Abstract
Modulation of the immune system to amplify anti-tumor immunity carries the risk of developing autoimmune diseases, including hypothyroidism, as seen with cancer patients undergoing clinical trials for immunotherapeutic regimens. Although there is a tendency to view autoimmunity as a positive indicator for cancer immunotherapy, some autoimmune manifestations can be life-threatening and necessitate prolonged medical intervention or removal from trial. We have established murine test models to assess such risks by monitoring, simultaneously, the immune reactivity to tumor-associated rat erbB-2 (neu) and another self Ag, mouse thyroglobulin (mTg). We previously reported that in wild-type, thyroiditis-resistant BALB/c mice that underwent regression of neu+ TUBO tumors following regulatory T cell (Treg) depletion, immune responses to rat neu and mTg with resultant autoimmune thyroiditis (EAT) were both enhanced. In this study, we tested the balance between tumor immunity and autoimmunity in neu-transgenic BALB NeuT female mice. First, growth and progression of neu+ tumor were compared in neu tolerant mice treated with either CD25 mAb to deplete Tregs and/or DNA vaccination. Only Treg depletion followed by neu DNA vaccination abrogated tolerance to neu, resulting in complete regression of neu+ tumors, as well as long-term protection from spontaneous tumorigenesis in 58% of mice. The risk of developing EAT was then assessed by incorporated mTg immunization with or without LPS as adjuvant. In mice with induced tumor regression, mTg response was enhanced with modest increases in EAT development. Therefore, tumor regression induced by Treg depletion and DNA vaccination can exacerbate autoimmunity, which warrants close monitoring during immunotherapy.
Modulation of the immune system to amplify anti-tumor immunity may be accompanied by the induction of autoimmunity. Autoimmune symptoms or Abs to self Ags, such as thyroid Ags, occurred in 26% of melanoma patients undergoing IFN-α-2b treatment, and this autoimmunity was associated with statistically significant improvements in both relapse-free survival and overall survival (1). In another trial testing, a Her-2 peptide vaccine combined with systemic Flt-3 ligand, 2 of 15 subjects developed autoimmune hypothyroidism with increased levels of thyroid-stimulating hormone and autoantibodies to thyroid Ags (2), further indicating the induction of autoimmune symptoms by systemic immune activation.
Consistent with the observation in cancer patients undergoing immunotherapy, the most prevalent autoimmune manifestations in humans is autoimmune thyroid disease, both hypothyroidism or Hashimoto’s thyroiditis, and hyperthyroidism or Graves’ disease. In the U.S. Caucasian population, 45% of women and 20% of men show focal thyroiditis at routine autopsy, indicating autoimmune hypothyroidism, and 1% of women and 0.05% of men exhibit clinical symptoms (3, 4). Focal thyroiditis is strongly correlated with circulating Abs to thyroglobulin and thyroid peroxidase (5). The disease is characterized by mononuclear cell infiltration and destruction of thyroid follicles, an elevation of thyroid-stimulating hormone, and a decrease in thyroid hormone (T3 and T4) levels. Both autoantibody and T cell responses to thyroglobulin and thyroid peroxidase are clinically sensitive indicators of autoreactivity (6, 7).
Although there is a tendency to view autoimmunity induced by immune modulation as a positive indicator of responsiveness to cancer immunotherapy, autoimmune diseases can be life-threatening. In patients with metastatic melanoma who received gp100 peptide vaccines along with an antagonist mAb to CTLA-4 (8–10), grade III/IV autoimmune manifestations were observed, including hypothyroidism, dermatitis, enterocolitis, hepatitis, and hypophysitis. The three patients in this study with objective cancer regression all developed severe autoimmune symptoms requiring intervention. Patients with autoimmune hypophysitis manifested hypocortisolism and low testosterone, requiring prolonged replacement therapy with steroids, thyroid hormone, and testosterone. Hence, the balance between cancer immunotherapy and autoimmunity becomes a critical issue.
To study the parameters that can influence this critical balance, we have established several test models to measure, simultaneously, the immune reactivity to tumor-associated erbB-2, as human Her-2 or the rat homolog neu, and a self-Ag, mouse thyroglobulin (mTg).3 First, we showed in wild-type, thyroiditis-resistant BALB/c mice that, when regulatory T cells (Tregs) were removed and neu+ TUBO tumors underwent regression, both immune responses to rat neu and mTg were increased (11). Next, we introduced HLA-DRB1*0301 (DR3), a susceptibility allele associated with thyroiditis (12) into Her-2 transgenic mice to simulate human immune reactivity to Her-2 and thyroglobulin. We reported that induction of Her-2 immunity by DNA vaccination was independent of HLA-DR3, which encodes susceptibility to mTg-induced experimental autoimmune thyroiditis (EAT), whereas Tregs control the responses to both self Ags (13). In this study, we further tested the balance between tumor immunity and autoimmunity in neu-transgenic BALB NeuT female mice, which develop spontaneous mammary tumors (14, 15). We describe the regression of established tumors and long-term protection from spontaneous tumorigenesis by Treg depletion and neu DNA vaccination, and the influence of induced tumor regression in enhancing mTg immunity and EAT development.
Materials and Methods
Mice and cell lines
BALB/c female mice at 6–8 wks of age were purchased from Charles River Laboratories. Heterozygous BALB NeuT mice, which expressed a transforming rat neu under the control of the mouse mammary tumor virus promoter, were maintained by breeding with BALB/c mice (14, 15). Transgene positive mice were identified by PCR. NeuT females developed up to 10 spontaneous mammary tumors starting at the age of 17–19 wks. All animal procedures were performed in accordance with the regulation of Wayne State University, Division of Laboratory Animal Resources, following the protocols approved by the Animal Investigation Committee.
The TUBO mammary tumor cell line was cloned from a spontaneous tumor in a NeuT female mouse and the cells expressed rat neu (16). The cells were maintained in DMEM supplemented with 5% cosmic calf serum (HyClone), 5% FCS (Sigma-Aldrich), 10% NCTC 109 medium (Invitrogen), 2 mM l-glutamine, 0.1 mM MEM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. All tissue culture reagents were purchased from Invitrogen unless otherwise specified. When injected s.c., TUBO cells grew progressively and gave rise to tumors that were histologically similar to those seen in NeuT mice (17).
APCs 3T3/NKB and 3T3/EKB were generated as previously described (18). In brief, BALB/c NIH 3T3 cells were transfected with Kd, B7.1 (CD80), and neu (NKB). Stable clones were selected, expression of their transgenes were verified by flow cyotometry, and the cells were maintained in supplemented DMEM (as above) with the addition of 0.8 mg/ml neomycin and 0.8 mg/ml zeocin.
Depletion of T cell subsets
Rat hybridoma line PC61 producing rat-anti-mouse CD25 mAb (ATCC) was propagated in SCID mice. To deplete CD25high T cells, mice were injected i.p. with 0.5 mg of CD25 mAb at the indicated times. Depletion of CD4+CD25high T cells was verified by flow cytometry (supplemental Fig. 1).4 The kinetics of Treg depletion and recovery after CD25 mAb treatment was previously reported (11, 29).
DNA immunization and tumor challenge
pcDNA 3.1 was purchased from Invitrogen. Construction of the pcDNA-neuTM plasmid encoding the extracellular and transmembrane domains of rat neu was previously described (19). The plasmid pEFBos-GM-CSF, encoding murine GM-CSF, was provided by Dr. N. Nishisaki at Osaka University, Osaka, Japan. Mice were injected in the quadriceps muscle with the combination of plasmid DNA as previously described (18, 20). DNA injection was followed immediately by square wave electroporation at the injection site using a BTX830 (BTX Harvard Apparatus). A tweezer electrode was used to deliver eight pulses at 100 V for 20 ms.
To measure tumor growth, mice were challenged s.c. with 2 × 105 TUBO cells in the flank. Tumor growth was monitored by weekly palpation, and mice were sacrificed when any one dimension reached 15 mm. Tumor volume was calculated by: (X2 × Y)/2. X and Y represent the short and long dimension, respectively, of the tumor. Tumor volume was determined at each week and Student’s t test used to determine difference between tumor-bearing groups. Statistical difference between tumor-free mice was determined by the log-rank test.
Measurement of anti-neu Ab by flow cytometry
For measurement of anti-neu Ab, 3T3/NKB cells were incubated with serially diluted immune mouse sera as we previously reported (21). PE-conjugated goat-anti-mouse Ab directed to the γ-chain of mouse IgG (Jackson Immunoresearch Laboratories) were used to detect bound Ab. The c-erbB2/c-neu mAb (Ab4, clone 7.16.4; Calbiochem), which recognized an extracellular domain of rat neu protein, was used as the positive control (EMD). A standard curve was established using serially diluted Ab4 and the concentration of anti-neu Ab in the test sera was calculated by regression analysis as we described (21). Normal mouse serum or isotype matched mAb was the negative control. Flow cytometric analysis was performed with a FACSCalibur (BD Biosciences). Differences in Ab concentration were analyzed by the Student’s t test.
Measurement of T cell response by ELISPOT assay
Neu-reactive T cells were measured by ELISPOT assay (18). Spleen cells were suspended in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. A total of 4 × 105 cells were added to each well of a 96-well HTS IP plates (Millipore) which were precoated with 2.5 μg/ml rat anti-mouse IFN-γ (IgG1, clone R4–6A2) and incubated for 48 h at 37°C in 5% CO2. To measure T cell response, the engineered APCs, 3T3/NKB were also added to the wells. The control 3T3/KB cells expressed Kd and B7.1. The ratio of spleen cells to APC was 10:1. Following a 48 h incubation, cells were removed and 2 μg/ml biotinylated rat anti-mouse-IFN-γ (IgG1, clone XMG 1.2) was added. All Abs were purchased from BD Pharmingen. Plates were incubated for another 12 h at 4°C, then washed to remove unbound Ab. Bound Ab was detected by incubating the plates with 0.9 μg/ml avidin-HRP (BD Pharmingen) for 2 h at room temperature. The substrate 3-amino-9-ethylcarbazole (BD Pharmingen) in 0.1 molar acetic acid and 0.003% hydrogen peroxide was added and the plate was incubated for 3 min. 3-amino-9-ethylcarbazole solution was discarded and the plates were washed six times with water. The visualized cytokine spots were enumerated with the ImmunoSpot analyzer (CTL) and the results expressed as number of cytokine producing cells per 106 cells. Data were analyzed using the Student’s t test.
Immunization with mTg
mTg was prepared from frozen thyroids by fractionation with a Sephadex G-200 column as we previously described (22, 23) and diluted in nonpyrogenic saline before use. The presence of LPS was measured by Limulus amebocyte assay (Associates of Cape Cod) (24). A 40 μg dose of mTg contained <0.5 ng of LPS. Mice were injected i.v. with 40 μg of mTg, followed in 3 h with 20 μg of Salmonella enteritidis LPS. The injections were repeated in 7 days. Alternatively, 40 μg of mTg was injected i.v. on 4 successive days followed by 3 days of rest (24). This treatment was repeated for 4 wk.
Measurement of anti-mTg Ab
Anti-mTg Ab titers were measured by ELISA as we described previously (25). In brief, Immulon I microtiter plates (Dynatech Laboratories) were coated with mTg at 1 μg/well and serially diluted test sera were added. After washing, bound Ab was detected with alkaline phosphatase-labeled goat-anti-mouse IgG and enzyme substrate. Sera pooled from EAT-susceptible CBA/J mice immunized with mTg and LPS were used as the control at a designated concentration of 10,000 U/ml. A standard curve of mTg binding was generated with serially diluted control sera and relative concentrations of test sera were determined based on this standard curve.
Measurement of mTg-reactive IFN-γ secreting T cells by ELISPOT assay
mTg-reactive T cells were measured with a two-step ELISPOT assay, as reported previously (11). Spleen cells were incubated with 80 μg/ml mTg in 96-well tissue culture plates for 3 days before the content of the wells was transferred to HTS IP plates and further incubated for 24 h. The detection and enumeration of cytokine spots were performed as described above.
Histological evaluation of EAT
Thyroid specimens were sectioned vertically through both lobes and 50–60 histological sections were prepared from 10 to 15 step levels. The extent of mononuclear cell infiltration was scored based on the pathology index scale of 0–4 and presented as percentage of thyroid infiltration: 0, no infiltration; 0.5, >0–10% thyroid infiltration consisting of perivascular foci without follicular destruction; 1.0, >10–20% thyroid infiltration with follicular destruction; 2.0, >20–40% diffuse thyroid infiltration; 3.0, >40–80% thyroid destruction; and 4.0, >80–100% thyroid destruction (23). The sections were scored without knowledge of the groups. Statistical differences were analyzed by the nonparametric Mann-Whitney U test.
Results
Tumor regression in BALB NeuT mice induced by both Treg depletion and pneuTM DNA electrovaccination
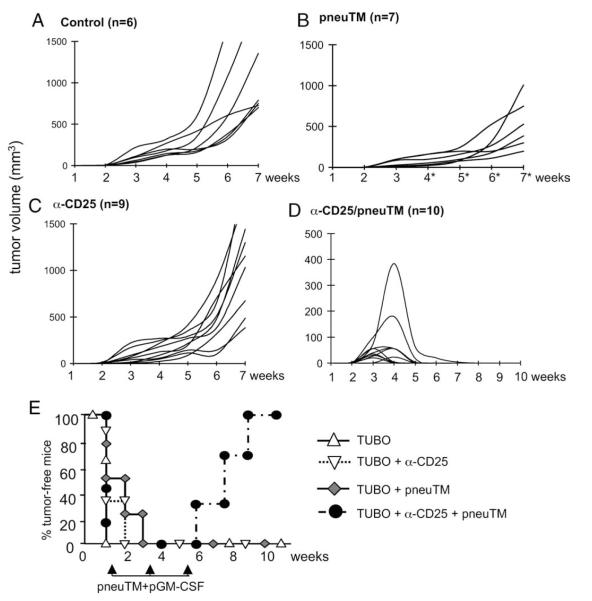
To test whether TUBO tumors growing in neu tolerant mice would regress after Treg depletion as we had previously observed in normal BALB/c mice (11), neu tolerant NeuT mice were injected with 0.5 mg CD25 mAb on days 1 and 3 after tumor inoculation (Fig. 1C). There was no change in tumor growth after Treg depletion, showing that immune stimulus in Treg-depleted NeuT mice was inadequate to initiate tumor rejection.
FIGURE 1.
Tumor regression in BALB NeuT mice induced by Treg depletion and pneuTM vaccination. NeuT females were inoculated s.c. with 2 × 105 TUBO cells. Mice were (A) left untreated, (B) treated with 0.5 mg anti-CD25 mAb on days 1 and 3 to deplete regulatory T cells, (C) electrovaccinated three times at 2 wk intervals with pneuTM and pGM-CSF, or (D) treated with anti-CD25 mAb and vaccinated with pneuTM and pGM-CSF. Tumor growth was monitored weekly and tumor volume was shown as mm3. E, A summary of percentage of tumor-free mice shown in A–D.
To test whether established tumors in neu tolerant mice can be eliminated by neu DNA vaccination, NeuT mice were inoculated s.c. with 2 × 105 TUBO cells. Ten days later, mice received i.m. electrovaccinations with pneuTM, encoding the extracellular and transmembrane domains of neu, together with pGM-CSF. The vaccination was repeated twice, 2 wk apart. The tumors in all test mice continued to grow, but at a significantly reduced rate when compared with untreated mice (Fig. 1B vs 1A, wk 3 through 7). Therefore, pneuTM vaccination delayed, but did not eliminate tumor growth.
To further test whether Treg depletion combined with neu vaccination would induce sufficient anti-tumor immunity, NeuT mice inoculated with TUBO cells were treated with CD25 mAb as described, followed by three time pneuTM electrovaccination, starting 10 days after tumor inoculation. Every mouse developed palpable tumors and all tumors regressed, even a tumor which was close to 400 mm3 in size (Fig. 1D). By wk 9, all 10 treated mice were completely free of tumor. Fig. 1E summarizes the percentages of tumor-free mice in each group over the 11 wk observation period. Therefore, established tumors in NeuT mice were rejected only by combined treatment with CD25 mAb and pneuTM vaccination.
Induction of neu-reactive Ab and T cells during tumor regression
To determine whether neu-reactive Ab and T cells were induced in the treated groups, sera were collected at wk 10 from mice that rejected tumor or at wk 8 from mice receiving single agent or control vector, due to their tumor burden. Significant levels of neu-binding Ab, ranging from 5 to 40 μg/ml, were observed only in mice that underwent complete tumor regression by the combined treatment of Treg depletion and neu vaccination (Fig. 2A). In contrast, mice immunized with pneuTM without Treg depletion developed low level Ab response at <5 μg/ml, and their tumors continued to grow, albeit at reduced rate (Fig. 1B). Ab to neu was not detectable in either CD25 mAb treated tumor-bearing mice or mice bearing progressing TUBO tumors without treatment (Fig. 2A).
FIGURE 2.
neu-reactive Ab and T cells induced during tumor regression. A, neu-binding Ab levels were measured at wk 10, at wk 8 for single agent treatment, or when mice were sacrificed due to tumor load, by flow cytometry. B, T cell response was determined by IFN-γ ELISPOT assay using splenocytes. Immunospots were enumerated with CTL plate reader and shown as number of IFN-γ-secreting cells per million splenocytes.
To analyze T cell response, splenocytes were prepared 8 wk after TUBO inoculation in mice with progressing tumors and at wk 10 from tumor-free mice. The cells were incubated for 2 days with the engineered APCs 3T3/NKB, expressing neu, Kd, and B7.1, as we previously described (11). IFN-γ-producing T cells were detected by ELISPOT in mice receiving both CD25 mAb and pneuTM vaccination (Fig. 2B). In contrast, T cell response was not detected in mice receiving either CD25 mAb or DNA vaccination alone. Therefore, tumor regression in vaccinated, Treg-depleted mice was associated with neu-specific humoral and cellular immunity.
Long-term protection against spontaneous tumorigenesis after tumor rejection
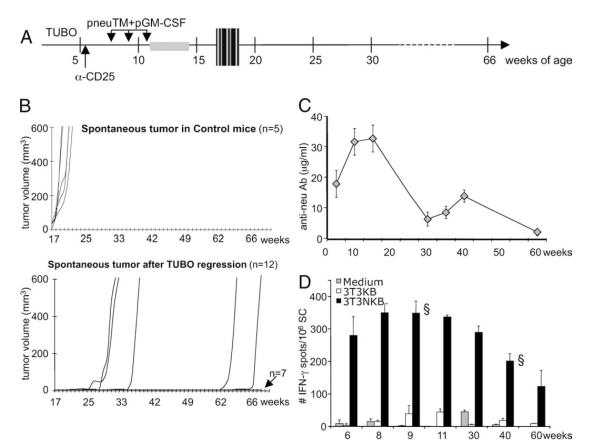
To determine whether long-term protection was established in NeuT mice after they rejected neu+ TUBO tumors, spontaneous tumorigenesis was monitored in 12 NeuT females which rejected implanted TUBO tumors until the mice were 66 wks old (Fig. 3A). Untreated NeuT females developed spontaneous mammary tumors between 17 and 19 wks of age with up to ten tumors arising from five pairs of mammary glands (15) (Fig. 3B, top). Of the 12 test mice, 7 mice were still free of spontaneous tumors when sacrificed at wk 66 (Fig. 3B, bottom). Mammary tumor development in the five remaining mice was delayed until they were 25, 27, 33, 62, and 66 wk of age. Thus, long-term protection against spontaneous tumorigenesis was established in 58% of the immunized mice which had rejected the TUBO tumor.
FIGURE 3.
Protection against spontaneous tumorigenesis after TUBO tumor regression. A, Experimental scheme. NeuT females at 5 wks of age were inoculated with TUBO cells s.c. followed by two i.p. injections with anti-CD25 mAb and then three times of i.m. electrovaccination with pneuTM and pGM-CSF (see Fig. 1 legend). TUBO tumors regressed after transient growth and mice were monitored weekly until they are 66 wks of age or when spontaneous tumors developed. The gray box indicates the window of TUBO tumor regression. The hatched box indicates the window of spontaneous tumor appearance. B, Growth of spontaneous tumors in all 10 mammary glands was monitored weekly, and total volume of the spontaneous tumors is expressed. There were 19 mice in the test group (bottom) and five control NeuT females were not implanted with TUBO, nor vaccinated (top). Seven test mice were randomly sacrificed for ELISPOT analysis and were not included in this graph. C, neu-binding Ab levels from test group. D, At indicated interval, one randomly selected mouse from the test group was sacrificed and anti-neu T cell response in the spleen was measured by IFN-γ ELISPOT assay, following in vitro stimulation with 3T3/NKB. §, Mice that were tumor-bearing at the time of analysis.
To determine whether neu immunity was maintained through the lifespan of the protected mice, serum Ab or IFN-γ-producing T cells were measured at wk 6, 8, 9, 10, 11, 15, 30, 35, 40, and 60 in randomly selected mice (Fig. 3, C and D). Both Ab and T cell responses were sustained for over a year. At age 60 wk, anti-neu Ab was still detectable at 2.0 ± 0.2 μg/ml and IFN-γ-secreting cells were at 123 ± 49/106 splenocytes. Therefore, anti-neu immunity induced by immune rejection of TUBO tumor was maintained for over a year in neu transgenic mice and was associated with the delay or complete elimination of spontaneous mammary tumorigenesis.
Concurrent induction of tumor regression and autoimmune thyroiditis
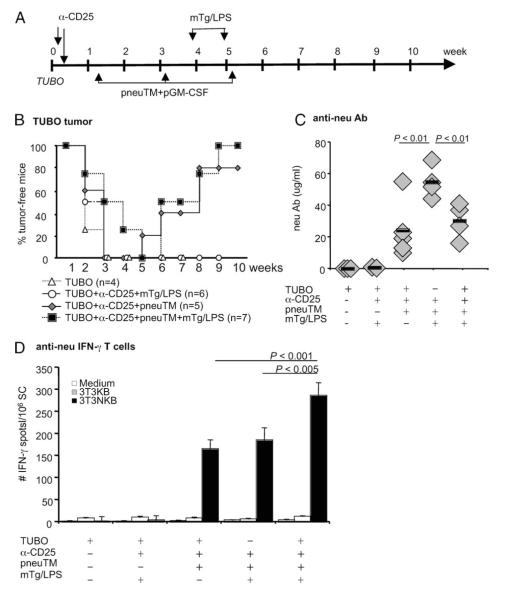
We previously showed an amplification of autoimmune response to mTg immunization in the presence of Her-2 immune response in Her-2-tolerant and DR3 double transgenic C57BL/6 mice after Treg depletion (13). This amplification had significant implications for cancer immunotherapy and was further tested in NeuT mice, which were likewise neu-tolerant. NeuT females inoculated with TUBO cells were treated with anti-CD25 mAb and vaccinated three times, at 2 wk intervals, with pneuTM and pGM-CSF as described (Fig. 4A). At 4 and 5 wk after tumor inoculation, mice received 40 μg mTg i.v, followed by 20 μg LPS, i.v., corresponding to the time when tumors in neu-vaccinated mice were starting to regress (Fig. 4B). All tumors eventually regressed. In mice similarly vaccinated with neu DNA, but without mTg immunization, tumors also regressed, as shown in Fig. 4B and Figs. 1E and 6B. The rate of tumor regression was not statistically different between the groups that received mTg/LPS or not (Fig. 4B). Thus, tumor regression in NeuT mice was achieved only after Treg depletion and neu vaccination, regardless of mTg/LPS immunization.
FIGURE 4.
Tumor regression and anti-neu immunity during concurrent immunization with mTg and LPS. A, Experimental scheme. NeuT female mice were inoculated with 2 × 105 TUBO tumor cells on day 0, followed by anti-CD25 mAb injection and pneuTM vaccination (see Fig. 1 legend). mTg and LPS were injected i.v. at wk 4 and 5. B, Tumor growth was monitored by weekly palpation and percentage of tumor-free mice are depicted. C, Immune sera were collected at wk 8 and neu-binding Ab was measured by flow cytometry. D, Spleen cells were harvested at wk 8 and neu-reactive T cells from individual mice were measured by ELISPOT assay.
FIGURE 6.
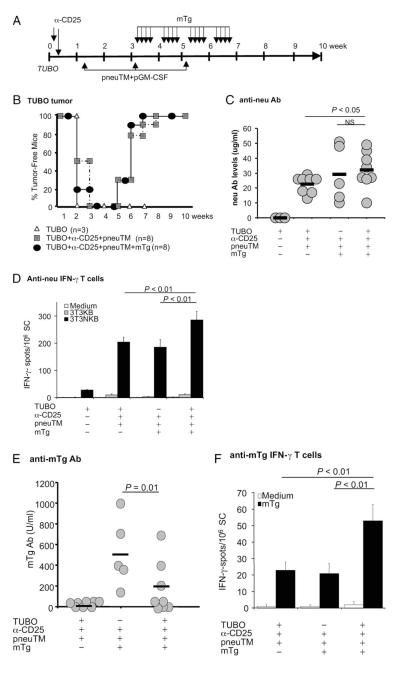
Effect of increased circulatory mTg levels during tumor regression on immune responses to neu and mTg. A, Experimental scheme. NeuT female mice were inoculated with TUBO cells and treated as described in Fig. 4A, with the modification that mTg was injected i.v. daily, without LPS, for 4 wk beginning wk 3 after tumor inoculation. B, Tumor growth was monitored by weekly palpation. C, Ab to neu was measured at wk 8. D, T cell response to neu was measured by IFN-γ ELISPOT assay. E, mTg Ab response as measured by ELISA. Sera were collected 3 wk following the final mTg immunization. F, mTg-specific IFN-γ-secreting T cells as measured by ELISPOT assay.
Anti-neu Ab and T cell responses were measured at wk 10. In neuTM-vaccinated mice which were not tumor-bearing, neu Ab level was 55 ± 5 μg/ml, while in vaccinated mice whose tumors underwent complete regression, neu Ab levels were comparable, at 30 ± 6 and 24 ± 8 μg/ml with or without mTg/LPS immunization (Fig. 4C). The lower neu Ab levels in mice with regressed tumors may reflect usage of Abs in aiding tumor clearance. Comparing T cell responses, neu-specific IFN-γ-secreting T cells were comparable; 165 ± 21 vs 185 ± 29 μg/ml in Treg-depleted, pneuTM-vaccinated mice which either rejected TUBO tumors or were non-tumor-bearing. But in mice with regressing tumors also given mTg and LPS for EAT induction, neu-specific T cells increased to 286 ± 28 ( p < 0.001) (Fig. 4D). Therefore, when EAT was induced during tumor regression, anti-neu T cell response was enhanced.
mTg immunity and autoimmune thyroiditis during concurrent tumor regression
The impact of tumor growth or regression on mTg reactivity and EAT development was also measured (Scheme, Fig. 4A). Sera collected at wk 10 showed ~1,500 U/ml mTg Ab in mice that were depleted of Tregs and immunized with mTg and LPS, whether they were covaccinated with pneuTM and undergoing tumor regression or not (Fig. 5A). Therefore, like neu Ab response, humoral immunity to mTg was not enhanced by concurrent tumor rejection and mTg immunization. Treg-depleted, mTg/LPS-immunized mice produced similar levels of mTg Ab as mice covaccinated with pneuTM and undergoing tumor regression (Fig. 5A).
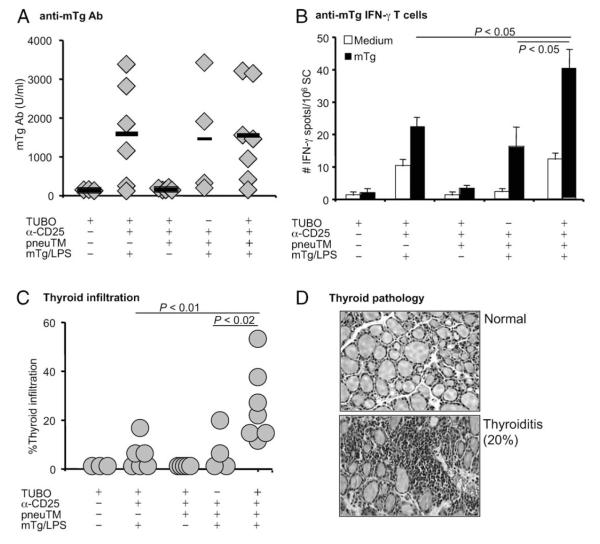
FIGURE 5.
mTg immunity and autoimmune thyroiditis during concurrent tumor regression. A, mTg Ab levels in the immune sera were measured by ELISA and Ab concentration calculated by regression analysis using pooled immune sera as a standard. Ab level is shown as relative U/ml. B, Splenic IFN-γ-secreting cells were enumerated after culture with mTg for 4 days. C, Thyroids were collected at wk 10 and mononuclear cell infiltration was analyzed by H&E staining. D, Representative thyroid sections showing normal thyroid vs a thyroid with 20% infiltration and destruction.
To compare T cell responses, spleen cells were incubated with mTg in a two-step ELISPOT assay and the secretion of IFN-γ measured. Treg-depleted, mTg/LPS immunized mice with progressing tumors generated 22 ± 3/106 IFN-γ-secreting cells (Fig. 5B) in their spleens. Mice that had received additional pneuTM/pGM-CSF-vaccination with consequent tumor regression generated 40 ± 6/106 IFN-γ-secreting cells to mTg, whereas similarly treated, but nontumor bearing mice generated only 18 ± 6 IFN-γ-secreting cells. Thus, mTg-specific, IFN-γ-secreting cell numbers were elevated in neu-vaccinated mice with regressing tumors. The immune responses to neu and mTg did not cross-react. Anti-mTg Ab did not recognize neu (Fig. 4C) and anti-neu Ab did not recognize mTg (Fig. 5A). The same is true for T cell response as shown in Figs. 4D and 5B. Therefore, there was no cross-reactivity between neu and mTg immunity.
Thyroid pathology was determined by mononuclear cell infiltration and is shown as the extent of individual thyroid involvement. In mice bearing progressing tumors or no tumors at all, thyroid infiltration ranged from 0–20%, while neu-vaccinated mice with regressing tumors developed 15–50% infiltration and more extensive destruction of thyroid follicles (Fig. 5C). Thus, elevated levels of thyroid destruction (Fig. 5D), as reflected by enhanced T cell response to mTg and thyroid infiltration, was observed in Treg-depleted, neu-vaccinated mice only during tumor regression.
Concurrent induction of tumor regression and anti-mTg response following prolonged elevation of circulating mTg levels
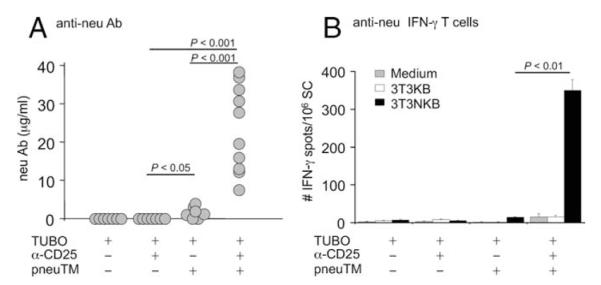
We reported earlier that TUBO rejection after Treg depletion in wild-type, thyroiditis-resistant BALB/c mice amplified mTg autoreactivity following repeated mTg doses, even without LPS as an adjuvant for EAT induction (11). To determine whether such an increase in the level of circulating mTg during tumor regression in NeuT mice would enhance the immune response to either neu or mTg, mTg was injected i.v. 16 times over a 4-wk period, beginning on day 22 after TUBO inoculation (Fig. 6A) when tumors would start to regress. Each week, four daily injections of 40 μg mTg were given, followed by 3 days of rest. Tumor growth was monitored at weekly intervals (Fig. 6B). As expected, TUBO tumors grew, then regressed in mice which had been vaccinated with neu following depletion of Tregs, as shown in Figs. 1 and 4, regardless of mTg injections. The increase in circulating mTg level had no impact on tumor growth or regression.
When measured at wk 9, mice with progressing tumors did not produce neu-specific Ab, while Treg-depleted and neu-vaccinated mice undergoing tumor regression averaged 23 ± 2 μg/ml neu Ab (Fig. 6C). Increasing the circulatory mTg levels increased neu Ab levels to 32 ± 6 μg/ml ( p < 0.05). However, this average level was not significantly different from mice without tumor challenge.
For neu-specific, IFN-γ-secreting T cells, untreated tumor-bearing mice averaged 28 ± 2/106 splenocytes. Treg-depleted, and neu-vaccinated mice showed ~200/106 IFN-γ-secreting cells, whether mice also received tumor challenge or mTg immunization. However, in the presence of elevated mTg level, anti-neu IFN-γ response further increased to 286 ± 31 in vaccinated mice undergoing tumor regression ( p < 0.01; Fig. 6D). Therefore, although there was minimal enhancement of neu Ab levels, significant enhancement of T cell response to neu was induced when concurrent autoreactivity to mTg was ongoing during tumor regression. Conversely, concurrent tumor regression might affect the response to circulating mTg. Anti-mTg Ab levels did not increase in mice with tumor regression; the levels were reduced in half the mice compared with those without tumor challenge (190 ± 82 vs 516 ± 150 U/ml) (Fig. 6E). In contrast, the levels of anti-mTg IFN-γ-secreting cell numbers of individual mice were significantly higher in mice undergoing concurrent tumor regression, whereas those without tumor challenge were at background levels as were mice without mTg injections (Fig. 6F). Thus, the response to elevated levels of circulating mTg during tumor vaccination and regression resulted in significant mutual amplification of neu-specific and mTgspecific T cell responses. The weaker mTg immunization regimen, initiated 3 wks after Treg depletion and in the absence of adjuvant LPS, did not result in thyroid pathology in any mTg-treated NeuT mice, including those which underwent tumor regression. Therefore, clinical exacerbation of autoimmune thyroiditis was spared when mTg was administered by chronic injection to EAT-resistant NeuT mice.
Discussion
We previously demonstrated that wild-type BALB/c mice rejected neu+ TUBO tumor when their Tregs were depleted with CD25 mAb (11). When mTg immunization was also conducted in this EAT-resistant strain during TUBO tumor regression, the immune response to neu and mTg was mutually amplified with enhanced thyroid pathology and follicular destruction in the thyroids of some mice, particularly when mTg was administered with LPS as an adjuvant. Because neu is a foreign Ag in normal BALB/c mice, rejection of TUBO tumor likely involved a more vigorous inflammatory response, promoting more severe autoimmunity than in mice with naturally arising tumors. Because the risk of developing autoimmune symptoms during cancer immunotherapy is a significant concern, we further examined the impact of tumor regression on the development of autoimmune thyroiditis in BALB NeuT mice which express neu as a self Ag. NeuT female mice develop spontaneous mammary tumors when they reach 17–19 wks of age, thus more closely mimic humans with Her-2 positive cancers.
In BALB NeuT mice carrying neu+ TUBO tumor s.c., self tolerance to neu was overcome by three-time electrovaccination with pneuTM following Treg depletion with CD25 mAb, resulting in tumor regression, whereas vaccination or Treg depletion alone did not induce significant immune response, or tumor regression. In 10 mice treated with CD25 mAb and DNA electrovaccination, TUBO tumors regressed completely and significant anti-neu Ab and IFN-γ-producing T cells were elicited, indicating that tumor rejection in neu-tolerant mice was mediated by substantial immunity. Moreover, anti-neu humoral and cellular immunity was sustained until the mice were at least 66 wks of age. In 12 mice whose implanted tumors regressed, seven were free of spontaneous tumors at 66 wks, while the remaining five showed a delay ranging from 25 to 66 wk, compared with 17 wk in control mice, a demonstration of long-term protection by anti-neu immunity.
The neu transgene is driven by the MMTV promoter in target organs such as the mammary and salivary glands, causing continuous transformation of the epithelial cells. Once immune priming had been initiated in mice by DNA vaccination followed by TUBO tumor regression, neu-specific T and B cells were subjected to continuous stimulation by neu Ag either expressed by or released from the transformed cells. Long-term protection from tumor recurrence in NeuT mice is strong evidence that sufficient immunity to the product of the powerful oncogene neu can be achieved by vaccination-induced tumor rejection.
Previously, we reported that electrovacination of NeuT mice with pneuTM induced a strong Ab response, which correlated with a delay in spontaneous tumorigenesis, although none of the treated mice were cured of the disease (18). Spadaro et al. (26) demonstrated that continuous depletion of Tregs by weekly injections of CD25 mAb also delayed the onset of spontaneous tumors from 20 to 32 wks of age; T cell response but little Ab was induced with this regimen. In the current study, we showed that the combination of neu DNA electrovaccination with Treg depletion was more effective than either treatment alone.
The effectiveness of DNA electrovaccination coupled with Treg depletion in inducing rejection of TUBO tumors, as well as preventing recurrence, in BALB NeuT mice that normally develop spontaneous tumors in 100% of mice provided a unique opportunity to evaluate the risk of autoimmunity during cancer immunotherapy. We selected EAT as a prototype autoimmune disease model because Hashimoto’s thyroiditis is one of the most prevalent autoimmune diseases (4) and because its MHC class II-based susceptibility in both mice and humans is well established (12, 23). Moreover, Tregs have been shown to mediate tolerance to EAT induction (27), and although Tregs can influence susceptibility, they do not supersede the strong influence of MHC class II (28). Indeed, when concurrent tumor immunity was induced in the presence of EAT in our previous reports, the severity of EAT is determined primarily by MHC haplotypes and whether Tregs were depleted without compromising anti-tumor immunity (13, 28). When mTg and LPS were administered 5 days after Treg depletion in otherwise EAT-resistant, wild-type BALB/c mice, moderate thyroid inflammation was detected in all the mice (11). When mTg was administered chronically without LPS adjuvant in Treg-depleted BALB/c mice, three of seven mice developed mild thyroiditis (11).
In the current study, the aim was to assess the impact of tumor regression on EAT development, not merely the impact of Treg depletion. Therefore, mTg with or without LPS was administered during tumor regression, ~4 wk after CD25 mAb treatment when Tregs were being replenished (11). There was a modest mutual amplification in neu and mTg immunity whether mTg was administered with LPS or not, although this response did not significantly alter tumor regression (Figs. 1B, 4B, and 6B). The increase in both neu and mTg reactivity was observed primarily in the T cell compartment. The severity of EAT in mTg/LPS-treated NeuT mice was comparable to that observed in conventional BALB/c mice undergoing TUBO rejection following Treg depletion (11). However, in NeuT mice with concurrent TUBO regression and chronic mTg injection without LPS, T cell response to mTg was lower than that in similarly treated BALB/c mice and none of the treated NeuT mice developed thyroid inflammation, despite amplified T cell reactivity. Taken together, it appears that only in mice undergoing tumor regression was there significant enhancement of autoimmune reactivity in the EAT-resistant strain. Autoimmunity risk may be lessoned with reduced stimulus, such as the absence of LPS and less vigorous anti-tumor response as in neu-tolerant mice. These conditions may be more in line with cancer patients undergoing immunotherapy. However, because cancer patients have difference MHC class II genes and susceptibility to autoimmune diseases is known to be MHC class II-restricted, it remains critical to also assess what effect tumor regression might have on autoimmunity risk in susceptible hosts. Until the mechanisms of mutual amplification are defined and counter measures developed, patients undergoing immunotherapy should be closely monitored for signs of autoimmune symptoms. The development or increase of autoantibodies to self Ags of prevalent autoimmune diseases, such as those from the thyroid, may be a sensitive indicator of treatment-induced autoimmunity and an early indication for intervention.
Supplementary Material
Acknowledgments
We thank Andi Cani for assistance with breeding and screening of transgenic mice, Laura Baksic for excellent care of the experimental animals, and John Zielinski for assistance with data collection. We also thank Renee Wilder for careful histologic preparation of thyroids and Dr. C. Jeffries for S. enteritidis LPS.
Footnotes
Disclosures The authors have no financial conflict of interest.
This work was supported by NIH CA125680 and CA76340 (to W.Z.W.), DK45960 (to Y.M.K.), and St. John Hospital and Medical Center (to Y.M.K.).
Abbreviations used in this paper: mTg, mouse thyroglobulin; Treg, regulatory T cell; EAT, experimental autoimmune thyroiditis; NeuT, neu transgenic mice; pneuTM, plasmid encoding the extracellular and transmembrane domains of neu.
The online version of this article contains supplemental material.
References
- 1.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N. Engl. J. Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 2.McNeel DG, Knutson KL, Schiffman K, Davis DR, Caron D, Disis ML. Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J. Clin. Immunol. 2003;23:62–72. doi: 10.1023/a:1021904432489. [DOI] [PubMed] [Google Scholar]
- 3.Okayasu I, Hara Y, Nakamura K, Rose NR. Racial and age-related differences in incidence and severity of focal autoimmune thyroiditis. Am. J. Clin. Pathol. 1994;101:698–702. doi: 10.1093/ajcp/101.6.698. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 5.Takamatsu J, Yoshida S, Yokozawa T, Hirai K, Kuma K, Ohsawa N, Hosoya T. Correlation of antithyroglobulin and antithyroid-peroxidase antibody profiles with clinical and ultrasound characteristics of chronic thyroiditis. Thyroid. 1998;8:1101–1106. doi: 10.1089/thy.1998.8.1101. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Kukes GD. Hashimoto’s thyroiditis: an organ-specific autoimmune disease: pathogenesis and recent developments. Lab. Invest. 1999;79:1175–1180. [PubMed] [Google Scholar]
- 7.Canonica GW, Cosulich ME, Croci R, Ferrini S, Bagnasco M, Dirienzo W, Ferrini O, Bargellesi A, Giordano G. Thyroglobulin-induced T-cell in vitro proliferation in Hashimoto’s thyroiditis: identification of the responsive subset and effect of monoclonal antibodies directed to Ia antigens. Clin. Immunol. Immunopathol. 1984;32:132–141. doi: 10.1016/0090-1229(84)90115-6. [DOI] [PubMed] [Google Scholar]
- 8.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J. Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YM. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res. 2005;65:8471–8478. doi: 10.1158/0008-5472.CAN-05-0934. [DOI] [PubMed] [Google Scholar]
- 12.Kong YM, Lomo LC, Motte RW, Giraldo AA, Baisch J, Strauss G, Hammerling GJ, David CS. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J. Exp. Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob JB, Kong YM, Meroueh C, Snower DP, David CS, Ho YS, Wei WZ. Control of Her-2 tumor immunity and thyroid autoimmunity by MHC and regulatory T cells. Cancer Res. 2007;67:7020–7027. doi: 10.1158/0008-5472.CAN-06-4755. [DOI] [PubMed] [Google Scholar]
- 14.Lucchini F, Sacco MG, Hu N, Villa A, Brown J, Cesano L, Mangiarini L, Rindi G, Kindl S, Sessa F, et al. Early and multifocal tumors in breast, salivary, harderian and epididymal tissues developed in MMTY-Neu transgenic mice. Cancer Lett. 1992;64:203–209. doi: 10.1016/0304-3835(92)90044-v. [DOI] [PubMed] [Google Scholar]
- 15.Boggio K, Nicoletti G, Di CE, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De GC, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. Inhibition of carcinogenesis by DNA vaccination. J. Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 17.Nanni P, Pupa SM, Nicoletti G, De GC, Landuzzi L, Rossi I, Astolfi A, Ricci C, De VR, Invernizzi AM, et al. p185(neu) protein is required for tumor and anchorage-independent growth, not for cell proliferation of transgenic mammary carcinoma. Int. J. Cancer. 2000;87:186–194. [PubMed] [Google Scholar]
- 18.Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, Wei W. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol. 2006;240:96–106. doi: 10.1016/j.cellimm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Miller F, Jones RF, Jacob J, Kong YM, Wei WZ. From breast cancer immunobiology to her-2 DNA vaccine and autoimmune sequelae. Breast Dis. 2004;20:43–51. doi: 10.3233/bd-2004-20106. [DOI] [PubMed] [Google Scholar]
- 20.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J. Immunol. 2003;170:1202–1208. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 21.Piechocki MP, Pilon SA, Wei WZ. Quantitative measurement of anti-ErbB-2 antibody by flow cytometry and ELISA. J. Immunol. Methods. 2002;259:33–42. doi: 10.1016/s0022-1759(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 22.Kong YM, David CS, Giraldo AA, Elrehewy M, Rose NR. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J. Immunol. 1979;123:15–18. [PubMed] [Google Scholar]
- 23.Kong YM. Experimental autoimmune thyroiditis in the mouse. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley and Sons; New York: 2007. pp. 15.7.1–15.7.21. [DOI] [PubMed] [Google Scholar]
- 24.Elrehewy M, Kong YM, Giraldo AA, Rose NR. Syngeneic thyroglobulin is immunogenic in good responder mice. Eur. J. Immunol. 1981;11:146–151. doi: 10.1002/eji.1830110216. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Flynn JC, Kong YM. IL-12 prevents tolerance induction with mouse thyroglobulin by priming pathogenic T cells in experimental autoimmune thyroiditis: role of IFN-γ and the costimulatory molecules CD40L and CD28. Cell Immunol. 2001;208:52–61. doi: 10.1006/cimm.2001.1767. [DOI] [PubMed] [Google Scholar]
- 26.Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sacchetti P, Curcio C, Amici A, Wei WZ, Musiani P, Lollini PL, et al. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin. Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 27.Morris GP, Chen L, Kong YM. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226:20–29. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Morris GP, Kong YM. Tolerance to autoimmune thyroiditis: CD4+CD25+ regulatory T cells influence susceptibility but do not supersede MHC class II restriction. Front. Biosci. 2006;11:1234–1243. doi: 10.2741/1876. [DOI] [PubMed] [Google Scholar]
- 29.Radkevich-Brown O, Jacob J, Kershaw M, Wei W-Z. Genetic regulation of the response to Her-2 DNA vaccination in human Her-2 transgenic mice. Cancer Res. 2009;69:212–218. doi: 10.1158/0008-5472.CAN-08-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.