Abstract
Phrenic long-term facilitation (pLTF) is a form of serotonin-dependent respiratory plasticity induced by acute intermittent hypoxia (AIH). pLTF requires spinal Gq protein-coupled serotonin-2 receptor (5-HT2) activation, new synthesis of brain-derived neurotrophic factor (BDNF) and activation of its high-affinity receptor, TrkB. Intrathecal injections of selective agonists for Gs protein-coupled receptors (adenosine 2A and serotonin-7; 5-HT7) also induce long-lasting phrenic motor facilitation via TrkB “trans-activation.” Since serotonin release near phrenic motor neurons may activate multiple serotonin receptor subtypes, we tested the hypothesis that 5-HT7 receptor activation contributes to AIH-induced pLTF. A selective 5-HT7 receptor antagonist (SB-269970, 5mM, 12μl) was administered intrathecally at C4 to anesthetized, vagotomized and ventilated rats prior to AIH (3, 5-min episodes, 11% O2). Contrary to predictions, pLTF was greater in SB-269970 treated versus control rats (80±11% vs 45±6% 60 min post-AIH; p<0.05). Hypoglossal LTF was unaffected by spinal 5-HT7 receptor inhibition, suggesting that drug effects were localized to the spinal cord. Since 5-HT7 receptors are coupled to protein kinase A (PKA), we tested the hypothesis that PKA inhibits AIH-induced pLTF. Similar to 5-HT7 receptor inhibition, spinal PKA inhibition (KT-5720, 100μM, 15μl) enhanced pLTF (99±15% 60 min post-AIH; p<0.05). Conversely, PKA activation (8-br-cAMP, 100μM, 15μl) blunted pLTF versus control rats (16±5% vs 45±6% 60 min post-AIH; p<0.05). These findings suggest a novel mechanism whereby spinal Gs protein-coupled 5-HT7 receptors constrain AIH-induced pLTF via PKA activity.
Keywords: Respiratory plasticity, motor neuron, phrenic, respiratory control, long-term facilitation, hypoxia
INTRDUCTION
Plasticity is a hallmark feature of the neural system controlling breathing, and is essential for its response to repeated or prolonged physiological or environmental challenges (Mitchell and Johnson 2003). One well-studied model of respiratory plasticity is phrenic long-term facilitation (pLTF) following acute intermittent hypoxia (AIH; Mitchell et al. 2001; Feldman et al. 2003; Mahamed and Mitchell 2007). AIH-induced pLTF is a progressive, serotonin-dependent increase in respiratory motor output lasting at least 60 min following episodic stimulation of the carotid sinus nerve (Millhorn et al. 1980; Millhorn et al. 1980) or AIH (Hayashi et al. 1993; Bach and Mitchell 1996; Baker and Mitchell 2000). In our working model, AIH activates medullary raphe neurons (Erickson and Millhorn 1994; Teppema et al. 1997) with spinal projections (Lalley 1986) resulting in serotonin (5-HT) release in or near the phrenic motor nucleus (Pilowsky et al. 1990; Kinkead et al. 2001; Morris et al. 2001). Subsequent 5-HT2 receptor activation on phrenic motor neurons (Fuller et al. 2001; Baker-Herman and Mitchell 2002; MacFarlane et al. 2011) elicits signaling cascades that underlie pLTF (Dale-Nagle et al. 2010).
Activation of Gq protein-coupled serotonin type-2 (5HT2) receptors (Kinkead and Mitchell 1999; MacFarlane et al. 2011) on or near phrenic motor neurons (Basura et al. 2001) is necessary and sufficient to elicit pLTF (Fuller et al. 2001; MacFarlane and Mitchell 2009). Moreover, episodic 5-HT receptor activation elicits LTF in synaptically isolated hypoglossal motor neurons of neonatal rat rhythmogenic brainstem slice preparations (Bocchiaro and Feldman 2004), and in spinal respiratory motor output from brainstem-spinal cord preparations (cervical and thoracic LTF; Lovett-Barr et al. 2006). Cervical spinal injections of serotonin and selective 5-HT2 receptor agonists induce long-lasting phrenic motor facilitation (pMF) by a mechanism that requires NADPH oxidase activity (MacFarlane and Mitchell 2008; MacFarlane et al. 2009).
Recently, spinal serotonin type-7 (5-HT7) receptors were also shown to elicit phrenic motor facilitation (PMF) in the absence of AIH (Hoffman and Mitchell 2011). 5-HT7 receptors are necessary for full expression of AIH-induced ventilatory LTF in rats pretreated with chronic intermittent hypoxia (McGuire et al. 2004). To further understand serotonergic mechanisms giving rise to pLTF, we tested the hypothesis that serotonin released during AIH activates multiple serotonin receptor subtypes (e.g. 5-HT2 and 5-HT7), and that both contribute to AIH-induced pLTF. However, contrary to our hypothesis, spinal 5-HT7 receptor inhibition enhanced pLTF, suggesting that 5-HT7 receptor activation constrains (versus contributes to) normal mechanisms of pLTF. This enhancement is similar to that observed following intrathecal adenosine 2A receptor inhibition (Hoffman et al. 2010).
Because 5-HT7 (and adenosine 2A) receptors are coupled to Gs proteins, initiating an adenylate cyclase/cAMP/protein kinase A (PKA) signal transduction cascade (Lovenberg et al. 1993; Krobert et al. 2001), we hypothesized that PKA inhibition would enhance, whereas PKA activation would suppress pLTF. Indeed, our findings support a mechanism whereby PKA constrains pLTF via “cross-talk” inhibition of the signaling cascade initiated by Gq protein-coupled 5-HT2 receptors. A more comprehensive understanding of 5-HT receptor “cross-talk” may provide insights concerning fundamental mechanisms and the significance of respiratory plasticity.
METHODS
Experimental Studies
All experiments were performed on male Sprague–Dawley rats (Harlan colony 218a, n=56; Harlan Inc., Indianapolis, IN, USA) aged 101 ± 14 days and weighing 384 ± 33 grams. Animals were doubly housed in a controlled environment (12h light/dark cycle, daily humidity and temperature monitoring). All protocols were approved by the Institutional Animal Care and Use Committee of the School of Veterinary Medicine at the University of Wisconsin, Madison.
In a first experimental series, we tested the hypothesis that spinal 5-HT7 receptors contribute to AIH-induced pLTF by intrathecal application of a selective 5-HT7 receptor inhibitor. Since spinal 5-HT7 receptor antagonist injections enhanced pLTF, a second experimental series was designed to test whether inhibition of “downstream” spinal protein kinase A contributed to the enhancement of pLTF; in this series we delivered a selective PKA inhibitor to the cervical spinal cord. In the final experimental series, we tested the hypothesis that increased PKA activity inhibits AIH-induced pLTF by intrathecally injecting a cAMP/PKA activator. Investigators were not blinded concerning the identity of intrathecal solutions applied during experiments.
Surgical Preparation
Surgical procedures have been described in detail elsewhere (Baker-Herman et al. 2002; Baker-Herman et al. 2004) and are summarized below. Anesthesia was induced with isoflurane; isoflurane was continued during surgical preparations initially with a nose cone, and then through a tracheal cannula (2.5–3.5% in 50% 02, balance N2). Once surgical procedures were complete, rats were slowly converted to urethane anesthesia over 15 min (1.75μg/kg). Adequate anesthetic depth was tested by lack of any pressor or respiratory neural response to toe pinch with a hemostat. After conversion to urethane anesthesia, a continuous intravenous infusion was initiated (4–6.5 ml*kg−1*hr−1) of a 1:4 mixture of 6% Hetastarch (artificial colloid composed dissolved in 0.9% normal saline) and lactated Ringer’s to maintain blood volume, fluid balance and acid-base status. A tracheal cannula was placed in the neck to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA; tidal volume = 2.5ml). A rapidly responding flow-through carbon dioxide analyzer (Capnogard, Novametrix, Wallingford, CT) was placed on the expired limb of a Y-tube connected to the tracheal cannula to enable measurements of end-tidal carbon dioxide partial pressures (PETCO2). The vagus nerves were cut in the mid-cervical region to prevent entrainment of respiratory neural activity with the ventilator. During ventilation, rats were paralyzed with pancuronium bromide (2.5mg/kg). A polyethylene catheter (PE-50, Intramedic) was placed in the right femoral artery and blood pressure was monitored with a pressure transducer (Gould, P23ID). A 3-way stop-cock, attached to the arterial catheter, was used to withdraw blood samples (0.2–0.4ml) for blood gas analysis (ABL-500, Radiometer; Copenhagen, Denmark); during an experiment, blood gas determinations were made during baseline conditions, the first hypoxic episode of an LTF protocol, and at 15, 30 and 60 min post-AIH. Body temperature was monitored with rectal thermometer (Fischer Scientific) and maintained (37.5±1°C) with a heated surgical table.
The left phrenic and hypoglossal nerves were isolated using a dorsal approach, cut distally, desheathed and placed on bipolar silver electrodes to record respiratory neural activity. Phrenic and XII nerve signals were amplified (100,000x), band-pass filtered (300–10,000 Hz Model 1800, A-M Systems, Carlsborg, WA), rectified and integrated (Paynter filter; time constant, 50ms, CWE Inc., MA-821; Ardmore, PA). The resulting integrated nerve bursts were digitized (8000 Hz) and analyzed using a WINDAQ data acquisition system (DATAQ Instruments, Akron, OH).
To test whether spinal 5-HT7 receptor activation contributed to pLTF, the spinal column was exposed dorsally, followed by laminectomy and partial durotomy at cervical level 2 (C2). A soft silicone catheter (2 French; Access Technologies, IL, USA) was inserted caudally below the dura until tip was located at approximately C4. The catheter was attached to a 50 μl Hamilton syringe containing either vehicle (saline and 20 % Dimethyl sulfoxide, DMSO) or drug (dissolved in vehicle).
In our studies, hypoglossal nerve activity served as an internal control to determine whether intrathecal injections near the phrenic motor nucleus resulted in unintended drug distribution to the brainstem, as described previously (Baker-Herman et al. 2002; MacFarlane et al. 2009). Since we did not observe any changes in hypoglossal nerve activity, at doses that augmented pLTF, we conclude that each drug exerted localized effects in the cervical spinal cord versus the brainstem.
Experimental protocol
At least one hour after conversion to urethane anesthesia, apneic and recruitment thresholds were determined by increasing ventilation and lowering PETCO2 until rhythmic nerve bursts ceased (apneic threshold). After approximately one minute, the ventilator rate was slowly decreased or inspired carbon dioxide was slowly increased until rhythmic nerve bursts resumed (i.e., recruitment threshold). Baseline conditions were then established by holding PETCO2 ~2 mmHg above the recruitment threshold and allowing ≥15 min for neural activity to stabilize. At this point, an arterial blood sample was taken to document baseline blood gas levels. Throughout a protocol, arterial PCO2 was maintained isocapnic (±1.5mmHg) with respect to its baseline level by manipulation of inspired CO2 and/or ventilator rate.
All rats received slow (over 1 min) intrathecal injections of either vehicle or drug (dissolved in vehicle) five min prior to first hypoxic episode (or equivalent time for controls). Acute intermittent hypoxia (AIH) consisted of three, five min episodes of isocapnic (±1.5mmHg) hypoxia (~11% inspired O2, PaO2 = 35–45mmHg), separated by five min intervals of baseline oxygen levels (~51% inspired O2, PaO2 ≥ 150mmHg). After the third hypoxic episode, rats were returned to baseline oxygen levels and maintained for the duration of an experiment.
To test the hypothesis that spinal 5-HT7 receptors contribute to AIH-induced pLTF, a selective 5-HT7 receptor antagonist (SB269970, Tocris Biosciences, Minneapolis, MN; Perez-Garcia and Meneses 2005) was injected via an intrathecal catheter over the cervical spinal cord. In this series of experiments, SB-269970 was dissolved in vehicle to an effective concentration determined in prior experiments (Hoffman et al. 2011), and delivered five min prior to AIH. Since SB-269970 enhanced pLTF, we sought to further characterize the signaling pathway involved. Therefore we used a cell permeable PKA inhibitor (KT-5720, an ATP-site inhibitor of phosphorylation, (100 μM), dosage determined from literature; Tocris Biosciences; Kase et al. 1987; Domenici et al. 2004) to determine if pLTF is similarly enhanced. To further characterize the role of PKA, we tested the hypothesis that PKA activation inhibits pLTF by intrathecally injecting a cell-permeable cAMP analogue (8-bromoadenosine-3′, 5′-cyclic monophosphate, sodium salt {8-br-cAMP}, 12μl, 100μM; Tocris Biosciences) known to activate PKA (Meyer and Miller 1974; Kajana and Goshgarian 2008). Control rats received either vehicle or drug (SB-269970, KT-5720, 8-br-cAMP) five min prior to initiating an experimental protocol, but these rats did not receive AIH.
Data Analysis
Integrated phrenic and hypoglossal nerve burst amplitudes were averaged over one min bins at each experimental time-point, (baseline, 15, 30 and 60 min) using custom software (Courtesy of Dr. Safraaz Mahamed; LabView 6.1, National Instruments, Austin, TX, USA). Changes in nerve burst amplitude were reported as % change from baseline. Burst frequencies were expressed as an absolute change from baseline (bursts per minute). All statistical comparisons between treatment groups for nerve amplitude, mean arterial pressures, PaCO2 and PaO2 (15, 30 and 60 min post-AIH) were made using a two-way ANOVA with a repeated measures design. Since no differences were detected between hypoxic exposures (episode 1 vs 2 vs 3) within treatment groups (data not shown), comparisons were made using two-way ANOVA of phrenic burst amplitude during the fifth minute of hypoxic episodes averaged from all three episodes (Hoffman et al. 2010; Hoffman et al. 2012). All individual comparisons were made using the Student-Neuman-Keuls post-hoc test (SigmaStat 2.03, Systat Software Inc., San Jose, CA, USA). Differences between groups were considered significant if p<0.05. All values are expressed as means ± 1 S.E.M.
RESULTS
Spinal 5-HT7 receptor inhibition enhances phrenic LTF
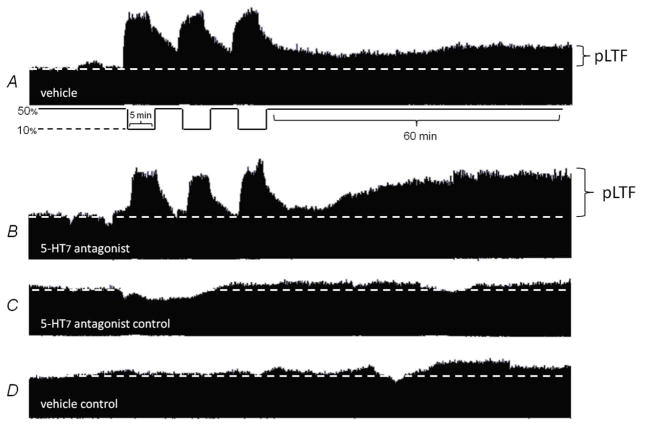
Since spinal 5-HT7 receptors have been implicated in respiratory plasticity (McGuire et al. 2004; Hoffman et al. 2011) we originally hypothesized that 5-HT7 receptors contribute to AIH-induced pLTF. Our results, however, indicate that 5-HT7 receptors actually inhibit pLTF. Since the peak amplitude of integrated inspiratory phrenic nerve bursts strongly correlates with tidal volume and respiratory muscle activity in spontaneously breathing animals (Eldridge 1976), we used these measures as an index of changes in respiratory motor output. Typical phrenic neurograms during experimental protocols are shown in Fig. 1.
Figure 1. Representative phrenic neurograms depicting experimental protocols in rats intrathecally treated with 5HT7 receptor antagonist and vehicle.
A, In vehicle treated rats acute intermittent hypoxia (AIH; 3, 5 minute episodes) elicited phrenic long term facilitation (pLTF) 60 minutes post-AIH. B, Intrathecal injection of 5-HT7 receptor antagonist (SB269970) prior to AIH greatly enhances pLTF. C, Intrathecal 5-HT7 antagonist alone and D, vehicle alone treated for equivalent experimental duration, respectively, did not elicit pLTF.
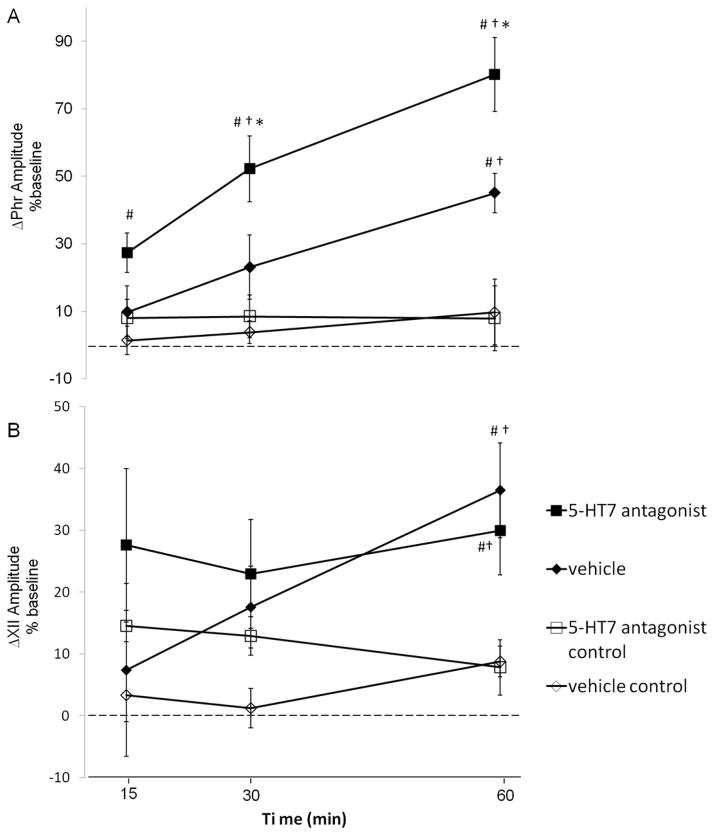
In vehicle treated rats (◆, n=8) exposed to AIH, phrenic amplitude was increased above baseline at 30 (◆, 23±10%, p=0.012) and 60 min post-AIH (◆, 45±6%, p<0.001; Fig 2a), indicating pLTF. Following intrathecal injections of the 5-HT7 receptor antagonist (5mM, SB-269970, 12μl) in rats exposed to AIH, phrenic amplitude was significantly elevated from baseline at 15, 30 and 60 min post-AIH (■, 15 min: 27±6%; 30 min: 52±10%; 60 min: 80±11%; all p<0.001, n=8; Fig 2a). This increase in phrenic nerve burst amplitude was significantly greater in rats that received SB-269970 versus vehicle treated rats by 30 min (p<0.05) post-AIH and beyond (60 min: p<0.001); thus, pLTF was enhanced (Fig 3a).
Figure 2. A,B. Spinal 5-HT7 receptor inhibition enhances phrenic, not hypoglossal pLTF.
Changes in phrenic (A) and XII (B) burst amplitudes (percent change from baseline) in rats receiving 5-HT7 antagonist + AIH (SB269970, ■, n = 8), vehicle + AIH (saline, ◆, n = 8) and control rats not receiving AIH (i.e., time controls) SB269970 (□, n=5) and vehicle (◇, n=7). Compared to vehicle, intrathecal SB269970 enhanced phrenic burst amplitude responses following AIH. However, similar enhancement of XII LTF (B) was not observed, suggesting that drug distribution was restricted to the spinal cord. Values are means±S.E.M. *different than vehicle + AIH, #different than vehicle control, †different than 5-HT7 antagonist control, RMANOVA, p<0.05.
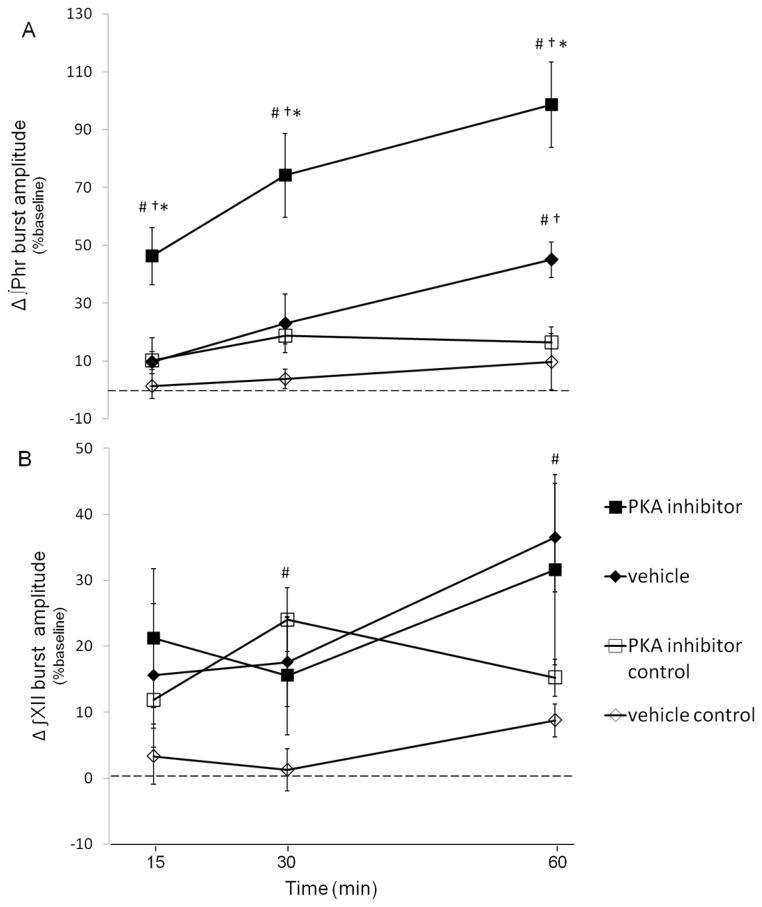
Figure 3. Representative phrenic neurograms depicting experimental protocols in rats intrathecally treated with PKA inhibitor and PKA activator.
A, intrathecal PKA inhibitor (KT5720) injected prior to AIH; B, KT5720 alone C, PKA activator (8-br-cAMP) prior to AIH and D, 8-br-cAMP alone treated for equivalent experimental duration respectively. Intrathecal PKA inhibition greatly enhances pLTF (A). PKA activation blunts expression of pLTF (C). Neither PKA activation nor inhibition augments phrenic motor activity (B&D).
To address concerns that spinal 5-HT7 receptor inhibition may induce phrenic motor facilitation in the absence of AIH, a group of rats received intrathecal SB-269970 without AIH (□, n=5); in these rats, no significant time-dependent changes in phrenic amplitude were observed throughout the duration of the experiment (15min 8±6%, 30min 8±6%, 60min 8±10%, all p>0.05 Fig 2a). Similarly, there were no time-dependent changes in phrenic burst amplitude in rats maintained in normoxia throughout the protocol (⋄, vehicle control; Fig. 2a; all p>0.05), confirming the stability of the preparation. Hence, spinal 5-HT7 receptor inhibition enhances AIH-induced pLTF, but this effect cannot be accounted for by non-specific drug effects independent from AIH.
Spinal 5-HT7 receptor inhibition enhances phrenic but not hypoglossal LTF
In our working model of pLTF, we hypothesize that plasticity arises in cervical spinal segments containing the phrenic motor nucleus (Baker-Herman et al. 2002). Thus, as an internal control, comparisons were made with hypoglossal (XII) burst amplitude to assess potential rostral drug distribution following intrathecal injections, as done previously (Baker-Herman et al. 2004; Kuraishi et al. 2008). In vehicle treated rats, XII burst amplitude was elevated from baseline at 60 min post-AIH (◆, 36±7%, n=8; p<0.001, Fig 2b), indicating XII LTF (Baker-Herman and Strey 2011). Similar XII LTF was observed following spinal 5-HT7 receptor inhibition (■, 30±9%, above baseline at 60 min post-AIH; n=8; p=0.005, Fig 2b). In rats not exposed to AIH, no treatment effects were observed between vehicle and SB-269970 at 60 min post-AIH (p=0.49). Furthermore, no time-dependent effects were observed in XII burst amplitude versus baseline in SB-269970 treated (□; 15 min: 15±3%, 30 min: 13±3%, 60 min: 8±4%; all p>0.05) or vehicle treated rats (⋄; 15 min: 3±4%, 30 min: 1±3, 60 min: 9±3%; all p>0.05) without AIH. Collectively, these data suggest that effective drug concentrations were restricted to the spinal cord.
Spinal protein kinase A inhibition enhances phrenic but not hypoglossal LTF
To test the hypothesis that PKA activity contributes to pLTF rats received an intrathecal injection of a cell permeable PKA inhibitor (KT-5720, 12μl 100μM). Representative phrenic neurograms are illustrated in Fig 3. Following AIH, phrenic burst amplitude in rats receiving KT-5720 (■; n=8) was elevated above baseline by 15 min and beyond (■; 15 min: 46±10; 30 min: 74±15%; 60 min: 99±15%; all p<0.001; Fig 4a), indicating pLTF. AIH-induced pLTF was significantly greater after KT-5720 versus vehicle injections at all time points (◆; 15 min: 10±8%, p=0.003; 30 min: 23±10%, p<0.001; 60 min: 45±6%, p<0.001; Fig 4a), suggesting that pLTF was enhanced by spinal PKA inhibition. In contrast, no time-dependent effects were observed in phrenic burst amplitude from rats treated with intrathecal KT-5720 (15 min: 10±3%, 30 min: 18±3%, 60 min: 16±5%, versus baseline; all p>0.05) without AIH.
Figure 4. A,B. Spinal Protein Kinase A (PKA) inhibition enhances phrenic, not hypoglossal pLTF.
Changes in phrenic (A) and XII (B) burst amplitudes (% change from baseline) in rats receiving PKA inhibitor +AIH (KT5720, ■, n = 8), vehicle +AIH (saline, ◆, n = 8) and control rats not receiving AIH (i.e., time controls) KT5720 −AIH (□, n = 7) and vehicle −AIH (◇, n = 7). Intrathecal KT5720 augmented phrenic burst amplitude responses following AIH, indicative of enhanced pLTF. However, similar enhancement of XII LTF was not observed, suggesting that drug distribution was restricted to the spinal cord. Values are means±S.E.M. *different than vehicle + AIH, #different than vehicle control, †different than PKA inhibitor control, RMANOVA, p<0.05.
XII burst amplitude was elevated over baseline at 60 min post-AIH in vehicle treated rats (◆; 36±7%, n=8, p< 0.001, Fig 4b), indicating XII LTF. Since XII burst amplitude in KT-5720 treated rats was elevated above baseline by a similar amount (■; 32±14% at 60 min; n=7; p=0.043), XII LTF was unaffected by spinal drug administration (p=0.658), again suggesting that effective drug concentrations were restricted to the spinal cord. Time control rats receiving intrathecal KT-5720 without AIH (□; n=6) exhibited no time-dependent changes in XII burst amplitude (□; 15 min: 12±4%; 30 min: 24±5%; 60 min: 15±3%; all p>0.05 Fig 4b).
Spinal PKA activation inhibits phrenic but not hypoglossal LTF
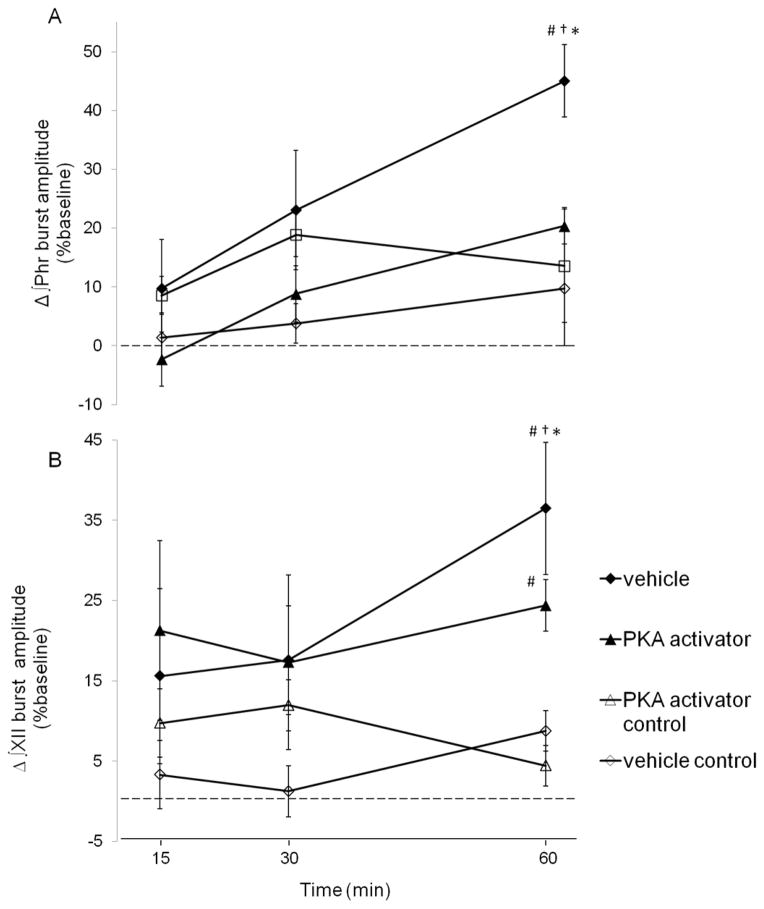
To further test the role of PKA involvement in pLTF, a subset of rats received intrathecal injections of the cell-permeable cAMP analogue (8-br-cAMP, 15μl, 100μM) prior to AIH. Representative phrenic neurograms are illustrated in Fig 3c/d. In rats receiving intrathecal 8-br-cAMP pLTF was observed 60 min post-AIH (▲; 20±3%, n=7; p=0.014; Fig 5a), demonstrating pLTF. However, the magnitude of pLTF was significantly lower in 8-br-cAMP versus vehicle treated rats 60 min post-AIH (◆; 45±6%, p<0.001, Fig 5a). In control rats receiving intrathecal 8-br-cAMP injections without AIH, phrenic burst amplitude did not exhibit any time-dependent effects (△; 15min: 10±7%; 30 min: 12±3%; 60min: 4±10%: all p>0.05, n=6; Fig 5a).
Figure 5. A,B. Spinal PKA activation attenuates phrenic, not hypoglossal pLTF.
Changes in phrenic (A) and XII (B) burst amplitudes (% change from baseline) in rats receiving PKA activator +AIH (8-br-cAMP, ■, n = 7), vehicle +AIH (saline, ◆, n = 8) and control rats not receiving AIH (i.e., time controls) 8-br-cAMP −AIH (□ n = 6) and vehicle −AIH (◇, n = 7). Intrathecal KT5720 augmented phrenic burst amplitude responses following AIH, indicative of enhanced pLTF. However, similar enhancement of XII LTF was not observed, suggesting that drug distribution was restricted to the spinal cord. Values are means±S.E.M. *different than vehicle + AIH, #different than vehicle control, †different than PKA activator control, RMANOVA, p<0.05.
In rats receiving intrathecal 8-br-cAMP, XII burst amplitude (n=5) was not different versus vehicle treated rats at 60 min post-AIH (24±3% and 33±8%, respectively, p=0.34; Fig 5b). Further, no time dependent effects were observed in XII burst amplitude in rats receiving 8-br-cAMP without AIH (△; 15 min: 10±7%, 30 min: 12±3%, 60 min: 4±10%; all p>0.05, n=6; Fig 5b).Together these results support a mechanism whereby activation of spinal 5-HT7 receptors and/or PKA constrains normal mechanisms of AIH-induced pLTF.
Short-term hypoxic phrenic and hypoglossal responses
In vehicle treated rats (n=8), hypoxia elicited a brisk increase in phrenic (i.e. the short term hypoxic response; ■; 107±9%, p<0.001) and hypoglossal nerve burst amplitudes (□; 197±14%, p<0.001; Figs 6&1). The short term hypoxic phrenic response observed in rats pretreated with a 5-HT7 receptor antagonist (SB-269970, n=8; 131±13%, p< 0.001), PKA inhibitor (KT-5720, n=8; 140±15%, p<0.001) and PKA activator (8-br-cAMP, n=7; 98±13%, p<0.001) were similar to vehicle treated rats (p=0.054).
Figure 6. Phrenic and hypoglossal (XII) responses during hypoxic episodes (i.e. short-term hypoxic response).
Changes in integrated phrenic and XII burst amplitudes during final minute of hypoxic exposures (average of 3) from intrathecal vehicle, 5-HT7 antagonist, PKA inhibitor and PKA activator. Phrenic and hypoglossal bursts amplitudes significantly increased above baseline in all rats exposed to AIH (data not shown). No significant differences were observed between treatment groups in phrenic (solid) or hypoglossal (open) burst amplitudes from rats receiving intrathecal SB269970, KT5720, vehicle or 8-Br-cAMP prior to AIH. Values are means±S.E.M.
Hypoglossal burst amplitude from rats treated with SB-269970 (n=8), KT-5720 (n=7) and 8-br-cAMP (n=5), were not different than vehicle treated rats (180±40%, 214±35% and 191±15%, respectively; p=0.662, Fig 6). Thus spinal 5-HT7 and PKA inhibition did not affect the phrenic or hypoglossal short-term hypoxic responses.
Mean arterial pressures and Blood Gases
During studies of hypoxia-induced respiratory plasticity, it is critical to maintain precise control of blood gases (Mitchell et al. 2003). PaCO2 measurements remained within (±)2 mmHg of baseline levels throughout an experiment; similar regulation was observed in all treatment groups (table 1). PaO2 remained above 200 mmHg throughout all experiments, except during hypoxic episodes (table 1). Thus, changes in chemoreceptor stimulation from baseline cannot account for pLTF or differences in pLTF expression caused by drug treatments.
Table 1.
Measurements of PaCO2, PaO2 and mean arterial pressure (MAP) during baseline, hypoxic exposure and 60 minutes post-hypoxia.
| Treatment groups (+AIH) | PaCO2 (mmHg) | PaO2 (mmHg) | MAP (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| baseline | hypoxia | 60min | baseline | hypoxia | 60min | baseline | hypoxia | 60min | |
| vehicle control
|
45.4±1.2 | 46.2±1.3 | 45.3±1.1 | 263±10 | 43±8† | 257±8 | 116±5 | 99±7† | 112±3 |
| 5-HT7 antagonist
|
45.6±0.6 | 46.8±0.9 | 46.1±0.6 | 288±9 | 33±5† | 273±11 | 112±3 | 87±8† | 103±5 |
| PKA inhibitor
|
46.2±1.1 | 45.8±0.7 | 46.9±1.0 | 252±12 | 37±2† | 244±8 | 112±5 | 91±6† | 111±4 |
| PKA activator | 46.7±0.6 | 47.0±1.1 | 46.8±0.6 | 276±4 | 39±2† | 255±6† | 109±4 | 86±6† | 100±4 |
|
| |||||||||
| (−AIH) groups | baseline | Equiv Time | 60min | baseline | Equiv Time | 60min | baseline | Equiv Time | 60min |
|
| |||||||||
| vehicle control
|
46.8±1.0 | 46.3±0.8 | 45.7±1.1 | 262±9 | 254±7 | 243±8 | 128±7 | 129±6 | 127±6 |
| 5-HT7 antagonist control
|
46.7±1.2 | 46.3±1.0 | 45.8±0.9 | 292±6 | 283±7 | 272±12 | 124±12 | 122±11 | 125±9 |
| PKA inhibitor control
|
46.8±0.7 | 47.1±0.8 | 47.5±0.8 | 280±5 | 273±7 | 269±6 | 120±5 | 122±5 | 122±4 |
| PKA activator control | 48.1±1.6 | 47.6±1.8 | 48.2±1.7 | 264±8 | 258±8 | 253±9 | 111±4 | 111±5 | 109±5 |
Values are means ± S.E.M.,
different than baseline, p<0.05.
Mean arterial pressures (MAP) were similar between groups during baseline conditions and 60 minutes post-AIH (table 1). All groups exhibited a similar, transient decrease in MAP (~45±15 mmHg) during hypoxia as is characteristic of anesthetized rats table 1).
DISCUSSION
Multiple lines of evidence support a critical role for spinal serotonin receptor activation in the initiation of pLTF (Millhorn et al. 1980; Fregosi and Mitchell 1994; Bach et al. 1996; Baker-Herman et al. 2002), predominantly via Gq protein-coupled 5-HT2 receptors (Fuller et al. 2001). However, little is known regarding interactions between Gq and Gs-coupled metabotropic serotonin receptors that are co-activated during AIH. Here we demonstrate that AIH-induced pLTF is constrained by coincident spinal 5-HT7 receptor activation. This inhibition arises via activation of a “downstream” kinase in the canonical 5-HT7 receptor PKA signaling cascade. Indeed, manipulation of PKA activity is sufficient to account for 5-HT7 receptor-dependent modulation of pLTF following AIH. Our results suggest a novel mechanism whereby Gs protein-coupled 5-HT7 receptors inhibit Gq-dependent plasticity via PKA activation.
Gs protein-coupled receptor involvement in respiratory plasticity
This is the first evidence that 5-HT7 receptor signaling modulates respiratory plasticity following AIH in normal rats. However, our finding that 5-HT7 receptors impose an inhibitory constraint on pLTF may at first appear to contradict our previous report that Gs protein-coupled 5-HT7 receptor activation without hypoxia elicits phrenic motor facilitation (PMF; Hoffman et al. 2011); we previously demonstrated that intrathecal delivery of a 5-HT7 agonist elicits long-lasting pMF (Hoffman et al. 2011). We postulate that AIH-induced pLTF (Baker-Herman et al. 2004) and 5-HT7 receptor agonist-induced pMF (Golder et al. 2008; Hoffman and Mitchell 2008) arise from distinct mechanisms that interact via “cross-talk inhibition” (Dale-Nagle et al. 2010).
We propose that serotonin receptors coupled to Gq and Gs proteins are both activated during AIH, but that Gq protein coupled receptor signaling normally predominates. This is supported by observations that AIH-induced pLTF is blocked by spinal PKC inhibition (Devinney and Mitchell, unpublished), inhibition of new BDNF synthesis or TrkB activation (Baker-Herman et al. 2004) and ERK-MAP kinase inhibition (Hoffman et al. 2012). In contrast, Gs protein coupled receptor induced pMF requires new synthesis of an immature TrkB isoform (not BDNF) (Golder et al. 2008; Hoffman et al. 2011) and PI3 kinase/Akt activation (Hoffman et al. 2011). However, partial activation of the Gs-pathway does not contribute to AIH-induced pLTF, but actually attenuates Gq-dependent AIH-induced pLTF. By preventing activation of a Gs protein-coupled serotonin receptor, in the present study, we release this cross-talk inhibition and enable full expression of pLTF.
For simplicity we have designated the AIH-induced pathway to pLTF as the “Q-pathway” (Dale-Nagle et al. 2010) because 5-HT2 receptors are coupled to Gq-proteins (Millan et al. 2008). Multiple Gq protein coupled receptors elicit similar pMF (Neverova et al. 2007). Since episodic 5-HT2 receptor activation on or near phrenic motor neurons is necessary and sufficient to elicit the Q-pathway (Baker-Herman et al. 2002; MacFarlane et al. 2009; MacFarlane et al. 2011) it was originally proposed to explain AIH-induced pLTF (Fuller et al. 2001; Bocchiaro et al. 2004; MacFarlane et al. 2008). 5-HT2 receptor activation triggers new BDNF synthesis and TrkB activation (Baker-Herman et al. 2004), followed by ERK-MAP kinase activation (Hoffman et al. 2012).
In contrast, the “S-pathway” is triggered by activation of Gs-PCRs (i.e. A2A and 5-HT7) which elicits pMF via new synthesis of an immature TrkB isoform that auto-phosphorylates and signals via protein kinase B (ie. Akt; Golder et al. 2008; Hoffman et al. 2011). Although the Q and S-pathways to pMF both require new protein synthesis, they differ in their requirement for new synthesis of BDNF versus TrkB (Baker-Herman et al. 2004; Golder et al. 2008; Hoffman et al. 2011), and by their requirement for ERK versus Akt signaling (Golder et al. 2008; Hoffman et al. 2012).
Regulation of phrenic long-term facilitation
The Q-pathway to pMF (i.e. pLTF) is tightly regulated. AIH-induced pLTF requires reactive oxygen species (ROS) formation via NADPH oxidase activity (MacFarlane et al. 2009), which, we hypothesize, inhibits spinal serine-theronine protein phosphatases (e.g., PP2A/5) and relieves an important constraint to pLTF (Macfarlane et al. 2008; Wilkerson et al. 2008). We postulate that ROS inhibition of protein phosphatase activity is a key factor differentiating intermittent versus sustained hypoxia in their capacity to elicit pLTF (Wilkerson et al. 2007; Macfarlane et al. 2008). Moreover, recent data suggest that a distinct serotonin receptor subtype (5-HT2B) activates the NADPH required for pMF (MacFarlane et al. 2011).
A major finding in the present study is that that Gs protein-coupled 5-HT7 receptors (and presumably A2A receptors; (Hoffman et al. 2010) constrain AIH-induced pLTF via PKA activation. Although mechanisms whereby PKA inhibits the Q-pathway are unclear at this time, one possibility is that PKA inhibits NADPH oxidase activity, a prerequisite for AIH-induce pLTF expression (MacFarlane et al. 2009). Indeed, PKA phosphorylates NADPH oxidase subunits and inhibits NADPH oxidase activity (Bengis-Garber and Gruener 1996; Kim et al. 2007). Diminished ROS generation would then suppress the capacity for Q-pathway-dependent pLTF. Considerably less is known about factors regulating the S-pathway, or how it is affected by the Q-pathway.
S-pathway regulation of pLTF through NADPH oxidase
We propose that 5-HT7 receptors activate PKA and modulate the “regulatory cassette” of the Q pathway by reducing the NADPH oxidase derived ROS formation necessary for pLTF (Macfarlane et al. 2008). A major consequence of diminished ROS formation is less inhibition of protein phosphatases known to constrain pLTF (Macfarlane et al. 2008; Wilkerson et al. 2008).
Neuronal NADPH oxidase consists of multiple subunits that form a functional protein complex (Infanger et al. 2006); NADPH oxidase complex formation is regulated by the phosphorylation state of key elements (Bedard and Krause 2007). Thus, when PKA phosphorylates the p47 subunit, complex formation is impaired, thereby limiting ROS formation (Nogueira-Machado et al. 2003; Kim et al. 2007). In pheochromocytoma cells (PC-12), serum deprivation increases ROS formation, an effect blunted by pretreatment with an A2A receptor (S-pathway) agonist (Huang 2003). Similarly, A2A receptor agonists inhibit ROS formation in activated neutrophils by suppressing NADPH oxidase activity (Revan et al. 1996). Conversely, PKC activation (i.e., Q-pathway) with phorbol esters increases ROS formation via NADPH oxidase, an effect negated by PKA activation (Bengis-Garber et al. 1996). Since AIH-induced pLTF requires NADPH oxidase activity and subsequent ROS formation (via NADPH oxidase; MacFarlane et al. 2009; 2011), we propose that spinal 5-HT7 receptor activation suppresses NADPH oxidase-dependent ROS formation via PKA activation, thereby constraining pLTF.
Complexity of neuromodulator induced phrenic motor facilitation
Hypoxia elicits the release of multiple neuromodulators (e.g., serotonin, norepinephrine, adenosine) that interact with multiple receptor subtypes and initiate diverse signaling cascades (Mitchell et al. 2003; Doi and Ramirez 2008). For example, extracellular serotonin and adenosine levels increase during hypoxia in several CNS respiratory centers (Richter et al. 1999; Gourine et al. 2002; Mitchell et al. 2003). These neuromodulators often induce and/or regulate plasticity (Mitchell et al. 2003). Thus, a detailed understanding of interactions between neuromodulator receptor systems is crucial to fully understand respiratory plasticity.
Similar to spinal 5-HT7 receptor inhibition, spinal A2A receptor inhibition enhances AIH-induced pLTF (Hoffman et al. 2010). Thus, although A2A receptors are not necessary for pLTF following moderate AIH, they attenuate the magnitude of serotonin-dependent pLTF. Conversely, severe AIH elicits phenotypically similar (although somewhat larger) pLTF, yet this pLTF is independent of serotonin receptor activation (Nichols et al. 2012). Instead, following severe AIH, pLTF is A2A receptor dependent, signifying a shift from predominant Q to predominant S pathways to pMF (Nichols et al. 2012). This shift may arise from greater extracellular adenosine accumulation during severe hypoxic episodes, and may account for XII LTF in in vitro rhythmogenic brainstem slice preparations (Blitz and Ramirez 2002). One potential benefit of having multiple mechanisms of pMF is flexibility as an animal responds to perturbations that may vary in severity, pattern and duration.
Significance
AIH elicits spinal plasticity, functionally strengthening neural pathways to phrenic motor neurons. Such plasticity has considerable potential to be harnessed for therapeutic benefit in multiple disorders that compromise breathing capacity, including spinal injury, motor neuron disease and even obstructive sleep apnea (Mitchell 2007). For example, we recently demonstrated the potential of repetitive exposure to AIH as a therapeutic approach in the treatment of chronic cervical spinal injury in animal models (Lovett-Barr et al. 2012) and humans with spinal injury (Trumbower et al. 2012). Thus, drugs, such as A2A or 5-HT7 receptor antagonists may enhance the functional benefits of repetitive AIH in the treatment of spinal injury and other disorders that compromise breathing may be of benefit. Conversely, A2A or 5-HT7 receptor agonists may be useful to restore respiratory and non-respiratory (somatic) motor function following spinal injury (Golder et al. 2008) or motor neuron disease by eliciting spinal plasticity. Factors that activate cAMP-PKA may trigger functional recovery (Kajana et al. 2008; 2009).
We have only begun to understand basic cellular/molecular mechanisms underlying pLTF (or other forms of respiratory plasticity); further discoveries may enable us to realize the potential of induced respiratory plasticity to treat important clinical disorders that compromise ventilatory capacity.
Highlights.
Spinal 5-HT7 receptor inhibition enhances pLTF after AIH
Spinal PKA inhibition enhances pLTF
Spinal PKA activation blunts pLTF
5-HT7 receptors constrain AIH-induced pLTF, acting via PKA
Acknowledgments
This work was funded by NIH grant R01 HL80209. MSH was supported by T32 HL07654 and F31-HL092785. Special appreciation is extended to Safraaz Mahamed for his custom computer program used for data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104(2–3):251–60. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1):48. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22(14):6239–46. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol. 2011;179(1):48–56. doi: 10.1016/j.resp.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–9. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169(2):255–63. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bengis-Garber C, Gruener N. Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell Signal. 1996;8(4):291–6. doi: 10.1016/0898-6568(96)00052-6. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87(6):2964–71. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101(12):4292–5. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol. 2010;669:225–30. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164(1–2):96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici MR, Pepponi R, Martire A, Tebano MT, Potenza RL, Popoli P. Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)-mediated effects in the striatum. J Neurochem. 2004;90(5):1276–9. doi: 10.1111/j.1471-4159.2004.02607.x. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest. 1976;70(1 Suppl):154–7. doi: 10.1378/chest.70.1_supplement.154. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348(2):161–82. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477(Pt 3):469–79. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90(5):2000–6. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28(9):2033–42. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Thomas T, Dale N, Spyer KM. Adenosine release in nucleus tractus solitarii does not appear to mediate hypoxia-induced respiratory depression in rats. J Physiol (Lond) 2002;544(1):161–170. doi: 10.1113/jphysiol.2002.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265(4 Pt 2):R811–9. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol. 2010;588(Pt 1):255–66. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Episodic Spinal 5-HT7 Receptor Activation Induces Phrenic Motor Facilitation. FASEB J. 2008;22(1_MeetingAbstracts):1232.8. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol. 2011;589(Pt 6):1397–407. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol. 2012;113(8):1184–93. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NK. Adenosine A2A receptors regulate oxidative stress formation in rat pheochromocytoma PC12 cells during serum deprivation. Neuroscience Letters. 2003;350(2):127. doi: 10.1016/s0304-3940(03)00860-7. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8(9–10):1583–96. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res. 2008;1232:206–13. doi: 10.1016/j.brainres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Systemic administration of rolipram increases medullary and spinal cAMP and activates a latent respiratory motor pathway after high cervical spinal cord injury. J Spinal Cord Med. 2009;32(2):175–82. doi: 10.1080/10790268.2009.11760769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142(2):436–40. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 Activity via Protein Kinase A-mediated Phosphorylation of NoxA1 and 14-3-3 Binding. J Biol Chem. 2007;282(48):34787–800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130(2):207–18. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol. 1999;277(3 Pt 2):R658–66. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363(6):620–32. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Yageta Y, Konno M, Andoh T, Yamaguchi-Miyamoto T, Nojima H. Intracisternal, but not intrathecal, injection of naloxone inhibits cutaneous itch-related response in mice. Biol Pharm Bull. 2008;31(11):2143–5. doi: 10.1248/bpb.31.2143. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986;380:373–85. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11(3):449–58. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32(11):3591–600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. NADPH oxidase activity is necessary for phrenic motor facilitation induced by 5HT2B receptor activation. FASEB J. 2008;22(1_MeetingAbstracts):1232.7. [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587(Pt 22):5469–81. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol. 2009;587(Pt 9):1931–42. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience. 2011;178:45–55. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008 doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92(1):27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R334–41. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- Meyer RB, Jr, Miller JP. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci. 1974;14(6):1019–40. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008 doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41(1):87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42(3):171–88. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. 1. Springer; New York: 2007. pp. 291–306. [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90(6):2466–75. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–74. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol. 2001;532(Pt 2):483–97. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27(16):4435–42. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol. 2012;112(10):1678–88. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Machado JA, Lima e Silva FC, Medina LO, Costa DC, Chaves MM. Modulation of the reactive oxygen species (ROS) generation mediated by cyclic AMP-elevating agents or Interleukin 10 in granulocytes from type 2 diabetic patients (NIDDM): a PKA-independent phenomenon. Diabetes Metab. 2003;29(5):533–7. doi: 10.1016/s1262-3636(07)70068-x. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia GS, Meneses A. Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav Brain Res. 2005;163(1):136–40. doi: 10.1016/j.bbr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10(4):1091–8. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revan S, Montesinos MC, Naime D, Landau S, Cronstein BN. Adenosine A2 receptor occupancy regulates stimulated neutrophil function via activation of a serine/threonine protein phosphatase. J Biol Chem. 1996;271(29):17114–8. doi: 10.1074/jbc.271.29.17114. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–78. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388(2):169–90. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26(2):163–72. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Macfarlane PM, Hoffman MS, Mitchell GS. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem Soc Trans. 2007;35(Pt 5):1269–72. doi: 10.1042/BST0351269. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic Acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci. 2008;28(11):2949–58. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]