Abstract
The urinary tract is a common site of infection in humans. During pregnancy, urinary tract infection (UTI) is associated with increased risks of maternal and neonatal morbidity and mortality, even when the infection is asymptomatic. By mapping available rates of UTI in pregnancy across different populations, we emphasize this as a problem of global significance. Many countries with high rates of preterm birth and neonatal mortality also have rates of UTI in pregnancy that exceed rates seen in more developed countries. A global analysis of the etiologies of UTI revealed familiar culprits as well as emerging threats. Screening and treatment of UTI have improved birth outcomes in several more developed countries and would likely improve maternal and neonatal health worldwide. However, challenges of implementation in resource-poor settings must be overcome. We review the nature of the barriers occurring at each step of the screening and treatment pipeline and highlight steps necessary to overcome these obstacles. It is our hope that the information compiled here will increase awareness of the global significance of UTI in maternal and neonatal health and embolden governments, nongovernmental organizations, and researchers to do their part to make urine screening and UTI treatment a reality for all pregnant women.
Key Words: Urinary tract infection, pregnancy, complications, prevention, women's health
摘要
尿路是人体中的常见感染部位。在怀孕期间,尿道感染 (urinary tract infection, UTI)与孕产妇和新生儿发病率和死亡率的高风险有关,即使该感染并无临床症状。我们针对不同人群怀孕期间的 UTI 发病率作图,突出强调了这一具有全球显著性的问题。在许多早产率和新生儿死亡率高企的国家中,怀孕期 UTI 患病率也超过较发达国家的患病率。一项 UTI 病因学整体分析发现,病因既包括人们所熟知的因素,也包含多项新出现的威胁。对 UTI 的筛选和治疗已经提高了多个较发达国家的出生结果,并且有可能改善全世界孕产妇和新生儿的健康状况。然而,在资源贫乏的环境中推行该等筛选和治疗,必须克服种种困难。我们回顾了在筛选和治疗过程每个步骤所遇到的阻碍的性质,并强调了克服这些阻碍所需采取的措施。我们希望,本文汇编的信息能够增强对 UTI 在孕产妇和新生儿健康方面所具有的全球显著性的意识,并鼓励政府、非政府组织和研究人员尽自己的一份力量,以实现所有妊娠女性的尿液筛选和 UTI 治疗。
SINOPSIS
El tracto urinario es un lugar frecuente de infección en los seres humanos. Durante el embarazo, la infección del tracto urinario (ITU) va asociada a un aumento del riesgo de morbilidad y mortalidad maternas y neonatales, incluso cuando la infección es asintomática. Al trazar un mapa de los índices disponibles de ITU durante el embarazo en diferentes poblaciones, subrayamos que se trata de un problema de importancia mundial. Muchos países con elevados índices de nacimientos prematuros y de mortalidad neonatal tienen también índices de ITU durante el embarazo superiores a los que se observan en los países más desarrollados. Un análisis a nivel global de las etiologías de la ITU puso en evidencia responsables ya conocidos, pero también amenazas emergentes. La detección y el tratamiento de la ITU han mejorado los resultados de los partos en varios de los países más desarrollados, y probablemente mejorarán en todo el mundo la salud materna y neonatal. Sin embargo, se han de superar los retos que plantea la puesta en práctica en entornos con bajos recursos. Revisamos la naturaleza de las barreras que aparecen en cada etapa del desarrollo de la detección y el tratamiento, y destacamos los pasos necesarios para vencer esos obstáculos. Tenemos la esperanza de que la información que aquí hemos reunido aumentará la conciencia de la importancia a nivel mundial de la ITU en la salud materna y neonatal, y animará a los gobiernos, las organizaciones no gubernamentales y los investigadores a poner de su parte para que la detección en la orina y el tratamiento de la ITU sean una realidad para todas las mujeres embarazadas.
GENITOURINARY INFECTION IS A LEADING CAUSE OF PREVENTABLE ADVERSE PREGNANCY OUTCOMES
In response to Millennium Development Goals 4 and 5 (MDG4 and MGD5)—to reduce childhood mortality and improve maternal health—a variety of global efforts have arisen to improve birth outcomes.
One example is a recent collaborative report from the March of Dimes, the Partnership for Maternal, Newborn and Child Health (MNCH), Save the Children and the World Health Organization (WHO). The “Born Too Soon: Global Action Report on Preterm Birth,” estimated that 15 million babies are born preterm every year, resulting in more than a million deaths annually.1 Complications of delivery can have lifelong effects on mother and child. For example, preterm infants often experience lung problems, diabetes, heart disease, mental retardation, hearing loss and visual impairment, learning disabilities, behavioral problems, and cerebral palsy. Authors of the “Born Too Soon” report stress that global efforts to reduce the death-toll attributable to PTB should begin with implementation of proven strategies such as antenatal corticosteroid treatment and “Kangaroo Mother Care.”
Indeed, prevention of deaths due to preterm delivery and other complications of pregnancy is a worthy primary goal. However, prevention of the complications that lead to neonatal deaths is likely to have an even larger health and economic impact and should be placed at the center of the research agenda. For example, prenatal care practices that can prevent life-threatening complications include the detection and treatment of preeclampsia, intermittent presumptive treatment of malaria, and immunization of pregnant women with tetanus toxoid.2-4 Maternal genitourinary infection during pregnancy is another leading cause of pregnancy complications such as preterm birth and stillbirth. This article will focus on infection (and in particular urinary tract infection) as a cause of maternal and neonatal morbidity and mortality that might be prevented with appropriate screening and treatment programs. For example, a variety of infectious conditions of the female genitourinary tract have been associated with higher risks of adverse birth outcomes, including syphilis, chlamydia, gonorrhea, bacterial vaginosis (BV), and urinary tract infection (UTI).2-4 Maternal infection is one of few causes of neonatal complications/deaths that are potentially preventable through routine screening and treatment. In fact, prevention and management of sexually transmitted infections both before and during pregnancy is recommended by the World Health Organization and the “Born Too Soon” report.1
Here we focus specifically on UTI as a potentially preventable cause of pregnancy complications. This article will (1) provide a historical and scientific overview of the link between UTI and adverse pregnancy outcomes, (2) overlay literature rates of bacteriuria (bacteria in urine at specific threshold levels) on a global map to estimate the scope of UTI in pregnancy as a global problem, (3) compile available information about the global patterns of bacterial species isolated from urines of pregnant women, and finally, (4) discuss the challenges associated with implementation of bacteriuria screening and treatment programs in low-resource settings. The goal of this effort is to foster communication between researchers and the global health community and to stimulate innovation and action for the benefit of pregnant women and their babies.
Urinary Tract Infections Are Common and Can Be Dangerous During Pregnancy
Urinary tract infections (UTI) are among the most common bacterial infections in humans. UTI is commonly diagnosed based on clinical findings of bacteriuria (bacteria in midstream urine) counts of > 105 colony forming units (cfu)/mL along with patient-reported symptoms. Lower bacterial counts are considered clinically significant when urine is collected by catheterization. Cystitis, or infection of the bladder, is typically accompanied by painful urination (dysuria), urgency, and frequent urination. A more severe infection of one or both kidneys, called pyelonephritis, is often accompanied by fever and flank pain, often in addition to symptoms of cystitis. When bacteria (at >105/ml) are observed in urine in the absence of UTI symptoms, a patient is diagnosed as having asymptomatic bacteriuria (ASB). ASB is a common finding, but is currently only considered clinically important during pregnancy, when physiological and hormonal changes increase the risk of ascending infections of the kidney.
Women are disproportionately affected by UTI compared to men, and risk of UTI increases further during pregnancy.5 Pregnant women are at increased risk of bacterial ascension to the kidneys and pyelonephritis,6 due partly to dilation of the renal pelvis and ureters by as early as the eighth week of pregnancy.7 Bacteriuria that progresses to pyelonephritis during pregnancy is associated with poor outcomes for both the mother and child, including maternal sepsis and anemia, preterm birth (PTB) low birth weight (LBW), and perinatal death. Even without progression to pyelonephritis, bladder infection during pregnancy is associated with increased risk of maternal hypertension, anemia, amnionitis, and premature labor, as well as PTB, and LBW (reviewed in Schnarr and Smaill8). However, in countries with rigorous screening and treatment of bacteriuria in pregnancy, only a small percentage of pregnant women progress to pyelonephritis.9
SCREENING AND TREATING ASYMPTOMATIC BACTERIURIA (ASB) PREVENTS ADVERSE PREGNANCY OUTCOMES
The first evidence that ASB may be associated with adverse pregnancy outcomes came in 1960, when Kass hypothesized that ASB, which persisted in 6% of pregnant women, was associated with the development of acute pyelonephritis.10 In a randomized, placebo controlled trial, he demonstrated that antibiotic treatment of ASB successfully eliminated bacteriuria and completely prevented acute pyelonephritis in pregnant women, while untreated ASB led to pyelonephritis in 40% of women receiving placebo11.11 Although not the topic of his inquiry, Kass' investigation led to the fortuitous discovery that, in addition to the increased risk of pyelonephritis, women with ASB in the placebo treated group had two- to three-fold higher incidence of LBW, neonatal death and prematurity compared to both nonbacteriuric women and to antibiotic treated women who cleared bacteriuria.10,11 Based on his findings, Kass made the first recommendation to screen and treat pregnant women for ASB, estimating that his strategy would prevent 10% of preterm births.10
During the next 50 years, the potential association between ASB and adverse pregnancy outcomes such as PTB was the subject of many studies, yet remained controversial (see Smaill12 for references of ASB in pregnancy). The advent of meta-analyses that combine the results of clinical trials offered a new opportunity to synthesize data from multiple studies. In 1989, Romero et al applied this approach to seventeen cohort studies from 1962 to 1975, revealing that the risk of LBW in non-bacteriuric patients was two-thirds that of ASB patients receiving no treatment, and moreover, that the risk of PTB in nonbacteriuric patients was about half that of ASB patients receiving no treatment.13 Additional meta-analysis of eight randomized clinical trials revealed that antibiotic treatment of bacteriuria reduced LBW risk by 6.4%.13 The authors concluded that a strong association exists between untreated ASB and LBW/PTB and that antibiotic treatment of ASB during pregnancy is effective at reducing LBW.13 A 2001 Cochrane Review including fourteen studies concluded that antibiotic treatment was effective against ASB, and reduced incidence of pyelonephritis, LBW and PTB.14 An update in 2007 confirmed the antibiotic effectiveness for pyelonephritis and LWB prevention, but failed to show a significant reduction in PTB with antibiotic treatment.15
Despite minor disagreements over the strength of the ASB-PTB relationship, treatment of bacteriuria in pregnancy has widespread support. In fact, screening and treatment for bacteriuria in pregnancy is currently common practice in several more developed countries and is currently recommended by the United States Preventive Services Task Force,16 the Infectious Diseases Society of America,17 the Canadian Task Force on Preventive Care,18 the Scottish Intercollegiate Guidelines Network,19 the European Association of Urology,12 and the National Collaborating Centre for Women's and Children's Health of the National Institute for Health and Clinical Excellence (UK).20 Although ASB screening and treatment was initially implemented to reduce PTB and LBW, several current recommendations cite prevention of maternal pyelonephritis as the primary goal. In agreement with the 2007 Cochrane review, the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) review group gave only a weak recommendation for preventing PTB by screening and treatment for asymptomatic bacteriuria,21 due to the low quality of evidence stemming from methodological issues in the studies addressing PTB risk. However, bacteriuria screening was strongly recommended by GAPPS for intervention of LBW and maternal morbidity (including pyelonephritis).
Recently, studies using a mouse model have provided even more compelling evidence for a causal relationship between UTI and adverse pregnancy outcomes. Experimental UTI in pregnant mice was sufficient to cause intrauterine growth restriction and resulted in significantly reduced litter size.22 This study is significant as it shows for the first time that there is a causal relationship between UTI and LBW in an experimental model of UTI in pregnancy. The authors provided evidence that the uropathogenic Escherichia coli inoculated into the bladder in these experiments did not gain access to the kidneys, nor could bacteria be isolated from blood, spleen, uterine horns or placentas. However, despite the absence of live bacteria from these compartments, the bladder-localized infections appeared to be sufficient to result in both fetal growth restriction and systemic immune activation, as evidenced by elevated inflammatory cytokines in kidney homogenates and serum. These data suggest that immune activation in response to a localized bladder infection may be responsible for the observed effects on birth weight.
In summary, while a direct association between ASB and PTB is still a point of disagreement in the literature, there is a strong consensus regarding the clear connection between untreated ASB and elevated risk of pyelonephritis (and subsequent complications of pregnancy, including PTB), with especially strong data in trials that carefully consider the effectiveness of treatment.9
BACTERIURIA IN PREGNANCY: A GLOBAL PROBLEM
Since screening and treatment of bacteriuria has resulted in improved birth outcomes in several more developed countries, many have reasoned that the same approach might help make tangible gains in prenatal care and birth outcomes worldwide. In their analysis of strategies to achieve MDG4 Millennium development goals, Adam et al cited screening for ASB as one of the most practical and cost-efficient means by which to improve maternal and neonatal health in developing countries.23 Moreover, of 46 treatment strategies reviewed, ASB screening was highlighted as one of sixteen evidence-based, cost-effective practices, along with similar interventions including screening and treatment for syphilis and malaria, that can reduce neonatal mortality in the developing world.24 ASB screening is thus included in the WHO recommended antenatal care (ANC) package. One study estimated that screening/ treatment of bacteriuria in pregnancy is likely to reduce the incidence of prematurity and low birth weight by 20% to 55% and reduce neonatal mortality due to PTB by 5% to 14%.24
To estimate the magnitude of bacteriuria (symptomatic or asymptomatic) in pregnancy as a global problem, we examined the literature for rates of bacteriuria, using “urinary tract infection,” “UTI” or “bacteriuria” along with “pregnant” or “pregnancy” and the country of interest. We focused on countries with rates of PTB >10% according to “Born Too Soon.”1 Using PubMed and Google Scholar, we were unable to find data on bacteriuria in pregnancy for most of the countries with the highest rates of PTB (countries in grey, Figure 1). Similarly, of the 11 countries with rates of PTB over 15% (Malawi, Congo, Comoros, Zimbabwe, Equatorial Guinea, Mozambique, Gabon, Pakistan, Indonesia, Mauritania, and Botswana) we found reports of ASB or UTI rates in only three (Congo, Pakistan, Botswana). This dearth of available information is likely a reflection of the overall lack of health care infrastructure for research, screening, and treatment programs in these areas. However, despite the paucity of data, viewing the results of this search on a world map (Figure 1, data provided in Table 1) emphasizes that ASB/UTI in pregnancy is, in fact, a global phenomenon, with median rates of 3% to 35% reported in a wide range of countries spanning five continents.
Figure 1.
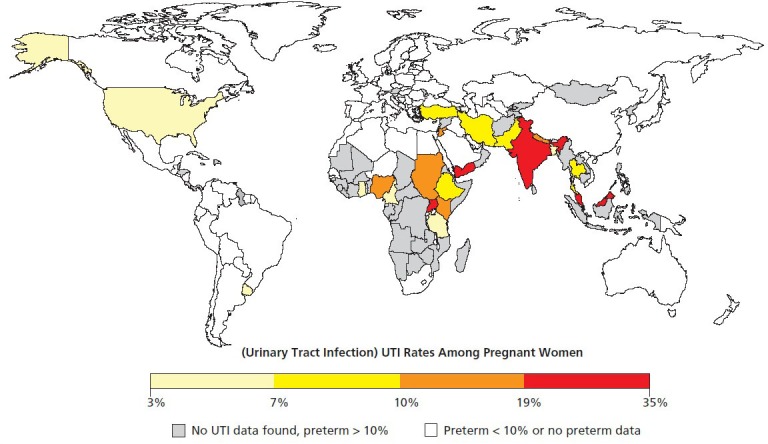
Prevalence of bacteria in urine of pregnant women among countries with preterm birth rates of >10%.
Worldwide rates of urinary tract infection (UTI) in pregnant women ranging from moderate (yellow) to high (red). Countries with >10% preterm birth rates but without available data on bacteriuria are colored gray. Countries with <10% preterm birth rates are shown in white. Despite improvements in pregnancy outcomes with treatment of UTI in more developed nations, high rates of UTI in many third-world countries likely contribute substantially to maternal and infant morbidity and mortality. While different methodologies were employed by the studies summarized here, this figure is meant to illustrate the scale of the problem of bacteriuria in pregnancy and the potential widespread impact of treating bacteriuria among pregnant women to prevent adverse health outcomes. The majority of studies used urine culture to determine bacterial titers. When multiple studies of bacteriuria in pregnancy were available, median values were calculated without weighting. Data were plotted using the Rworldmap package in the R project for statistical computing.105 The data and references used to generate this figure appear in Table 1.
Table 1.
Urinary Tract Infection Rates in Pregnancy Among Countries With Preterm Birth Rates >10%a
| Country | Bacteriuria Rate (%) | No. Pregnant Women | Year | Reference no. |
|---|---|---|---|---|
| Bangladesh | 1 | 300 | 1977 | 57 |
| Bangladesh | 4 | 600 | 2012 | 58 |
| Bangladesh | 7 | 115 | 2012 | 59 |
| Bangladesh | 12 | 216 | 2007 | 60 |
| Congo | 24 | 1535 | 1992 | 61 |
| Ethiopia | 7 | 326 | 1998 | 62 |
| Ethiopia | 9 | 367 | 2012 | 33 |
| Ethiopia | 10 | 173 | 2007 | 63 |
| Ethiopia | 10 | 385 | 2012 | 32 |
| Ethiopia | 20 | 414 | 2008 | 31 |
| Ghana | 7 | 220 | 2007 | 30 |
| India | 4 | 161 | 2005 | 64 |
| India | 8 | 500 | 2002 | 65 |
| India | 10 | 300 | 2012 | 66 |
| India | 20 | 161 | 2005 | 64 |
| India | 25 | 8379 | 2011 | 67 |
| India | 26 | 200 | 2010 | 68 |
| Iran | 5 | 389 | 2009 | 35 |
| Iran | 6 | 1100 | 2007 | 69 |
| Iran | 9 | 1505 | 2008 | 36 |
| Iran | 13 | 322 | 2007 | 70 |
| Jamaica | 9 | — | 2007 | 71 |
| Jordan | 14 | 260 | 2003 | 72 |
| Jordan | 20 | 4501 | 2012 | 73 |
| Kenya | 15 | 270 | 2009 | 74 |
| Malaysia | 19 | 1661 | 2002 | 75 |
| Nepal | 12 | — | 2007 | 76 |
| Nigeria | 4 | 1000 | 1989 | 77 |
| Nigeria | 9 | 352 | 2008 | 78 |
| Nigeria | 11 | — | 2011 | 79 |
| Nigeria | 11 | 205 | 2011 | 80 |
| Nigeria | 11 | — | 2010 | 81 |
| Nigeria | 26 | 357 | 2010 | 28 |
| Nigeria | 21 | 300 | 2006 | 82 |
| Nigeria | 24 | 510 | 1993 | 83 |
| Nigeria | 29 | 473 | 2011 | 84 |
| Nigeria | 38 | 352 | 2008 | 78 |
| Nigeria | 40 | 125 | 2012 | 26 |
| Nigeria | 45 | 1228 | 2010 | 27 |
| Nigeria | 87 | 500 | 2001 | 25 |
| Pakistan | 4 | 232 | 2010 | 85 |
| Pakistan | 5 | 1579 | 1994 | 67 |
| Pakistan | 9 | 1000 | 2006 | 86 |
| Pakistan | 29 | 250 | 2000 | 87 |
| Pakistan | 3–7 | 580 | 2006 | 88 |
| Sudan | 14 | 235 | 2011 | 29 |
| Thailand | 5 | 24 430 | 2009 | 89 |
| Thailand | 10 | 360 | 2009 | 90 |
| Turkey | 4 | 250 | 2006 | 91 |
| Turkey | 5 | 406 | 2003 | 92 |
| Turkey | 8 | 110 | 2005 | 93 |
| Turkey | 9 | 2011 | 2011 | 38 |
| Turkey | 9 | 270 | 2002 | 94 |
| Turkey | 16 | 159 | 2005 | 95 |
| Uganda | 35 | 120 | 1971 | 96 |
| United Republic of Tanzania | 5 | 5153 | 2005 | 37 |
| United Republic of Tanzania | 6 | 1007 | 1983 | 97 |
| United Republic of Tanzania | 18 | 247 | 2009 | 98 |
| United States | 3 | 4200 | 2004 | 99 |
| United States | 6 | 8000 | 2008 | 100 |
| United States | 17 | — | 2008 | 101 |
| Uruguay | 4 | 885338 | 2000 | 102 |
| Yemen | 39 | 2256 | 2002 | 103 |
| Yemen | 30 | 137 | 2005 | 104 |
PubMed and Google Scholar were searched using the terms bacteriuria, urinary tract infection, or UTI and pregnancy or pregnant along with the name of the country. Countries with >10% preterm births not appearing on this table are those for which we could find no reported bacteriuria rates in pregnancy using the given search criteria.
It has been estimated in many studies that about 2% to 10% of women in more developed countries will experience ASB or UTI in pregnancy.12 However, as shown in Table 1 and illustrated in Figure 1, many studies indicate that pregnant women in developing countries have as high or higher rates of bacteriuria than their counterparts in more developed nations. For example, several countries in Africa, the Middle East, and Asia appear to have higher rates of UTI during pregnancy than the United States (Figure 1). In fact, it was not uncommon to find reported rates of asymptomatic bacteriuria in excess of 25%. These high rates did not appear to be due to differences in the definition of ASB, since most of these studies used standard methods of quantitative bacterial culture with the accepted cutoff of 105cfu/ml for reporting a positive result. It is routine for clinical labs to discard plates with three or more different bacterial species, since this result is most often due to contamination of the urine with vaginal bacteria. However, many of the studies of ASB in the developing world identified a single bacterial species (i.e. monoculture) in the vast majority of ASB cases, strongly suggesting that the high rates of ASB were not due to false positives as a result of contamination of urine with vaginal bacteria.
One striking example of the high rates of ASB observed in some developing countries was a study of 500 consecutive pregnant women in Benin, Nigeria.25 The study used standard methods in clinical microbiology and the accepted definition of “significant bacteriuria” as >105 cfu bacteria per ml of midstream urine. Authors of the study described a striking 86.6% of these women as having asymptomatic bacteriuria with >90% of the cultures resulting in growth of a single organism. Urine microscopy further revealed that 72.4% of ASB-positive women also had pyuria (white blood cells in urine). S aureus was isolated from the urines of nearly one in three of these ASB-positive women and was the most frequent organism isolated (29.9%), followed closely by E coli (29.1%). Vaginal swabs taken from the ASB-positive women revealed S aureus in only 15.8% of those sampled. Taken together these studies seem to suggest that vaginal contamination is an unlikely explanation for the high rate of S aureus–associated ASB in this population.
GLOBAL SIGNIFICANCE OF STAPHYLOCOCCSUS AUREUS AS A UROPATHOGEN
S aureus is not considered a typical uropathogen, although S aureus UTI is known to occur in patients with urinary catheters and is associated with the development of invasive infection in these patients. To determine if the findings of S aureus in the Nigerian study were a curiosity of a single cohort, or if they were characteristic of Nigeria or developing countries in general, we examined other reports of urine microbiology, using the studies presented in Figure 1. In fact, three independent studies conducted in different Nigerian cities also reported high rates of S aureus ASB in pregnancy, ranging from 21% to 36% of all ASB-positive women (Figure 2).26-28 S aureus was also reported as one of the major etiologies of pregnancy-associated ASB in Sudan (39% of isolates)29 and Ghana (31% of isolates).30 Studies suggest it may also be endemic in areas of Ethiopia (9-20% of isolates),31-33, Uganda,34 and Iran (0% to 20% of isolates).35,36 In contrast, S aureus was identified only rarely as the etiology of pregnancy-associated ASB in Tanzania (1.9% of isolates)37 and Turkey (0.6% of isolates) 38,38 and was not found at all among pregnant women in South Africa39 or India40 (see Figure 2 for a summary of these findings). Thus, it appears that a growing body of evidence is demonstrating that S aureus is quite common in some parts of the world and is a major cause of pregnancy-associated ASB. Further discussion about the significance of S aureus genitourinary colonization can be found in the section “Urine sample collection: contamination, confounders, and other considerations.”
Figure 2.
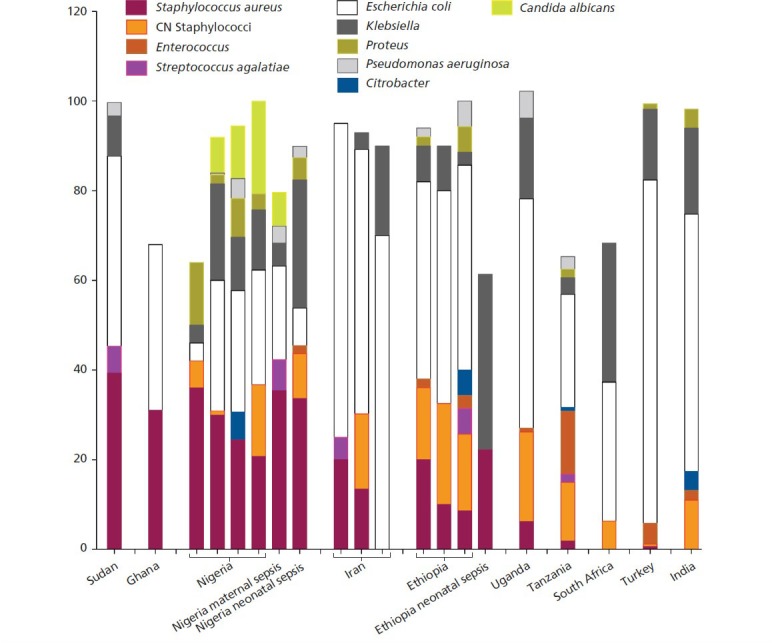
Etiologies of bacterial urinary tract infection (UTI) in pregnancy with an emphasis on Staphylococcus aureus.
A comparison of clinical studies that performed screening of pregnant women for bacteriuria and a breakdown of the etiologies involved. Studies included pregnant women studied in 10 countries.25-40 Bars farthest to the right provide a comparison to the microbial etiologies of neonatal sepsis in Nigeria and Ethiopia.50,106 Note in particular the striking similarity between etiologies of maternal UTI and neonatal sepsis in Nigeria. Studies that are short of 100% did not provide detailed identifications for the remaining isolates.
Abbreviation: CN, coagulase-negative.
BACTERIURIA SCREENING AND TREATMENT IN THE DEVELOPING WORLD: OPPORTUNITIES AND CHALLENGES
In many areas of the world suffering from high rates of pregnancy complications, it is likely that modern ante-natal urine screening combined with appropriate antibiotic treatment could significantly improve maternal and neonatal health. As with many infectious diseases, it appears that the burden of ASB/UTI in pregnancy is greatest in some of the poorest nations. Implementing bacteriuria screening of pregnant women in resource-poor settings presents significant challenges, which we discuss further below. We also attempted to identify countries that might benefit most from the implementation of ASB screening and treatment and/or those that are best prepared to overcome some of the challenges.
Screening for bacteriuria in the United States typically occurs as follows: (1) A pregnant woman visits a clinic to receive ANC; (2) a midstream urine specimen is collected, appropriately contained and stored, followed by any transport required for analysis; (3) a clinical microbiology lab receives urine and uses standard aerobic culture techniques to measure potential uropathogens in the sample, performs antibiotic susceptibility testing and identification of any uropathogens and reports the findings to the clinician; and finally (4) if necessary, the clinician prescribes or provides antibiotic treatment for the woman, along with sufficient patient education to help ensure compliance. Implementation of bacteriuria screening in resource-poor settings will face hurdles at each of these four steps.
1. The Problem of Available Antenatal Care
The currently accepted methods for bacteriuria screening and antibiotic treatment (eg, doctor in a clinic with access to a microbiology lab) can only be successful if pregnant women within a region of interest have access to ANC and seek it out. The World Health Organization's “Opportunities for Africa's Newborns” report classified screening and treatment of asymptomatic UTIs using standard approaches as a “more complex and expensive” intervention, but still considered it a desirable addition to an ANC package once basic care has become available in the region of interest.41
The best opportunities for antenatal ASB screening to be effective are countries with high rates of perinatal morbidity and existing ANC programs with minor “gaps in coverage” (ie, most women receive care). To identify some of the top candidates for implementing ASB screening and treatment, we analyzed reports from the WHO, including “Born Too Soon,”1 “Opportunities for Africa's Newborns,”41 and “Countdown to 2015.”42 We considered PTB rate, progress toward MDG4, presence of a Partnership for MNCH task force and ANC utilization as measures of overall maternal and child health. We also included “gap in coverage” data, which indicates differences in coverage between the poorest and the least poor, thereby serving as an indicator of whether ASB screening could be universally implemented. Here we focused on the eleven countries with >15% preterm birth (compiled in Table 2); however, women in many countries likely would benefit from implementation of ASB screening and treatment. We refer readers to the resources listed above for additional potential candidates.
Table 2.
Antenatal Care and Gaps in Coverage Among Countries With Preterm Birth Rates >15%
| Country | ANC42 | ANC Gap in coverage42 | ANC (4+)42 | ANC(4+) Gap in coverage42 | % NND due to PTB42 | MNCH task force41 | PTB rate1 | Trending toward MDG441,42 |
|---|---|---|---|---|---|---|---|---|
| Malawi | 95 | 3.1 | 46 | 10.3 | 30 | Yes | 18.1 | Yes |
| DR Congo | 88 | 17.8 | 45 | 21.5 | 38 | No | 16.7 | No |
| Comoros | 75 | 28 | 52 | 44.5 | 38 | Yes | 16.6 | Yes |
| Zimbabwe | 90 | 4.4 | 57 | 15.4 | 34 | Yes | 16.6 | Yes |
| Equatorial Guinea | 86 | nd | nd | nd | 34 | Yes | 16.5 | Yes |
| Mozambique | 92 | 31.3 | 53 | 39.9 | 25 | No | 16.4 | Yes |
| Gabon | 94 | 13.7 | 63 | 38.9 | 37 | No | 16.3 | No |
| Pakistan | 61 | 55 | 28 | 53.9 | 37 | Yes | 15.8 | Yes |
| Indonesia | 93 | 17 | 82 | 35.3 | 44 | No | 15.5 | Yes |
| Mauritania | 75 | 40.6 | 16 | nd | 26 | No | 15.4 | No |
| Botswana | 94 | nd | 73 | nd | 37 | in process | 15.1 | No |
Superscript numbers indicate the references from which data were taken. Data from “Countdown to 2015”42 were taken from the “Health Data-2012 Profile” for each country. In columns two and four, “ANC” and “ANC(4+)” refers to the overall average percentage of women who had at least 1 or more than 4 antenatal care visits, respectively. As found in the “Health Data-2012 Equity Profile” for each country, gaps in coverage with respect to socioeconomic status were estimated by dividing the population into 5 wealth quintiles and determining the average coverage in each group. The data listed in columns three and six refer to the difference in the percentage of coverage between the wealthiest and poorest quintiles. Gaps in coverage were taken from the “Health Data-2012 Equity Profile” for each country. %NND due to PTB is the percentage of neonatal deaths that are due to preterm birth, calculated based upon “Causes of under-five deaths, 2010” data found in each “Health Data-2012 Profile.” MNCH task force indicates whether the country has a Partnership for Maternal, Newborn and Child Health Task Force in place. Trending toward MDG4 indicates whether the under-5 mortality rate is decreasing since 1990.
Malawi and Zimbabwe are good examples of countries that have shown significant progress in the area of women's and children's health during the past two decades (Table 2), with ≥90% of women attending at least one ANC visit and ~50% attending the recommended four or more ANC visits (ANC 4+). Gaps in coverage between the poorest and least poor are also relatively low in Malawi and Zimbabwe. This is not the case for several other countries in Table 2, as discussed below. However, Malawi and Zimbabwe still have among the highest rates of PTB in the world. In their April 2007 planning meeting report, the Maternal Health working group of the Malawi MNCH emphasized that in low-resource settings there is an urgent need to prioritize interventions. We encourage this group to consider ASB screening and treatment in their package of ANC services.
Botswana and Equatorial Guinea also appear to have made great strides recently in achieving greater availability and utilization of ANC within their populations. For example, coverage in Equatorial Guinea rose from 37% to 86% between 1994 and 2012. However, there was no available data for either country regarding gaps in coverage. This is important to consider when estimating accessibility to ASB screening and treatment. For example, while the percentage of women receiving ANC appears to be high in Mozambique, a large gap in coverage indicates that there is still more to be done to make care available to the poorest women. In this regard, Gabon and Indonesia fare well with respect to coverage for one ANC visit; however, the gap in coverage for the optimal ANC (4+) remains large. The problem of inaccessible ANC is even more pronounced in Pakistan, Comoros and Mauritania, where the realities of poverty and lack of available services are at present, an insurmountable obstacle for implementing universal healthcare during pregnancy.
2. Genital Mutilation as an Additional Risk Factor for UTI and Poor Pregnancy Outcomes
It is estimated that more than 140 million women today have endured some type of genital mutilation, which, despite bans, continues to be common in at least 28 African countries.43 There are several types of female genital mutilation (FGM), often performed on girls aged 4 to 9 years, ranging from clitoridectomy (removal of the clitoris, type I FGM) to infibulation (type III FGM). Infibulation often entails the complete excision of the clitoris, labia minora, and labia majora, followed by surgical closure of the remaining tissue with narrowing and sometimes near-complete closure of the vaginal opening, which may require surgical re-opening (de-infibulation) to facilitate intercourse. Infibulation is associated with a variety of adverse uro-logic, gynecologic, and obstetric outcomes including obstructed labor, stillbirth, hemorrhage, and fistula.44
FGM not only permanently alters a girl's urogenital anatomy; data suggest it may fundamentally change the way her genitourinary mucosa interacts with her genitourinary microbiota.45,46 Anecdotal reports describe long-term sequelae of type III FGM to the uri-nary tract, including difficult and/or painful urination, urinary stasis, and heightened susceptibility to repeated urinary tract infections.45-48 In one prospective study that examined UTI among 255 consecutive girls aged 4 to 9 years in Sudan, FGM was associated with a five-fold higher risk of UTI in girls under 7 years of age and a three-fold increase among those who had a type III FGM (infibulation).49 Unfortunately, studies have not adequately addressed the impact of FGM on the risks of bladder and kidney infections in nonpregnant and pregnant women. However, if the three-fold increased risk of UTI among infibulated girls is more widely applicable to infibulated women, it is likely that FGM-associated UTI contributes significantly to adverse pregnancy outcomes.
It is recommended by the WHO that health care providers ascertain the status of pregnant women with respect to FGM at the first antenatal visit and when necessary, to perform culturally sensitive counseling regarding the complications associated with infibulation during pregnancy and childbirth. To avoid such complications, which have been associated with 1 to 2 additional deaths per 100 deliveries,44 de-infibulation is often recommended in the second trimester, allowing the tissue to heal prior to delivery. Studies regarding the timing of bacteriuria screening and treatment among infibulated women are needed.
3. Urine Sample Collection: Contamination, Confounders, and Other Considerations
The implementation of urine screening requires collection of a urine specimen that does not come into contact with excess bacteria from other genitourinary sources such as periurethral areas or the vagina. Contamination of urine with vaginal bacteria during collection is a frequent cause of inconclusive urine screening. Since the number of formal ANC visits attended by women in resource-poor settings is limited (Table 2), care must be taken to obtain urine specimens from which a clear positive or negative result can be obtained. To address this issue, patient education regarding urine collection procedures needs to be performed by trained personnel using validated education materials.
Urine contamination may be particularly difficult to avoid in women who have undergone infibulation. In this situation, the urethral opening is covered by infibulated scar tissue and urine exits the body from the vaginal neointroitus. It is curious that S aureus, a bacterium most often associated with skin infections, is a common cause of ASB in regions that practice FGM (see Figure 2 and “global significance of S aureus” section, above). The presence of keratinized scar tissue over the urethral opening might lead to a more habitable environment for skin-associated bacteria such as S aureus in a niche directly adjacent to the urethral opening. We were unable to assess rates of ASB/UTI in pregnancy in many resource-poor settings where FGM is common. Moreover, none of the published studies that examined ASB in pregnancy in regions known to practice FGM offer any information about the possible association of S aureus ASB and FGM. It is possible that the high rates of ASB in some areas, (Figure 1), is an artifact of urine “contamination” due to high rates of type III FGM. As mentioned above, most of the studies were carried out using accepted methods and definitions and suggest that S aureus ASB is occurring as a monomicrobial infection at titers >105 cfu/ml. Unfortunately, most of the published datasets do not provide adequate details to formally exclude the possibility that S aureus may be coming from a genitourinary niche other than the bladder. Future studies in populations where FGM is endemic should provide a better understanding of the possible relationships between UTI, FGM, and adverse pregnancy outcomes and better illuminate the possible contribution of S aureus in this context.
Whether the presence of S aureus in urine is an indication of bladder colonization or heavy vaginal colonization is an important distinction. Perhaps even more important is whether maternal genitourinary colonization with S aureus is associated with pregnancy complications and/or fetal infection. S aureus is an important peri-natal pathogen in many areas of the developing world. For example, in Nigeria, it is both the leading cause of early onset neonatal sepsis50 and the leading cause of maternal sepsis in childbirth.51 In fact, the microbiological profiles of the causes of maternal and neonatal sepsis around the time of birth in Nigeria are remarkably similar to the causes of maternal UTI (Figure 2). Future studies should examine whether S aureus, in the developing world, has a similar transmission route as Group B Streptococcus (GBS). GBS is the most common cause of neonatal sepsis in the United States and many other more developed countries. GBS is known to colonize the female genitourinary tract and to be transmitted vertically to the neonate before, during, or after birth. Maternal vaginal GBS colonization status has been linked with risk of neonatal infection. Moreover, presence of GBS in urine is an independent risk factor for GBS neonatal disease.12 Similar studies should be carried out to examine whether S aureus presence in urine is associated with a higher risk of maternal or neonatal sepsis. Screening and intrapartum prophylaxis has been very effective in reducing morbidity and mortality of perinatal streptococcal infections and may also be an effective strategy for controlling perinatal staphylococcal infections.
4. Detecting Bacteria in Urine: The Need for Clinical Microbiology Labs or Innovative Alternatives
Urine culture is considered the gold standard for ASB screening, and should be the first choice for any setting with adequate clinical microbiology resources. Unfortunately, laboratory microbiology resources are not always readily available in developing world settings, even when ANC services may be present. Despite being the gold standard, standard culture techniques are inconvenient for low-resource settings. As discussed above, few women have access to multiple ANC visits. Routine bacterial culture of urine specimens requires sterile media and 24 hours incubation at 37° C for bacterial growth to occur. Bacterial antibiotic susceptibility testing can take additional time to complete. Typically, women in more developed countries are contacted by their obstetrician's office within 24 to 48 hours of their visit when urine cultures are positive, and antibiotics are prescribed. However, women in resource-limited settings do not have access to neighborhood retail pharmacies. Thus, point of care tests would simplify the logistics and timelines associated with urine microbiology and treatment in resource-poor settings.
Various attempts have been made to replace standard culture techniques, but none have been shown to be as effective as urine culture for identifying ASB.8 For example, a number of studies have attempted to use commercially available urine dipsticks that measure nitrite (a product of nitrate reductase, expressed by some uropathogens) and leukocyte esterase activity (evidence of the host inflammatory process). However, this method is not sensitive enough to detect many cases of asymptomatic bacteriuria and often misses some of the less common uropathogens (eg, enterococci and streptococci) that do not express nitrate reductase. We thus emphasize that reported values of specificity and sensitivity using this method will depend on the specific population tested and the specific range of uropathogens causing UTI in that population. Another approach commonly used in place or in addition to urine culture is microscopic examination of gram-stained urine sediments. This method is advantageous from the perspective that it can identify bacteriuria even when standard aerobic culture techniques are insufficient to cultivate certain less common uropathogens. However, it has been reported that this method is highly sensitive to error when performed as a point of care test rather than by trained laboratory personnel. Dip paddles or dipslides52 are another way of culturing bacteria from urine that can be used outside the clinical microbiology lab. Dipslides contain two different bacterial growth media and facilitate CFU determination in a convenient format that assists with bacterial identification. However, the use of these alternative methods under “real” conditions (in a general practice office) showed a sensitivity of 74% and a specificity of 94%.53 We note that while these alternatives to urine culture may not be ideal, they may still be useful in limited resource settings and could promote meaningful gains in the prevention of pregnancy complications in target populations.
Clearly, there is a real need for portable methods that can accurately measure bacteriuria and distinguish potential uropathogens from contaminating vaginal bacteria. In the field, urine tests must be stable to local conditions, or convenient to store. Simplifying the clinical/microbiology workflow would allow flexible use by community health workers, perhaps expanding access to urine screening outside the traditional clinic setting.
5. Antibiotics: Treatment, Risks, Compliance, Susceptibility, and Resistance
UTI is one of the most common reasons for antibiotic use and is thus one of the main drivers of antibiotic resistance. As such, it is important that antibiotics not be given if they are not needed and that when needed, correct antibiotics are given to eliminate the infecting strain. Certain antibiotics, for example metronidazole, given during pregnancy have been associated with increased frequency of preterm birth.54-56 Antibiotics for UTI with moderately low risk in pregnancy (category B) include: cephalexin, erythromycin, nitrofurantoin, amoxicillinclavulanate, fosfomycin. Patient education is important for compliance, particularly in the context of asymptomatic UTI. Antibiotic resistance is more likely to develop in patients that do not comply with the full duration of antibiotic therapy. Alternative treatment options such as probiotics, functional food products and vaccines, may help circumvent the antibiotic resistance issue, but further studies are needed to establish their efficacy in the pregnant population. As discussed above, portable tests, particularly those that might be used within a community-based ANC setting, would greatly enhance the ability of healthcare workers to effectively treat UTI.
SUMMARY AND CONCLUSIONS
In summary, a substantial body of evidence in the United States and other more developed nations indicates that UTI in pregnancy (whether symptomatic or asymptomatic) is a risk factor for adverse outcomes that endanger the health of both mother and fetus. Multiple sources of evidence strongly support screening and treatment of UTI as a valuable approach for improving birth outcomes. In most industrialized nations, E coli is the predominant cause of UTI in nonpregnant reproductive-age women. Other bacterial genera such as Klebsiella, Enterococcus, Streptococcus, Proteus, coagulase-negative Staphylococci, and Pseudomonas also cause UTI. Studies conducted in more developed countries have found that this same collection of organisms are also responsible for the vast majority of UTIs (symptomatic or asymptomatic) in the pregnant host.8 However, based on our analysis of the literature, a large body of evidence is beginning to suggest that a Gram-positive bacterium that has been widely considered as an atypical cause of UTI—S aureus—actually is quite common in many parts of the developing world. Resource availability appears to be the primary hurdle for eventual reductions in adverse pregnancy outcomes associated with UTI. Where the resources are available, we encourage maternal and child health organizations and local governments to consider, or reconsider ASB screening and treatment as part of a package of infection prevention strategies (including sexually transmitted infections) to reduce pregnancy complications. Finally, we hope that the information outlined here serves to bring screening and treatment of UTI in pregnancy into the forefront of “essential” antenatal care.
Disclosures The authors completed the ICMJE Form for Disclosures of Potential Conflicts of Interest, and Drs Hultgren, Macones, and A. Lewis disclosed the receipt of grants by their institution from the National Institutes of Health (grant numbers P50 DK064540-11 and R21 DK092586-01A1). Drs Gilbert and O'Brien disclosed the receipt of grants by their institution from the American Heart Association and the National Science Foundation, respectively. Dr W. Lewis had no potential conflicts to disclose.
Contributor Information
Nicole M. Gilbert, Department of Molecular Microbiology, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States.
Valerie P. O'Brien, Department of Molecular Microbiology, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States
Scott Hultgren, Department of Molecular Microbiology, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States.
George Macones, Department of Obstetrics and Gynecology, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States.
Warren G. Lewis, Department of Medicine, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States.
Amanda L. Lewis, Departments of Molecular Microbiology, Obstetrics and Gynecology, Center for Women's Infectious Disease Research, Washington University School of Medicine, St Louis, Missouri, United States.
REFERENCES
- 1.March of Dimes P, Save the Children, WHO Born too soon: the global action report on preterm birth Geneva: World Health Organization;2012 [Google Scholar]
- 2.Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS medicine. 2013;10(2):e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rours GI, Duijts L, Moll HA, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol. 2011;26(6):493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis. 2011;38(3):167–71 [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113Suppl 1A:5S–13S [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE, Friesen D, Harding GK, Roos LL. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis. 1996;22(6):1051–6 [DOI] [PubMed] [Google Scholar]
- 7.Fried AM. Hydronephrosis of pregnancy: ultrasonographic study and classification of asymptomatic women. Am J Obstet Gynecol. 1979;135(8):1066–70 [DOI] [PubMed] [Google Scholar]
- 8.Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008;38 Suppl 2:50–7 [DOI] [PubMed] [Google Scholar]
- 9.Gratacos E, Torres PJ, Vila J, Alonso PL, Cararach V. Screening and treatment of asymptomatic bacteriuria in pregnancy prevent pyelonephritis. J Infect Dis. 1994;169(6):1390–2 [DOI] [PubMed] [Google Scholar]
- 10.Kass EH. Bacteriuria and pyelonephritis of pregnancy. Archives of internal medicine. 1960;105:194–8 [DOI] [PubMed] [Google Scholar]
- 11.Kass EH. Pyelonephritis and bacteriuria. A major problem in preventive medicine. Ann Intern Med. 1962. Jan;56:46–53 [DOI] [PubMed] [Google Scholar]
- 12.Smaill F. Asymptomatic bacteriuria in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):439–50 [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73(4):576–82 [PubMed] [Google Scholar]
- 14.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001;(2):\5. [DOI] [PubMed] [Google Scholar]
- 15.Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2007;(2):\5. [DOI] [PubMed] [Google Scholar]
- 16.US Preventive Services Task Force Screening for asymptomatic bacteriuria in adults: reaffirmation recommendation statement. Am Fam Physician. 2010;81(4):505. [PubMed] [Google Scholar]
- 17.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–54 [DOI] [PubMed] [Google Scholar]
- 18.Nicolle LE. Screening for asymptomatic bacteriuria in pregnancy Canadian guide to clinical preventative health care. Ottowa: Health Canada; 1994:100–6 [Google Scholar]
- 19.Scottish Intercollegiate Guidelines Network (SIGN) Management of suspected bacterial urinary tract infection in adults Edinburgh: SIGN; 2012 [Google Scholar]
- 20.National Collaborating Centre for Women's and Children's Health Antenatal care: routine care for the healthy pregnant woman London: RCOG Press; 2008 [PubMed] [Google Scholar]
- 21.Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC pregnancy and childbirth. 2010;10Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton M, Horvath DJ, Jr, Li B, et al. Intrauterine growth restriction is a direct consequence of localized maternal uropathogenic Escherichia coli cystitis. PloS one. 2012;7(3):e33897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adam T, Lim SS, Mehta S, et al. Cost effectiveness analysis of strategies for maternal and neonatal health in developing countries. BMJ. 2005;331(7525):1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365(9463):977–88 [DOI] [PubMed] [Google Scholar]
- 25.Akerele J, Abhulimen P, Okonofua F. Prevalence of asymptomatic bacteriuria among pregnant women in Benin City, Nigeria. J Obstet Gynaecol. 2001;21(2):141–4 [DOI] [PubMed] [Google Scholar]
- 26.Ajayi AB, Nwabuisi C, Aboyeji AP, Ajayi NS, Fowotade A, Fakeye OO. Asymptomatic bacteriuria in antenatal patients in Ilorin, Nigeria. Oman Med J. 2012;27(1):31–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imade PE, Izekor PE, Eghafona NO, Enabulele OI, Ophori E. Asymptomatic bacteriuria among pregnant women. N Am J Med Sci. 2010;2(6):263–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oli AN, Okafor CI, Ibezim EC, Akujiobi CN, Onwunzo MC. The prevalence and bacteriology of asymptomatic bacteriuria among antenatal patients in Nnamdi Azikiwe University Teaching Hospital Nnewi; South Eastern Nigeria. Niger J Clin Pract. 2010;13(4):409–12 [PubMed] [Google Scholar]
- 29.Hamdan HZ, Ziad AH, Ali SK, Adam I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Ann Clin Microbiol Antimicrob. 2011. Jan 18;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turpin C, Minkah B, Danso K, Frimpong E. Asymptomatic bacteriuria in pregnant women attending antenatal clinic at komfo anokye teaching hospital, kumasi, ghana. Ghana Med J. 2007;41(1):26–9 [PMC free article] [PubMed] [Google Scholar]
- 31.Assefa A, Asrat D, Woldeamanuel Y, Y GH, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008;46(3):227–35 [PubMed] [Google Scholar]
- 32.Alemu A, Moges F, Shiferaw Y, et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2012. Apr 25;5:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demilie T, Beyene G, Melaku S, Tsegaye W. Urinary bacterial profile and antibiotic susceptibility pattern among pregnant women in north west ethiopia. Ethiop J Health Sci. 2012;22(2):121–8 [PMC free article] [PubMed] [Google Scholar]
- 34.Andabati G, Byamugisha J. Microbial aetiology and sensitivity of asymptomatic bacteriuria among ante-natal mothers in Mulago hospital, Uganda. Afr Health Sci. 2010;10(4):349–52 [PMC free article] [PubMed] [Google Scholar]
- 35.Kasraeian M, Asadi N, Ghaffarpasand F. Prevalence of asymptomatic bacteriuria among pregnant women in Shiraz, Iran. Saudi Med J. 2009;30(7):917–20 [PubMed] [Google Scholar]
- 36.Enayat K, Fariba F, Bahram N. Asymptomatic bacteriuria among pregnant women referred to outpatient clinics in Sanandaj, Iran. Int Braz J Urol. 2008;34(6):699-704;discussion –7 [DOI] [PubMed] [Google Scholar]
- 37.Blomberg B, Olsen BE, Hinderaker SG, et al. Antimicrobial resistance in urinary bacterial isolates from pregnant women in rural Tanzania: implications for public health. Scand J Infect Dis. 2005;37(4):262–8 [DOI] [PubMed] [Google Scholar]
- 38.Celen S, Oruc AS, Karayalcin R, et al. Asymptomatic bacteriuria and antibacterial susceptibility patterns in an obstetric population. ISRN Obstet Gynecol. 2011;2011:721872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich M, Hoosen AA, Moodley J, Moodley S. Urogenital tract infections in pregnancy at King Edward VIII Hospital, Durban, South Africa. Genitourin Med. 1992;68(1):39–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayalakshmi J, Jayaram VS. Evaluation of various screening tests to detect asymptomatic bacteriuria in pregnant women. Indian J Pathol Microbiol. 2008;51(3):379–81 [DOI] [PubMed] [Google Scholar]
- 41.The Partnership for Maternal NaCH Opportunities for Africa's newborns: practical data, policy and programmatic support for newborn care in Africa Geneva, Switzerland: WHO on behalf of The Partnership for Maternal Newborn and Child Health; 2006 [Google Scholar]
- 42.Requejo J, Bryce J, Victora C. Countdown to 2015. [Author: Who is the publisher of this document?] Geneva, Switzerland; 2012
- 43.WHO Classification of female genital mutilation Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 44.Banks E, Meirik O, Farley T, Akande O, Bathija H, Ali M. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367(9525):1835–41 [DOI] [PubMed] [Google Scholar]
- 45.Iavazzo C, Sardi TA, Gkegkes ID. Female genital mutilation and infections: a systematic review of the clinical evidence. Archives of gynecology and obstetrics. 2013;287(6):1137–49 [DOI] [PubMed] [Google Scholar]
- 46.Consequences of genital mutilation Women's Health Newsletter. 1998;(36):\5 [Author: I can't verify this reference. Are there authors of this article?] [PubMed]
- 47.Knight R, Hotchin A, Bayly C, Grover S. Female genital mutilation—experience of The Royal Women's Hospital, Melbourne. Aust N Z J Obstet Gynaecol. 1999;39(1):50–4 [DOI] [PubMed] [Google Scholar]
- 48.El Dareer A. Complications of female circumcision in the Sudan. Trop Doct. 1983;13(3):131–3 [DOI] [PubMed] [Google Scholar]
- 49.Almroth L, Bedri H, El Musharaf S, et al. Urogenital complications among girls with genital mutilation:a hospital-based study in Khartoum. Afr J Reprod Health. 2005;9(2):118–24 [PubMed] [Google Scholar]
- 50.Ogunlesi TA, Ogunfowora OB, Osinupebi O, Olanrewaju DM. Changing trends in newborn sepsis in Sagamu, Nigeria:bacterial aetiology, risk factors and antibiotic susceptibility. J Paediatr Child Health. 2011;47(1-2):5–11 [DOI] [PubMed] [Google Scholar]
- 51.Bako B, Audu BM, Lawan ZM, Umar JB. Risk factors and microbial isolates of puerperal sepsis at the University of Maiduguri Teaching Hospital, Maiduguri, North-eastern Nigeria. Arch Gynecol Obstet. 2012;285(4):913–7 [DOI] [PubMed] [Google Scholar]
- 52.Kazemier BM, Schneeberger C, De Miranda E, et al. Costs and effects of screening and treating low risk women with a singleton pregnancy for asymptomatic bacteriuria, the ASB study. BMC Pregnancy Childbirth. 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winkens R, Nelissen-Arets H, Stobberingh E. Validity of the urine dipslide under daily practice conditions. Fam Pract. 2003;20(4):410–2 [DOI] [PubMed] [Google Scholar]
- 54.Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2003;189(5):1398–400 [DOI] [PubMed] [Google Scholar]
- 55.Shennan A, Crawshaw S, Briley A, et al. A randomised controlled trial of metro-nidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study. BJOG. 2006;113(1):65–74 [DOI] [PubMed] [Google Scholar]
- 56.Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345(7):487–93 [DOI] [PubMed] [Google Scholar]
- 57.Razzaque SM, Rahman KM. Bacteriuria and urinary tract infection in pregnancy. Bangladesh Medical Research Council bulletin. 1977;3(2):145–8 [PubMed] [Google Scholar]
- 58.Ullah A, Barman A, Ahmed I, Salam A. Asymptomatic bacteriuria in pregnant mothers: a valid and cost-effective screening test in Bangladesh. J Obstet Gynaecol. 2012;32(1):37–41 [DOI] [PubMed] [Google Scholar]
- 59.Hamadani JD, Tofail F, Hilaly A, et al. Association of postpartum maternal morbidities with children's mental, psychomotor and language development in rural Bangladesh. J Health Popul Nutr. 2012;30(2):193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullah MA, Barman A, Siddique MA, Haque AK. Prevalence of asymptomatic bacteriuria and its consequences in pregnancy in a rural community of Bangladesh. Bangladesh Med Res Counc Bull. 2007;33(2):60–4 [DOI] [PubMed] [Google Scholar]
- 61.Kalenga MK, Mutach K, Nsungula K, Kabyla I, Odimba FK. [Epidemiological considerations on the deliveries of stillbirths in the maternity unit of Gecamines Sendwe of Lubumbashi (Zaire)]. Rev Fr Gynecol Obstet. 1992;87(1):26–9 [PubMed] [Google Scholar]
- 62.Gebre-Selassie S. Asymptomatic bacteriuria in pregnancy: epidemiological, clinical and microbiological approach. Ethiop Med J. 1998;36(3):185–92 [PubMed] [Google Scholar]
- 63.Tadesse A, Negash M, Ketema LS. Asymptomatic bacteriuria in pregnancy: assessment of prevalence, microbial agents and their antimicrobial sensitivity pattern in Gondar Teaching Hospital, north west Ethiopia. Ethiop Med J. 2007;45(2):143–9 [PubMed] [Google Scholar]
- 64.Bandyopadhyay S, Thakur JS, Ray P, Kumar R. High prevalence of bacteriuria in pregnancy and its screening methods in north India. J Indian Med Assoc. 2005;103(5):259–62, 266 [PubMed] [Google Scholar]
- 65.Lavanya SV, Jogalakshmi D. Asymptomatic bacteriuria in antenatal women. Indian J Med Microbiol. 2002;20(2):105–6 [PubMed] [Google Scholar]
- 66.Thakre SS, Dhakne SS, Thakre SB, Thakre AD, Ughade SM, Kale P. Can the Griess Nitrite Test and a Urinary Pus Cell Count of>/=5 Cells Per Micro Litre of Urine in Pregnant Women be Used for the Screening or the Early Detection of Urinary Tract Infections in Rural India? J Clin Diagn Res. 2012;6(9):1518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi RN, Khan KS, Darr O, Khattak N, Farooqui BJ, Rizvi JH. Bacteriuria and pregnancy outcome:a prospective hospital-based study in Pakistani women. J Pak Med Assoc. 1994;44(1):12–3 [PubMed] [Google Scholar]
- 68.Lata I, Pradeep Y, Sujata Jain A. Estimation of the incidence of bacterial vaginosis and other vaginal infections and its consequences on maternal/fetal outcome in pregnant women attending an antenatal clinic in a tertiary care hospital in North India. Indian J Community Med. 2010;35(2):285–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazhir S. Asymptomatic bacteriuria in pregnant women. Urol J. 2007;4(1):24–7 [PubMed] [Google Scholar]
- 70.Sharami SH, Afrakhteh M, Shakiba M. Urinary tract infections in pregnant women with bacterial vaginosis. Am J Obstet Gynecol. 2007;27(3):252–4 [DOI] [PubMed] [Google Scholar]
- 71.Young J, Trotman H, Thame M. The impact of antenatal care on pregnancy performance between adolescent girls and older women. West Indian Med J. 2007;56(5):414–20 [PubMed] [Google Scholar]
- 72.Mawajdeh SM, Al-Qutob R, Schmidt A. Measuring reproductive morbidity: a community-based approach, Jordan. Health Care Women Int. 2003;24(7):635–49 [DOI] [PubMed] [Google Scholar]
- 73.Quteitat A, Shraideh I, Malek AM, Gowieri A, Alnashash H, Amarin ZO. Maternal morbidity: results of a country-wide review. Arch Gynecol Obstet. 2012;286(6):1357–62 [DOI] [PubMed] [Google Scholar]
- 74.Ukachukwu VE, Unger H, Onoka C, Nduka C, Maina S, Ngugi N. Maternal morbidity and mortality in peri-urban Kenya–assessing progress in improving maternal healthcare. East Afr J Public Health. 2009;6(2):112–8 [PubMed] [Google Scholar]
- 75.Mohammad M, Mahdy ZA, Omar J, Maan N, Jamil MA. Laboratory aspects of asymptomatic bacteriuria in pregnancy. Southeast Asian J Trop Med Public Health. 2002;33(3):575–80 [PubMed] [Google Scholar]
- 76.Kayastha S, Tuladhar H. Study of low birth weight babies in Nepal Medical College. Nepal Med Coll J. 2007;9(4):266–9 [PubMed] [Google Scholar]
- 77.Nnatu S, Essien EE, Akinkugbe A, Odum CU. Asymptomatic bacteriuria in pregnant Nigerian patients. Clin Exp Obstet Gynecol. 1989;16(4):126–9 [PubMed] [Google Scholar]
- 78.Onakoya JA, Amole OO, Ogunsanya OO, Tayo O. Comparing the specificity and sensitivity of nitrate and leucocyte tests on multistick in screening for urinary tract infections amongst pregnant women at Lagos State University Teaching Hospital Ikeja, Nigeria. Nig Q J Hosp Med. 2008;18(2):61–3 [DOI] [PubMed] [Google Scholar]
- 79.Awonuga DO, Dada-Adegbola HO, Fawole AO, Olala FA, Onimisi-Smith HO. Asymptomatic bacteriuria among an obstetric population in Ibadan. West Afr J Med. 2011;30(2):89–93 [PubMed] [Google Scholar]
- 80.Awonuga DO, Fawole AO, Dada-Adegbola HO, Olola FA, Awonuga OM. Asymptomatic bacteriuria in pregnancy:evaluation of reagent strips in comparison to microbiological culture. Afr J Med Med Sci. 2011;40(4):377–83 [PubMed] [Google Scholar]
- 81.Awonuga DO, Fawole AO, Dada-Adegbola HO, Olola FA, Awonuga OM. Predictors of asymptomatic bacteriuria among obstetric population in Ibadan. Niger J Med. 2010;19(2):188–93 [DOI] [PubMed] [Google Scholar]
- 82.Akinloye O, Ogbolu DO, Akinloye OM, Terry Alli OA. Asymptomatic bacteriuria of pregnancy in Ibadan, Nigeria: a re-assessment. Br J Biomed Sci. 2006;63(3):109–12 [DOI] [PubMed] [Google Scholar]
- 83.Olusanya O, Ogunledun A, Fakoya TA. Asymptomatic significant bacteriuria among pregnant and non-pregnant women in Sagamu, Nigeria. West Afr J Med. 1993;12(1):27–33 [PubMed] [Google Scholar]
- 84.Kehinde AO, Adedapo KS, Aimaikhu CO, Odukogbe AT, Olayemi O, Salako B. Significant bacteriuria among asymptomatic antenatal clinic attendees in Ibadan, Nigeria. Trop Med Health. 2011;39(3):73–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;60(3):213–6 [PubMed] [Google Scholar]
- 86.Hanif S. Frequency and pattern of urinary complaints among pregnant women. J Coll Physicians Surg Pak. 2006;16(8):514–7 [PubMed] [Google Scholar]
- 87.Sheikh MA, Khan MS, Khatoon A, Arain GM. Incidence of urinary tract infection during pregnancy. East Mediterr Health J. 2000;6(2-3):265–71 [PubMed] [Google Scholar]
- 88.Fatima N, Ishrat S. Frequency and risk factors of asymptomatic bacteriuria during pregnancy. J Coll Physicians Surg Pak. 2006;16(4):273–5 [PubMed] [Google Scholar]
- 89.Lumbiganon P, Villar J, Laopaiboon M, et al. One-day compared with 7-day nitrofurantoin for asymptomatic bacteriuria in pregnancy: a randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):339–45 [DOI] [PubMed] [Google Scholar]
- 90.Kovavisarach E, Vichaipruck M, Kanjarahareutai S. Risk factors related to asymptomatic bacteriuria in pregnant women. J Med Assoc Thai. 2009;92(5):606–10 [PubMed] [Google Scholar]
- 91.Kacmaz B, Cakir O, Aksoy A, Biri A. Evaluation of rapid urine screening tests to detect asymptomatic bacteriuria in pregnancy. Jpn J Infect Dis. 2006;59(4):261–3 [PubMed] [Google Scholar]
- 92.Kutlay S, Kutlay B, Karaahmetoglu O, Ak C, Erkaya S. Prevalence, detection and treatment of asymptomatic bacteriuria in a Turkish obstetric population. J Reprod Med. 2003;48(8):627–30 [PubMed] [Google Scholar]
- 93.Tugrul S, Oral O, Kumru P, Kose D, Alkan A, Yildirim G. Evaluation and importance of asymptomatic bacteriuria in pregnancy. Clin Exp Obstet Gynecol. 2005;32(4):237–40 [PubMed] [Google Scholar]
- 94.Uncu Y, Uncu G, Esmer A, Bilgel N. Should asymptomatic bacteriuria be screened in pregnancy? Clin Exp Obstet Gynecol. 2002;29(4):281–5 [PubMed] [Google Scholar]
- 95.Gunes G, Gunes A, Tekiner S, Karaoglu L, Kaya M, Pehlivan E. Bacteriuria and socioeconomic associations among pregnant women in Malatya, Turkey. Public health. 2005;119(11):1039–41 [DOI] [PubMed] [Google Scholar]
- 96.Lightman SL, Powell-Jackson PR. The incidence of trichomoniasis and dysuria in pregnant women in Eastern Uganda. Trop Geogr Med. 1971;23(1):113–4 [PubMed] [Google Scholar]
- 97.Mtimavalye LA, Runyoro DE, Massawe FN, Mhalu FS, Kanyawana JZ. Asymptomatic bacteriuria and concomitant presence of other micro-organisms in urine of pregnant women in Dar es Salaam—Tanzania. J Obstet Gynaecol East Cent Africa. 1983;2(3):108–12 [PubMed] [Google Scholar]
- 98.Masinde A, Gumodoka B, Kilonzo A, Mshana SE. Prevalence of urinary tract infection among pregnant women at Bugando Medical Centre, Mwanza, Tanzania. Tanzan J Health Res. 2009;11(3):154–9 [DOI] [PubMed] [Google Scholar]
- 99.Prakalapakorn SG, Rasmussen SA, Lambert SR, Honein MA. Assessment of risk factors for infantile cataracts using a case-control study:National Birth Defects Prevention Study, 2000-2004. Ophthalmology. 2010;117(8):1500–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cleves MA, Malik S, Yang S, Carter TC, Hobbs CA. Maternal urinary tract infections and selected cardiovascular malformations. Birth Defects Res A Clin Mol Teratol. 2008;82(6):464–73 [DOI] [PubMed] [Google Scholar]
- 101.Whitehead NS, Callaghan W, Johnson C, Williams L. Racial, ethnic, and economic disparities in the prevalence of pregnancy complications. Matern Child Health J. 2009;13(2):198–205 [DOI] [PubMed] [Google Scholar]
- 102.Conde-Agudelo A, Belizan JM, Lindmark G. Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol. 2000;95(6 Pt 1):899–904 [PubMed] [Google Scholar]
- 103.Makki AM. Risk factors for low birth weight in Sana'a City, Yemen. Ann Saudi Med. 2002;22(5-6):333–5 [DOI] [PubMed] [Google Scholar]
- 104.Al-Haddad AM. Urinary tract infection among pregnant women in Al-Mukalla district, Yemen. East Mediterr Health J. 2005;11(3):505–10 [PubMed] [Google Scholar]
- 105.Andy South JS-P, Barry Rowlingson, Roger Bivand, Pru Foster. rworldmap: Mapping global data, vector and raster. R project for statistical computing. 1.02 ed; 2012. p. Enables mapping of country level and gridded user datasets.
- 106.Shitaye D, Asrat D, Woldeamanuel Y, Worku B. Risk factors and etiology of neonatal sepsis in Tikur Anbessa University Hospital, Ethiopia. Ethiop Med J. 2010;48(1):11– [PubMed] [Google Scholar]


