ≤ 0.001). The pindolol (32 mg/kg) and ondansetron (0.01 μg/kg) pretreatments also enhanced the anti-immobility activity of S 20098 at doses of 4, 16 and 64 mg/kg (p < 0.01 and p < 0.01, respectively; H7,72 = 57.28, p ≤ 0.001 for pindolol, H7,72 = 50.32, p ≤ 0.001 for ondansetron). Pretreatment with anpirtoline (1 mg/kg) and ritanserin (4 mg/kg) enhanced the effects of 16 and 64 mg/kg S 20098 in the FST (p < 0.05; H7,72 = 37.08, p ≤ 0.001 for anpirtoline, and p < 0.05; H7,72 = 36.53, p ≤ 0.001 for ritanserin).
Table 2
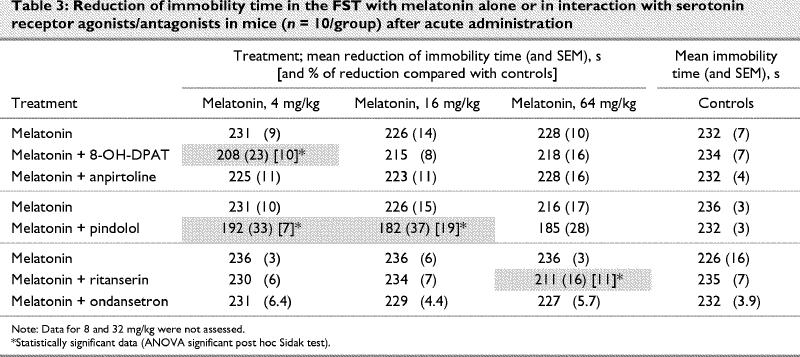
Moreover, whereas acute administration of melatonin to mice was inactive in the FST, pretreatments with 8-OH-DPAT or pindolol revealed an anti- immobility effect (Table 3). Pretreatment with 8-OH-DPAT enhanced significantly (p < 0.05) the antidepressant-like activity of melatonin for only 4 mg/kg (F7,72 = 5.76, p ≤ 0.001). Pretreatment with pindolol induced antidepressant-like activity when administered with melatonin at 2 doses (4 and 16 mg/kg, p < 0.01; F7,72 = 10.60, p ≤ 0.001). Pretreatment with ritanserin induced a decrease in duration of immobility only at the dose of 64 mg/kg (F7,72 = 5.52, p ≤ 0.001). Pretreatment with anpirtoline and ondansetron did not have any effect (F7,72 = 1.39, p ≤ 0.22 and F7,72 = 4.24, p ≤ 0.001).
Table 3
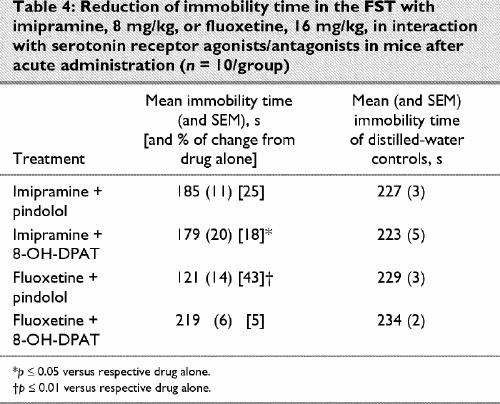
Finally, pretreatment with 8-OH-DPAT, but not with pindolol, enhanced the antidepressant-like activity of imipramine at the normally subactive dose of 8 mg/kg (p ≤ 0.05). On the other hand, pindolol, but not 8-OH-DPAT, enhanced the effect of fluoxetine in the FST, at a normally subactive dose of 16 mg/kg (p ≤ 0.01; Table 4).
Table 4
Discussion
The present study shows that, when administered repeatedly, S 20098 exerts an antidepressant-like activity in the FST. Moreover, this antidepressant effect is observed both in rats and mice, but is less marked in the latter species.
Acute administration of S 20098 in rats is active and repeated administration significantly decreases the duration of immobility in a dose-dependent manner when administered during the artificial lighting period. In mice, repeated treatment in the evening is active, and the combination of S 20098 with the 5-HT1A or 5-HT1B receptor agonists (8-OH-DPAT, anpirtoline) and the 5-HT1A/1B, 5-HT2A/2C or 5-HT3 antagonists (pindolol, ritanserin, ondansetron) enhances the morning effects of S 20098 given alone. On the other hand, acute melatonin alone is inactive in the FST, and only pretreatments with 8-OH-DPAT or pindolol facilitate the anti-immobility effect of the neurohormone. The 5-HT receptor subtypes that may support the activity of S 20098 in the FST differ from those of prototypical antidepressants, because only 8-OH-DPAT and pindolol can enhance the effects of imipramine and fluoxetine, respectively.
In mice, repeated, but not acute, administration of S 20098 induces antidepressant-like effects in the FST. This effect is related neither to a change in locomotor activity as demonstrated here, nor to a sedative effect because no sedation is observed in mice with S 20098 up to the dose of 128 mg/kg (R.P., unpublished observations, 1989). These results are similar to those of Raghavendra et al,21 who found that melatonin (2.5–10 mg/kg) administered acutely to BALB/c and C57BL/6J mice failed to induce any antidepressant effects, whereas its daily administration reversed the increase in immobility period in the FST. Melatonin or certain melatonin agonists also reduce the immobility of rats in the FST after repeated, but not acute, administration.20,22,28 Melatonin also shows antidepressant activity in the mouse chronic mild stress model,29 although with a less marked effect than fluoxetine.
Interestingly, the activity of S 20098 in the FST does not depend on the time of the administration (morning or evening), which has been already shown in the rat chronic mild stress test.15 This strongly suggests that properties other than its chronobiotic ones5,8,9,10,30,31 may sustain the activity of S 20098 in the FST. Raghavendra et al21 have also reported that circadian variations (noon, early dark, midnight) do not influence the effect of melatonin treatment on the duration of the immobility period in mice, which suggests that the FST may be not sufficiently sensitive to appropriately study compounds with chronobiotic properties in this species.
In mice, the comparison of the efficacy of S 20098 and melatonin was tested under the same conditions, but S 20098 seemed to be more efficacious in the FST than the endogenous ligand. This also suggests that the nonmelatoninergic (or nonchronobiotic) properties of S 20098 are involved in its antidepressant activity. In line with this, manipulation of several serotonergic receptor subtypes in mice can influence the antidepressant-like effects of S 20098 and lead to more powerful effects on the duration of immobility with S 20098 than with melatonin. This indicates that the activity of S 20098 is partly dependent on its interaction with the serotonergic system. S 20098 possesses antagonistic activity at 5-HT2C receptors11,12 that could explain, at least partly, its efficacy in the FST. In line with this, the pretreatment with the 5-HT2A/2C antagonist ritanserin significantly enhanced the effect of S 20098 at doses of 16 and 64 mg/kg. Although no study with specific antagonists at the 5-HT2C receptor subtype has been performed in the FST to our knowledge, clinically effective antidepressants that exhibit 5-HT2C antagonist properties are active in the FST.18,19
The 5-HT1A agonist, 8-OH-DPAT, and ritanserin also favour an antidepressant-like activity of melatonin, suggesting that the potential antidepressant-like property of melatonin can only be revealed in the FST following manipulation of the serotonergic system.
It has been shown that the 5-HT1A receptors that mediate increased mobility in the FST are located postsynaptically.32 The additive effects of 8-OH-DPAT with S 20098 and imipramine suggest that postsynaptic 5-HT1A receptors play a role in the ability of these drugs to reduce immobility in the FST. The potentiating effects of pindolol with S 20098 also suggest that antagonism of presynaptic 5-HT1A receptors plays a role in the ability of this compound to reduce immobility in the FST, unlike imipramine, whose action seems to be mediated by postsynaptic 5-HT1A receptors. However, the involvement of 5-HT1A receptors in the mechanism of action of S 20098 is questionable, because it has been demonstrated that this compound, as is the case for melatonin, does not modify the 5-HT1A autoradiographic signal, the guanosine-5́-O-(3-thiotriphosphate) (GTP-γ-S) labelling or the electrophysiologic properties of dorsal raphe and CA1 neurons perfused with 5-HT1A agonists (ipsapirone and 5-carboxamido-tryptamine) or antagonist (WAY 100635).33
The present study also suggests that 5-HT1B and 5-HT3 receptor subtypes may play a role in the activity of S 20098 in the mouse FST and, indeed, there has been some interest, for example, in the antidepressant-like effects of 5-HT3 receptor antagonists.34 However, given the absence of affinity of S 20098 for the 5-HT1B and 5-HT3 receptors, the involvement of these subtypes in its antidepressant effect appears rather unlikely.
Finally, the results of the present study suggest a role for 5-HT2A/C and 5-HT3 receptors in the action of imipramine and fluoxetine, respectively. Whereas the antagonism of 5-HT2A/C receptors is important in the action of tricyclic antidepressants in the FST, the antagonism of 5-HT3 receptors may play a partial role in the anti-immobility effects of the SSRIs.23
In conclusion, repeated treatment with S 20098 exerts an antidepressant-like activity in the FST both in rats and mice, with a mechanism of action that differs from that of traditional antidepressants.
The antidepressant-like effect of S 20098 depends, at least partially, on its melatonin agonist properties. Furthermore, manipulation of the serotonergic system can influence its effects in the mouse FST and, by comparison, the weaker impact of melatonin in combination with 5-HT agonists and antagonists indicates that the activity of S 20098 is also based on its interaction with the 5-HT system. Finally, while exhibiting similar antidepressant-like effect in the FST, S 20098, imipramine and fluoxetine have clearly distinct profiles regarding the 5-HT receptor subtypes that may support this activity.
We suggest that the efficacy of S 20098 in the FST involves a combination of both its melatonin agonist and 5-HT2c receptor antagonist properties.
Footnotes
Competing interests: Dr. Bourin has received sponsorship from Laboratoires Servier. Dr. Mocaër has been employed by the Institut de Recherches Internationales Servier (IRIS). Dr. Porsolt has had a paid consultancy with IRIS.
Correspondence to: Dr. Michel Bourin, EA 3256, Neurobiologie de l'anxiété et de la dépression, Faculté de Médecine, 1, rue Gaston Veil, BP 53508, F 44035 Nantes Cedex 1, France; fax 33 02 40 41 28 56; mbourin@sante.univ-nantes.fr
Submitted Nov. 12, 2002; Revised Apr. 17, 2003; June 27, 2003; July 21, 2003; Accepted Aug. 11, 2003
References
- 1.Yous S, Andrieux J, Howell HE, Morgan PJ, Renard P, Pfeiffer B, et al. Novel naphthalenic ligands with high affinity for the melatonin receptor. J Med Chem 1992;35:1484-585. [DOI] [PubMed]
- 2.Ying SW, Rusak B, Delagrange P, Mocaër E, Renard P, Guardiola-LemaÎtre B. Melatonin analogues as agonists and antagonists in the circadian system and other brain areas. Eur J Pharmacol 1996;296:33-4 [DOI] [PubMed]
- 3.Conway S, Canning SJ, Edward Howell HH, Mowat ES, Barrett P, Drew JE, et al. Characterisation of human mt1 MT2 receptors by CRE-luciferase reporter assay. Eur J Pharmacol 2000; 390: 15-24. [DOI] [PubMed]
- 4.Bonnefond C, Martinet L, Lesieur D, Adam G, Guardiola-LemaÎtre B. Characterization of S 20098, a new melatonin analogue. In: Touitou Y, Arendt J, Pevet P, editors. Melatonin and the pineal gland. New York: Elsevier; 1993. p. 123-6.
- 5.Armstrong SM, McNulty OM, Guardiola-LemaÎtre B, Redman JR. Successful use of S20098 and melatonin in an animal model of delayed sleep-phase syndrome (DSPS). Pharmacol Biochem Behav 1993;46:45-9. [DOI] [PubMed]
- 6.Redman JR, Guardiola-LemaÎtre B. The melatonin agonist, S 20098: effects on the rat circadian system. In: Touitou Y, Arendt J, Pévet P, editors. Melatonin and the pineal gland. From basic to science to clinical application. Proceedings of the International Symposium on Melatonin and the Pineal Gland: From Basic Science to Clinical Application; 1992 Sept 6-9; Paris. New York: Excerpta Medica; 1993. p. 127-30.
- 7.Tobler I, Jaggi K, Borbély A. Effects of melatonin and the melatonin agonist S 20098 on the vigilance states, EEG spectra, and cortical temperature in the rat. J Pineal Res 1994;16:26-32. [DOI] [PubMed]
- 8.Redman JR, Guardiola-LemaÎtre B, Brown M, Delagrange P, Armstrong SM. Dose-dependent effects of S 20098, a melatonin agonist, on the direction of re-entrainment of rat circadian rythms. Psychopharmacology 1995;118:385-90. [DOI] [PubMed]
- 9.Martinet L, Guardiola-LemaÎtre B, Mocaër E. Entrainment of circadian rythms by S 20098, a melatonin agonist, is dose and plasma concentration dependent. Pharmacol Biochem Behav 1996; 54:713-8. [DOI] [PubMed]
- 10.Van Reeth O, Olivares Y, Zhang FW, Turek FW, Defrance R, Mocaer E. Comparative effects of a melatonin agonist on the circadian system in mice and syrian hamsters. Brain Res 1997; 762: 185-94. [DOI] [PubMed]
- 11.Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman- Tancredi A, Pasteau V, et al. The novel melatonin agonist, agomelatine (S20098), is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharm Exp Ther 2003;306(3):954-64. [DOI] [PubMed]
- 12.Protais P, Chagraoui A, Mocaër E. S 20098 (agomelatine) antagonizes the penile erections induced by the stimulation of 5-HT2C receptors in Wistar rats. Int J Neuropsychopharmacol 2002; 5 (Suppl 1):S65. [DOI] [PubMed]
- 13.Chagraoui A, Protais P, Filloux T, Mocaër E. Agomelatine (S 20098) antagonizes the penile erections induced by the stimulation of 5-HT2C receptors in Wistar rats. Psychopharmacology (Berl) 2003;170:17-22. [DOI] [PubMed]
- 14.Bertaina-Anglade V, Mocaër E, Drieu la Rochelle C. Antidepressant-like action of S 20098 (agomelatine) in the learned helplessness test. Int J Neuropsychopharmacol 2002;5(Suppl.1): S65.
- 15.Papp M, Gruca P, Boyer PA, Mocaër E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology 2003;28:694-703. [DOI] [PubMed]
- 16.Barden N, Labbé M, Vacher R, Mocaër E. Antidepressant action of S 20098 (agomelatine) in a transgenic mouse model. Int J Neuropsychopharmacol 2002;5(Suppl. 1):S64.
- 17.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn 1977;229:327-36. [PubMed]
- 18.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379-91. [DOI] [PubMed]
- 19.Danysz W, Plaznik A, Kostowski W, Malatynska E, Jarbe TU, Hiltunen AJ, et al. Comparison of desipramine, amitriptyline, zimeldine and alaproclate in six animal models used to investigate antidepressant drugs. Pharmacol Toxicol 1988;62:42-50. [DOI] [PubMed]
- 20.Overstreet DH, Pucilowski O, Retton MC, Delagrange P, Guardiola-Lemaitre B. Effect of melatonin receptor ligands on swim test immobility. Neuroreport 1998;9:249-53. [DOI] [PubMed]
- 21.Raghavendra V, Kaur G, Kulkarni SK. Antidepressant action of melatonin in chronic forced swimming-induced behavioral despair in mice, role of peripheral benzodiazepine receptor modulation. Eur Neuropsychopharmacol 2000;10:473-81. [DOI] [PubMed]
- 22.Shaji AV, Kulkarni SK. Central nervous system depressant activities of melatonin in rats and mice. Ind J Exp Biol 1998; 36: 257-63. [PubMed]
- 23.Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol 1997;325:129-35. [DOI] [PubMed]
- 24.Redrobe JP, MacSweeney CP, Bourin M. The role of 5-HT1A and 5-HT1B receptors in antidepressant drug actions in the mouse forced swimming test. Eur J Pharmacol 1996;318:213-20. [DOI] [PubMed]
- 25.Redrobe JP, Bourin M, Colombel MC, Baker GB. Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology 1998;138:1-8. [DOI] [PubMed]
- 26.Boissier JR, Simon P. Action de la caféine sur la motilité spontanée de la souris. Arch Int Pharmacodyn Ther 1965;158:212-21. [PubMed]
- 27.Armitage P, Berry G. Comparison between groups using the a posteriori Sidak test. In: Armitage P, Berry G, editors. Statistical methods in medical research. 2nd ed. Oxford: Blackwell Publishing; 1987. p. 203-5.
- 28.Brotto LA, Barr AM, Gorzalka BB. Sex difference in forced-swim and open-field test behaviour after chronic administration of melatonin. Eur J Pharmacol 2000;402:87-93. [DOI] [PubMed]
- 29.Kopp C, Vogel E, Rettori MC, Delagrange P, Misslin R. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behav Pharmacol 1999; 10: 73-83. [DOI] [PubMed]
- 30.Van Reeth O, Weibel L, Olivares E, Maccari S, Mocaër E, Turek FW. Melatonin or a melatonin agonist correct age- related changes in circadian response to an environmental stimulus. Am J Physiol 2001;280:1582-91. [DOI] [PubMed]
- 31.Weibel L, Turek FW, Mocaër E, Van Reeth O. A melatonin agonist facilitates circadian clock resynchronisation in old hamsters after abrupt shifts in the light-dark cycle. Brain Res 2000; 880: 207-11. [DOI] [PubMed]
- 32.Luscombe GP, Martin KF, Hutchins LJ, Gosden J, Heal DJ. Mediation of the antidepressant-like effect of 8-OH-DPAT in mice by post-synaptic 5-HT1A receptors. Br J Pharmacol 1993; 108: 669-77. [DOI] [PMC free article] [PubMed]
- 33.Lanfumey L, Hanoun N, Boyer PA, Mocaër E. The novel antidepressant agomelatine (S 20098) acts independently of 5-HT1A receptors in the rat. Eur Neuropsychopharmacology 2003; 13(Suppl):S237.
- 34.Greenshaw AJ. Behavioural pharmacology of 5-HT3 receptor antagonists: a critical update on therapeutic potential. Trends Pharmacol Sci 1993;14:265-70. [DOI] [PubMed]


