Abstract
Background
Mitral valve (MV) enlargement is a compensatory mechanism capable of preventing functional mitral regurgitation (FMR) in dilated ventricles. Total leaflet area and its relation with closure area measured by 3D-echocardiography have been related to FMR. Whether these parameters can be assessed with other imaging modalities is not known. Our objectives are to compare cardiac CT-based measurements of MV leaflets with 3D-echocardiography and determine the relationship of these metrics to the presence of FMR.
Methods and Results
We used two cohorts of patients who had cardiac CT to measure MV total leaflet, closure and annulus areas. In cohort 1 (26 patients), we validated these CT metrics to 3D-echocardiography. In cohort 2 (66 patients), we assessed the relation of MV size with the presence of FMR in three populations: heart failure with FMR, heart failure without FMR, and normal controls. Cardiac CT and 3D-echocardiography produced similar results for total leaflet (R2=0.97), closure (R2=0.89) and annulus areas (R2=0.84). MV size was largest in heart failure without FMR compared with controls and FMR patients (9.1±1.7 vs 7.5±1.0 vs 8.1±0.9 cm2/m2, p<0.01). FMR patients had reduced ratios of total leaflet:closure areas and total leaflet:annulus areas when compared to patients without FMR (p<0.01).
Conclusions
MV size measured by CT is comparable to 3D-echocardiography. MV enlargement in cardiomyopathy suggests leaflet adaptation. Patients with FMR have inadequate adaptation as reflected by decreased ratios of leaflet area and areas determined by ventricle size (annulus and closure areas). These measurements provide additional insight into the mechanism of FMR.
Keywords: mitral regurgitation, cardiac computed tomography, 3-dimensional echocardiography
Mitral regurgitation (MR) is a common and morbid condition that represents a major health burden1. MR can result from mitral leaflet disease (organic MR) or secondary to left ventricle (LV) remodeling (functional MR). The latter is the complex result of global or local (typically after infero-posterior infarction) LV remodeling with papillary muscle displacement, and/or mitral annulus dilatation2–12. These LV geometric alterations can be isolated or coexist, with secondary mitral apparatus distortion and incomplete closure of the mitral valve. Understanding the mechanisms underlying MR is of highest importance to choose the appropriate therapy13, and echocardiography is currently the central tool used for that purpose. The complex three-dimensional (3D) shape of the mitral apparatus makes its evaluation challenging by 2D planes, generating a potential role for 3D imaging11, 12, 14. Recently, echocardiography based three-dimensional reconstructions of the mitral leaflets provided new insight into the mechanisms of functional MR, in particular by demonstrating the capacity of adaptation of the mitral leaflets, which can actively enlarge in response to mechanical stretch, therefore compensating for LV dilatation and prevent MR15–17. This compensatory enlargement can explain at least in part why some patients have variable severity of MR despite similar degree of LV remodeling.
MV reconstruction algorithms allowing total leaflet area measurement have been validated with 3D echocardiography. However, transthoracic ultrasound can be limited in patients with poor echocardiographic windows, and transesophageal echocardiography is invasive. For that reason, whether or not these 3D parameters can be obtained from other non-invasive imaging modality need to be evaluated. Cardiac computed tomography (CT) is a growing imaging modality, and the recent CT scanners are able to acquire 3D datasets with excellent spatial resolution and enhanced temporal resolution.
Our first aim is to validate the three-dimensional measurements obtained by contrast-enhanced cardiac CT to those obtained by 3D echocardiography. To assess the role of these measurements to explore MV changes in heart failure and their potential contribution to functional MR, we subsequently measured CT-derived mitral valve leaflet area in heart failure patients with and without functional MR as well as normal controls.
Methods
Validation study between CT and 3D Echo
In cohort 1, we searched our institution echocardiography and radiology databases for patients who had both a three-dimensional echocardiographic study (transthoracic or transesophageal) and a retrospectively gated cardiac CT between January 2006 and June 2011. Patients were considered if they had both studies within a period of 6 months. Patients with a delay more than 48h between the two exams were enrolled if their clinical condition was stable and if serial standard 2D echocardiograms were available to show stable cavity dimensions, systolic and valve functions at both timepoints. Patients with history of previous mitral surgery or insufficient image quality on one or both imaging modality were excluded. In addition to LV volume and function, 2D and 3D echocardiography images were used to evaluate the presence, mechanism and severity of MR, which was graded by one observer as trace, mild, moderate or severe by integrating color Doppler jet area and vena contracta width18, 19.
Assessment of mitral valve adaptation in heart failure with CT
In cohort 2, we identified three groups of patients who had a retrospectively gated cardiac CT and a standard 2D echocardiography for MR quantification on the same day: (1) FMR group consisted of patients with heart failure as defined by LVEF < 50% and at least moderate functional MR, (2) patients without significant MR (mild or less) but with similar LV size and function compared to the FMR group were identified (no FMR group), and (3) a group consisting of normal patients with no known cardiac abnormality.
Imaging Protocols
Cardiac CT acquisition
CT scans were performed with contrast and retrospective-gating with tube current modulation. The scanners used were 128-slice dual source scanner (Siemens Definition Flash, 2 × 64 × 0.6 mm detectors, gantry rotation time 280 ms, temporal resolution 75 ms), 64 slice dual source scanner (Siemens Definition, 2 × 32 × 0.6 mm detectors, gantry rotation time 330 ms, temporal resolution 83 ms), and 64-slice single source scanner (Siemens Sensation, 1 × 32 × 0.6 mm detectors, gantry rotation time 330 ms, temporal resolution 165 ms). For non-dual source CT scanners (70% of the exams), intravenous beta-blocker was given immediately before the exam as needed to achieve heart rate below 65 beats per minute.
3D echocardiography acquisition
Full volume datasets were acquired over 4–7 heart beats with a Philips iE33 scanner. The 3D datasets were obtained from the apical window (transthoracic echocardiogram) using an X3-1 matrix-array transducer (Philips) or a mid-esophageal view centered on the mitral valve (transesophageal echocardiogram) using an X7-2t matrix-array transducer (Philips).
Image Analysis
3D datasets from the CT and 3D echocardiography were imported and processed in dedicated software (Omni4D, MD Handschumacher). Observer 1 performed the reconstructions and measurements on all patients once. For inter- and intra-observer reproducibility, Observer 2 performed the reconstructions and measurements in 10 randomly selected patients and Observer 1 repeated this process in 10 patients one month later, respectively.
Total leaflet area has to be measured in diastole, when there is no tension on the leaflets (up to 15% increase in mitral surface has been shown during systole20, 21) and no coaptation surface. On both datasets, the best diastolic frame was selected (mid to late diastole), and the mitral leaflets were traced in multiple planes and reconstructed to obtain the total leaflet area as described previously15(Figure 1). The closure area, representing the minimal surface that needs to be covered by the leaflets to prevent MR, was traced in mid-systole (Figure 2). The annulus area (projected on its least-square plane) was also measured in mid-systole. The ratios of total leaflet area/annulus area and total leaflet area/closure area were then calculated. A reduction in these ratios (representing a proportionally smaller valve compared to LV and annulus size) has been previously associated with the presence of functional MR15. Other FMR key parameters such as tenting area, tenting volume, tethering distances (distance between each papillary muscle and contralateral annulus) were measured. End-systolic and end-diastolic LV volumes were measured from the CT datasets by the Simpson method of disks. The study was approved by our institutional review committee and the subjects gave informed consent.
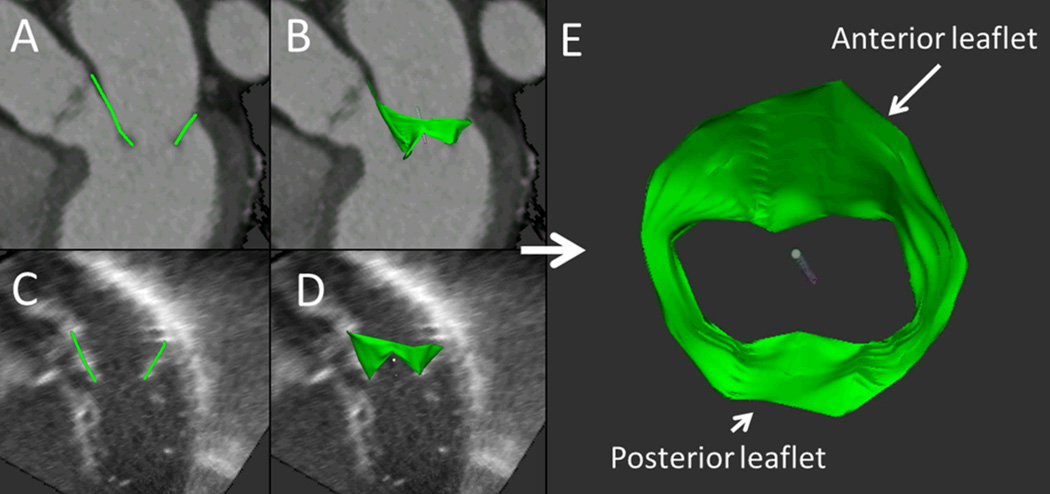
Figure 1.
Total leaflet area measurement. Anterior and posterior mitral leaflets are traced in multiple planes on CT datasets (A), allowing for three-dimensional reconstruction of the entire leaflet surface (B). Same method was utilized on 3D Echo datasets (C, D). E: Reconstructed mitral valve viewed from the left atrium obtained from the CT dataset.
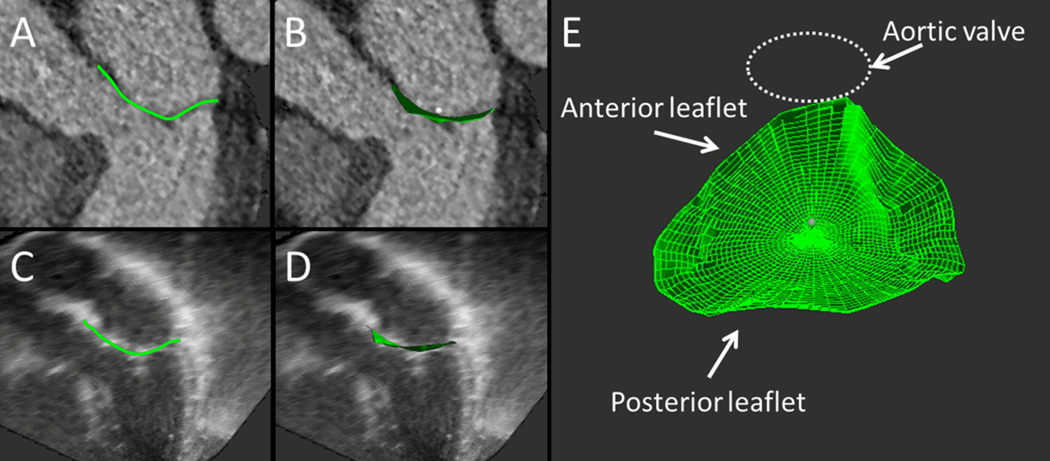
Figure 2.
Mitral valve closure area measurement. The mid-systolic closed mitral valve surface is traced in multiple planes on CT datasets (A) and then reconstructed to compute the closure surface area (B). Same method was utilized on 3D Echo datasets (C, D). E: Projection of the resulting area obtained from the CT dataset, representing the minimal surface that needs to be covered by the leaflets to prevent mitral regurgitation. As the leaflet tips need to bend to form a coaptational seal in systole, an excess of total leaflet tissue in relation to that closure area is needed for adequate valve closure.
Statistical Analysis
Continuous variable were expressed as mean ± standard deviation or median with interquartile range [IQR] as appropriate. Categorical variables were expressed as percentages. We compared the differences between two tests using Student’s t-tests or Wilcoxon rank sum test for continuous variables and Chi-square or Fisher’s Exact test for categorical variables, as appropriate. All volumetric and area measurements were indexed for body surface area. For the validation part of this study, inter- and intra-observer agreements of CT total leaflet area, closure area and annulus area were assessed using a single measure, two-way random effect intraclass correlation coefficient (ICC) model. The paired t-test was used to determine the significance for mean absolute and relative differences. We used linear regression and Bland-Altman analysis to compare CT and 3D echocardiography measurements of total leaflet area, closure area and annulus area of the mitral valve. For mitral valve characterization with CT in heart failure (cohort 2), the significance of differences amongst the three groups (normal, FMR and no FMR) was determined using analysis of variance (ANOVA) test. A two-sided p-value <0.05 was considered to indicate statistical significance for all tests. Statistical analysis was performed with Stata/IC 11.2 (StataCorp LP, Tx).
Results
Validation study between CT and 3D Echo
A total of 32 patients were initially assessed for validation between CT and 3D echo. Six patients were excluded for poor image quality (all had poor apical echocardiographic window with non-interpretable 3D Echo datasets, 1 patient also had insufficient image quality on CT). Therefore, 26 patients with CT and 3D Echo (22 transthoracic, 4 transesophageal) were included in this analysis. Table 1 shows the patient demographics. Median time between the 2 exams was 46 [20–179] days. 7 patients had normal valve and LV size and function, 5 had intrinsic mitral valve disease (2 mitral valve prolapse, 1 mitral cleft, 1 leaflet perforation and 1 parachute mitral valve), and 14 had evidence of cardiomyopathy (5 with functional MR, 9 without).
Table 1.
Characteristics of Cohort 1
| Age | 55 ± 18 |
| Female gender, n (%) | 18 (69%) |
| Body mass index (Kg/m2) | 24.8 ± 4.4 |
| Median time between echo and CT (days) | 46 [20–179] |
| Body surface area (m2) | 1.8 ± 0.2 |
| LV ejection fraction (%) | 56 ± 15 |
| Groups, n (%) | |
| Normal | 7 (27) |
| Cardiomyopathy with functional MR | 5 (19) |
| Cardiomyopathy without functional MR | 9 (35) |
| Organic MV disease | 5 (19) |
CMP: Cardiomyopathy; MV: mitral valve; MR: mitral regurgitation; LV: Left Ventricle.
Intra- and interobserver reproducibility of CT measurements of total leaflet area, closure area and annulus area are displayed in Table 2.
Table 2.
Inter- and intra-observer reproducibility of CT mitral valve metrics
| Average difference* (cm2) |
Relative difference (%) |
ICC | p value | |
|---|---|---|---|---|
| Interobserver | ||||
| Total leaflet area | 0.6 | 4.4 | 0.974 | <0.01 |
| Closure area | 0.9 | 7.6 | 0.922 | <0.01 |
| Annulus area | 0.7 | 6.3 | 0.944 | <0.01 |
| Intraobserver | ||||
| Total leaflet area | 0.6 | 4.2 | 0.979 | <0.01 |
| Closure area | 0.5 | 5.1 | 0.977 | <0.01 |
| Annulus area | 0.5 | 5.3 | 0.976 | <0.01 |
ICC: Intraclass Correlation Coefficient.
All p-values non-significant (>0.05).
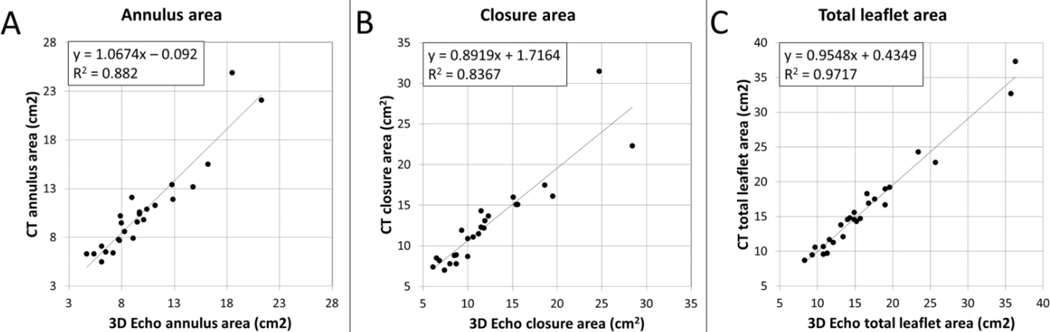
Correlations of mitral valve total leaflet area, annulus area and closed leaflet area between CT and 3D echocardiography are illustrated in Figure 3. There was excellent correlation between both modalities for open leaflet area (R2 = 0.97, p<001, SEE 1.18), with an average absolute difference between 3D echocardiography and CT of 0.9 ± 0.8 cm2 (relative difference: 5.6 ± 4.3 %). Good correlation was also found for the closure area (R2 = 0.84, p<0.01, SEE 2.24), average absolute difference of 1.5 ± 1.7 cm2 (average relative difference: 11.2 ± 8.4 %), as well as annulus area (R2 = 0.89, p<0.01, SEE 1.44), average absolute difference of 1.1 ± 1.3 cm2 (average relative difference: 10 ± 9%). There was no difference in agreement with 3D echocardiography for patients scanned with single source (n=8) vs Dual-Source CT (n=18; p=0.75 for total leaflet area, 0.84 for closure area and 0.85 for annulus area). Measurements of tenting area, tenting volume and tethering distances also yielded comparable results with both modalities (R2=0.85 for tenting area and tenting volume, R2=0.90 and 0.84 for anterolateral and posteromedial tethering distances, respectively). Similar results to CT were seen whether the 3D echo was transthoracic or transesophageal (p>0.50 for leaflet, closure and annulus areas). In this validation cohort, patients with cardiomyopathy had larger valve size than normal patients (8.7 vs 7.0 cm2/m2, p=0.02 by CT; similar results with 3D Echo). Patients with FMR had decreased ratios of total leaflet area / closure area (1.1 ± 0.1 vs 1.4 ± 0.1, p = 0.002) and total leaflet area / annulus area (1.4 ± 0.1 vs 1.7 ± 0.1, p=0.006).
Figure 3.
CT and 3D Echo measurements validation. Comparison of CT and 3D Echo derived measurements of annulus area (A), closure area (B) and total leaflet area (C), showing excellent correlation between the two modalities for the three parameters.
Assessment of mitral valve adaptation in heart failure with CT
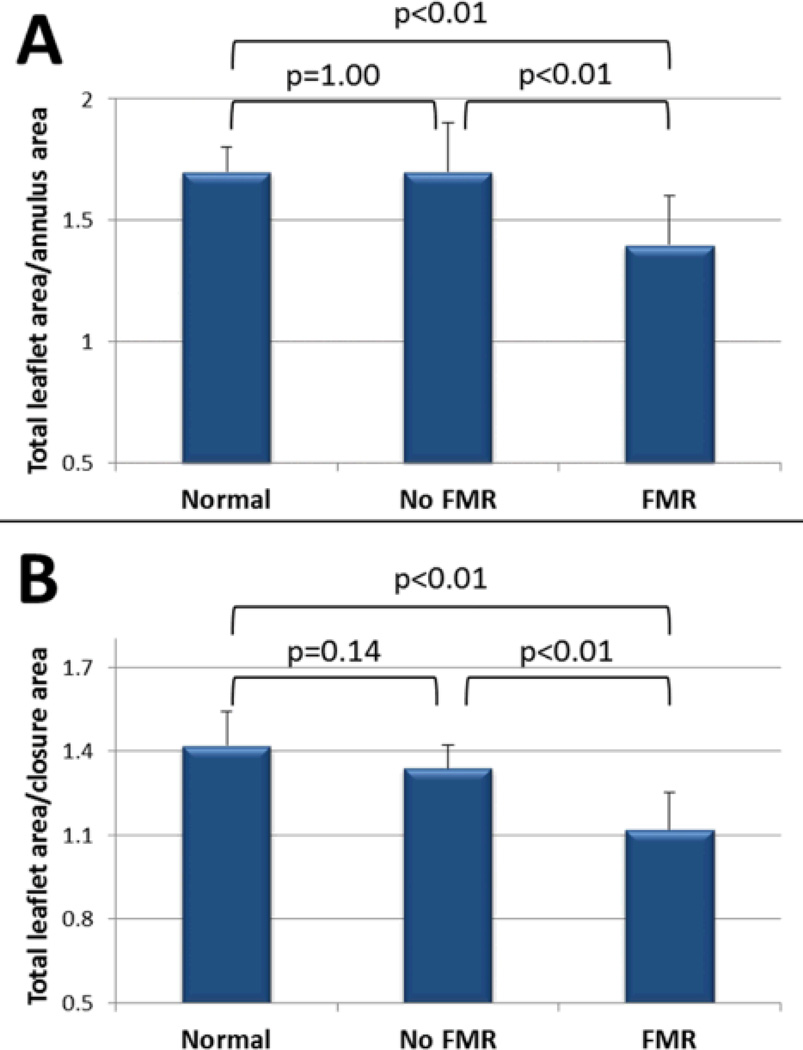
A total of 68 patients who had a retrospectively gated cardiac CT and standard 2D echocardiography on the same day were studied; 2 patients were excluded for CT artifacts caused by rapid or irregular rhythm. Sixty-six patients are therefore included and divided into three groups: 25 heart failure patients with FMR, 25 heart failure patients without FMR, and 16 normals. Both FMR and no-FMR groups had significantly increased LV volumes, annulus and closure areas as well as decreased LVEF compared with controls (Table 3). These two groups were similar for age, gender, prevalence of ischemic vs non-ischemic cardiomyopathy, LV volumes and LV function. Total leaflet area index derived from CT was significantly larger in the no-FMR group compared to both normal and FMR patients (9.1±1.7 vs 7.5±1.0 vs 8.1±0.9 vs cm2/m2 for no-FMR, normal, and FMR groups, p<0.01). In patients without functional MR, MV enlargement was proportional to LV and annular dilatation, maintaining a normal ratio of total leaflet area / closure area (1.4±0.1 vs 1.3±0.1, p=0.14) and total leaflet area / annulus area (1.7±0.1 vs 1.7±0.2, p=1.00). In contrast, both ratios were reduced in the FMR group (total leaflet area / closure area: 1.1±0.1 vs 1.4±0.1, p<0.01; total leaflet area / annulus area: 1.4±0.2 vs 1.7±0.2, p<0.01). These reduced ratios indicate inadequate leaflet enlargement to compensate for LV dilatation in patients with functional MR (Figure 4). In this cohort of patients of similar LV size and function, the only variables significantly different between patients with and without FMR were total leaflet area (p=0.03), ratio of leaflet area / closure area (<0.01) and ratio of leaflet area / annulus area (p<0.01). The average radiation dose for all patients was 12.6± 4.9 mSv.
Table 3.
Clinical and CT characteristics in normal and cardiomyopathy patients (Cohort 2)
| Normal (n=16) |
FMR (n=25) |
No FMR (n=25) |
p value |
|
|---|---|---|---|---|
| Clinical Parameters | ||||
| Age | 47 ± 17 | 66 ± 17* | 60 ± 13* | <0.01 |
| Female gender, n (%) | 10 (63) | 8 (32) | 7 (28) | 0.09 |
| Body mass index | 28 ± 7 | 27 ± 5 | 27 ± 4 | 0.56 |
| Ischemic cardiomyopathy, n(%) | 0 | 16 (64)* | 12 (48)* | <0.01 |
| CT LV volume and function | ||||
| LVEDVi (ml/m2) | 65 ± 13 | 105 ± 30* | 115 ± 37* | <0.01 |
| LVESVi (ml/m2) | 25 ± 10 | 71 ± 26* | 75 ± 29* | <0.01 |
| LV ejection fraction (%) | 63 ± 8 | 29 ± 15* | 34 ± 12* | <0.01 |
| CT Mitral Valve metrics | ||||
| Distance PPM-annulus (mm) | 40 ± 6 | 45 ± 5* | 45 ± 6* | <0.01 |
| Distance LPM-annulus (mm) | 37 ± 5 | 41 ± 3* | 43 ± 4* | <0.01 |
| Total leaflet area (cm2) | 14.0 ± 2.8 | 15.7 ± 2.2 | 18.3 ± 4.3*† | <0.01 |
| Total leaflet area index (cm2/m2) | 7.5 ± 1.0 | 8.1 ± 0.9 | 9.1 ± 1.7*† | <0.01 |
| Closure area (cm2) | 9.9 ± 2.0 | 13.9 ± 1.9* | 13.6 ± 3.2* | <0.01 |
| Closure area index (cm2/m2) | 5.3 ± 0.7 | 7.2 ± 0.7* | 6.7 ± 1.4* | <0.01 |
| Annulus area (cm2) | 8.4 ± 1.7 | 11.3 ± 1.5* | 11.1 ± 2.6* | <0.01 |
| Annulus area index (cm2/m2) | 4.5 ± 0.6 | 5.9 ± 0.6* | 5.5 ± 1.1* | <0.01 |
| Tenting area (cm2) | 1.4 ± 0.6 | 2.9 ± 1.0* | 2.7 ± 1.2* | <0.01 |
| Tenting volume (cm3) | 1.5 ± 0.6 | 3.7 ± 1.5* | 3.2 ± 2.0* | <0.01 |
LVEDVi: Left ventricle end-diastolic volume index; LVESVi: Left ventricle end-systolic volume index; LV: Left ventricle; CMP: Cardiomyopathy; MR: Mitral regurgitation.
p<0.05 vs normal group.
p<0.05 vs FMR group.
Figure 4.
Adequacy of leaflet adaptation. Comparisons of ratios of total leaflet area to annulus and closure areas in cardiomyopathy patients with and without MR versus normals. Total leaflet area / annulus area (A) and total leaflet area / closure area (B) ratios are preserved in cardiomyopathy patients without MR, but abnormal in those with functional MR, indicating inadequate leaflet compensation in that case. CMP: Cardiomyopathy; MR: Mitral regurgitation.
Discussion
In this study, we show that detailed reconstruction of the mitral valve leaflets is possible by cardiac CT, with excellent intra- and inter-observer variability and correlation with 3D echocardiography in patients with either normal or abnormal (organic or functional regurgitation) mitral valve. Both interobserver variability and correlation with 3D ultrasound was better for total leaflet area (measured in diastole) than for annulus and closure area (mid-systolic measurements). This might be related to the use of current tube modulation algorithm with subsequent increased noise in systole. Finally, we also show that in the setting of cardiomyopathy and dilated LV, total leaflet area increases, suggesting compensatory enlargement of the MV. Patients without functional MR have typically larger mitral valve which stays proportional to LV size (as reflected by preserved total leaflet area / closure area ratio). Conversely, patients with functional MR have insufficient MV enlargement to match the LV dilatation. These findings are in accordance with what was previously shown with 3D echocardiography and animal studies suggesting that mitral valve size is not fixed, but rather can enlarge and adapt to prevent MR in patients with abnormal LV function15–17.
The classical definition and evaluation of functional MR relies only on LV and annulus changes, overlooking the importance of mitral valve size and adaptation, which should be considered as one of the most important variables. Functional MR genesis should not be linked to LV remodeling alone, but rather seen as a mismatch between LV remodeling and mitral valve adaptation. An interesting finding is that some but not all patients demonstrate adequate MV enlargement, suggesting that there might be processes to limit this adaptation. The capacity to measure changes in total leaflet area in targeted populations will be important to explore the underlying mechanisms and limiting factors of MV compensatory enlargement. The systolic closure and annulus areas are also helpful: these parameters can be influenced by either annular or ventricular changes, and therefore represent the sum of LV alterations affecting MV closure. The adequacy of adaptation can be evaluated by comparing the total leaflet area to closure area ratio, lower ratios representing insufficient valve compensation and more functional MR.
Cardiac CT is a promising tool for evaluation of mitral valve heart disease, especially in patients with poor echocardiographic windows. It can adequately evaluate the anatomical structure of the valve, presence of calcium or leaflet thickening, and identify patients with organic valve disease22. Valve deformation and tethering in functional MR has been also evaluated23. Moreover, it can accurately evaluate the repercussion of MR, such as cavity dilatation, including left atrial volume24. Cardiac CT has shown encouraging early results to assess MR severity, although its performance in this area is still currently limited: comparison between right and left stroke volumes to determine regurgitant volume and fraction can be useful, but rely on the assumptions that there is no shunt and other valves are competent25, 26. Direct planimetry of regurgitant orifice area has been described with variable results compared to other quantitative parameters27, 28, but this method is inherently limited by the dynamic nature of mitral regurgitation throughout systole5, 29 and might not be practicable for patients with complex and/or multiple regurgitant orifices. The use of 3D parameters such as closure, annulus and total leaflet areas can add a comprehensive evaluation of the mitral valve in the setting of cardiomyopathy, taking into account the impact of LV geometry changes on the valve as well as the leaflet adaptive response to these changes. Although echocardiography remains the modality of choice to assess mitral valve, we show here that cardiac CT can provide reliable and advanced information on valve size and geometry; this modality can therefore be considered in patients with limited echocardiographic windows. Cardiac CT can also add useful information on MR mechanism and valve geometry in patients who have CT for coronary artery evaluation.
Limitations
Despite the majority (88%) of the retrospectively-gated CT studies were performed with ECG-tube modulation, we found excellent reproducibility and correlation between CT and 3D Echo. In the validation study, some patients had delays between 3D echo and CT which could affect the results. However all patients with heart failure had their CT and standard 2D echo for MR quantification the same day. Cardiac CT represents a potential risk from ionizing radiation; however the radiation doses are expected to be consistently lower in the near future as the technology evolves. Advantages of cardiac CT over either 2D or 3D Echo are less operator dependency, no issues with adequate acquisition windows and better spatial resolution. Because FMR and no-FMR patients were selected on the basis of similar LV size and function, the present study cannot assess the relative contributions of other key parameters of FMR such as annulus size, contraction or tethering distances. This selection can also explain the lack of significant difference in tenting volume and area between patients with and without FMR. However, our study design allows us to explore the key question of why patients with similar LV remodeling have variable MR severity. Although the use of 3D derived area such as total leaflet area and mitral closure area are useful in understanding mechanisms of MV disease and find new biological phenomenon such as mitral valve adaptation, their role in routine clinical practice needs to be defined.
Conclusion
Cardiac CT can be used to provide comprehensive and detailed reconstructions of the mitral valve. These measurements are helpful to understand why some patients have different severity of functional MR despite similar LV dilatation. Further clinical and experimental studies looking at mechanisms leading to or limiting mitral valve compensatory enlargement are needed.
Clinical perspective.
Functional mitral regurgitation is a common and morbid complication of ischemic and non-ischemic cardiomyopathies. Its mechanisms have been attributed to left ventricle and mitral annulus remodeling, while the mitral leaflets have been typically considered normal and only passively involved. However, recent data suggest that leaflet tissue is able to actively enlarge in response to mechanical stretch. This suggests that functional mitral regurgitation should not be viewed only as a disease of the left ventricle, but rather a mismatch between the remodeling ventricle and compensatory leaflet enlargement. Since valve size is dynamic and has the potential to become a therapeutic target, it is important to develop tools to assess this variable in addition to left ventricle parameters when studying this disease. In this manuscript, we show that mitral leaflet size, geometry and their relation to left ventricular and annular parameters can also be assessed reliably with cardiac CT. We also use cardiac CT to demonstrate that despite similar LV size and function, some patients have variable leaflet compensation, leading to different degree of mitral regurgitation. Three-dimensional imaging will play a key role in future studies, trying to find the mechanisms and factors underlying valve adaptation.
Acknowledgments
Sources of Funding
Dr Beaudoin is funded by an AHA-Founders Affiliate post-doctoral fellowship grant 10POST4580055, Dallas, Tx. Dr. Truong received support from NIH grants K23HL098370 and L30HL093896. Dr Levine received support from grant 07CVD04 of the Leducq Foundation, Paris, France, for the Leducq Transatlantic MITRAL Network, and by NIH K24 HL67434 and R01 HL109506.
Disclosures
Dr. Truong receives research grants from Qi Imaging, LLC (formerly Ziosoft) and St. Jude Medical.
Abbreviations list
- MR
Mitral regurgitation
- LV
Left ventricle
- 3D
Three-dimensional
- MV
Mitral valve
- CT
Computed Tomography
- CMP
Cardiomyopathy
- ICC
Intraclass Correlation Coefficient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: A population-based study. The Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Godley RW, Wann LS, Rogers EW, Feigenbaum H, Weyman AE. Incomplete mitral leaflet closure in patients with papillary muscle dysfunction. Circulation. 1981;63:565–571. doi: 10.1161/01.cir.63.3.565. [DOI] [PubMed] [Google Scholar]
- 3.Gorman RC, McCaughan JS, Ratcliffe MB, Gupta KB, Streicher JT, Ferrari VA, St John-Sutton MG, Bogen DK, Edmunds LH. Pathogenesis of acute ischemic mitral regurgitation in three dimensions. The Journal of thoracic and cardiovascular surgery. 1995;109:684–693. doi: 10.1016/S0022-5223(95)70349-7. [DOI] [PubMed] [Google Scholar]
- 4.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation : Leaflet restriction versus coapting force: In vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 5.Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: Physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol. 1999;33:538–545. doi: 10.1016/s0735-1097(98)00570-1. [DOI] [PubMed] [Google Scholar]
- 6.Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation. An experimental evaluation. Circulation. 1991;84:2167–2180. doi: 10.1161/01.cir.84.5.2167. [DOI] [PubMed] [Google Scholar]
- 7.Komeda M, Glasson JR, Bolger AF, Daughters GT, MacIsaac A, Oesterle SN, Ingels NB, Miller DC. Geometric determinants of ischemic mitral regurgitation. 1997 [PubMed] [Google Scholar]
- 8.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. Journal of the American College of Cardiology. 1992;20:1594–1598. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 9.Kono T, Sabbah HN, Stein PD, Brymer JF, Khaja F. Left ventricular shape as a determinant of functional mitral regurgitation in patients with severe heart failure secondary to either coronary artery disease or idiopathic dilated cardiomyopathy. The American Journal of Cardiology. 1991;68:355–359. doi: 10.1016/0002-9149(91)90831-5. [DOI] [PubMed] [Google Scholar]
- 10.Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: Quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. The Journal of thoracic and cardiovascular surgery. 2003;125:135–143. doi: 10.1067/mtc.2003.78. [DOI] [PubMed] [Google Scholar]
- 11.Otsuji Y, Handschumacher MD, Liel-Cohen N, Tanabe H, Jiang L, Schwammenthal E, Guerrero JL, Nicholls LA, Vlahakes GJ, Levine RA. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: Three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. Journal of the American College of Cardiology. 2001;37:641–648. doi: 10.1016/s0735-1097(00)01134-7. [DOI] [PubMed] [Google Scholar]
- 12.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song J-K, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation : Direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 13.Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, Lessana A, D'Allaines C, Blondeau P, Piwnica A, Dubost C. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg. 1980;79:338–348. [PubMed] [Google Scholar]
- 14.Levine R, Triulzi M, Harrigan P, Weyman A. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75:756–767. doi: 10.1161/01.cir.75.4.756. [DOI] [PubMed] [Google Scholar]
- 15.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: Occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaput M, Handschumacher MD, Guerrero JL, Holmvang G, Dal-Bianco JP, Sullivan S, Vlahakes GJ, Hung J, Levine RA for the Leducq Foundation MTN. Mitral leaflet adaptation to ventricular remodeling: Prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120:S99–S103. doi: 10.1161/CIRCULATIONAHA.109.844019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, Levine RA, Carpentier A. Active adaptation of the tethered mitral valve: Insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120:334–342. doi: 10.1161/CIRCULATIONAHA.108.846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by doppler color flow mapping predicts severityof mitral regurgitation : Clinical studies. Circulation. 1995;91:746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. Journal of the American Society of Echocardiography. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 20.Kunzelman KS, Cochran RP. Stress/strain characteristics of porcine mitral valve tissue: Parallel versus perpendicular collagen orientation. Journal of Cardiac Surgery. 1992;7:71–78. doi: 10.1111/j.1540-8191.1992.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 21.May-Newman K, Yin FC. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol Heart Circ Physiol. 1995;269:H1319–H1327. doi: 10.1152/ajpheart.1995.269.4.H1319. [DOI] [PubMed] [Google Scholar]
- 22.Killeen RP, Arnous S, Martos R, Abbara S, Quinn M, Dodd JD. Chronic mitral regurgitation detected on cardiac mdct: Differentiation between functional and valvular aetiologies. European radiology. 2010;20:1886–1895. doi: 10.1007/s00330-010-1760-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Kaji S, An Y, Yoshitani H, Takeuchi M, Levine RA, Otsuji Y, Furukawa Y. Mechanism of asymmetric leaflet tethering in ischemic mitral regurgitation: 3d analysis with multislice ct. JACC. Cardiovascular imaging. 2012;5:230–232. doi: 10.1016/j.jcmg.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avelar E, Durst R, Rosito GA, Thangaroopan M, Kumar S, Tournoux F, Chan RC, Hung J, Hoffmann U, Abbara S, Brady T, Cury RC. Comparison of the accuracy of multidetector computed tomography versus two-dimensional echocardiography to measure left atrial volume. Am J Cardiol. 2010;106:104–109. doi: 10.1016/j.amjcard.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y-K, Yang Z-G, Ning G, Rao L, Dong L, Pen Y, Zhang T-M, Wu Y, Zhang X-C, Wang Q-L. Isolated mitral regurgitation: Quantitative assessment with 64-section multidetector ct—comparison with mr imaging and echocardiography1. Radiology. 2009;252:369–376. doi: 10.1148/radiol.2522081714. [DOI] [PubMed] [Google Scholar]
- 26.Lembcke A, Borges AC, Dohmen PM, Hoffmann U, Hermann KG, Kroencke TJ, Fischer T, Hamm B, Enzweiler CN. Quantification of functional mitral valve regurgitation in patients with congestive heart failure: Comparison of electron-beam computed tomography with cardiac catheterization. Investigative radiology. 2004;39:728–739. doi: 10.1097/00004424-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Arnous S, Killeen RP, Martos R, Quinn M, McDonald K, Dodd JD. Quantification of mitral regurgitation on cardiac computed tomography: Comparison with qualitative and quantitative echocardiographic parameters. Journal of computer assisted tomography. 2011;35:625–630. doi: 10.1097/RCT.0b013e31822d28b8. [DOI] [PubMed] [Google Scholar]
- 28.Alkadhi H, Wildermuth S, Bettex DA, Plass A, Baumert B, Leschka S, Desbiolles LM, Marincek B, Boehm T. Mitral regurgitation: Quantification with 16–detector row ct—initial experience1. Radiology. 2006;238:454–463. doi: 10.1148/radiol.2381042216. [DOI] [PubMed] [Google Scholar]
- 29.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: Clinical data and experimental testing. Circulation. 1994;90:307–322. doi: 10.1161/01.cir.90.1.307. [DOI] [PubMed] [Google Scholar]