Abstract
Background:
Type 2 diabetes (T2D) and coronary heart disease (CHD) are prevalent chronic diseases from which military personnel are not exempt. While many genetic markers for these diseases have been identified, the clinical utility of genetic risk testing for multifactorial diseases such as these has not been established. The need for a behavioral intervention such as health coaching following a risk counseling intervention for T2D or CHD also has not been explored. Here we present the rationale, design, and protocol for evaluating the clinical utility of genetic risk testing and health coaching for active duty US Air Force (AF) retirees and beneficiaries.
Primary Study Objectives:
Determine the direct and interactive effects of health coaching and providing genetic risk information when added to standard risk counseling for CHD and T2D on health behaviors and clinical risk markers.
Design:
Four-group (2 X 2 factorial) randomized controlled trial.
Setting:
Two AF primary care clinical settings on the west coast of the United States.
Participants:
Adult AF primary care patients.
Intervention:
All participants will have a risk counseling visit with a clinic provider to discuss personal risk factors for T2D and CHD. Half of the participants (two groups) will also learn of their genetic risk testing results for T2D and CHD in this risk counseling session. Participants randomized to the two groups receiving health coaching will then receive telephonic health coaching over 6 months.
Main Outcome Measures:
Behavioral measures (self-reported dietary intake, physical activity, smoking cessation, medication adherence); clinical outcomes (AF composite fitness scores, weight, waist circumference, blood pressure, fasting glucose, lipids, T2D/CHD risk scores) and psychosocial measures (self-efficacy, worry, perceived risk) will be collected at baseline and 6 weeks, and 3, 6, and 12 months.
Conclusion:
This study tests novel strategies deployed within existing AF primary care to increase adherence to evidence-based diet, physical activity, smoking cessation, and medication recommendations for CHD and T2D risk reduction through methods of patient engagement and self-management support.
Key Words: Health coaching, genomics, chronic disease, behavior change, diabetes, coronary heart disease
抽象
背景: 2 型糖尿病 (T2D) 及冠心 病 (CHD) 是军人普遍不能幸免的 慢性疾病。虽然已经确定这些疾 病的许多遗传标记,但尚未确立 对这些多因性疾病进行遗传风险 检测的临床应用。同时,尚未探 索进行行为干预(如在 T2D 或 CHD 风险咨询干预之后进行健康辅 导)的必要。我们在此提出对现 役美国空军 (AF) 退休人员和受益 者的遗传风险检测和健康辅导的 临床应用进行评估的理论依据、 设计和方案。
主要研究目标:将健康辅导和提 供遗传风险信息加入 CHD 和 T2D 健康行为和临床风险标记的标准 风险咨询中,确定其直接和相互 影响。
设计: 4 组(2 X 2 阶乘)随机 对照试验。
环境: 美国西海岸的两处 AF 基 础护理临床设施。
参与者:成年 AF 基础护理患者。
干预: 所有参加者将到临床提供 者处进行风险咨询就诊,讨论个 人 T2D 和 CHD 的风险因素。一半 的参与者(两组)也将在风险咨 询期间得知其 T2D 和 CHD 的遗传 风险测试结果。随机分配至接受 健康辅导两组的参与者随后将接 受逾 6 个月的电话健康辅导。
主要结果测量指标: 行为测量指 标(自我报告的饮食摄入、体力 活动、戒烟、药物依从性);临 床结果(AF 综合健身分数、体 重、腰围、血压、空腹血糖、血 脂、T2D/CHD 风险分数)以及心理 测量指标(自我效能、忧虑、感 知风险)将于基线期及 6 周、3 、6 和 12 个月内收集。
结论: 本研究对部署在现有 AF 基础护理中的新策略进行测试, 通过患者参与和自我管理支持的 方法,加强坚持有据
SINOPSIS
Fundamentación:
La diabetes de tipo 2 (DT2) y las cardiopatías coronarias (CC) constituyen enfermedades crónicas muy extendidas de las cuales no está exento el personal militar. Aunque se han encontrado numerosos marcadores genéticos para estas enfermedades, aún se desconoce la utilidad clínica de las pruebas de riesgo genético para enfermedades multifactoriales como estas. Tampoco se ha explorado la necesidad de una intervención conductual como la formación sanitaria tras una intervención de asesoramiento sobre el riesgo de DT2 o CC. Aquí presentamos la justificación, el diseño y el protocolo para evaluar la utilidad clínica de las pruebas de riesgo genético y la formación sanitaria de miembros en activo, jubilados y beneficiarios de las fuerzas aéreas (FF. AA.) estadounidenses.
Objetivos principales del estudio:
Conocer los efectos directos e interactivos de la formación sanitaria y proporcionar información sobre el riesgo genético, cuando se combina con el asesoramiento estándar acerca del riesgo de CC y de DT2, sobre las conductas relacionadas con la salud y los marcadores de riesgo clínico.
Diseño:
Ensayo controlado y aleatorizado con cuatro grupos (factorial de 2 x 2).
Entorno:
Dos entornos clínicos de atención primaria de las FF. AA. situados en la costa oeste de Estados Unidos.
Participantes:
Pacientes adultos de atención primaria de las FF. AA.
Intervención:
Todos los participantes acudirán a una visita de asesoramiento sobre riesgos con un profesional sanitario para hablar de los factores personales de riesgo de DT2 y CC. La mitad de los participantes (dos grupos) conocerá también los resultados de sus pruebas de riesgo genético de DT2 y de CC en esta sesión de asesoramiento sobre riesgos. Los participantes asignados aleatoriamente a los dos grupos, que reciban formación sanitaria, recibirán formación sanitaria telefónica a lo largo de 6 meses.
Criterios de valoración principales:
En el inicio, la sexta semana y al cabo de 3, 6 y 12 meses se obtendrán mediciones conductuales (ingesta dietética declarada, actividad física, abandono del tabaco, cumplimiento del tratamiento farmacológico), resultados clínicos (puntuaciones combinadas de la condición física de las FF. AA., peso, perímetro de cintura, presión arterial, glucosa en ayunas, lípidos, puntuaciones de riesgo de DT2 y CC) y mediciones psicosociales (autoeficacia, preocupación, riesgo percibido).
Conclusiones:
En este estudio, se examinan algunas estrategias nuevas adoptadas dentro de la atención primaria existente de las FF. AA. para mejorar el cumplimiento de la dieta basada en evidencias, la actividad física, el abandono del tabaco y la medicación para reducir el riesgo de DT2 y CC, a través de métodos de compromiso de los pacientes y de apoyo de la autogestión.
INTRODUCTION
While coronary heart disease (CHD) and type 2 diabetes (T2D) are leading causes of morbidity and mortality in the United States, they are also two of the most preventable chronic diseases.1,2 They are linked: T2D and its precursor, pre-diabetes, increase risk for CHD and respectively affect about 10% and 25% of US adults.1,2 Military personnel and their dependents are not exempt from this preventable chronic disease epidemic.3-6 Professional guidelines establish standard risk assessment for CHD and T2D and risk reduction via pharmacotherapy and recommended health behavior change,7–9 yet the difficulty of sustainably changing behavior is well-known.10,11 Emerging approaches to support behavior change include health coaching12,13 and provision of genetic information to patients.14–19 Patient interest in both approaches13,20–22 to moderating risk for CHD and T2D may present healthcare providers with the opportunity to construct an effective “teachable” moment. Though risk for both CHD and T2D remains modifiable with lifestyle changes such as diet and physical activity,9,23 neither the feasibility nor the clinical utility of integrating genetic risk information into primary care patients' global risk assessment for these diseases is known. It is also not known how health coaching or risk counseling using genetic information, by themselves or in combination, will fare against standard risk counseling alone in facilitating lifestyle change for primary care patients at risk for CHD and T2D.
MILITARY RELEVANCE
Military personnel are not exempt from increasing rates of overweight and obesity or the associated rising burden of CHD and T2D seen in the US population. Cigarette smoking, even among young, active-duty military personnel, has been associated with a significant number of lost workdays and even hospitalizations.3 Healthy behaviors are critical to reducing T2D and CHD risk and are lacking among some active-duty Air Force (ADAF) personnel. Although there has been an increase in the military operational tempo, 30% of ADAF personnel reported less than 20 minutes of moderate intensity exercise at least 3 days per week, and only 23% reported 60 or more minutes at least 3 days per week.4 ADAF personnel also reported low intake of fruits, vegetables, whole grains, and lean protein and fairly high consumption of fast food.4 Smoking was reported by 32% of military personnel. Improvement in these health behaviors could reduce short- and long-term health risks from T2D and CHD and improve mission readiness.
Another issue for ADAF personnel is failure of one or more components of the required annual fitness test (in accordance with Air Force Instruction 36-2905 dated July 1, 2012, and amended on January 3, 2013). Overweight, obesity, and frequency of aerobic exercise are the most significant predictors of low physical fitness among ADAF personnel, regardless of gender.3 Individualized behavioral approaches such as our proposed interventions of risk counseling and telephonic health coaching have successfully promoted healthy behaviors such as physical activity in other studies of ADAF personnel,24 yet they have not been integrated into settings such as AF primary care clinics. This study addresses human performance by increasing the capacity of AF primary care to support achievement of fitness goals in an integrated manner, using novel tools, namely T2D and CHD risk counseling including genetic risk information and subsequent health coaching.
Rates of overweight and obesity are also high among military retirees and dependents, reaching 80% in men and 60% in women in 2003, with correspondingly high rates of diabetes (11% men, 8% women), hypertension (39% men, 37% women), and high cholesterol (49% men, 39% women).5 The prevalence of T2D among military personnel is similar to that in the civilian population, affecting over 38 000 personnel, retirees, and dependents.6 It is well known that these conditions are preventable with lifestyle modifications and early treatment.25,26 It is also known that behavioral interventions have been effective in risk reduction in military settings.27,28 By integrating into primary care individualized chronic disease risk counseling and a behavioral intervention to motivate and support behavior change, this research evaluates a novel approach to reducing risk of chronic disease in AF personnel and their dependents, groups that are strikingly similar to the general US population in terms of chronic disease risk.
RISK ASSESSMENT TOOLS
Clinical Risk tools for Coronary Heart disease and type 2 diabetes
The most widely used global CHD risk assessment is the Framingham Risk Score (FRS). The FRS predicts 10-year mortality or myocardial infarction in those without existing heart disease29 by incorporating gender, age, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), smoking status, and blood pressure (BP) into an epidemiology-based algorithm. Ten-year CHD risk levels based on the FRS are estimated as high (>20%), intermediate (10%-20%), or low (<10%) risk.29 The clinical validity of the FRS is well established, and it is included in clinical guidelines for prescribing therapies to reduce CHD risk, particularly lipid levels.9,29,30 The FRS also is used to communicate CHD risk to patients in clinical care in order to shape risk perception and motivate participation in risk-reducing therapy and/or behaviors.8,9 A systematic review of the efficacy of global risk assessment as a behavior change tool revealed that the few studies incorporating risk presentation with education resulted in a small improvement in CHD risk (–0.75%; P=<.001).31 With repeated counseling, risk score (FRS) was reduced over 10 to 12 months slightly more (–0.2 to –2%), but one-time intervention showed small, if any, effects on risk score.31 None of these studies included a comparison group or used a longitudinal intervention to accompany the risk information.
Risk assessment for T2D typically includes age, gender, race/ethnicity, BP, body mass index (BMI) or weight, indicators of glucose metabolism (eg, glucose intolerance or insulin resistance), family history, and history of gestational diabetes. Using these factors, a risk score for T2D can be calculated based on validated calculations such as the American Diabetes Association risk score.32 There is clear evidence that lifestyle change (diet, physical activity) and/or medication therapy can effectively reduce risk of progression to T2D, even in the face of genetic risk for T2D.23,25
Genetic Risk for Coronary Heart disease and type 2 diabetes
Incorporation of genetic information into risk scores for CHD may improve their ability to accurately predict risk, but findings of clinical utility to date are equivocal. Since 2005, more than 100 genetic variants, or single nucleotide polymorphisms (SNPs), have been associated with increased risk for heart disease; the best-validated of these are SNPs linked to the 9p21 locus of chromosome 9.33–38 One study incorporating genetic risk information from 101 SNPs associated with cardiovascular disease (CVD) did not improve risk prediction,38 and another study that used only the 12 SNPs associated with actual CVD diagnoses did improve risk prediction, albeit only slightly.33,38 Similarly, a small but significant improvement in risk prediction occurred when 9p21 genetic testing results were incorporated into the prediction model.39 Even though addition of genetic information has not greatly improved the prediction accuracy in terms of CHD as compared to that estimated by clinical risk factors alone, incorporation of genetic information may help reclassify individuals into more appropriate risk strata. For example, 12% of one sample39 and 14% of another37 were reclassified into more accurate risk categories when genetic risk information was incorporated.37
Incorporation of genetic information into risk scores for T2D has been less studied. At least 40 markers have been associated with T2D risk.40 However, genetic risk information has not yet been shown to add much to the statistical accuracy of T2D risk prediction.41,42 Nonetheless, incorporating genetic information into T2D risk counseling has shown some suggestion of clinical utility. Early research into the utility of T2D genetic risk information has reportedly increased patient intentions to change behaviors,15,16 enhanced reported motivation for behavior change,43–45 and led to small but not significant changes in actual behavior (dietary intake)16 and clinically relevant changes in clinical outcomes such as weight.15,16 To further test the possibility that genetic risk information may have clinical utility in effecting behavior change, we selected three T2D-related risk markers that have been highly validated in multiple ethnic groups (rs7903146, rs1801282, rs5219) to incorporate into patient feedback on risk.46
Risk Counseling/Health Coaching
Given the apparent gap between clinical utility, the changes in behavioral precursors, and actual behavior shifts, we propose that patients may need a more personalized intervention to use risk information to support personal goals for health. In other words, the risk counseling seems to evoke in some people a desire or motivation to improve health behaviors and overall health but does not clearly lead to actual behavior change and clinical outcomes in many. When the risk counseling is combined with genetic risk information, the counseling may be personalized enough to enact greater change than does the standard risk counseling alone. Moreover, given the significant changes in both behavior and clinical markers noted in recent studies of health coaching for both T2D13,47,48 and those at risk for CHD,12,47 there is good reason to believe that providing health coaching after risk counseling for T2D and CHD is likely to be more effective than standard risk counseling alone on both behavior change and clinical markers. Finally, the two burgeoning literature bases of genomics and health coaching each present the possibility that incorporating genetic information into risk counseling may interact with health coaching to create a still larger effect (whether additive or synergistic). We thus will evaluate the effects of adding health coaching and/or genetic information to standard CHD and T2D risk counseling in primary care.
CONCEPTUAL FRAMEWORK
The Common Sense Model (CSM) of self-regulation of health and illness adapted by Marteau and Weinman49 to explain patient responses to health risk information (Figure 1) will serve as the framework for the study. Our working hypothesis is that the mechanism of effect of CHD and T2D genetic risk information is that it increases perceived risk (ie, perceived risk of harm if no action is taken; also vulnerability, likelihood, or susceptibility), which in turn increases motivation to engage in preventive health behaviors. Self-regulation refers to efforts to reduce the discrepancy between one's current status (eg, presence of health threat) and desired status (eg, reduction in threat of disease). According to the CSM, self-regulation is a dynamic process. Health risk information activates the cognitive representation of a health threat and/or the emotion associated with a health threat, which in turn activates a coping plan, which is then followed by an appraisal of the coping plan.50 The appraisal feeds back to update the representation and coping plan.
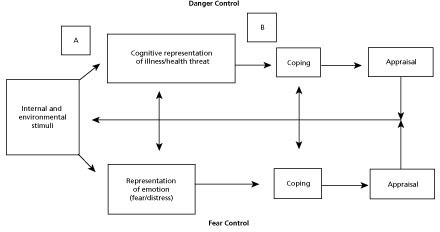
The novelty of genetic risk information for CHD and T2D may inform the cognitive representation of CHD and T2D as health threats in sharper relief than other, more familiar forms of risk information. The emotional expression of this altered representation would be manifested in heightened perceived risk regarding CHD and T2D, which then could interact with the cognitive representation to formulate a coping plan that is re-appraised from time to time depending upon the attention paid to CHD and T2D risk. We argue that the re-appraisal and attention to CHD and T2D may depend on the depth of the impression made when the issue of these risks is first discussed. Genetic testing (or any discussion of risk) may be most effective in increasing preventive behaviors if supplemented with health coaching, which may increase the effect of the perceived health threat on health behaviors by promoting self-awareness of current health status in contrast to desired health state, increasing self-efficacy, and providing support and skill building for health behavior change in the period following the receipt of risk information. Coaching brings about change in individuals through the active participation of the client, a supportive alliance between the coach and client, evaluation of the client's perception of the problem, and the client's generation of solutions to the problem.51–54 Importantly, while the risk counseling focuses on the perceived future threat, integrative health coaching (IHC) focuses on the discrepancy between that threat and the desired future state of the client,55 extending the CSM using additional theories of goal setting and motivation for sustainable behavior change.
INTEGRATIVE HEALTH COACHING
Although it shares theoretical roots with psychology and personal development, the health coaching approach is distinct from traditional health education and psychotherapy.55 IHC is based on 4 decades of behavioral science focused on how human motivation for change works. Based upon self-determination theory,56 self-concordance theory,57 positive psychology,58,59 and multiple aspects of goal-setting theory,60,61 integrative health coaches work to support clients' fulfillment of their desired future self. This is in contrast to their current self whose behavior creates a perceived threat to a desired future and ability to behave in a way that expresses core values. The more goals are self-congruent and aligned with core values, the more they are associated with a willingness to strive toward the goal.62,63 While most medical professionals are well intentioned in desiring change for their patients, they operate from a model that is incongruent with human motivation for change, and they are not trained to adapt their approach to the nature of behavior change. Integrative health coaches, on the other hand, help clients access the motivation needed to initiate and maintain change, offering a variety of perspectives and recognizing that many factors contribute to achieving goals.64 IHC has been shown to improve outcomes in both CHD risk12 and T2D.13 In a study conducted by members of our study team, the FRS of those who received health coaching for CHD risk reduction decreased significantly more at 10 months (P = .04) than in the control group FRS.12 The IHC group also showed significant improvements in number of days of exercise per week (P = .002) and weight loss (P = .06). In this study, IHC will be provided telephonically by IHC certified health coaches, all of whom have master's degrees in allied health professions (health promotion, clinical social work, and health psychology), at least 100 hours of coach-specific training, and a minimum of 8 years of health coaching experience.
PRECAUTIONS IN THE USE OF GENETIC RISK INFORMATION FOR LIFESTYLE CHANGE
Some concerns have been noted in the literature around presentation of genetic information to facilitate lifestyle change. One long-standing concern about genetic risk information is that genetic risk for disease may be interpreted by patients as deterministic (ie, a guarantee for the development of the disease), and this genetic fatalism may lead to perceived inability to act against a genetic threat. However, this does not appear to be a typical response, and often individuals undergo genetic testing in order to gain a sense of control.65,66 Another common concern is the potential for genetic tests to offer false reassurance to those with low or “no” genetic risk, giving them “permission” to avoid attending to healthy behavior.67,68 We have not noted these responses in our preliminary study leading up to this work.16,17 In addition, risk counseling materials (eg, the Standard Risk Assessment [SRA]) have been created to give study participants a complete view of their risk factors (both modifiable and nonmodifiable) and reduce potential misconceptions.
STUDY DESIGN AND SPECIFIC AIMS
Using a four-group (2 X 2 factorial) randomized controlled design, we will determine the direct and interactive effects of health coaching and the provision of multiple-marker genetic risk information when added to standard risk counseling for CHD and T2D. The intervention effects will be evaluated using changes in physical fitness, health behaviors, clinical risk factors, and potential mediators in ADAF and retirees and beneficiaries. Given the short duration of the intervention (6 mo), the primary outcomes of interest are specific risk-related health behaviors (physical activity, dietary intake, smoking cessation, medication adherence). Nonetheless, we ultimately are interested in morbidity; hence, the trial also will collect clinical markers (weight, waist circumference, BP, fasting glucose, total cholesterol, HDL, calculated LDL, and triglycerides) to use in predicting future morbidity and CHD and T2D risk status, as well as fitness status per new (2013) AF fitness scores. Secondary outcomes include multiple potential mediators of behavioral change, including variables that may be affected by the presentation of genetic risk (perceived risk, worry, and self-efficacy specific to CHD and T2D), patient activation, stages of change for behaviors of interest, and psychosocial risk factors for CHD and/or T2D (depression, unmanaged stress, and social isolation). Provision of the interventions through AF primary care clinics will provide us with generalizable samples and venues (primary care). This study design will allow data collection to address the following four specific aims.
Determine the main and interactive effects of an established, telephonic health coaching intervention and multiple-marker genetic risk information incorporated into standard CHD and T2D risk counseling (SRA) on health behavior (dietary intake, physical activity habits, smoking cessation, medication adherence) over 12 months among ADAF and retirees and beneficiaries. Determine the main and interactive effects of a telephonic health coaching intervention and genetic risk information incorporated into standard CHD and T2D risk counseling on clinical outcomes (AF composite fitness scores, weight, waist circumference, BP, fasting glucose, total cholesterol, HDL, calculated LDL, triglycerides, and CHD and T2D risk scores) over 12 months in this AF primary care cohort.
Determine the effects of potential mediators of primary outcomes, including perceived risk, patient activation, stages of change for behaviors of interest, and psychosocial risk factors for CHD and/or T2D (depression, unmanaged stress, and social isolation).
Determine the differential effects of level of CHD and T2D genetic risk (number of risk alleles) on behavior change (dietary intake, physical activity habits, smoking cessation, medication adherence) and AF fitness scores at 12 months after baseline.
Augment the implementation science on personalized interventions (genetic and behavioral) for risk reduction in primary care. By conducting this trial in two AF primary care clinics, following the implementation of similar protocols in two Duke primary care clinics,17 we will be able to provide specific lessons learned on strategies for implementation within primary care.
As seen in Figure 2, participants in this 2×2 factorial design will be randomized to one of four groups: (1) risk counseling based on standard CHD/T2D risk assessment (SRA) alone; (2) SRA plus CHD/T2D genetic risk (G) information (SRA+G); (3) SRA plus health coaching (HC) intervention (SRA+HC); and (4) SRA plus genetic risk (G) information plus health coaching intervention (SRA+G+HC). Following baseline measures, the risk counseling, with or without genetic risk information, will occur at a one-time visit (about 30 minutes) with a clinic provider specifically trained in risk counseling. For those randomized to the telephonic health coaching intervention, it will begin after the risk counseling visit and be carried out biweekly over 6 months. Follow-up measures of primary outcomes will be collected at 3, 6, and 12 months to determine long-term effects and map trajectories of outcomes by treatment group over time. Secondary measures that may serve as mediators of change will occur by survey at 6 weeks and 3 months.
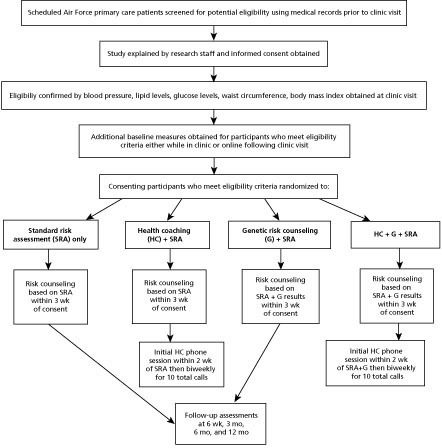
Figure 2 Study schema.
Participants
Primary care patients (N = 400) will be recruited from two participating AF outpatient clinic sites. Potential participants who are completing an annual preventive health assessment (PHA) or annual physical will be informed about the study and asked about interest in participating. With a Health Insurance Portability and Accountability Act (HIPAA) waiver, screening of medical records can be run on those with scheduled annual physicals to estimate likelihood of eligibility based on previous clinical and laboratory values. Eligibility screening will include BMI, systolic BP, and waist circumference, which are obtainable at the time of baseline visit, and lab results obtained within the last 6 months. Inclusion criteria are (1) age 18 years or older; (2) presence of at least one of the following cardiometabolic risk factors: fasting plasma glucose (FPG) ? 100 mg/dL, hemoglobin A1c (HbA1c) >5.7%, SBP ? 130 mmHg, DBP >85 mmHg, total cholesterol ? 200 mg/dL, LDL ? 129 mg/ dL, triglycerides ? 150 mg/dL, BMI ? 25 kg/m2, or waist circumference ? 102 cm in men or ? 88 cm in women; (3) able to speak, write, and understand English; and (4) able and willing to give informed consent. Exclusion criteria include diagnosed CHD or T2D, as this trial is a secondary prevention trial; inability to participate in physical activity; or serious medical complications or conditions that would threaten participation and/or undermine interpretation of the outcomes (cancer, renal failure, stroke). In order to protect intervention integrity, no two members of the same household will be admitted to the study. By targeting recruitment of those with scheduled annual physicals, we hope to obtain genetic testing samples and clinical and lab values that are typically obtained under standard of care without participants incurring additional testing. The priority will be to enroll ADAF members, but as dictated by recruitment rates and clinic populations, the trial may be opened to retirees and other adult AF beneficiaries.
PROCEDURES
Baseline data Collection
After screening, informed consent, and confirmation of eligibility, baseline measures are collected, as displayed in Table 1. Note that the same clinical and lab values may be used for confirmation of eligibility as well as baseline data. Immediately prior to or after the routine physical exam, the clinical research staff will measure height, weight, and waist circumference if they were not obtained during the exam. Staff will obtain weight using a calibrated scale with the participant wearing light indoor clothes without shoes. Height is measured at baseline only, using a scale or wall-mounted stadiometer. BMI is later calculated with the Quetelet index, (weight [kg]/height [m2]). Using standardized procedures, waist circumference is measured twice at the iliac crest with a metric tape measure and recorded to the nearest half centimeter, and the two values are later averaged. BP measurements are taken on the right arm (unless contraindicated) with an appropriate size cuff while participants are seated with the right arm supported at mid-atrial level. Participants are asked to refrain from eating, smoking, and exercising for at least 30 minutes and to sit quietly for at least 2 minutes before the BP measurement. Two measurements of BP will be taken and averaged. Following the visit, the clinical research staff will extract relevant baseline measures from the medical record. Similarly, lab values will be obtained from the medical record once assays are completed. Either at the visit or within 2 weeks of the visit, participants also will complete survey instruments through an appropriately firewalled online platform that meets standards set by the Office of Human Research Protection (OHRP) and the Federal Information Security Management Act of 2002 (FISMA) to ensure participant privacy, confidentiality, and data security. Once baseline data are complete, participants are scheduled for their risk counseling visit. In our pilot trials, 3 weeks between the baseline visit and the counseling visit allowed adequate time for participants to complete online measures and for necessary genetic results to be processed. See the section on genetic testing procedures.
Table 1.
Study Measures
| Study Measures | Baseline | 6 wks | 3 mo | 6 mo | 12 mo |
|---|---|---|---|---|---|
| Eligibility screen and informed consent | X | ||||
| Demographic questionnaire | X | ||||
| Family history of CHD and T2D | X | ||||
| Blood draw for genetic testing | X | ||||
| Primary Outcomes: Behavioral | |||||
| Dietary intake (NCI Screener) | X | X | X | X | X |
| Physical activity (SBAS) | X | X | X | X | X |
| Smoking status | X | X | X | X | X |
| Medication adherence (Morisky 4) | X | X | X | X | X |
| Primary Outcomes: Clinical | |||||
| Height for BMI calculation | X | ||||
| Weight | X | X | X | ||
| Waist circumference | X | X | X | ||
| Blood pressure | X | X | X | ||
| Medications | X | X | X | X | X |
| Laboratory tests (fasting glucose, total cholesterol, HDL, LDL, triglycerides) | X | X | |||
| Fitness status (Air Force Fitness Score) | X | X | X | X | X |
| Risk status (FRS and T2D Risk Score) | X | X | X | X | X |
| Potential mediators | |||||
| Perceived risk for CHD and T2D | X | X | X | X | X |
| Worry, self-efficacy | X | X | X | X | X |
| Patient activation | X | X | X | X | X |
| Stages of change | X | X | X | X | X |
| Psychosocial risk factors (depression, stress level, social isolationa) | X | X | X | X | X |
While hostility has strong predictive power for CHD,69 we have chosen not to assess for hostility given the participant burden of multiple surveys. Abbreviations: BMI, body mass index; CHD, coronary heart disease; FRS, Framingham Risk Score; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NCI, National Cancer Institute; SBAS, Stanford Brief Activity Survey; T2D, type 2 diabetes.
Randomization
Assessment staff and risk counselors need to remain blinded to protect against bias. Hence, all baseline data must be completed before randomization assignments are made. Once baseline data are complete, individuals are randomized into one of the following four groups: SRA only; SRA plus genetic risk information (SRA + G); SRA plus health coaching (SRA + HC); or SRA, genetic risk information, and health coaching (SRA + G + HC). Random assignments are generated using a standard random number generator with uniform distribution to the four groups. The research staff will then let participants know of their group assignment.
Risk Counseling Visit
Within 3 weeks after the baseline visit, all participants receive risk counseling with trained provider(s) at the clinic site. The aim of the risk counseling intervention is to inform patients' perceptions of their CHD and T2D risk. During the visit, the provider will review each aspect of the SRA plus/minus genetic testing results depending on their random group assignment. The provider will use standard educational materials to counsel participants about what they can do to lower their risk, particularly through diet, physical activity, smoking cessation, and, if relevant, medication adherence.
Standard Risk Assessment for Coronary Heart Disease and Type 2 Diabetes
The SRA has been developed for this study based on the FRS, diabetes risk score, and other known risk factors for both CHD and T2D. The FRS for 10-year risk of hard coronary disease is based upon age, gender, BP, total cholesterol, HDL, and smoking status. Before the risk counseling visit, FRS and diabetes risk score will be calculated for each patient and presented along with other well-validated CHD and T2D risk factors, including family history (number of first-degree relatives who have CHD or T2D), fasting glucose or HbA1c, waist circumference, BMI, calculated LDL, triglycerides, and fitness status using standard AF fitness scores. Additionally, results of the psychosocial risk factors for CHD and T2D will be presented, pointing out the association between these factors and disease risk. Psychosocial risk factors reviewed include depression, perceived stress, and social isolation. At the risk counseling visit, all risk factors are reviewed with patients, and their risk calculations are presented as the likelihood of a coronary event in the next 10 years or diabetes in the next 5 years. The materials describing the contribution of environmental risk and genetic risk for these multifactorial diseases are presented in lay language from the National Academy of Sciences.70 Similar procedures were used in two pilot trials of more than 400 clinical study patients17,71 and in more than 200 primary care patients seen in integrative medicine lifestyle trials.12,72 The process of providing predictive information based upon psychosocial risk factors has been used with more than 2000 executive primary care patients over the past 15 years (K. Waters, personal communication, March 18, 2013).
Genetic Testing for Coronary Heart Disease and Type 2 Diabetes Risk
Because participants and assessment staff members need to remain blinded until after the collection of all baseline data, genetic testing cannot occur until after randomization. However, it is inconvenient for participants to schedule an additional study visit only to provide the blood sample. Therefore, our goal is to recruit participants who are having blood drawn for routine lab testing and draw the extra vial for the genetic testing at that time to avoid additional venipuncture. Following randomization, the blood samples will be sent to a Clinical Laboratory Improvement Amendments (CLIA)–certified lab to run the real-time polymerase chain reaction (PCR) assay for allelic discrimination incorporating the relevant SNPs. For CHD risk, the rs10757274 9p21 SNP will be genotyped.33 For participants randomized to the two groups receiving genetic CHD and T2D risk information, the results will be used to modify the FRS39 (+1 point for two higher-risk alleles, 0 points for one higher-risk allele, and –1 point for no higher-risk alleles), and participants will be informed if the addition of their genetic information leads them to be reclassified at a different risk level than predicted by the FRS alone. The results of the T2D genetic risk testing for SNPs rs7903146, rs1801282, and rs5219 will be presented to participants as the total number of higher-risk alleles out of all six T2D risk alleles tested.
Health Coaching Intervention
Participants randomized to the two groups that include health coaching (n = 200) will be assigned to a trained health coach, and the first session will occur within 2 weeks of the risk counseling visit (and within 5 weeks of enrollment). Specifically, IHC sessions are provided by telephone using a structure that has evolved in multiple trials and clinical programs at Duke Integrative Medicine.12,13,73,74
Initial Call.
In the initial coaching call (∼ 60 minutes in length), the coach discusses the role of a health coach and the logistics of the sessions and guides the participant in a self-assessment of health perceptions and goals. The process begins with the participant envisioning a future self that represents optimal health and manifests the core values of the individual. In this session, as well as throughout the coaching experience, participants are guided in self-discovery processes in which they clarify their values and goals and the link between their values and goals and optimal health. The coaches assist participants in an iterative self-assessment to compare their desired futures to their current state in multiple domains of their lives.12,13,75 Areas that are discrepant (highly important, but low current satisfaction ratings) are further explored for readiness to change.76 Participants then prioritize where to begin. They are encouraged to make decisions that are congruent with their individual values, long-term vision of health, and sense of purpose. Participants are more likely to create self-sustaining agendas for themselves when they have considered the greater perspective of their lives. The coaching agenda, priorities, and specific goals are set by the participant; however, the coach regularly asks participants about self-assessment in terms of other risk reduction areas such as diet, exercise, smoking status, and medication adherence. Committing to small action steps on a biweekly basis, participants move toward self-identified health goals. Results of the self-assessment provide motivation for lifestyle behavior change. During the initial call, participants are also taught how to prepare for the IHC sessions. They are given tools for preparation, including a “coaching prep form.” The form is organized to assist clients in briefly noting discussion points for the call, including successes since last call, obstacles encountered, problem-solving approaches tried, outcomes, upcoming potential obstacles, resources needed, and small action-step commitments to be accomplished before the next call.
Additional Nine Integrative Health Coaching Calls.
As planned, and as demonstrated empirically in other trials, IHC calls follow the general template of the coaching prep form. The sessions begin with a brief check-in, with the participant reporting on specific action steps from the preceding week(s). Participants are trained to note success first, then to problem-solve and explore solutions for obstacles. Importantly, they learn from failed problem-solving attempts, which are nonjudgmentally framed as “experiments” necessary for true learning.77 Nonjudgmental framing is essential to maintaining rapport and encouraging clients to experiment with solutions without fear of failure. Participants commit to new action steps toward goals to be accomplished before the next call and clarify the resources they need to accomplish them. Of note, the health coaches are blinded to the participants' genetic risk results; however, some participants choose to disclose them to the coaches, mimicking how individuals may use this information in nonstudy settings.
Outcome Measures
Outcome measures and timepoints at which they are collected are indicated in Tables 1 and 2.
Table 2.
Instruments for Outcome Measures
| Concept measured | Details | No. of Items | Validity and reliability |
|---|---|---|---|
| Demographic data (researcher- developed instrument) | Age, gender, race/ethnicity, educational level, marital status, living arrangements, smoking, alcohol use, history of being overweight, family history of heart disease (siblings, parents, grandparents, aunts, and uncles). | Used in other studies regarding chronic disease risk (T2D) with participants from the preliminary studies leading to this protocol. | |
| Dietary intake: NCI Multifactor Screener | Assesses frequency of intake of various foods over the last month (by d, wk, mo). The screener asks respondents to report how frequently they consume foods in 16 categories. | 16 | Multifactor screener has demonstrated correlations of 0.5-0.8 with estimated true intake.78 |
| Physical activity: Stanford Brief Activity Survey | Assesses two categories of physical activity—work and leisure. Five options for degree of activity to choose from in each of the two areas of activity. | 2 | Test-retest reliability demonstrated (r = 0.62) and construct validity shown through significant inverse correlations with stress, anxiety, and depression and positive correlations with mental and physical well-being.79,80 Has been correlated inversely with CHD risk.79,80 |
| Coronary and diabetes risk perception | Assesses level of personal perceived risk, fear, anger, worry regarding T2D and CHD risk. | 52 | Used in two prior studies by investigative team. |
| Perceived control over T2D | Personal control subscale of the Brief IPQ (adapted for type 2 diabetes and CHD).81 Sample item: “Whether or not I get diabetes depends on me.” Response scale 1 (strongly disagree) to 5 (strongly agree). | 6 | Demonstrated good test–retest reliability and concurrent validity with relevant measures. The discriminant validity of the Brief IPQ was supported by its ability to distinguish between different illnesses.81 |
| Patient activation | Assesses the following 4 stages of patient activation: (1) believing the patient role is important, (2) having the confidence and knowledge necessary to take action, (3) actually taking action to maintain and improve one's health, and (4) staying the course even under stress.82 | 13 | Cronbach's ? of 0.87 as well as established criterion and construct validity. |
| Readiness for change | Assesses stage of change based upon the Transtheoretical Model75 for 5 health behavior domains (dietary intake, exercise, weight loss, smoking cessation, and medication adherence). Individual items are validated,83 aggregated by co-PI, and used in prior studies. | 5 | Used in prior studies involving health coaching.12,13 |
Abbreviations: CHD, coronary heart disease; IPQ, illness perception questionnaire; NCI, National Cancer Institute; PI, principal investigator; T2D, type 2 diabetes.
Analysis Plan
Demographic characteristics of the sample (income level, age, gender, race/ethnicity, educational level) will be provided using descriptive statistics to describe the overall sample and by group to ensure that randomization was effective. Distribution of variables will be determined before further statistical analysis takes place. SAS software (9.2 edition, SAS Institute Inc, Cary, North Carolina) will be used for all analyses.
To address the primary aims, mixed models will be used. The first aim is to determine the direct and interactive effects of genetic risk information incorporated with standard CHD/T2D risk counseling and a health coaching intervention for CHD/T2D on behavior change (diet, physical activity) in AF primary care patients over 12 months. The goals of the statistical analysis will be to quantify (1) the extent to which genetic risk counseling influences the trajectory of change in physical activity and diet measures from baseline to 3, 6, and 12 months and (2) the extent to which the genetic risk information augments the trajectory of the effects of health coaching on diet and physical activity measures over the same time period. We will fit two general linear mixed models to these data,84 with physical activity and diet measures at each of the five points in time. For either model, there will be three independent variables: genetic risk information (G) with values of 1 or 0, health coaching (HC) also with values of 1 or 0, and time since randomization with values of 0, 3, 6, and 12 months. Within the context of the linear mixed model, we should observe a statistically significant three-way interaction of time by genetic information by health coaching. The particular type of longitudinal model selected for analysis will depend upon preliminary analyses of the data. For example, a subject-specific (also called hierarchical) model with random intercept and slope assumes that within-subject variances increase or decrease over time.84 A model hypothesizing only a random intercept does not assume that within-subject variances show this pattern. Another consideration will be the extent of missing data; some longitudinal models are better than others at handling missing data.84
The second part of the primary analysis will determine the direct and interactive effects of genetic risk information incorporated with standard CHD/T2D risk counseling and a health coaching intervention for CHD/ T2D on metabolic outcomes (fasting blood glucose, SBP, BMI, waist circumference, LDL, triglycerides, total cholesterol) over 12 months. The statistical analysis will be the same as described above except that the dependent variables will be fasting blood glucose, SBP, BMI, waist circumference, LDL, triglycerides, and total cholesterol.
To address aim 2, we will examine the mediating effects of level of CHD/T2D genetic risk (number of risk alleles) and consequent reclassification of FRS in the case of CHD risk (decreased, neutral, or increased) on behavior change (diet, physical activity) at 6 months. The goal of this analysis is to quantify the extent to which subgroups of patients exhibit different levels of change in diet and physical activity behavior at 6 months after randomization depending on reclassification of FRS and T2D risk level. We also want to know if the differences depend on whether or not the genetic results were delivered in the context of health coaching. For these analyses, we will use results from only the two groups of patients randomized to receive genetic testing (n = 200). Because we will have only two points in time, we will use the simpler linear regression model, with the dependent variable being either diet or physical activity collected at 6 months after randomization. There will be six independent variables: the outcome measured at baseline (a covariate), the results of the genetic testing (entered as two dummy coded variables), a dummy coded variable to indicate the presence or absence of health coaching, and two dummy coded variables representing the interaction of health coaching and the results of genetic testing. We are particularly interested in comparing the diet and physical activity changes of patients whose genetic test results move their Framingham risk or T2D levels either up or down (based on number of higher-risk alleles) to those whose genetic results do not. In our regression analysis, this comparison will be facilitated by creating two dummy coded variables: Dummy 1 will be 1 if the patient has “low” risk based on number of risk alleles and 0 otherwise, and dummy 2 will be 1 for patients with “high” risk based on number of risk alleles and 0 otherwise. When group membership is coded in this way, the regression coefficients (parameters) fitted to the data represent differences in adjusted group means. “Adjusted” refers to group differences on outcome measures obtained at 6 months while statistically eliminating any differences between groups on outcomes at baseline. Thus, the regression coefficient for dummy 1 will reflect the differences in average adjusted outcome at 6 months between patients whose genetics results shifted their risk down and patients whose results did not affect their risk. Similarly, the regression coefficient for dummy 2 will reflect differences in adjusted outcome means comparing patients whose risk shifted up to those whose risk was unaffected by their genetic test results. The interaction components of this model will allow us to assess whether or not these comparisons significantly differ depending upon whether or not the patient received health coaching.
Analyses for aim 3 will examine the mediating effects of perceived CHD/T2D risk, self-efficacy, worry, patient activation, and readiness for change on the effects of genetic risk information on diet and physical activity at 6 months after randomization. The analytic strategy for this aim will be to determine the extent to which genetic risk treatment effects on diet and physical activity occur subsequent to treatment effects on perceived CHD/T2D risk, self-efficacy, worry, patient activation, and readiness for change. For example, one set of analyses will estimate the direct relationship between genetic risk counseling and changes in physical activity at 6 months (independent of evaluation of the effects of treatment on perceived risk). Additionally, the extent to which genetic risk counseling influences perceived risk at 6 weeks and 3 months postrandomization, which in turn affects behavior change at 6 months, will be assessed. This analysis will be repeated using diet as the dependent variable. Then both will be repeated using self-efficacy, worry, patient activation, and readiness for change as mediators. In each analysis, mediation will be estimated via a series of linear regressions. Mediation will be tested statistically by examining the empirically generated sampling distribution of the parameters involved in the mediational hypothesis: the effect of treatment on the mediators at 6 weeks and 3 months, the effect of the mediators at 3 months on the dependent variable at 6 months, and the product of these effects. We will use bootstrapped sampling distributions of the parameters involved in each meditational hypothesis.85 Recent work86–88 supports this method rather than the more traditional Sobel tests, especially with smaller samples.
Sample Size/Power Calculation
The objective of the power analysis was to determine the minimum sample size needed to reject the null hypothesis that the three-way genetic information by health coaching by time interaction was equal to 0 in the context of a longitudinal general linear mixed model. The model was assumed to have two levels of genetic information (present or absent), two levels of health coaching (present or absent), and five levels of time (0, 1.5, 3, 6 and 12 months after randomization). In producing the required sample-size estimates, we further assumed that each of the four group means over time would take on standardized mean values. The type I error rate was set to be no greater than .025 as there will be two primary analyses. Controlling each one at no greater than .025 controls the overall experiment type I error rate to be no greater than .05. Calculations89 indicated that with a sample size of 99 subjects per group, or 396 total, the power to reject the null hypothesis concerning the three-way interaction was .80. Assuming an attrition rate of at most 15% and the pilot T2D study attrition of 11%, over the 12 month time period we calculated a required sample size of (396/.86) = 460 to attain the target sample of 400. Sample size calculations are not provided for detecting the direct effects or interactive effects of the two interventions pooled over time, primarily because we expect the genetic information by health coaching interaction to change over time, as reflected in the three-way interaction.
DISCUSSION
This study tests novel strategies, deployable within existing AF primary care, to increase adherence to evidence-based diet, physical activity, and smoking cessation recommendations for CHD and T2D risk reduction through patient engagement and self-management support. This study seeks to increase our understanding of two personalized interventions, one informational (standard and/or genetic CHD and T2D risk counseling) and one behavioral (telephonic health coaching), that are increasingly available but relatively little studied. This study is translational in nature, positioning the interventions within primary care to provide patients with consistent reinforcing messages and assistance to make necessary lifestyle changes to reduce their CHD and T2D risk. We are also laying the groundwork for an important future phase in genomic-based risk marker evaluation, ie, assessing their cost-effectiveness in prevention.
Moreover, we will address a much discussed but little studied concern regarding the use of genetic testing for chronic disease risk—that of unintended psychological consequences, such as perceptions of genetic determinism or false reassurance. By using a single well-validated genetic marker for CHD risk, 9(p21), a “negative” genetic CHD risk result and a downward movement in level of CHD risk are possible. Also, by incorporating the most highly validated T2D markers in terms of number of risk alleles, we provide patients with a well-informed perception of level of genetic risk. Doing so enables us to examine whether in fact we see evidence of false reassurance or determinism, assessing risk perception, worry, and self-efficacy as well as behavior change.
In keeping with our focus on translation, we made a deliberate decision not to use a genetic counselor to deliver the results of genetic testing to study participants. Instead, understanding that (1) a referral model for a single piece of information that informs a much broader clinical risk assessment conducted in a primary care setting was unlikely to be practical, (2) it will be important for healthcare providers to have a working understanding of emerging genomic information, and (3) counseling about risk reduction overall is as important as expert explanations of each risk factor. We will have an experienced genetic counselor and nurse practitioner with experience with risk counseling in our prior studies train the AF providers who will perform the risk counseling. An overarching goal of the Duke University–US Air Force Medical Service partnership is the expansion of the latter's ongoing “train the trainer” program in genomics-based medicine. This effort, which aims to standardize the translation and utility of genetic-based risk information, will afford precision care and personalized medicine to beneficiaries while minimizing treatment failure rates and detrimental side effects.
Finally, we are integrating an innovative telephonic health coaching intervention into prevention of CHD and T2D in primary care. This formal health psychology– based coaching program is based on extensive research in behavior change. Used in executive coaching for almost 30 years, the approach has only recently been piloted in healthcare.12,13,55,72 We propose that health coaching may have an additive effect in the context of risk information or counseling, making this risk information more “potent” in activating participants to reduce their risk for CHD and T2D.
Acknowledgments
This material is based on pilot studies supported through the Duke Center for Personalized Medicine Small Grants Program. We would like to thank Alex Cho, MD, MBA; Dana Baker, MS; Mary Lou Bembe, PA, MHS; Teji Rakhra-Burris, MA; Michael Musty, BA; Janet Levy, PhD; and the health coaches who participated in the pilot studies: Jessica Wakefield, MA; Linda Duda, MSW; Elizabeth Bechard, BA; Paige O'Luanaigh, BSN, MBA; and Anne Doster, EdD. Additionally, we appreciate the support of Cecili Sessions, MD, MPH, FAAP, Lt Col USAF, MC, and USAF Chief of Personalized Medicine for facilitating the translation of this project to the USAF.
The published protocol will be used in research sponsored by Air Force Medical Support Agency, Medical Research and Innovation Directorate AFMSA/SG5I under agreement number FA8650-12-2-6374. The US Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon.
Disclosures The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and disclosed the grant specified in the Acknowledgments section of this article. No other potential conflicts of interest were disclosed.
Disclaimer The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Medical Support Agency, Medical Research and Innovation Directorate AFMSA/SG5I or the US Government.
Contributor Information
Allison A. Vorderstrasse, Duke University School of Nursing, United States.
Geoffrey S. Ginsburg, Institute for Genome Sciences & Policy, United States.
William E. Kraus, Division of Cardiology, Duke Schools of Medicine and Nursing, United States.
Maj Carlos J. Maldonado, David Grant USAF Medical Center, Travis Air Force Base, California, United States.
Ruth Q. Wolever, Duke Integrative Medicine, Department of Psychiatry & Behavioral Science, Duke School of Medicine, Center for Personalized Medicine, Duke University Health System, United States.
REFERENCES
- 1.CDC National diabetes fact sheet 2007. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf Accessed April 22, 2013. [Google Scholar]
- 2.CDC Heart Disease. http://www.cdc.gov/nchs/fastats/heart.htm Accessed April 22, 2013. [Google Scholar]
- 3.Robbins A, Chao S, Fonseca V, Snedecor M, Knapik J. Predictors of low physical fitness in a cohort of a active-duty U.S. air force members. AM J Prev Med. 2001;20(2):90–6 [DOI] [PubMed] [Google Scholar]
- 4.Bray R, Hourani L, Olmstead K. 2002 Department of Defense survey of health related behaviors among military personnel. Research Triangle Park, NC: RTI International; 2005. Contract No.: RTI/7841/106-FR. http://www.dtic.mil/cgibin/GetTRDoc?AD=ADA431566 Accessed April 22, 2013. [Google Scholar]
- 5.Kress A, Hartzel M, Peterson M. Burden of disease associated with overweight and obesity among U.S. military retirees and their dependents, aged 38-64, 2003. Prev Med. 2005;41:63–9 [DOI] [PubMed] [Google Scholar]
- 6.Paris R, Bedno S, Krauss M, Keep L, Rubertone M. Weighing in on type 2 diabetes in the military: characteristics of U.S. military personnel at entry who develop type 2 diabetes. Diabetes Care. 2001;24(11):11894–8 [DOI] [PubMed] [Google Scholar]
- 7.Standards of medical care in diabetes—2013. Diabetes Care. 2013;36Suppl 1:S11–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105(18):2231–9 [DOI] [PubMed] [Google Scholar]
- 9.Redberg RF, Benjamin EJ, Bittner V, et al. AHA/ACCF [corrected] 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on performance measures (writing committee to develop performance measures for primary prevention of cardiovascular disease): developed in collaboration with the American Academy of Family Physicians; American Association of Cardiovascular and Pulmonary Rehabilitation; and Preventive Cardiovascular Nurses Association: endorsed by the American College of Preventive Medicine, American College of Sports Medicine, and Society for Women's Health Research. Circulation. 2009;120(13):1296–336 [DOI] [PubMed] [Google Scholar]
- 10.Muller-Riemenschneider F, Reinhold T, Nocon M, Willich SN. Long-term effectiveness of interventions promoting physical activity: a systematic review. Prev Med. 2008;47(4):354–68 [DOI] [PubMed] [Google Scholar]
- 11.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41–50 [DOI] [PubMed] [Google Scholar]
- 12.Edelman D, Oddone EZ, Liebowitz RS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21(7):728–34 Epub 2006/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ. 2010;36(4):629–39 [DOI] [PubMed] [Google Scholar]
- 14.Voils CI, Coffman CJ, Edelman D, et al. Examining the impact of genetic testing for type 2 diabetes on health behaviors: study protocol for a randomized controlled trial. Trials. 2012;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant RW, O'Brien KE, Waxler JL, et al. Personalized genetic risk counseling to motivate diabetes prevention: a randomized trial. Diabetes Care. 2013;36(1):13–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho A, Vorderstrasse A, Suchindran S, et al. Preliminary 3-month outcomes of study of genetic risk testing for Type 2 diabetes in primary care settings. Society of General Internal Medicine Annual Meeting; May7, 2012; Orlando, Florida [Google Scholar]
- 17.Cho AH, Killeya-Jones LA, O'Daniel JM, et al. Effect of genetic testing for risk of type 2 diabetes mellitus on health behaviors and outcomes: study rationale, development and design. BMC Health Serv Res. 2012;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen KD, Roberts JS, Uhlmann WR, Green RC. Changes to perceptions of the pros and cons of genetic susceptibility testing after APOE genotyping for Alzheimer disease risk. Genet Med. 2011;13(5):409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron LD, Sherman KA, Marteau TM, Brown PM. Impact of genetic risk information and type of disease on perceived risk, anticipated affect, and expected consequences of genetic tests. Health Psychol. 2009;28(3):307–16 [DOI] [PubMed] [Google Scholar]
- 21.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson SC, Wardle J, Jarvis MJ, Humphries SE. Public interest in genetic testing for susceptibility to heart disease and cancer: a population-based survey in the UK. Prev Med. 2004;39(3):458–64 [DOI] [PubMed] [Google Scholar]
- 23.Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin SN, Crownover BK, Kovach FE. Clinical inquiries. What's the best way to motivate patients to exercise? J Fam Pract. 2010;59(1):43–4 [PubMed] [Google Scholar]
- 25.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bairey Merz CN, Alberts MJ, Balady GJ, et al. ACCF/AHA/ACP 2009 competence and training statement: a curriculum on prevention of cardiovascular disease. J Am Coll Cardiol. 2009September29;54(14):1336–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowles S, Picano J, Epperly T, Myer S. The LIFE program: a wellness approach to weight loss. Mil Med. 2006;171(11):1089–94 [DOI] [PubMed] [Google Scholar]
- 28.Nelson M, Robbins A, Thornton J. An intervention to reduce excess body weight in adults with or at risk for type 2 diabetes. Mil Med. 2006;171(5):409–14 [DOI] [PubMed] [Google Scholar]
- 29.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7 [DOI] [PubMed] [Google Scholar]
- 30.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E–9E [DOI] [PubMed] [Google Scholar]
- 31.Sheridan SL, Viera AJ, Krantz MJ, Ice CL, Steinman LE, Peters KE, et al. The effect of giving global coronary risk information to adults: a systematic review. Arch Intern Med. 2010;170(3):230–9 [DOI] [PubMed] [Google Scholar]
- 32.Mochan E, Ebell M. Risk-assessment tools for detecting undiagnosed diabetes. American family physician. 2009;80(2):175–8 [PubMed] [Google Scholar]
- 33.Palomaki GE, Melillo S, Bradley LA. Association between 9p21 genomic markers and heart disease: a meta-analysis. JAMA. 2010;303(7):648–56 [DOI] [PubMed] [Google Scholar]
- 34.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–3 [DOI] [PubMed] [Google Scholar]
- 35.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromo-some 9 associated with coronary heart disease. Science. 2007;316(5830):1488–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talmud PJ, Cooper JA, Palmen J, et al. Chromosome 9p21.3 coronary heart disease locus genotype and prospective risk of CHD in healthy middle-aged men. Clin Chem. 2008;54(3):467–74 [DOI] [PubMed] [Google Scholar]
- 38.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med. 2009;150(2):65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brautbar A, Ballantyne CM, Lawson K, et al. Impact of adding a single allele in the 9p21 locus to traditional risk factors on reclassification of coronary heart disease risk and implications for lipid-modifying therapy in the Atherosclerosis Risk in Communities study. Circ Cardiovasc Genet. 2009June;2(3):279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–50 [DOI] [PubMed] [Google Scholar]
- 41.Dreyfuss JM, Levner D, Galagan JE, Church GM, Ramoni MF. How accurate can genetic predictions be? BMC Genomics. 2012;13:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prudente S, Dallapiccola B, Pellegrini F, Doria A, Trischitta V. Genetic prediction of common diseases. Still no help for the clinical diabetologist! Nutr Metab Cardiovasc Dis. 2012;22(11):929–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassy JL, Donelan K, Hivert MF, Green RC, Grant RW. Genetic susceptibility testing for chronic disease and intention for behavior change in healthy young adults. J Community Genet. 2013;4(2):263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haga SB, Barry WT, Mills R, et al. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers. 2013;17(4):327–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markowitz SM, Park ER, Delahanty LM, O'Brien KE, Grant RW. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care. 2011;34(3):568–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3(10):e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreusicke MH, Fenster A, Skinner B, et al. Pharmacy claims data support the effectiveness of integrative health coaching for patients with type II diabetes. Poster presented at the 71st Scientific Sessions of the American Diabetes Association. San Diego, CA: June2011 [Google Scholar]
- 49.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62(6):1360–8 [DOI] [PubMed] [Google Scholar]
- 50.Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interaction. Cognitive Ther Res. 1992;16(2):143–63 [Google Scholar]
- 51.Kaufman C. Positive psychology: the science at the heart of coaching. In: Stober DR, Grant AM, editors. Evidence-based coaching handbook. Hoboken NJ: John Wiley & Sons; 2006 [Google Scholar]
- 52.DeShazer S. Keys to solution in brief therapy. New York: Norton; 1985 [Google Scholar]
- 53.O'Hanlon B. Guide to possibility land. New York: Norton; 1999 [Google Scholar]
- 54.O'Hanlon B. Do one thing different. New York: Morrow; 1999 [Google Scholar]
- 55.Wolever RQ, Caldwell KL, Wakefield JP, et al. Integrative health coaching: an organizational case study. Explore (NY). 2011;7(1):30–6 [DOI] [PubMed] [Google Scholar]
- 56.Deci E, Ryan R. Intrinsic motivation and self-determination in human behavior. New York: Plenum; 1985 [Google Scholar]
- 57.Sheldon KM, Elliot AJ. Goal striving, need satisfaction, and longitudinal well-being: the self-concordance model. J Pers Soc Psychol. 1999;76(3):482–97 [DOI] [PubMed] [Google Scholar]
- 58.Cohn MA, Fredrickson BL. In search of durable positive psychology interventions: Predictors and consequences of long-term positive behavior change. The journal of positive psychology. 2010;5(5):355–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stober DR, Grant AM, editors. Evidence based coaching handbook: putting best practices to work for your clients. Hoboken, NJ: Wiley; 2006:219–53 [Google Scholar]
- 60.Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. A 35-year odyssey. Am Psychol. 2002;57(9):705–17 [DOI] [PubMed] [Google Scholar]
- 61.Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32–42 [DOI] [PubMed] [Google Scholar]
- 62.Sheldon K. The self-concordance model of healthy goal-striving: when personal goals correctly represent the person. In: Deci E, Ryan R, editors. Handbook of self-determination research. Rochester, NY: University of Rochester Press; 2002 [Google Scholar]
- 63.Sheldon K, Kasser T. Pursuing personal goals: skills enable progress but not all progress is beneficial. Pers Soc Psychol Bull. 1998;24:1319–31 [Google Scholar]
- 64.Stober D, Grant A, editors. Evidence-based coaching handbook. Hoboken, NJ: John Wiley & Sons; 2006 [Google Scholar]
- 65.Marteau T, Senior V, Humphries SE, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: a randomized controlled trial. Am J Med Genet. 2004;128A(3):285–93 [DOI] [PubMed] [Google Scholar]
- 66.Bates BR, Templeton A, Achter PJ, Harris TM, Condit CM. What does “a gene for heart disease” mean? A focus group study of public understandings of genetic risk factors. Am J Med Genet. 2003;119A(2):156–61 [DOI] [PubMed] [Google Scholar]
- 67.Leighton JW, Valverde K, Bernhardt BA. The general public's understanding and perception of direct-to-consumer genetic test results. Public Health Genom. 2012;15(1):11–21 [DOI] [PubMed] [Google Scholar]
- 68.McPherson E. Genetic diagnosis and testing in clinical practice. Clin Med Res. 2006;4(2):123–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychol Bull. 1996;119(2):322–48 [DOI] [PubMed] [Google Scholar]
- 70.Williams SC. Epigenetics. Proc Natl Acad Sci USA. 2013;110(9):3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vorderstrasse A, Cho A, Wolever R. Clinical utility of genetic risk assessment in common chronic diseases: T2D & CHD—feasibility & preliminary data. International Society of Nurses in Genetics Annual Conference; October27, 2012; Philadelphia, PA [Google Scholar]
- 72.Wolever RQ, Webber DM, Meunier JP, Greeson JM, Lausier ER, Gaudet TW. Modifiable disease risk, readiness to change, and psychosocial functioning improve with integrative medicine immersion model. Altern Ther Health Med. 2011;17(4):38–47 [PMC free article] [PubMed] [Google Scholar]
- 73.Smith LL, Lake NH, Simmons LA, Perlman A, Wroth S, Wolever RQ. Integrative health coach training: a model for shifting the paradigm toward patient-centricity and meeting new national prevention goals. Global Adv Health Med. 2013;2(3):52-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldwell KL, Grey J, Wolever RQ. The process of patient empowerment in integrative health coaching: How does it happen? Global Adv Health Med. 2013;2(3):34-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liebowitz R, Smith L, editors. The Duke encyclopedia of new medicine: conventional and alternative medicine for all ages. New York: Rodale; 2006 [Google Scholar]
- 76.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48 [DOI] [PubMed] [Google Scholar]
- 77.Berger J. Adult development theory and executive coaching practice. In: Stober D, Grant A, editors. Evidence based coaching handbook. Hoboken, NJ: Wiley; 2006. p. 77-102 [Google Scholar]
- 78.Thompson FE, Midthune D, Subar AF, Kahle LL, Schatzkin A, Kipnis V. Performance of a short tool to assess dietary intakes of fruits and vegetables, percentage energy from fat and fibre. Public Health Nutr. 2004;7(8):1097–105 [DOI] [PubMed] [Google Scholar]
- 79.Taylor-Piliae RE, Haskell WL, Iribarren C, et al. Clinical utility of the Stanford brief activity survey in men and women with early-onset coronary artery disease. J Cardiopulm Rehabil Prev. 2007;27(4):227–32 [DOI] [PubMed] [Google Scholar]
- 80.Taylor-Piliae RE, Fair JM, Haskell WL, et al. Validation of the Stanford Brief Activity Survey: examining psychological factors and physical activity levels in older adults. J Phys Act Health. 2010;7(1):87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–7 [DOI] [PubMed] [Google Scholar]
- 82.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prochaska JO, Velicer WF, Redding C, et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Prev Med. 2005;41(2):406–16 [DOI] [PubMed] [Google Scholar]
- 84.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of longitudinal data. 2nd ed: Oxford: Oxford University Press; 2002 [Google Scholar]
- 85.Efron B, Tibshirani R. An introduction to the bootstrap. London: Chapman & Hall/CRC; 1994 [Google Scholar]
- 86.MacKinnon DPL, C M, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2004;7(1):83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–45 [PubMed] [Google Scholar]
- 89.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17(14):1643–58 [DOI] [PubMed] [Google Scholar]


