Abstract
Objective
The dopaminergic system is associated with feelings of pleasure and reward and with positive hedonic processes related to food, sexual activity and certain substances. Because it is recognized that patients who have eating disorders with binge-eating behaviour have a high comorbidity of substance dependence, we examined the association between the variable number of tandem repeats (VNTR) polymorphism in the 3́ untranslated region of the dopamine transporter gene (DAT1) and eating disorders with binge-eating behaviour.
Methods
The subjects were 90 female Japanese patients with eating disorders diagnosed using DSM-IV; they were compared with 115 healthy female controls. Genomic DNA was extracted from whole blood, and standard polymerase chain reaction testing was performed. We compared the frequencies of a short allele (7 or 9 repeats) and a long allele (10 or 11 repeats) in both groups.
Results
In the group who had an eating disorder with binge-eating behaviour, the frequency of a short allele was significantly higher compared with the control group.
Conclusion
It seems plausible that the association between the DAT1 VNTR and binge-eating behaviour indicates that dysregulation of dopamine reuptake may act as a common pathophysiologic mechanism in eating disorders with binge-eating behaviour and in disorders related to substance use.
Medical subject headings: case-control studies, dopamine, eating disorders, genetic predisposition to disease
Abstract
Objectif
Le système dopaminergique est associé à des sentiments de plaisir et de récompense ainsi qu'aux processus hédoniques positifs liés à l'alimentation, à l'activité sexuelle et à certaines substances. Comme il est reconnu que les patients atteints de troubles de l'alimentation avec frénésie alimentaire présentent une comorbidité élevée de dépendance à une substance, nous avons examiné l'association entre le polymorphisme de répétitions variables (VNTR) dans la région non traduite de 3́ du gène transporteur de la dopamine (DAT1) et un trouble de l'alimentation accompagné de frénésie alimentaire.
Méthodes
Le groupe-sujet se composait de 90 patientes japonaises ayant reçu un diagnostic de trouble de l'alimentation selon le DSM-IV; on les a comparées à 115 témoins en santé, également de sexe féminin. On a extrait l'ADN génomique du sang total puis effectué un test standard de réaction par polymérisation en chaÎne. On a comparé les fréquences d'un allèle court (7 ou 9 répétitions) et d'un allèle long (10 ou 11 répétitions) dans les deux groupes.
Résultats
Dans le groupe qui présentait un trouble de l'alimentation avec frénésie alimentaire, la fréquence d'un allèle court a été significativement plus élevée que dans le groupe-témoin.
Conclusion
Il semble plausible que l'association entre le DAT1 VNTR et la frénésie alimentaire indique qu'un dysfonctionnement du recaptage de la dopamine puisse être un mécanisme pathophysiologique courant des troubles de l'alimentation avec frénésie alimentaire ainsi que des troubles liés à une toxicomanie.
Introduction
It is widely recognized that the cause of eating disorders is likely to involve a complex mix of multiple genes and multiple specific environmental risk factors.
Various pieces of evidence have supported the contribution of genetic factors to the origin of eating disorders. Devlin et al1 performed linkage analysis of anorexia nervosa incorporating behavioural covariates. They found several regions of suggestive linkage in patients with anorexia nervosa who had a drive for thinness and obsessiveness. The same group2 detected significant linkage on chromosome 10 in families of patients with bulimia nervosa.
There have been extensive association studies of the serotonin-2A receptor gene,3,4,5,6 and analysis of the serotonin transporter-linked polymorphism in anorexia nervosa has been reported by several groups.7,8,9 Recently, several association studies related to leptin, neuropeptide Y receptor, estrogen receptor, catechol-o-methyltransferase and norepinephrine transporter genes have been carried out.10,11,12,13,14 Branson et al15 indicated that binge-eating behaviour is a major phenotypic characteristic of subjects with a mutation in the melanocortin 4 receptor gene, which is a candidate gene for the control of eating behaviour. In the dopaminergic system, association studies with the D3 and D4 dopamine receptor genes that yielded negative associations have been reported.16,17 However, the dopaminergic system, besides its well-known role in the regulation of appetite, is also considered important for such behaviour as reward and reinforcement.18 In accordance with neurochemical studies, the high comorbidity between substance- related disorders and eating disorders with binge- eating behaviour is well established clinically.19,20,21 In addition, several groups have reported that shorter alleles of the variable number of tandem repeats (VNTR) polymorphism in the 3' untranslated region of the dopamine transporter (DAT1) gene were associated with substance dependence.22,23,24,25 These data suggest that binge-eating behaviour and substance dependence may share a genetic causative mechanism associated with the dopaminergic system.
Based upon these data, we postulated that DAT1 might play a key role in eating behaviour and the development of eating disorders. We investigated the association between the VNTR polymorphism in the 3' untranslated region of DAT1 and eating disorders with binge-eating behaviour.
Methods
The subjects were 90 female Japanese patients with eating disorders, aged 15–36 (mean 22.1, standard deviation [SD] 4.0) years. The diagnoses were made according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)26 by 1 psychiatrist (H.M.) who specialized in eating disorders and had run an eating-disorder clinic for more than 3 years. In addition, symptoms were assessed by the Eating Disorder Inventory.27 The details of the diagnostic assessment have been described in a previous report.28 The patients were classified as having anorexia nervosa binge-eating/purging type (n = 35) and bulimia nervosa (n = 55). A clinical trait common to both groups was binge-eating behaviour. These diagnoses had been stable over at least a 3-year follow-up period.
The healthy controls were 115 women aged 18–26 (mean 18.7, SD 1.1) years, whose current body weight was stable with fluctuations within 20% of the standard body weight (normal range of body mass index). Written informed consent of the patients and healthy controls was obtained after the purpose and methods of this study had been fully explained. The Ethics Committee of the University of Yamanashi approved this study.
Peripheral blood was drawn from the antecubital vein, and genomic DNA was extracted from whole blood using a QIAamp DNA Blood Mini Kit (QIAGEN Hilden, Germany).
Standard polymerase chain reaction (PCR) testing was performed in a total volume of 20 μL containing 4 ng genomic DNA, 1.5 mmol/L MgCl2, 0.2 mmol/L of each deoxyribonucleotide, 0.5 μmol/L of each primer (sense, 5'-TGTGGTGTAGGGAACGGCCTGAG, antisense, 5'-CTTCCTGGAGGTCACGGCTCAAGG), 10% dimethyl sulfoxide and 2 U Taq DNA polymerase.29 The conditions for 35 cycles consisted of denaturation at 95°C for 30 seconds, annealing at 65°C for 1 minute and extension at 72°C for 30 seconds, before a final extension step at 72°C for 4 minutes. Cycling amplification was performed by standard methods in a thermal cycler 9700 (PE Applied Biosystems, Norwalk, Conn.). The PCR products were separated on 2% agarose gel with ethidium bromide staining and visualized under ultraviolet illumination.
Identification of the bands was performed independently by 2 researchers who had not been informed of the clinical diagnosis of the patients, and their results were found to agree with each other.
The χ2 test was used for statistical analysis to evaluate allele frequencies. The agreement of genotype frequencies with the Hardy–Weinberg equilibrium was also tested using χ2.
Results
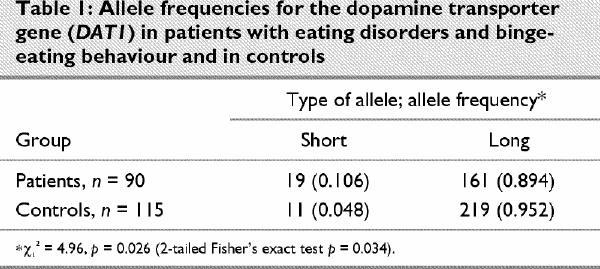
In the control group, alleles of 7 (360 base pairs [bp]), 9 (440 bp), 10 (480 bp) and 11 repeats (520 bp) were observed with frequencies of 0.9%, 3.9%, 93.9% and 1.3%, respectively. The frequency of the 10-repeat allele was overwhelmingly the highest. This frequency distribution is in accordance with that reported by Nakatome et al,30 but different from data from a white population.31
Based on previous reports described earlier,22,23,24,25we compared the frequencies of a short allele (7 or 9 repeats) and a long allele (10 or 11 repeats) in both groups (Table 1). In the group with eating disorders and binge-eating behaviour, the frequency of a short allele was significantly higher as compared with the control group (χ12 = 4.96, p = 0.026; 2-tailed Fisher's exact test, p = 0.034). Genotype distributions were in accordance with the Hardy–Weinberg equilibrium for patients (χ32 = 0.12, p < 0.99) and controls (χ32 = 0.05, p > 0.99).
Table 1
Discussion
In the present study, the short allele was observed at a significantly higher frequency in the patients with eating disorders and binge-eating behaviour, as compared with the control group.
Heinz et al32 reported genotype influences on in-vivo dopamine transporter availability in the human striatum using [iodine 123]β-CIT single-photon emission computed tomography. Subjects who carried a genotype containing a short allele demonstrated significantly lower dopamine transporter binding in the striatum than carriers of a long allele. This finding is in accordance with the biochemical findings of Fuke et al.33 They reported that a short allele had lower transcriptional activity. Furthermore, in clinical studies, associations between shorter alleles and substance dependence, such as alcohol and nicotine dependence, have been observed by several groups.22,23,24,25
The high comorbidity between eating disorders and substance-related disorders is well recognized clinically,19,20,21 and especially that between eating disorder with binge-eating behaviour and alcohol-related disorder.34,35 Dopaminergic transmission plays a key role in both appetite regulation and addiction. Because DAT1 modulates dopaminergic transmission, it seems plausible that the association between the DAT1 VNTR and binge-eating behaviour indicates that dysregulation of dopamine reuptake may act as a common pathophysiologic mechanism in both eating disorders with binge-eating behaviour and substance-related disorders.
A clinically important issue is the validity of diagnosis of subtypes of eating disorders, because this influences the treatment and prognosis of patients with these disorders. The DAT1 VNTR polymorphism could be used as a biologic marker to identify patients with binge-eating behaviour among individuals with eating disorders.
Our results were obtained from a case–control association study with a relatively small sample. Although there is apparent ethnic similarity between the control and patient groups because the Japanese population is fairly homogeneous, we still cannot completely rule out genetic heterogeneity between the 2 groups. Thus, the results may not be completely free of the possibility of stratification. Thus, this study needs to be extended to samples from different ethnic backgrounds. Confirmation of the results in independent samples or with family-based controls will be necessary to further clarify the subgroups of eating disorders.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Shigenobu Kanba, Department of Neuropsychiatry, Faculty of Medicine, University of Yamanashi, 1110 Tamaho, Nakakoma, Yamanashi 409-3898, Japan; fax 81 552 73 6765; skanba@yamanashi.ac.jp
Submitted Mar. 6, 2003; Revised Sept. 25, 2003; Accepted Oct. 9, 2003
References
- 1.Devlin B, Bacanu SA, Klump KL, Bulik CM, Fichter MM, Halmi KA, et al. Linkage analysis of anorexia nervosa incorporating behavioral covariates. Hum Mol Genet 2002;11:689-96. [DOI] [PubMed]
- 2.Bulik CM, Devlin B, Bacanu SA, Thornton L, Klump KL, Ficher MM, et al. Significant linkage on chromosome 10p in families with bulimia nervosa. Am J Hum Genet 2003;72:200-7. [DOI] [PMC free article] [PubMed]
- 3.Collier DA, Arranz MJ, Li T, Mupita D, Brown N, Treasure J. Association between 5-HT2A gene promoter polymorphism and anorexia nervosa. Lancet 1997;350:412. [DOI] [PubMed]
- 4.Nishiguchi N, Matsushita S, Suzuki K, Murayama M, Shirakawa O, Higuchi S. Association between 5HT2A receptor gene promoter region polymorphism and eating disorders in Japanese patients. Biol Psychiatry 2001;50:123-8. [DOI] [PubMed]
- 5.Gorwood P, Ades J, Bellodi L, Cellini E, Collier DA, Di Bella D, et al. The 5-HT2A–1438G/A polymorphism in anorexia nervosa: a combined analysis of 316 trios from six European centers. Mol Psychiatry 2002;7:90-4. [DOI] [PubMed]
- 6.Ricca V, Nacmias B, Cellini E, Di Bernardo M, Rotella CM, Sorbi S. 5-HT2A receptor gene polymorphism and eating disorders. Neurosci Lett 2002;323:105-8. [DOI] [PubMed]
- 7.Di Bella D, Catalano M, Cavallini MC, Riboldi C, Bellodi L. Serotonin transporter linked polymorphic region in anorexia nervosa and bulimia nervosa. Mol Psychiatry 2000;5:233-4. [DOI] [PubMed]
- 8.Sundaramurthy D, Pieri LF, Gape H, Markham AF, Campbell DA. Analysis of the serotonin transporter gene linked polymorphism (5-HTTLPR) in anorexia nervosa. Am J Med Genet 2000; 96:53-5. [PubMed]
- 9.Fumeron F, Betoulle D, Aubert R, Herbeth B, Siest G, Rigaud D. Association of a functional 5-HT transporter gene polymorphism with anorexia nervosa and food intake. Mol Psychiatry 2001; 6:9-10. [DOI] [PubMed]
- 10.Hinney A, Bornscheuer A, Depenbusch M, Mireke B, Tolle A, Middeke K, et al. No evidence for involvement of the leptin gene in anorexia nervosa, bulimia nervosa, underweight or early onset extreme obesity: identification of two novel mutations in the coding sequence and a novel polymorphism in the leptin gene linked upstream region. Mol Psychiatry 1998;3:539-43. [DOI] [PubMed]
- 11.Rozenkranz K, Hinney A, Ziegler A, von Prittwitz S, Barth N, Roth H, et al. Screening for mutations in the neuropeptide Y Y5 receptor gene in cohorts belonging to different weight extremes. Int J Obes Relat Metab Disord 1998;22:157-63. [DOI] [PubMed]
- 12.Eastwood H, Brown KMO, Markovic D, Piery LF. Variation in the ESR1 and ESR2 genes and genetic susceptibility to anorexia nervosa. Mol Psychiatry 2002;7:86-9. [DOI] [PubMed]
- 13.Frisch A, Laufer N, Danziger Y, Michaelovsky E, Leor S, Carel C, et al. Association of anorexia nervosa with the high activity allele of the COMT gene: a family based study in Israeli patients. Mol Psychiatry 2001;6:243-5. [DOI] [PubMed]
- 14.Urwin RE, Bennetts B, Wilcken B, Lampropoulos B, Beumont P, Clarke S, et al. Anorexia nervosa (restricting type) is associated with a polymorphism in the novel norepinephrine transporter gene promoter polymorphic region. Mol Psychiatry 2002; 7:652-7. [DOI] [PubMed]
- 15.Branson R, Potoczna N, Kral JG, Lentes KU, Hoeche MR, Horber FF. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 2003;348:1096-103. [DOI] [PubMed]
- 16.Bruins-Slot L, Gorwood P, Bouvard M, Blot P, Ades J, Feingold J, et al. Lack of association between anorexia nervosa and D3 dopamine receptor gene. Biol Psychiatry 1998;43:76-8. [DOI] [PubMed]
- 17.Hinney A, Schneider J, Ziegler A, Lehmkuhl G, Poustka F, Schmidt MH, et al. No evidence for involvement of polymorphisms of the dopamine D4 receptor gene in anorexia nervosa, underweight, and obesity. Am J Med Genet 1999;88:594-7. [PubMed]
- 18.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol 1989;40:191-225. [DOI] [PubMed]
- 19.Wiederman MW, Pryor T. Substance use among women with eating disorders. Int J Eat Disord 1996;20:163-8. [DOI] [PubMed]
- 20.Matsunaga H, Kaye WH, McConaha C, Plotnicov K, Pollice C, Rao R, et al. Psychopathological characteristics of recovered bulimics who have a history of physical or sexual abuse. J Nerv Ment Dis 1999;197:472-7. [DOI] [PubMed]
- 21.Daniels ES, Masheb RM, Berman RM, Mickley D, Grio CM. Bulimia nervosa and alcohol dependence. A case report of a patient enrolled in a randomized controlled clinical trial. J Subst Abuse Treat 1999;17:163-6. [DOI] [PubMed]
- 22.Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, et al. Association of smoking and personality with a polymorphism of the dopamine transporter gene: results from a community survey. Am J Med Genet 2000;96:331-4. [DOI] [PubMed]
- 23.Gelnerter J, Kranzler HR, Satel Sl, Rao PA. Genetic association between dopamine transporter protein alleles and cocaine- induced paranoia. Neuropsychopharmacology 1994;11:195-200. [DOI] [PubMed]
- 24.Muramatsu T, Higuchi S. Dopamine transporter gene polymorphism and alcoholism. Biochem Biophys Commun 1995;211: 28-32. [DOI] [PubMed]
- 25.Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, et al. Allelic association of a dopamine transporter gene polymorphism in alcohol dependence with withdrawal seizures or delirium. Biol Psychiatry 1997;41:299-304. [DOI] [PubMed]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington: American Psychiatric Association; 1994.
- 27.Garner DM, Olmsted MP. The Eating Disorder Inventory manual. Odessa (FL): Psychological Assessment Resources; 1984.
- 28.Mizushima H, Ono Y, Asai M. TCI temperamental scores in bulimia nervosa patients and normal women with and without diet experiences. Acta Psychiatr Scand 1998;98:228-30. [DOI] [PubMed]
- 29.Nakatome M, Honda K, Tun Z, Kato Y, Harihara S, Omoto K, et al. Genetic polymorphism of the 3' VNTR region of the human dopaminergic function gene DAT1 (human dopamine transporter gene) in the Mongolian population. Hum Biol 1996; 68: 509-15. [PubMed]
- 30.Nakatome M, Honda K, Islam MN, Terada M, Yamazaki M, Kuroki H, et al. Amplification of DAT1 (human dopamine transporter gene) 3' variable region in the Japanese population. Hum Hered 1995;45:262-5. [DOI] [PubMed]
- 31.Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics 1992; 14:1104-6. [DOI] [PubMed]
- 32.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000;22:133-9. [DOI] [PubMed]
- 33.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphisms of the human dopamine transporter (DAT1) gene affect gene expression. Pharmacogenomics J 2001;1:152-6. [DOI] [PubMed]
- 34.Schuckit MA, Tipp JE, Anthenelli RM, Bucholz KK, Hesselbrock VM, Nurnberger JI. Anorexia nervosa and bulimia nervosa in alcohol-dependent men and their relatives. Am J Psychiatry 1996;153:74-82. [DOI] [PubMed]
- 35.Dansky BS, Brewerton TD, Klipatrick DG. Comorbidity of bulimia nervosa and alcohol use disorders: results from the National Women's Study. Int J Eat Disord 2000;27:180-90. [DOI] [PubMed]


