Abstract
The present study was conducted to determine whether fetuin-A, a dominant serum protein plays a role in chemo-attraction and chemo-invasion of carcinoma cells in vitro. Serum is normally used as positive chemotaxis control in Boyden chamber motility assays, prompting the need to identify the factor/s in serum that contributes the bulk of chemo-taxis and invasion. Serum has a plethora of chemotactic factors including stromal derived factor 1 also known as CXCL12. Using highly purified fetuin-A, we compared its chemo-attraction potential to culture medium containing 10% fetal bovine serum. We also investigated its ability to attract tumor cells through a bed of Matrigel (invasion assay). We demonstrated, using similar concentration range of fetuin-A found in blood, that it robustly supports both directed chemo-attraction and invasion of breast tumor cells. More importantly, we showed that at low concentrations (fetuin-A coated wells) itinteracts synergistically with CXCL12 to promote chemotaxis. The presence of plasminogen (PL) blunted the fetuin-A mediated chemotaxis. Taken together, the data suggest an in vivo chemotaxis/invasion role for fetuin-A.
Keywords: Fetuin-A, Chemo-attraction, Chemo-invasion, Gradient, Exosomes, Chemotaxis
1. Introduction
Fetuin-A (Ahsg) is one of the negative acute phase proteins in humans, mainly produced by the liver and then secreted into the blood where its concentration in adult serum is approximately 0.5 mg/ml [1,2]. It can also be synthesized in other organs such as kidneys and tongue [3]. Its established physiological role is the inhibition of ectopic calcification [4], but mounting evidence suggests that it is a multifunctional protein capable of modulating a number of critical signaling pathways. For example, it has a motif that has a striking similarity with TGF-β receptor II [5], and can function as an extracellular decoy to block TGF-β signaling [6]. It also interferes with insulin signaling suggesting a role in the etiology of diabetes and other metabolic diseases [7].
We have followed the potential role of fetuin-A in breast cancer initiation and progression for a number of years. We previously demonstrated that in fetuin-A knockout and polyoma middle-T (PyMT) transgenic mice, TGF-β signaling using phospho-SMAD2/3 as readouts is active and the mice are protected from developing mammary tumors [6]. We also demonstrated that colonization of lungs by Lewis lung carcinoma cells is blunted in the fetuin-A null C57/BL-6 mice [8]. Furthermore we demonstrated that purified fetuin-A mediates the adhesion of breast carcinoma cells in vitro which then activates growth and survival signals [9]. Lastly, we demonstrated that fetuin-A mediates adhesion via a novel mechanism involving cellular exosomes [10].
In the presentstudy, we questioned whether or not fetuin-A (a serum protein) is a chemo-attractant in directed in vitro chemo-taxis assays. In addition we questioned whether it would attract tumor cells through a bed of Matrigel (chemo-invasion) when it is in the bottom wells of Boyden assay plates. Serum or culture mediums containing 5–10% fetal bovine serum (commonly referred to as complete medium) are routinely used in Boyden chamber motility assays as positive control. Serum consists of a myriad of chemokines and extracellular matrix adhesion proteins like fibronectin and laminin that are known to attract tumor cells [11]. The concentration of fetuin-A in complete medium containing 10% fetal bovine serum is approximately 2 mg/ml. Interestingly, the fetuin-A mediated adhesion via cellular exosomes, is blunted by plasminogen [10]. We have therefore exploited this biochemical property of plasminogen to suggest a mechanism for fetuin-A mediated chemo-attraction.
2. Materials and methods
2.1. Materials
Boyden chambers (motility and invasion assay kits) were purchased from BD Biosciences (Bedford, MA). Pedersen fetuin-A purchased from Sigma–Aldrich (St. Louis, MO) was further purified using a glycerol step gradient to remove alpha 2-macroglobulin and other contaminants as described [9]. Antibiotic–antimycotic and cell media were purchased from Life Technologies (Grand Island, NY). All other reagents were purchased from Sigma–Aldrich (St. Louis, MO) unless otherwise stated.
2.2. Cells
The breast carcinoma cell line, a sub-clone of BT-549 that expresses high levels of galectin-3 and has a high metastatic potential, was kindly donated to us by Dr. Avraham Raz of the Karmanos Cancer Research Institute, Detroit, MI. The colon cancer cell lines, DKO-1 and DKs-8 were kindly donated to us by Dr. Robert Coffey of Vanderbilt University. The cells were routinely maintained in Dulbeccos modified Eagles medium/nutrient F-12 (DMEM/F12) supplemented with 10% heat inactivated fetal bovine serum (FBS), antibiotic–antimycotic (50 units/ml), and incubated at 37 °C in 5% CO2 and 95% air. Where indicated, serum-free medium (SFM) consisting of DMEM/F12 supplemented with only the antibiotic–antimycotic, and 0.1% BSA was used.
2.3. Fetuin-A purification
The Pedersen fetuin-A, purchased from Sigma was further purified by glycerol step gradient as described [9]. Briefly, glycerol gradients (10–15–30–45–60%) were made in 13-ml ultracentrifuge tubes from 60% glycerol buffered with 10 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 0.5 mM MgCl2. About 10 mg of Pedersen fetuin-A in a volume of 1 ml was layered on the step gradient. This was centrifuged at 35,000 rpm (Beckman, SW40Ti rotor) for 18 h at 4 °C. Gradients were developed from the top and resolved in 4–12% SDS–PAGE gels.
2.4. Chemotaxis assays
In these assays, fetuin-A and other chemo-attractants were added to the lower wells of a Boyden plates. As positive controls, the lower wells had 500 μl of DMEM/F12 culture medium supplemented with 10% v/v of fetal bovine serum. This mix is referenced throughout the text as ‘complete medium’ (CM). The negative control lower wells contained 500 μl of SFM containing 0.1% BSA and hereafter only referred to as SFM. The cells were then added to the upper wells (25,000 cells in 500 μl of SFM) and incubated at 37 °C for 24 h. During this incubation, migration competent cells migrated through the 8 μm pores of the polycarbonate filter inserts to the underside of the filters in direct contact with the contents of the lower wells. The non-migrated cells on the top surface of the polycarbonate filter were scrapped off with a cotton swab, while cells attached to the lower surface or the underside were fixed in 4% formalin for 30 min and stained with 0.5% (w/v) crystal violet in 25% methanol. Migrated cells on the lower surface of the filter were enumerated under a microscope and data reported as mean number of migrated cells/field (200×).
To determine whether fetuin-A can synergize with stromal derived factor-1 (SDF-1)/CXCL12 to mediate motility, lower wells were coated with fetuin-A at 0.3 mg/ml or 0.6 mg/ml overnight at 4 °C. Other lower wells had 50, 100 or 150 ng/ml of CXCL12 in SFM (500 μl/well). The fetuin-A coated wells were washed once and reconstituted with either SFM (500 μl) alone or SFM containing 100 ng/ml of SDF-1. The positive control wells contained 500 μl of complete medium and negative control wells SFM.
To demonstrate that the movement of cells to the lower wells was driven by the fetuin-A gradient, the assays were repeated in the presence and absence of fetuin-A gradient. To collapse the gradient (absence of gradient), increasing concentrations of fetuin-A were added only to the upper wells of the Boyden plates. The lower wells in this case contained 500 μl of serum-free medium.
We previously demonstrated that fetuin-A mediates cellular adhesion via exosomes. This adhesion mechanism can be blunted by plasminogen (PL) [10]. Since chemotaxis is mainly driven by adhesion interactions, we therefore assessed the role of PL in fetuin-A mediated chemotaxis. PL (50 and 500 μg/ml) was added to the upper wells in SFM together with the cells while the lower wells contained either fetuin-A (1 mg/ml) or complete medium and assays performed as described above.
Chemo-invasion assays, were performed using invasion wells (BD Biosciences, Bedford, MA), according to manufacturer’s protocols. Briefly, the Matrigel coated wells were removed from storage at −20 °C and re-hydrated with warm SFM for 2 h at 37 °C in a 5% CO2 humidified incubator. Cells (25,000/well) were added to the top wells in SFM. The bottom wells contained either SFM, complete (CM) or fetuin-A (1 mg/ml). The cells were allowed to invade for 24 h and the cells penetrating to the underside of the filters enumerated as above. We also compared the chemo-invasion potential of three serum proteins, fibronectin, fetuin-A and laminin.
3. Results
3.1. Fetuin-A is a major chemo-attractant for breast and colon cancer cells
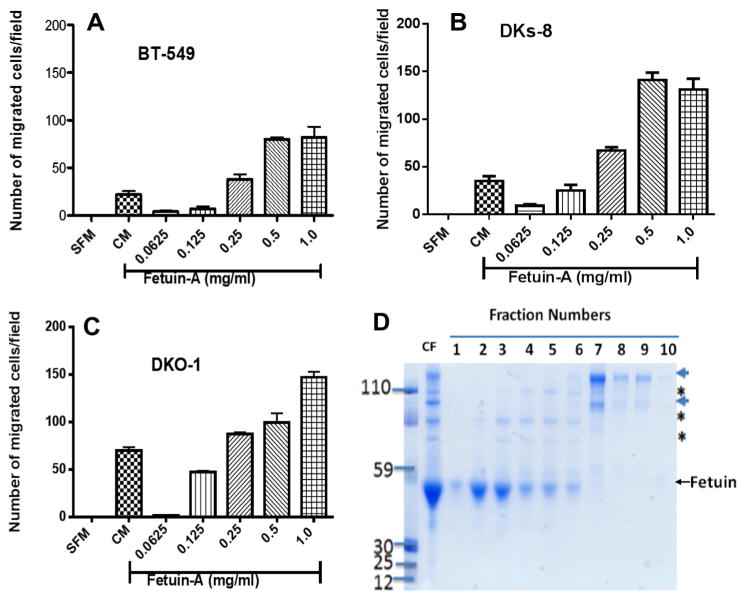
Having demonstrated that fetuin-A is a significant adhesion protein in serum [9,10], we questioned whether it could attract tumor cells from the upper wells when it is in the lower wells of the Boyden chemotaxis plates. We reasoned that fetuin-A from the lower wells would diffuse to the upper wells according to the concentration gradients through the pores, enter the cells and trigger the release of fetuin-A exosomes from them. The adhesion competent exosomes [10], would then mediate adhesion of the cells followed by motility, moving towards a higher concentration of fetuin-A in the lower wells. As shown in Fig. 1A, purified fetuin-A in SFM at 1 mg/ml is better at chemo-attraction of BT-549 cells when compared to complete medium (CM) containing 10% FBS. To confirm this observation in other cell types, we demonstrated that colon cancer cell lines, DKs-8 (Fig. 1B) or DKO-1 (Fig. 1C) also responded by moving towards higher concentrations of fetuin-A. The colloidal Coomassie gel showing fractions (1 and 2) from glycerol step gradient of Pedersen fetuin-A which were used in the assays is shown in Fig. 1D.
Fig. 1.
Fetuin-A is a chemo-attractant for breast and colon carcinoma cells. The cells, BT-549 (Panel A) or Colon Cancer (Panels B and C) were added to the upper wells (25,000 cells/well in 500 μl of SFM) of a Boyden chamber plates. The lower wells contained 500 μl of either SFM (negative control) or SFM containing 10% fetal bovine serum (CM), representing positive control. The experimental wells contained 500 μl of fetuin-A (0.0625–1 mg/ml) dissolved in SFM. The cells were incubated overnight at 37 °C in a humidified incubator. The cells remaining on the top wells/chambers were gently scraped off using cotton swabs and the cells that migrated to the underside of the inserts were fixed in 4% formalin and then stained with crystal violet at 37 °C for 4 h. The inserts were then washed in water, air dried and read under an inverted microscope equipped with a digital camera. The graphs represent the mean number of cells/field of three separate experiments ± SD The fetuin-A used in these experiments was the Pedersen fetuin-A, purified by glycerol step gradient [9] (Panel D). The arrow-heads and stars represent impurities which include alpha-2-macroglobulins (arrows) and inter-α(globulin) inhibitor H2 (ITIH2) represented by asterisks as determined by proteomics analysis. Only pure fractions #1 or #2 were used in these experiments.
3.2. Fetuin-A interacts synergistically with CXCL12 to attract tumor cells
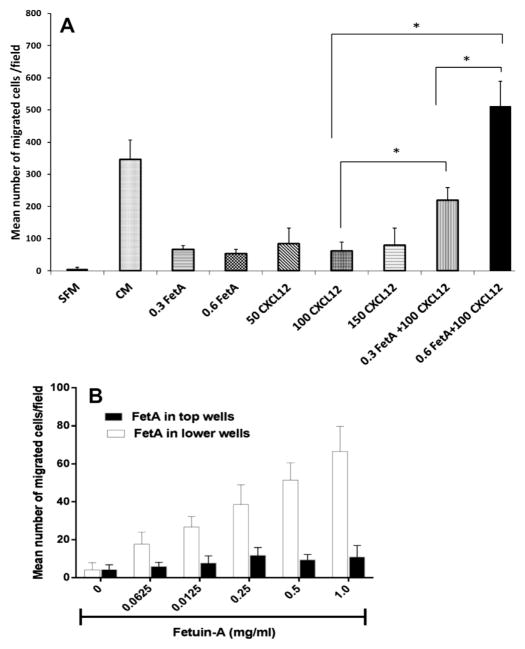
We questioned whether Boyden wells coated with fetuin-A (0.3–0.6 mg/ml), signifying a lower fetuin-A gradient compared to wells containing only CXCL12 (50–150 ng/ml) would produce enough gradient to attract tumor cells. We determined that wells coated with this concentration range of fetuin-A as well as wells containing only CXCL12 (50–150 ng/ml) supported modest but significant chemo-attraction in comparison to SFM control. Interestingly, wells coated with fetuin-A (0.3 mg/ml or 0.6 mg/ml) containing 100 ng/ml of CXCL12 demonstrated a dramatic increase in the attraction of cells, suggesting synergy (Fig. 2A). This experiment suggested that fetuin-A modulates SDF-1/CXCL12 signaling.
Fig. 2.
Fetuin-A interacts synergistically with CXCL12 to attract tumor cells. In panel A, chemo-attraction assay was performed as described in Section 2 using the breast carcinoma cell line, BT-549. The lower wells were coated with either 0.3 or 0.5 mg/ml of fetuin-A in SFM overnight at 4 °C, washed and the wells reconstituted with either 500 μl of SFM or CXCL12 (100 ng/ml) in SFM. Other lower wells contained only indicated concentrations of CXCL12 (50–150 ng/ml) in SFM. The BT-549 cells (25,000 cells/well) in 500 μl of SFM were then added to the upper wells and their attraction towards the respective lower wells monitored. The bars represent the means of three separate experiments under the same conditions ± SD (one way ANOVA *P < 0.001). In panel B, the chemo-attraction assay was repeated with increasing concentrations of fetuin-A (0–1 mg/ml) in 500 μl of SFM in the lower wells (open bars). BT-549 cells (25,000 cells/chamber) were added to the upper wells in 500 μl of SFM without (open bars) or containing fetuin-A (0–1 mg/ml) (solid bars). When fetuin-A was in the top wells with cells, the lower wells contained 500 μl of SFM. The bars represent mean number of cells per microscope field of three separate experiments.
It was determined that the movement of cells from the upper wells through the 8 μm pores was strictly a directed motility with minimal to negligible random movement (Fig. 2B).
3.3. Abrogation of fetuin-A mediated chemotaxis by plasminogen
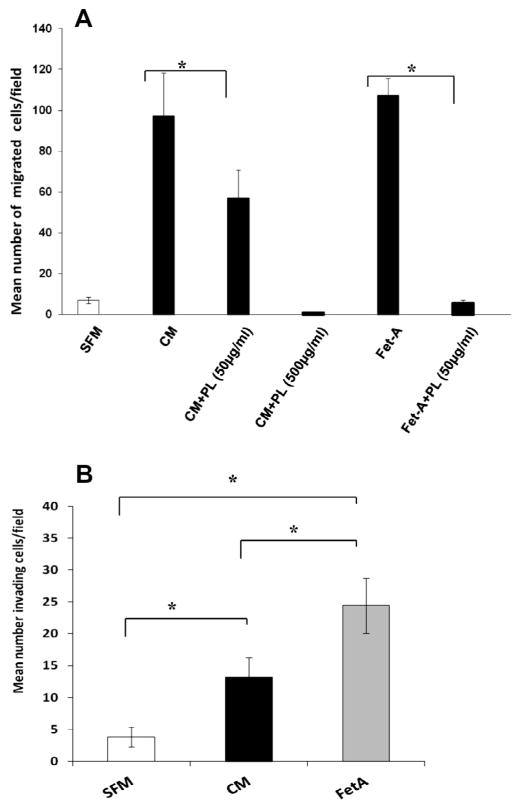
We previously demonstrated that plasminogen (PL) blunted both fetuin-A and exosomal mediated adhesion [10], and so we reasoned here that if exosomes are involved in chemo-attraction, addition of PL to the upper Boyden wells in the assay should blunt the motility. The data indeed show that PL at a concentration of 50 μg/ml in the upper wells blunted the migration of cells in the presence of either complete medium (CM) or fetuin-A (Fet-A) (1 mg/ml) in SFM in the lower wells (Fig. 3A). The inhibition was more drastic in the case of fetuin-A where this concentration of PL eliminated nearly all motility. In the case of CM, motility was completely abrogated at a PL concentration of 500 μg/ml (Fig. 3A).
Fig. 3.
Regulation of fetuin-A mediated chemo-attraction by plasminogen and the dual abilities of fetuin-A to attract and promote invasion of tumor cells through Matrigel. Panel A, the chemo-attraction assays were done essentially as described in Section 2. The BT-549 cells (25,000 cells/well) were added to the upper wells of Boyden chemotaxis wells in 500 μl of SFM without or with the indicated concentrations of plasminogen (PL) and placed over lower wells containing either 500 μl of CM or fetuin-A (1 mg/ml) respectively. Panel B, the lower wells of Boyden invasion chambers contained either 500 μl of SFM, CM, or purified fetuin-A (1 mg/ml) in SFM. The BT-549 cells (25,000 cells/well in 500 μl of SFM) were added to the upper wells containing Matrigel. The error bars in A and B represent the means of three separate experiments under the same conditions ± SD (one way ANOVA *P < 0.001).
3.4. Fetuin-A supports robust chemo-invasion of tumor cells through a Matrigel bed
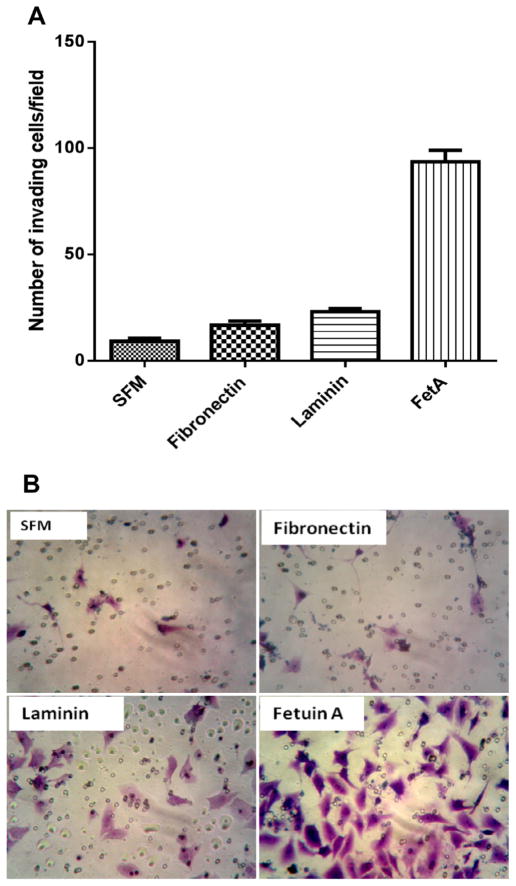
We next questioned whether fetuin-A had the dual potential of attracting and aiding the cells in their migration through Matrigel (representing basement membrane). We initially compared the ability of cells to invade Matrigel bed when SFM, CM, or fetuin-A (FetA) were in the lower wells. Interestingly, fetuin-A was better than complete medium (CM) in mediating directed chemo-invasion (Fig. 3B). Since other serum proteins such as fibronectin and laminin also have the ability to mediate directed chemotaxis due to their adhesive roles, we questioned their abilities to support chemo-invasion when placed in the lower wells. Of the three proteins, only fetuin-A was able to promote robust chemo-invasion (Fig. 4).
Fig. 4.
Fetuin-A promotes robust chemo-invasion of tumor cells through Matrigel bed while fibronectin and laminin do not. In Panel A, the lower wells of chemoinvasion assay plates had 500 μl of SFM (control), 500 μl of SFM containing fibronectin (30 μg/ml), laminin (30 μg/ml) and fetuin-A (1 mg/ml) The BT-549 cells were then added to the top wells (25,000 cells/well in 500 μl of SFM) and placed on top of the lower wells. The cells were then allowed to penetrate the Matrigel bed for 24 h and the cells invading and migrating to the underside of the coated filters enumaraged as described in Section 2. The bars represents the average number of invasive cells per field from two separate experiments. Panel B is a representative image of cells that successfully invaded the Matrigel and migrated to the underside of coated filters when the three proteins were in the bottom wells of the chemomoinvasion assay plate.
4. Discussion
In the present studies, we show for the first time, the ability of fetuin-A to attract tumor cells along its concentration gradient and to support invasion of cells through Matrigel coated inserts. Whereas its purported role in cellular adhesion was controversial for a number of years due to the presence of contaminating proteins in fetuin-A preparations [1], we recently demonstrated that highly purified form of the Pedersen fetuin-A retains cellular adhesiveness and promotes the growth of tumor cells through a novel mechanism which involves cellular exosomes [10].
Previous data from our laboratory demonstrated that fetuin-A could be internalized by breast carcinoma cells [9]. The uptake or endocytosis of fetuin-A by vascular smooth muscle cells was also reported by others [12]. Based on the present work, we hypothesize that exosomes that are secreted subsequent to the fetuin-A uptake by tumor cells, promote adhesion, motility and invasion of these cells towards regions of high fetuin-A concentration such as blood vessels in vivo. In support of this idea, exosomes derived from colon cancer cells that contain amphiregulin have been shown to mediate adhesion, motility and invasion of these cells [13]. Furthermore, there could be another fetuin-A gradient established in organs such as the liver and bone [14] that may have a higher concentration of fetuin-A relative to the blood vessels. This gradient would then attract tumor cells from the vessels to theirnew home (liver or bone marrow). Thus fetuin-A could be a vital component of the ‘good soil’ that prepares metastatic niches for tumor cells [15].
Another interesting mechanism(s) suggested by the data, is that fetuin-A even at a very low concentration, can synergize with SDF-1/CXCL12 to enhance the latter’s chemotactic ability [16]. This may involve the trafficking and or desensitization of the CXCR4 (the SDF-1/CXCL12 receptor) by fetuin-A [17]. The treatment of kidney HEK 293 cells with 10 nM of SDF1/CXCL12 elicited an increase in intracellular Ca2+ and phosphorylation of cortactin only at its Tyr421 [18]. Interestingly, we have also shown that fetuin-A is capable of eliciting similar increases in intracellular Ca2+ in breast tumor cells [10]. We previously demonstrated that some of the signaling pathways activated when fetuin-A interacts with cells are PI3/Akt and MAP kinase [9]. So far, no cell surface receptor that may transmit these signals has been identified for fetuin-A. It may activate these signaling pathways as a consequence of its ability to increase intracellular calcium levels once it is taken up by tumor cells [10]. Thus, fetuin-A even at very low concentrations, may activate more than one pathways germane to chemotaxis such as secretion of adhesion and motility competent exosomes and regulation of CXCL12–CXCR4 axis [19]. This further underscores the need to fully understand these mechanisms to enable us design small molecules that can abrogate themovement of met-astatic cells.
The inhibition of fetuin-A mediated chemo-attraction/motility by PL is significant in that it supports the hypothesized role of exosomes in the chemo-attraction and invasion mechanisms reported herein. We previously reported that fetuin-A as well as exosomes secreted by tumor cells in the presence of fetuin-A, had the capacity to mediate adhesion that could be abrogated by plasminogen [10]. This then suggested that fetuin-A even at low concentrations approaching 0.1 mg/ml could be taken-up by tumor cells and signal the secretion of adhesion competent exosomes which subsequently mediate adhesion and cell spreading. The blood plasminogen concentration of ~0.1 mg/ml [20], would interfere with exosomal mediated adhesion or motility according to the data presented herein. Interestingly, once metastatic tumor cells enter blood vessels during intravasation, they normally come together and form clumps sometimes with the aid of platelets (platelet aggregation) necessary to protect them from the hostile environment in the blood vessels [21,22]. However, during the process of extravasation, the tumor emboli has to somehow adhere to the endothelial cells in much the same as neutrophils do when they extravasate from the blood vessels to the site of tissue injury. Here most likely the tumor cells use either lectins such as selectins and or integrins [23].
The data demonstrate that fetuin-A is not only a powerful chemo-attractant for tumor cells, more importantly it accelerates the invasion of the Matrigel coated inserts by the cells. A number of serum proteins including laminin and fibronectin are excellent chemo-attractants [11]. However, the present data suggest that fetuin-A is the dominant serum chemo-attractant that also supports invasion through extracellular matrix, and warrants further in vivo studies to establish it as a major player in tumor metastasis. We previously demonstrated that one of the functions of fetuin-A and other members of the cystatin family is to protect matrix metalloproteinases (MMPs) from autolysis enabling them to remain active for a long time outside cells [24]. We believe the established fetuin-A gradient protected and maintained the activity of MMPs secreted at the invasive front of the cells and which in turn accelerated their invasion.
In summary our data suggest that fetuin-A is a major chemo-attractant in sera or blood and that tumor cells have the potential to follow its concentration gradient from the primary sites to the nearest blood vessel and most likely from the blood vessels to the secondary sites of growth during extravasation in the metastatic process. Furthermore, fetuin-A may also synergize with the traditional chemo-attractants such as SDF-1/CXCL12 to mediate the chemotaxis of tumor cells. Lastly, fetuin-A is not only a chemo-attractant, it also supports robust invasion of tumor cells through extracellular matrices represented by Matrigel. This suggested dual roles of fetuin-A would make it an ideal attractant for metastatic cells into and from blood vessels through extracellular matrices.
Acknowledgments
This study was supported by National Institutes of Health grants: 3SC1CA134018-04S1 (J.O.); P50 CA095103; G12 MD007586; 5 T32 HL007735-15.
References
- 1.Nie Z. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992;263:C551–C562. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- 2.Brown WM, Saunders NR, Mollgard K, Dziegielewska KM. Fetuin – an old friend revisited. Bioessays. 1992;14:749–755. doi: 10.1002/bies.950141105. [DOI] [PubMed] [Google Scholar]
- 3.Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J. 2003;376:135–145. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 6.Guillory B, Sakwe AM, Saria M, Thompson P, Adhiambo C, Koumangoye R, Ballard B, Binhazim A, Cone C, Jahanen-Dechent W, Ochieng J. Lack of fetuin-A (alpha2-HS-glycoprotein) reduces mammary tumor incidence and prolongs tumor latency via the transforming growth factor-beta signaling pathway in a mouse model of breast cancer. Am J Pathol. 2010;177:2635–2644. doi: 10.2353/ajpath.2010.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasul S, Wagner L, Kautzky-Willer A. Fetuin-A and angiopoietins in obesity and type 2 diabetes mellitus. Endocrine. 2012;42:496–505. doi: 10.1007/s12020-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 8.Kundranda MN, Henderson M, Carter KJ, Gorden L, Binhazim A, Ray S, Baptiste T, Shokrani M, Leite-Browning ML, Jahnen-Dechent W, Matrisian LM, Ochieng J. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005;65:499–506. [PubMed] [Google Scholar]
- 9.Sakwe AM, Koumangoye R, Goodwin SJ, Ochieng J. Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J Biol Chem. 2010;285:41827–41835. doi: 10.1074/jbc.M110.128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson K, Koumangoye R, Thompson P, Sakwe AM, Patel T, Pratap S, Ochieng J. Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading. FEBS Lett. 2012;586:3458–3463. doi: 10.1016/j.febslet.2012.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy JB, Furcht LT. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984;98:1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen NX, O’Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–F606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 13.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triffitt JT, Gebauer U, Ashton BA, Owen ME, Reynolds JJ. Origin of plasma alpha2HS-glycoprotein and its accumulation in bone. Nature. 1976;262:226–227. doi: 10.1038/262226a0. [DOI] [PubMed] [Google Scholar]
- 15.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–2287. [PubMed] [Google Scholar]
- 16.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 17.Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 18.Luo C, Pan H, Mines M, Watson K, Zhang J, Fan GH. CXCL12 induces tyrosine phosphorylation of cortactin, which plays a role in CXC chemokine receptor 4-mediated extracellular signal-regulated kinase activation and chemotaxis. J Biol Chem. 2006;281:30081–30093. doi: 10.1074/jbc.M605837200. [DOI] [PubMed] [Google Scholar]
- 19.Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 20.Cederholm-Williams SA. Concentration of plasminogen and antiplasmin in plasma and serum. J Clin Pathol. 1981;34:979–981. doi: 10.1136/jcp.34.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss L, Ward PM. Cell detachment and metastasis. Cancer Metastasis Rev. 1983;2:111–127. doi: 10.1007/BF00048965. [DOI] [PubMed] [Google Scholar]
- 22.Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:189–198. doi: 10.2183/pjab/84.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray S, Lukyanov P, Ochieng J. Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim Biophys Acta. 2003;1652:91–102. doi: 10.1016/j.bbapap.2003.08.004. [DOI] [PubMed] [Google Scholar]