Abstract
Health coaching is an emerging behavioral intervention to improve outcomes in chronic disease management and prevention; however, no studies have investigated its utility in postpartum women who have gained excess weight during pregnancy. A 32-year-old primigravida woman who was overweight at conception and gained 23 lbs more than Institute of Medicine recommendations for her pre-pregnancy body mass index participated in a 6-month personalized health planning with integrative health coaching (PHPIHC) intervention. The intervention included a baseline health risk assessment review with a healthcare provider and eight biweekly, 30-minute telephonic health coaching sessions. The participant demonstrated improvement in physical activity, energy expenditure, knowledge, and confidence to engage in healthpromoting behaviors. Although the participant did not reach the target weight by completion of the health coaching sessions, follow up 8 months later indicated she achieved the target goal (within 5% of prepregnancy weight). This case report suggests that PHP-IHC can support postpartum women in returning to pre-pregnancy weight after gaining excess gestational weight. Future research and clinical trials are needed to determine the best timing, length, and medium (online, in-person, telephonic) of PHP-IHC for postpartum women.
Key Words: Personalized health planning, health risk assessments, integrative health coaching, postpartum weight retention, obesity prevention, pregnancy weight gain, women's health
摘要
健康辅导是一种新兴的行为 干预方法,用以改善慢性疾病管 理和预防的成
果;然而,并无研究已调查 其对产后女性(在怀孕过程中超 重)的效用。一名 32 岁怀孕时超 重的初孕女性(增重 23 磅,超过 医学研究所 (IOM) 对其建议的怀 孕前身体质量指数)已参与应用 综合健康辅导 (PHP-IHC) 干预的 6 个月个性化健康计划。此干预包 括由医疗护理提供者进行的基线 健康风险评估检查,以及两周一 次共 8 次,每次 30 分钟的电话健 康辅导疗程。参与者展示出体力 活动、能量消耗、知识改善,并 对形成有利于身心健康的行为习 惯充满信心。尽管参与者完成健 康辅导疗程前并未达到目标体 重,但此后 8 个月的跟进结果显 示,其已达到既定目标(怀孕前 体重的 5% 范围内)。此病例报告 表明,PHP-IHC 可支持产后女性 在妊娠期超重后恢复至怀孕前的 体重。未来研究和临床试验需要 确认 PHP-IHC 治疗产后女性的最 佳时间、时长和方法(网上、亲 身、电话辅导)。
SINOPSIS
La formación de salud es una nueva intervención conductual para mejorar los resultados en la prevención y el tratamiento de la enfermedad crónica; sin embargo, ningún estudio ha examinado su utilidad en las mujeres puérperas que han aumentado excesivamente de peso durante el embarazo. Una mujer primigesta de 32 años de edad que presentaba sobrepeso en el momento de la concepción y había engordado 10,5 kg más que las recomendaciones establecidas por el Instituto de Medicina (IOM, por sus siglas en inglés) para su índice de masa corporal previo al embarazo participó en un plan sanitario de 6 meses de duración con una intervención de formación de salud integradora (PHP-IHC, por sus siglas en inglés). La intervención incluyó una revisión de evaluación basal del riesgo para la salud con un profesional sanitario y 8 sesiones telefónicas de formación de salud de 30 minutos de duración cada quince días. La participante demostró mejorías en la actividad física, el consumo de energía, el conocimiento y en la confianza a la hora de implicarse en comportamientos que favorecían su salud. A pesar de que la participante no logró alcanzar el peso objetivo al finalizar las sesiones de formación de salud, una visita de seguimiento 8 meses después confirmó que lo había alcanzado (dentro del 5 % del peso previo al embarazo). Este caso clínico sugiere que el PHP-IHC puede ayudar a las mujeres puérperas a recuperar el peso previo al embarazo tras el aumento de peso excesivo durante la gestación. Se necesitan investigaciones y ensayos clínicos futuros para determinar el momento, la duración y el método (en línea, en persona o telefónico) idóneos del PHP-IHC para la mujeres puérperas.
INTRODUCTION
Childbearing is a commonly cited cause of weight gain for women, and studies have shown 45% of normal, 40% to 50% of overweight, and 60% of obese women retain weight at 6 months postpartum.1 Studies of women 10 years and 15 years after their last childbirth show that compared to women who lost all pregnancy weight by 6 months postpartum, women who retained any weight at 6 months postpartum had increased odds of experiencing midlife obesity, coronary heart disease, and diabetes.2,3 Thus, pregnancy and the postpartum period are critical points in a woman's lifespan where interventions to prevent excess pregnancy weight gain and postpartum weight retention can promote midlife health and well-being.
Personalized health planning with integrative health coaching (PHP-IHC) is a strategic intervention that applies a holistic and individualized approach to optimizing physical, mental, and social health.4,5 PHPIHC is showing promising results in the reduction of disease risk, including improving exercise behaviors, body mass index (BMI), waist circumference, social support, and mood in patients of both sexes with multiple risk factors for cardiovascular disease, diabetes, and stroke.6-9 The PHP is tailored to the individual, taking into consideration the patient's unique risk factors, resources, and readiness for change, and it is formulated with input from both the individual and the healthcare provider.4,5 It provides a roadmap for improving health and well-being and serves as the platform through which patients and providers collaborate on the patient's care and track the patient's progress in 3-, 6-, and 12-month increments.5 IHC supports implementation of the PHP by working with individuals to articulate their vision of health and the values that are most important to them, developing goals aligned with those values, and supporting behavior changes to achieve self-chosen goals.7 PHP-IHC marks a shift away from current healthcare provision, which is often reactive and provider-directed, involves little patient engagement, and fails to consider individual receptiveness to specific behavioral interventions, to an approach that is patient-centric, proactive, and personalized.4,10
The primary purpose of the Personalized Medicine in Women's Obesity Prevention study (Simmons PI), a pilot randomized controlled trial still in the data-collection phase, is to test the feasibility and effectiveness of PHP-IHC to prevent 6-month postpartum weight retention (PPWR). PPWR is defined as weighing >5% more than one's pre-pregnancy weight. To be eligible, all participants have to be primiparous, have had gestational weight gains ≥10% (1.0 lbs-3.5 lbs) of Institute of Medicine (IOM) recommendations for pre-pregnancy BMI to account for scale variations across clinical sites and ensure excess weight gain occurred, no preexisting health conditions, and uncomplicated pregnancies and deliveries. Participants are recruited 1 to 5 days after delivery from the postpartum unit of one of two hospital-based birthing centers. Participants are randomized into one of three study conditions: (1) intervention (PHP-IHC): completion and physician review of a health risk assessment (HRA) with participant, individualized plan of care based on HRA results, and support in plan implementation through integrative health coaching (n=20); (2) active control (HRA only): completion and physician review of HRA with participant and provision of standardized written recommendations (n=20); or (3) usual-care control: completion of HRA data but participant does not receive results with standardized recommendations until the end of the study (n=20). Here we provide a case review of a participant who completed the PHP-IHC intervention.
THE CASE
Presenting Concerns
A 32-year old white, non-Hispanic primigravida woman consented to and was enrolled into the Personalized Medicine in Women's Obesity Prevention study 2 days postpartum after gaining a total of 48 lbs during pregnancy (total weight, 219 lbs). She was overweight (height, 65 in; weight, 171 lbs; BMI, 28.45 kg/m2) prior to pregnancy. Her total weight gain during pregnancy exceeded IOM recommendations for pregnancy weight gain for overweight women by 23 lbs (maximum recommended weight gain, 25 lbs), her BMI increased by 28.1% during pregnancy, and she had a postpartum BMI of 36.44 kg/m2.
Clinical Findings
The participant had no preexisting health conditions. She reported being a nonsmoker and receiving routine screenings and examinations (eg, annual physicals, vision tests, dental exams). Her singleton pregnancy and delivery were uncomplicated. The infant was delivered at 39 weeks, 4 days' gestation, weighed 7.46 lbs, and did not have any chronic diseases or major congenital abnormalities.
Diagnostic Focus and Assessment
The participant presented for her first study visit at 3 weeks, 2 days postpartum. She had lost 17.4 lbs since giving birth (weight = 201.6 lbs, BMI = 33.5 kg/m2). Her blood pressure was within normal limits (106/67 mmHg), and her waist circumference was 39 inches.
The participant's health behaviors were assessed during this visit using a comprehensive HRA designed by the study team based on factors in the literature that have been shown to be associated with not losing pregnancy weight. Because it was designed for the study, a total risk score with cut-off values has not been established to date. The HRA was administered via a webbased application on an iPad (Apple, Inc, Cupertino, California) and took approximately 45 minutes to complete. HRA results indicated that the participant had poor protein intake (servings = 2) and moderate fiber intake (score = 11, Nutrition Questionnaire About Eating Practices11). She reported no daytime sleepiness (Epworth Sleepiness Scale12), minimal stress (Perceived Stress Scale, PSS13), no depression (Edinburgh Postnatal Depression Scale14), good social support (Interpersonal Support Evaluation List, ISEL15), high weekly energy expenditure (Pregnancy Physical Activity Questionnaire [PPAQ]16), primarily internal locus of control around her weight (Weight Locus of Control17), and limited or no fast food intake (Nutrition Questionnaire about Eating Practices11). The majority of her physical activity was of moderate intensity (40.3%), while 30.2% was of light intensity and 3% was of vigorous intensity. Sedentary activity accounted for 26.6% of all activity.
The study physician reviewed these results with the participant, explaining what they meant in relation to her risk for weight retention after pregnancy, reviewed ways the participant may mitigate that risk, and provided standardized handouts on nutritional intake.
Therapeutic Focus and Assessment
PHP-IHC was employed to help the participant reduce her risk factors for retaining weight after pregnancy. She was partnered with an integrative health coach who is certified by the International Coaching Federation at the professional certified coach level. The coach has completed more than 100 hours of training and has more than 10 years of coaching experience. The coaching sessions began 1 month postpartum. Eight biweekly telephonic coaching sessions were provided over 3.5 months. Each session lasted approximately 30 minutes, except for the first session, which by design lasted 47 minutes. The participant attended all sessions.
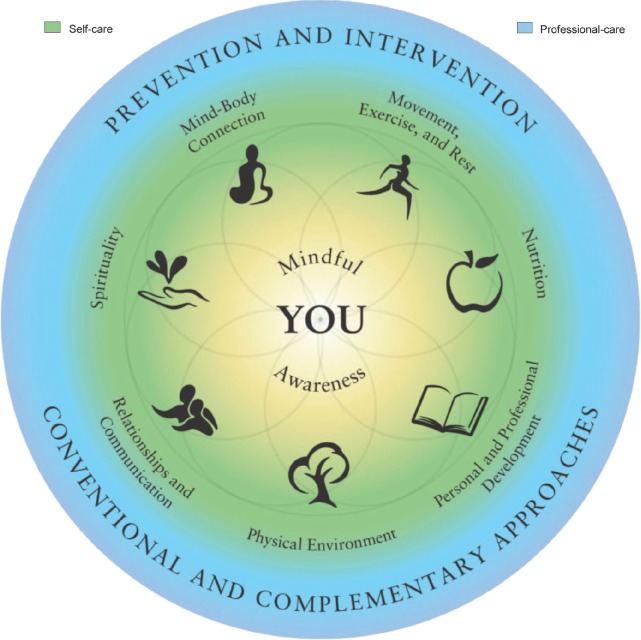
The Duke Integrative Medicine Wheel of Health (Figure 1) was administered to the participant prior to the initial coaching session and was used to help guide the coaching process. The participant reported on a scale of 1 to 10 how satisfied she was in each domain and what improvements, changes, or enhancements she would need achieve complete satisfaction. Based on this evaluation, the participant identified priority areas to focus on during coaching and used these priority areas to establish the coaching agenda, including goals and action steps.
Figure 1.

Timeline.
Abbreviations: BP, blood pressure; HRA, health risk assessment; MD, medical doctor; PHP-IHC, personalized health planning with integrative health coaching; RN, registered nurse.
During the initial telephone coaching session, the participant was asked what was important to her in terms of her health, how well she was managing her health currently, and what she perceived as challenges or areas that needed support. She was further guided in creating a vision of health, identifying her values, and establishing a personalized health plan (PHP). The PHP included short-term and long-term goals that aligned with the participant's vision and values and an individualized plan of action to accomplish these goals.
Over the remaining coaching sessions, the participant revisited the Wheel of Health and was encouraged to create specific, measurable, attainable, realistic, and timely (SMART) goals18 in the context of her vision of health and based upon her values. The coach regularly asked the participant to report on her accomplishments and challenges, both in writing via a weekly phone call preparation form and during the coaching sessions. The participant took the initiative to adjust her goals accordingly, although the coach collaborated with the participant to strategize solutions to challenges when needed.
Outcomes
The participant indicated that she was more aware of her current and projected health and health risks after completing and reviewing the HRA results with the physician. After the first health coaching session, the participant had identified that her first priority was to improve on her movement, exercise, and rest. She noted that she was “so out of shape that [she] was not sure what workout to do to get back into shape” but wanted to get on a workout schedule. Her second priority was nutrition, noting that she “ate a lot of chocolate and carbs” and was aware that she “needed to eat more proteins and veggies.” In addition to exercise and nutrition, the participant identified relationships and communication as a top priority area. By the end of the first session, the participant also had identified her optimal vision of health, which included weighing 140 lbs to 145 lbs, being active at least 6 days a week, having a clear mind to be able to make smart decisions for her family, having a happy and healthy family, honoring alone time to re-energize, and generally having “honesty and respect in all areas of [her] life.” Her long-term goal was to lose 60 lbs in 9 months.
Figure 2.
Duke Integrative Medicine Wheel of Health.
Copyright © 2010 by Duke University on behalf of Duke Integrative Medicine. Reprinted with permission. All rights reserved. MCOC-8720
After the second session, the participant had made significant accomplishments in adjusting to motherhood and making time for her husband and child. She decided to use a notebook to assist with scheduling chores and appointments for the family. The participant also noted that verbalizing her goals and weekly actions “made her commitments real” and helped with accountability. She also joined Weight Watchers for further accountability and to assist in food tracking. The participant used Weight Watchers' tracking templates in lieu of the Duke study templates.
After the third session, the participant reported that she had lost 5 lbs but faced difficulties achieving her goal of exercising for 30 minutes 6 days a week. The coach guided the participant to revise her exercise goal by asking what getting enough exercise would look like realistically. The participant decided to reduce her physical activity goal to 30-minutes of exercise 4 to 5 days per week. She also achieved her goal of eliminating dairy from her diet and allowed herself one piece of dark chocolate per day. She continued to make significant improvements in her mindful awareness and on her relationships with her child and husband, noting that she “felt so present with her baby and husband” and was “able to be in the moment.”
During the fourth session, the participant planned for her return to work, which presented new obstacles to getting enough exercise. She noted that she did not want to leave her child in daycare for long periods of time, which made it difficult to go to the gym. With encouragement from the coach, she set four specific, timely, and achievable goals to facilitate exercising during the week.
During the fifth session, the participant informed the coach that she was making progress in being kind to herself as she dealt with competing values for her time (eg, exercising for health and family time) and that coaching “helped her remember self-compassion.” She had continued to journal to help her identify and deal with her emotions. By the sixth session, the participant reported that she was “being very mindful of being honest with and kind [to herself], which had really eliminated the stress in [her] life.”
After the sixth session, the participant self-reported that she had lost another 5 lbs and had continued to exercise four times a week for 30-minutes each in “any way she could.” She revised her exercise plan to include more running or walking after work instead of going to the gym. She noted that she felt “really great about life right now.”
At the eighth session, the participant noted that she had been “very pleased with [her] success in losing weight, exercising, and being present with [her] family.” She hoped to continue health coaching outside of the study but was concerned about the cost.
Final examination in clinic at 24 weeks postpartum showed that the participant had lost 22.6 lbs (total weight = 196.4 lbs, BMI = 32.7 kg/m2). Her blood pressure continued to be normal (118/65 mmHg), and her waist circumference was 37 in (compared to 39 in at the first clinic visit). There was no significant change in her health risk factors as assessed by the HRA. Her protein intake did not improve and all other assessments (ie, physical activity, breastfeeding, sleep, stress levels, social support, depression, and weight locus of control) stayed within the normal range. Although the participant remained within the normal range for all assessments, she did show improvements in daytime sleepiness (Epworth Sleepiness Scale12 score 2 vs 4), stress (PSS13 score 8 vs 10), depression (Edinburgh Postnatal Depression Scale14 score 4 vs 6), social support (ISEL15 score 96 vs 111), and total average weekly energy expenditure (PPAQ16 score 657.83 vs 520.22). She increased her moderate intensity activity, which accounted for 60% of all her activity (vs 40.3% at baseline) and decreased her sedentary activity (26.6% vs 17.4%). She also reported more engagement in managing her health, including increased knowledge of health-promoting behaviors and greater confidence to enact these behaviors.
DISCUSSION
This case study provides additional support for PHP-IHC as a viable approach for risk reduction and more specifically, improving weight-related outcomes. In addition to a 22.6-lb weight loss, the participant reported increasing her moderate-intensity activity while decreasing sedentary activity, resulting in an overall increase in energy expenditure. Studies show that increases in physical activity are associated with decreased risk for cardiovascular disease and type II diabetes, and these reductions show a dose-response relationship.19 Research also has shown that moderate physical activity is associated with an annual $250 decrease in healthcare expenditures independent of BMI and a $450 annual reduction among obese individuals.20 Additionally, the participant reported greater engagement in managing her health, including increased knowledge of her health risks, improved confidence in the ability to engage in health-promoting behaviors, and taking actions to support health, with a specific focus on diet and exercise. Greene and Hibbard21 have reported that increases in knowledge and confidence around managing health are associated with a higher likelihood of engaging in health-promoting behaviors, such as obtaining preventive screenings and avoiding unhealthy behaviors (eg, smoking). Similarly, Hibbard et al22 found that patients who did not have the confidence or skills to engage in their care cost on average 8% more than patients who did. Thus, while the participant did not achieve the target endpoint for weight, she did experience other benefits in activity levels and engagement that suggest improved health over time, especially if they are sustained.
Our findings are consistent with those of Edelman et al,6 who documented increased days of exercise and greater weight loss among those who participated in PHP-IHC compared to those receiving usual care. Likewise, Wolever, et al8 demonstrated increased patient activation, social support, and exercise frequency among patients with type II diabetes who received PHP-IHC compared to those receiving usual care. By considering individual health risk factors, a vision of health, key values, resources, and readiness to engage in specific behavioral changes, PHP-IHC provides a model of care that may allow for optimization of patients' resources and increase their participation in their care.7 Further, this process facilitated the patient's ability to establish priority areas for goal setting. The Wheel of Health provided a framework from which the participant could connect this new understanding of her health from the HRA to the areas in her life where she most wanted—and was most ready—to make changes. Emerging research suggests that patient-centered goal setting creates a sense of ownership and accountability that does not emerge with provider-directed goals.23 Moreover, the PHP-IHC model is especially suitable for individuals trying to make behavioral changes during a major life change, such as the transition to parenthood, because telephonic coaching permits a degree of flexibility in scheduling, allowing for appropriate accommodation of a new mother's various other responsibilities and obligations.
A final key point to address is the role of the health coach and how background and training may influence outcomes of health coaching interventions. In our model, the health coach serves as a partner in the participant's healthcare while the participant is considered the expert in her life and in the personal strategies for behavior change that will work for her.7 The participant is both encouraged and empowered to solve problems and pursue goals consistent with her values and vision for health so that changes are not only initiated but are sustained over time. This approach is not consistent across health coaching interventions. Currently, there are no national standards for health coaches, and both the practices and strategies used and the training/credentialing required vary widely.24 The coach in our study was highly experienced and held an international certification in coaching. The issues of which strategies constitute health coaching and the minimum training/credentialing in coaching necessary to deliver such services will need to be addressed in future models assessing health coaching.24
This case study has some limitations. First, the participant enrolled in the Weight Watchers program while participating in the study, noting that the added accountability and tracking were very helpful. Her participation in Weight Watchers confounds the intervention results, and we will need to control for participation in additional programs when we analyze these pilot data. However, given that PHP-IHC is a time-limited, personalized approach to care, obtaining additional support as through Weight Watchers demonstrates a potential pathway for transitioning individuals from individual coaching to ongoing primary care. That is, one of the goals of PHP-IHC is to help participants identify necessary supports to sustain behavioral changes beyond the coaching period. Such pathways to independence will be critical when considering how to feasibly employ integrative health coaching on a large scale. Second, although the participant did lose nearly half of the weight gained during pregnancy, she did not return to within 5% of her pre-pregnancy weight by 6 months postpartum, which was a goal of the intervention. Third, the participant noted that although she was interested in continuing coaching beyond the study, cost was a concern, and she did not continue on her own. Fees for private practice coaches vary greatly, from $40 to $200 per hour, and at least one study of a self-pay coaching program reported that cost was indeed a barrier to participation.25 However, cost analyses of health coaching provided free of charge as part of a comprehensive model of care have demonstrated both cost savings26-28 and cost neutrality29 with improved health outcomes, suggesting it is a sustainable model that aligns with quality and expenditure improvement initiatives. A larger trial of our approach should include a cost analysis to assess its financial feasibility. Despite these limitations, the positive changes in exercise, energy expenditure, and engagement in managing health suggest a role for PHP-IHC in helping postpartum women to lose weight gained during pregnancy.
Excess pregnancy weight gain and postpartum weight retention are common and place women at increased risk of obesity and associated comorbidities. PHP-IHC may offer a solution to help women prevent postpartum weight retention and mitigate the current obesity epidemic. Further research is needed to determine whether PHP-IHC is a feasible, cost-effective approach for childbearing women, including research on the timing, length, and scope of the intervention. Studies that investigate the effects of longer duration, different media (eg, online, in-person), and beginning the coaching during pregnancy should be considered.
PATIENT PERSPECTIVE
The patient provided the following commentary on her case approximately 8 months after her final study visit:
I am 8 lbs away from my pre-pregnancy weight [now within 5% of her prepregnancy weight, or the target end point]. Being part of this study gave me confidence to seek help where I needed it. It encouraged a healthier lifestyle. And [the coach] was just a joy to work with—I am honored that I was able to work with her.
Disclosures The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and disclosed a grant from the Duke Center for Personalized Medicine. No other relevant potential conflicts of interest were disclosed.
Contributor Information
Nancy Y. Yang, Duke University School of Nursing, Durham, North Carolina..
Shelley Wroth, Duke Integrative Medicine, Durham, North Carolina..
Catherine Parham, Take Care Health Systems (Walgreens Health and Wellness), Conshohocken, Pennsylvania..
Melva Strait, Duke Integrative Medicine, Durham, North Carolina..
Leigh Ann Simmons, Duke University School of Nursing and Duke Centre for Research on Prospective Health Care, Durham, North Carolina..
REFERENCES
- 1.Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutr. 2008;28:411–23 [DOI] [PubMed] [Google Scholar]
- 2.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106(6):1349–56 [DOI] [PubMed] [Google Scholar]
- 3.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: One decade later. Obstet Gynecol. 2002;100(2):245–52 [DOI] [PubMed] [Google Scholar]
- 4.Simmons LA, Dinan MA, Robinson TJ, Snyderman R. Personalized medicine is more than genomic medicine: Confusion over terminology impedes progress towards personalized healthcare. Pers Med. 2012;9(1):85–91 [DOI] [PubMed] [Google Scholar]
- 5.Burnette R, Simmons LA, Snyderman R. Personalized health care as a pathway for the adoption of genomic medicine. J Pers Med. 2012;2(4):232–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelman D, Oddone EZ, Liebowitz RS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21(7):728–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolever RQ, Caldwell KL, Wakefield JP, et al. Integrative health coaching: An organizational case study. Explore (NY). 2011;7(1):30–6 [DOI] [PubMed] [Google Scholar]
- 8.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes. Diabetes Educ. 2010;36(4):629–39 [DOI] [PubMed] [Google Scholar]
- 9.Wolever RQ, Webber DM, Meunier JP, Greeson JM, Lausier ER, Gaudet TW. Modifiable disease risk, readiness to change, and psychosocial functioning improve with integrative medicine immersion model. Altern Ther Health Med. 2011;17(4):38–47 [PMC free article] [PubMed] [Google Scholar]
- 10.Snyderman R. Personalized health care: from theory to practice. Biotechnol J. 2012;7(8):973–9 [DOI] [PubMed] [Google Scholar]
- 11.Personal Wellness Profile Nutrition questionnaire about eating practices. Rockville, MD: Parklawn Federal Occupational Health Unit; 2000 [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–5 [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:386–96 [PubMed] [Google Scholar]
- 14.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782. [DOI] [PubMed] [Google Scholar]
- 15.Delistamati E, Samakouri MA, Davis EA, Vorvolakos T, Xenitidis K, Livaditis M. Interpersonal Support Evaluation List (ISEL)-college version: Validation and application in a greek sample. Int J Soc Psychiatry. 2006;52(6):552. [DOI] [PubMed] [Google Scholar]
- 16.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36:1750–60 [DOI] [PubMed] [Google Scholar]
- 17.Saltzer EB. The Weight Locus of Control (WLOC) Scale: A specific measure for obesity research. J Pers Assess. 1982;46(6):620–8 [DOI] [PubMed] [Google Scholar]
- 18.Strecher VJ, Seijts GH, Kok GJ, et al. Goal setting as a strategy for health behavior change. Health Educ Behav. 1995;22(2):190–200 [DOI] [PubMed] [Google Scholar]
- 19.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: Review and meta-analysis. Am J Prev Med. 2004;26(5):407. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, McDonald T, Champagne LJ, Edington DW. Relationship of body mass index and physical activity to health care costs among employees. J Occup Environ Med. 2004;46(5):428–36 [DOI] [PubMed] [Google Scholar]
- 21.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients' ‘scores’. Health Aff (Millwood). 2013;32(2):216–22 [DOI] [PubMed] [Google Scholar]
- 23.Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patientreported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolever RQ, Eisenberg DM. What is health coaching anyway? Standards needed to enable rigorous research: Comment on “evaluation of a behavior support intervention for patients with poorly controlled diabetes.” Arch Intern Med. 2011;171(22):201. [DOI] [PubMed] [Google Scholar]
- 25.Rice J, Thombs D, Leach R, Rehm R. Successes and barriers for a youth weight-management program. Clin Pediatr. 2008;47(2):143–7 [DOI] [PubMed] [Google Scholar]
- 26.Murphy BM, Schoenman JA, Pirani H. Health insurers promoting employee wellness: strategies, program components, and results. Am J Health Promot. 2010;24(5):e1–10 [DOI] [PubMed] [Google Scholar]
- 27.Long DA, Sheehan P. A case study of population health improvement at a Midwest regional hospital employer. Popul Health Manage. 2010;13(3):163–73 [DOI] [PubMed] [Google Scholar]
- 28.Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Légaré F. Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision aids. Medical Decis Making. 2012;32(3):E22–33 [DOI] [PubMed] [Google Scholar]
- 29.Meng H, Liebel D, Wamsley BR. Body mass index and the impact of a health promotion intervention on health services use and expenditures. J Health Aging. 2011;23(4):743–63 [DOI] [PubMed] [Google Scholar]