Abstract
Defatted soybean flour (DSF) can sorb and concentrate blueberry anthocyanins and other polyphenols, but not sugars. In this study blueberry polyphenol-enriched DSF (BB-DSF) or DSF were incorporated into very high fat diet (VHFD) formulations and provided ad libitum to obese and hyperglycemic C57BL/6 mice for 13 weeks to investigate anti-diabetic effects. Compared to the VHFD containing DSF, the diet supplemented with BB-DSF reduced weight gain by 5.6%, improved glucose tolerance, and lowered fasting blood glucose levels in mice within 7 weeks of intervention. Serum cholesterol of mice consuming the BB-DSF-supplemented diet was 13.2% lower than mice on the diet containing DSF. Compounds were eluted from DSF and BB-DSF for in vitro assays of glucose production and uptake. Compared to untreated control, doses of BB-DSF eluate containing 0.05 – 10 μg/μL of blueberry anthocyanins significantly reduced glucose production by 24% - 74% in H4IIE rat hepatocytes, but did not increase glucose uptake in L6 myotubes. The results indicate that delivery of blueberry polyphenols stabilized in a high-protein food matrix may be useful for the dietary management of pre-diabetes and/or diabetes.
Keywords: metabolic syndrome, diabetes, blueberry, soybean, anthocyanins, polyphenols
1. Introduction
Metabolic syndrome is characterized by central obesity, hyperglycemia, hyperinsulinaemia, dyslipidemia and hypertension. Individuals with any three of these five metabolic abnormalities are considered positive for the syndrome and are at increased risk for developing type 2 diabetes and cardiovascular disease [1]. In the US 34% of men and women display the early clinical symptoms of metabolic syndrome [2]. The worldwide prevalence of obesity among men and women doubled between 1980-2008 [3] while glycemia continued to rise and contribute to a more than twofold increase in global cases of adult type 2 diabetes within this time frame [4]. Current remedial options for metabolic syndrome involve lifestyle modification and poly-pharmacological drug treatment; however, improved therapeutic and preventive approaches are needed. Growing evidence indicates that polyphenols contained in berries, fruits, vegetables, nuts and teas possess many health-promoting and disease-preventing properties [5-6]. As a result, increased consumption of plant-based foods has been recommended by health agencies worldwide in an effort to prevent chronic diseases.
Blueberry fruits (Vaccinium angustifolium Aiton and Vaccinium corymbosum L.) have been used in traditional medicine, especially for the secondary complications of diabetes [7-8]. Blueberries contain a wide array of polyphenol compounds including up to 27 different anthocyanin pigments [9] as well as proanthocyanidins, quercetin, and quercetin glycosides and chlorogenic acids which all contribute to the fruit’s high antioxidant activity [10-11]. Apart from high antioxidant activity, research shows that blueberry polyphenols have anti-diabetic and cardio-protective properties. Two recent clinical studies where participants consumed blueberries formulated into a beverage have demonstrated improved insulin sensitivity in insulin-resistant subjects [12] and decreased blood pressure and markers of lipid oxidation in metabolic syndrome patients [13] compared to the control intervention. Compared to a control diet, supplementation with blueberries improved vascular tone and decreased blood pressure in spontaneously hypertensive rats [14-15], at least partially due to inhibition of ACE activity in blood [16]. A single dose of an anthocyanin-enriched blueberry fraction administered to diet-induced obese and diabetic C57BL/6 mice significantly lowered blood glucose levels [17]. In studies where C57BL/6 mice were fed whole blueberry powder or purified blueberry anthocyanins only the latter, which lacked sugars and lipid components, was effective for correcting dyslipidemia and reducing body weight gain [18-19]. These data suggest that sugars or lipid components in whole blueberries may counteract the anti-obesity and possibly other anti-diabetic effects of blueberry anthocyanins and other polyphenols.
We have recently reported that defatted soybean flour (DSF), sorbs and concentrates anthocyanins and other polyphenols, but not sugars, from blueberry, cranberry and grape juices [20-21]. Anthocyanins and other polyphenols were greatly stabilized in the blueberry polyphenol-enriched DSF (BB-DSF) matrix and a single oral dose of BB-DSF formulated in Labrasol® significantly lowered blood glucose levels in obese and hyperglycemic C57BL/6 mice 6 h post-administration [20]. In this study we formulated BB-DSF or DSF into the high fat diet of diabetic C57BL/6 mice and measured differential effects on body weight, food intake, blood glucose metabolism and serum markers of dyslipidemia, antioxidant activity and inflammation over a 13-week intervention period. Polyphenols eluted from BB-DSF or DSF alone were also prepared and tested in cell-based in vitro assays to investigate possible mechanisms of action.
2. Research Design and Methods
2.1. Preparation of rodent diets
Blueberry (Vaccinium corymbosum) juice concentrate (65 Brix; Fruit Smart, WA) produced from cultivated highbush blueberries was diluted 4× in water. DSF (Hodgson Mill Inc., IL) was added to 20 L of the diluted juice at 100 g/L and mixed for 30 min. The blueberry juice-flour mixture (pH 3.7) was allowed to settle and the excess juice was decanted. The remaining juice-flour mixture was centrifuged for 10 min at 4000 rpm (Eppendorf, model 5810R) and the BB-DSF was collected after decanting the juice. The DSF control was prepared by mixing DSF with water and acidifying the mixture to pH 3.7 with citric acid. The DSF solids were separated from the water as described above. BB-DSF and control DSF matrices were freeze-dried and powdered. The very high fat diet (VHFD; D12492) containing 61% kcal fat was used as the basis for formulating the BB-DSF and DSF intervention diets. Nutritional analysis was performed on BB-DSF and DSF by Medallion Labs (WA) and this information was used by Research Diets (New Brunswick, NJ) to formulate VHFDs containing approximately 40% BB-DSF or DSF while keeping all diets equivalent in terms of the percentage of kcalories (% kcals) contributed by protein, carbohydrate, fiber, and fat.
2.2. Quantification of anthocyanins and total polyphenols
Blueberry juice supernatants were passed through a 2 μm filter prior to quantification of blueberry anthocyanins and total polyphenols. Total monomeric anthocyanins, calculated as cyanidin 3-O-glucoside equivalents, were measured using the pH differential method [22] using a UV/VIS spectrophotometer (Synergy HT Multi-Detection Microplate Reader, BioTek). Total polyphenols were quantified by the Folin-Ciocalteu method [23] and samples were read at 726 nm against a gallic acid (Sigma) standard curve. The amount of anthocyanins or total polyphenols sorbed per gram of DSF was calculated by subtracting their concentration in the DSF-treated juice supernatants from that measured in untreated juice samples and dividing by the concentration of DSF used for sorption.
2.3. Mice and treatment protocol
The protocol was approved by Rutgers University Institutional Care and Use Committee and followed federal and state laws. Five-week-old male C57/Bl6 mice (10-20 g) were purchased from Jackson Laboratory (Bar Harbor, Maine) and fed a regular diet ad libitum (Purina, #5015) during their one week acclimatization period. Animals were housed, five per cage, with free access to water in a room with a 12:12 h light-dark cycle (7 am – 7 pm) and a temperature of 24 ± 1 °C. At 6 weeks of age all mice were placed on the VHFD for 12 weeks to induce obesity, insulin resistance and hyperglycemia. Mice were then divided into 3 groups (based on the baseline OGTT results) and placed on intervention diets for an additional 13 weeks. One group of 15 mice was placed on the VHFD containing BB-DSF (BB-DSF diet) and a second group of 15 mice were placed on the VHFD containing DSF alone (DSF diet). A third group of mice (n=5) stayed on the VHFD during the course of the study and served mainly as a control group to monitor weight gain and food intake in comparison to mice consuming the DSF and BB-DSF diets.
2.4. Body weights and food intake
The body weight of each mouse was recorded every week. Food intake per mouse per day was calculated as follows: [total food intake per cage] / [mice per cage] × [days of food consumption]. The mean ± SD were calculated for BB-DSF and DSF groups as they were each composed of three cages of mice whereas only the mean could be calculated for the VHFD group, which comprised a single cage.
2.5. Oral glucose tolerance test (OGTT) and fasting blood glucose test
For OGTTs mice in the BB-DSF and DSF groups were fasted overnight for 13 hours and body weights were measured the next morning. Fasting blood glucose levels were tested prior to glucose administration (T=0) using a glucometer (AlphaTRAK® 32004-02, Abbott Animal Health). Mice were then gavaged with 2 g/kg of glucose (500 mg/mL) and blood glucose was tested every 30 min up to 180 min to follow clearance of glucose from the blood. Oral glucose tolerance tests performed at week 0 (i.e. after the 12 week VHFD period and prior to the introduction of intervention diets) were used for randomizing the animals into BB-DSF or DSF diet groups. For fasting blood glucose tests the blood glucose levels of all mice were measured in the morning prior to the fasting period (T=0). Food was then removed from cages and blood glucose levels were measured during the day time after a 6 h and 9 h fast period.
2.6. Blood Chemistry
Mice were sacrificed after the 13-week intervention period by CO2 asphyxiation and trunk blood was collected into microfuge tubes and allowed to clot. Samples were centrifuged at 5000 rpm for 10 min and serum was collected for biochemical analysis, which was performed at Pennington Biomedical Research Center. Serum samples obtained from mice in the BB-DSF diet group (n=6) and mice in the DSF diet group (n=6) were individually subject to triglyceride, total cholesterol, insulin, adiponectin, RBP4 protein and total antioxidant activity (ferric reducing antioxidant power (FRAP) method) measurements. Triglycerides, total cholesterol and total antioxidant activity were quantified using a Beckman-Coulter DXC 600 Pro (Beckman-Coulter Inc, Brea, CA) using standard spectrophotometric assays. Serum insulin (CrystalChem Inc.) and RBP4 (AdipoGen International) were quantified by ELISA kits. Adiponectin was measured using Milliplex™ MAP single plex adiponectin kit (Millipore). The concentration of inflammatory cytokines IL-6, IL-1β and TNFα were quantified in individual serum samples obtained from the BB-DSF (n=9) and DSF (n=8) diet groups using the Milliplex™ MAP mouse cytokine/chemokine kit (Millipore).
2.7. Preparation of eluate samples for in vivo and in vitro experiments
BB-DSF, made with 30 g/L of DSF and 4× diluted blueberry juice (BBJ), and DSF control matrices were prepared as described above. Two grams of each freeze-dried powdered matrix was eluted 6 times with 20 mL volumes of acidic methanol (methanol: water: acetic acid, 75:20:5), vortexing for 20 sec and then sonicating for 5 min. The eluates of each respective sample were pooled and solvent was evaporated under vacuum to a final volume of 15-20 mL. Remaining solvent was removed by freeze drying (Labconco). Samples were subjected to C18 SPE column-purification to remove any sugars that would interfere with glucose release or uptake experiments. Briefly, C18 SPE cartridge columns were pre-conditioned with 2 volumes (2 × 5 mL) of ethyl acetate, 2 volumes of acidified methanol (methanol, 1% acetic acid) and 2 volumes of 1% acetic acid. The dried eluates were re-dissolved in water and applied to C18 SPE columns, which were washed 3 times with 1% acetic acid. The bound anthocyanins and other polyphenols were eluted from the column with acidified methanol. After adding 5 mL of water to each sample, methanol was removed by rotary evaporation and each sample was freeze-dried to obtain post-C18 eluates i.e., BB-DSF-e and DSF-e. The dry weight of each eluate was obtained and the percent difference was used to determine the dose of DSF-e used in subsequent experiments. BB-DSF-e was dissolved in water (10 mg/mL) to determine anthocyanin content, which was estimated to be 7-8% anthocyanins (wt/wt) of the BB-DSF-e material. BBJ was also used in glucose release and uptake experiments. BBJ was also subject to C18 SPE column purification to remove sugars. After the methanol solvent was evaporated and replaced by water, the anthocyanin concentration in the liquid sugar-free BBJ-c18 sample was determined prior to in vitro experiments.
2.8. Cytotoxicity assay
Cytotoxicity of BB-DSF-e and DSF-e samples were tested in L6 myotubes and H4IIE cells. Cells were prepared for glucose uptake or glucose release assays and BB-DSF-e, DSF-e or BBJ-c18 were added to the medium at various concentrations and incubated for 6 h as described in detail below. After the treatment period membrane integrity and cell viability were assessed by Calcein-306 AM (Ca-AM) incorporation. Ca-AM was added to each well at 3 μM, incubated for 1 h at 37 °C, and fluorescence was measured in a plate reader (BIOTEK Synergy II, Biotek Instruments Inc., Winooski, VT) at excitation wavelength at 485 nm and emission wavelength of 525 nm. Triplicate wells were used for each treatment. Doses that did not show toxicity (at least 90 % viability compared to untreated control) were used in subsequent assays.
2.9. Glucose production assay
H4IIE hepatoma cells were incubated at 37 °C in 5% CO2, 95% air in 24-well tissue culture plates (Greiner Bio-One, Monroe, NC) in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS), penicillin (100 IU/mL) and gentamicin (50 μg/mL). Upon confluence cells were starved overnight (14 h) in glucose production medium (glucose-free Dulbecco’s modified essential medium, pH 7.4 containing 2 mM sodium pyruvate, 20 mM sodium lactate and 22 mM sodium bicarbonate without phenol red). After the starvation period glucose production medium in each well was replaced alone (Ctl) or treated with indicated doses of BB-DSF-e, BBJ-c18 or DSF-e. Insulin (Humalog, Eli Lilly) or Metformin (Sigma) was used as a positive control for repression of glucose release. To test whether compounds from BB-DSF-e or DSF-e enhanced insulin sensitivity, cells were treated with medium alone, insulin (10 nM), or insulin plus indicated concentrations of BB-DSF-e or DSF-e. Each treatment condition was administered to triplicate wells of cells for 6 hours. Glucose released into the cell culture medium was quantified with the Amplex® Red Glucose assay kit (Invitrogen). The percentage of glucose released in the medium is expressed relative to the untreated control (100%).
2.10. Glucose uptake assay
L6 myoblasts from rat skeletal muscles [24] were grown and differentiated into myotubes as previously described [25]. Myotubes were then incubated in FBS-free DMEM for 6 h in the absence or presence of treatments (BB-DSF-e, DSF-e or insulin). Cells were subjected to glucose uptake assay as previously described [25]. Corrections for cell number were made on the basis of protein concentration measured using the BCA protein assay kit (Pierce Biotechnology). Glucose uptake results were expressed relative to the untreated control (100%).
2.11. Statistical Analysis
Statistics were performed with STATISTICA v.10 (StatSoft). One-way ANOVA was used to determine significance among three or more groups followed by the indicated post-hoc test. Paired t-tests were performed within groups (before vs. after treatment) and unpaired t-tests were used for independent groups.
3. Results
3.1. Diets, body weight and food intake
The VHFD, BB-DSF and DSF diets were formulated to be isocaloric, each delivering 20-21 kJ of energy per gram of diet (Table 1). Our previous data indicated that DSF does not sorb or concentrate sugars from fruit juices [20-21]; however, nutritional analysis data showed that BB-DSF contained 10.4% glucose and 11.9% fructose, which can be attributed to residual blueberry juice that remained with the moist BB-DSF pellet after centrifugation and decanting of the juice. Protein levels in BB-DSF powder was 37% compared to 50% in the DSF control powder. Glucose and fructose were added to the DSF control and additional soy protein isolate was added to the BB-DSF diet to adjust for these differences in the powders so that they contained comparable levels of fiber and nutrients (protein, carbohydrate, fat), contributing equivalent amounts of energy (% kcals) to each diet (Table 1).
Table 1.
Nutritional Composition of Diets
| VHFD diet |
BB-DSF diet |
DSF diet |
|
|---|---|---|---|
| Ingredient (g/kg) | |||
| Casein | 258.5 | 0 | 0 |
| L-Cystine | 3.9 | 0 | 0 |
| Soy protein, Supro 661 | 0 | 68.3 | 7.7 |
| DL- methionine | 0 | 3.6 | 3.8 |
| BB-DSF | 0 | 407 | 0 |
| DSF | 0 | 0 | 433.9 |
| Corn starch | 0 | 0 | 0 |
| Maltodextrin 10 | 161.5 | 136.6 | 145.5 |
| Sucrose | 88.9 | 0 | 0 |
| Cellulose | 64.6 | 0 | 0 |
| Lard | 316.6 | 296.6 | 312.7 |
| Soybean oil | 32.3 | 22.2 | 23.6 |
| Mineral mix S10026 | 12.9 | 12.0 | 12.8 |
| Dicalcium phosphate | 16.8 | 15.6 | 16.6 |
| Calcium carbonate | 7.1 | 6.6 | 7.0 |
| Potassium citrate | 21.3 | 19.8 | 21.1 |
| Vitamin Mix V10001 | 12.9 | 12.0 | 12.8 |
| Choline bitartrate | 2.6 | 2.4 | 2.6 |
| Red dye #40, FD&C | 0 | 0 | 0.1 |
| Blue dye #1, FD&C | 0.1 | 0 | 0 |
| Yellow dye #5, FD&C | 0 | 0.1 | 0 |
| Total (g) | 1000 | 1000 | 1000 |
| Gram% | |||
| Protein | 23.1 | 21.4 | 22.8 |
| Carbohydrate | 26.3 | 24.5 | 26.1 |
| Dextrose | 0 | 4.2 | 4.5 |
| Sucrose | 10.2 | 1.5 | 1.6 |
| Fructose | 0 | 4.8 | 5.2 |
| Maltodextrin | 16.2 | 13.7 | 14.6 |
| Raffinose & Stachyose | 0 | 0.2 | 0.2 |
| Fat | 34.9 | 32.4 | 34.5 |
| Fiber | 6.5 | 7.5 | 7.9 |
| DSF | 0 | 0 | 43.4 |
| BB-DSF | 0 | 40.7 | 0 |
| kcal (%) | |||
| Protein | 18 | 18 | 18 |
| Carbohydrate | 21 | 21 | 21 |
| Dextrose | 0 | 4 | 4 |
| Sucrose | 8 | 1.3 | 1.3 |
| Fructose | 0 | 4 | 4 |
| Maltodextrin | 12.6 | 11.5 | 11.5 |
| Fat | 61 | 61 | 61 |
| Total | 100 | 100 | 100 |
| kJ/g (calculated) | 21.4 | 19.9 | 21.2 |
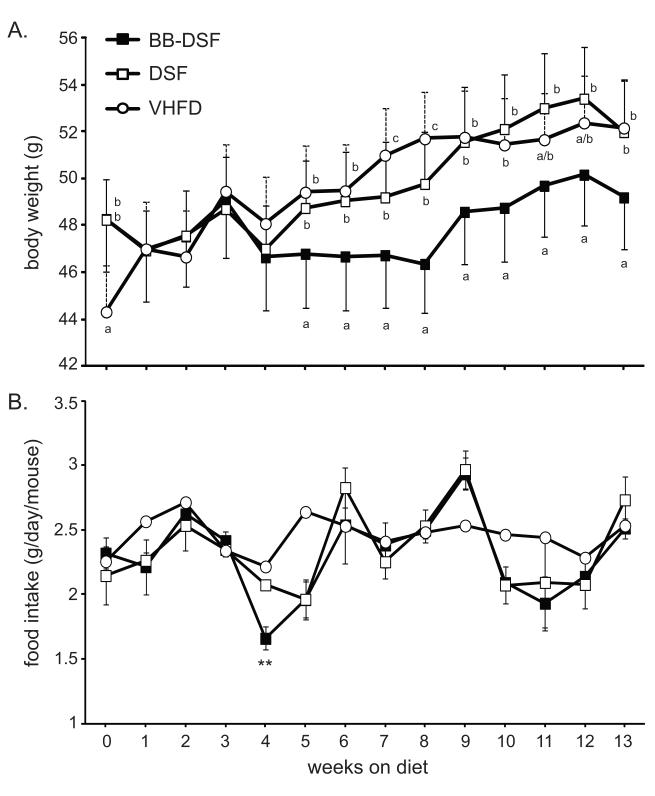
All mice initially consumed the VHFD for 12 weeks to induce the diabetic profile before being assigned to the BB-DSF or DSF intervention diets. Group assignments were made based on baseline OGTT data (see section 3.2). The BB-DSF diet contained anthocyanins at a concentration of 1.9 mg/g of diet and a total 6.3 mg/g of blueberry polyphenols. Mice in the BB-DSF group consumed an average of 2.2 g of diet per day, which provided 4.4 mg of anthocyanins and 13.9 mg of blueberry polyphenols per day. At week 0, mice in the VHFD group initially had lower body weights than mice assigned to the BB-DSF or DSF diet groups, but this difference disappeared over the next 4 weeks (Fig 1A). With the exception of a transient difference at weeks 7 and 8 post-intervention, there were no differences in body weights of mice in the VHFD or DSF diet groups (Fig 1A). Compared to the VHFD or DSF diet groups, mice consuming the BB-DSF diet began showing a significant decrease in weight gain starting at 5 weeks post-intervention and this effect continued for an additional 8 weeks until the end of the experimental period (Fig 1A). From week-5 to week-13, mice treated with the BB-DSF diet had an average of 5.6% less weight gain compared to mice on the control DSF diet or an average of 5.8% less weight gain compared to the VHFD. Except at week 4, there was no significant difference in the amount of food consumed between mice in the BB-DSF and DSF diet groups (Fig. 1B). The average food consumption per day (but not standard deviation) could be calculated for the VHFD diet group and was very similar to the BB-DSF and DSF groups (Fig. 1B) indicating that all diets were equally palatable.
Figure 1.
BB-DSF diet reduces weight gain, but not food intake in C57BL/6 mice. (A) Body weights (g) of mice (mean ± SD) consuming the indicated diets for the 13-week intervention period. At each time point one-way ANOVA followed by unequal N HSD post-hoc test was used. Significant difference between groups for each week is signified by letter a, b or c; different letters indicate significant difference (p <0.05) between groups while the same letter or absence of a letter indicates no difference. (B) Food intake of mice on indicated diets during the intervention period. Data are expressed as the mean for the VHFD group and mean ± SD for BB-DSF and DSF groups. T-tests (2-tailed) were performed between BB-DSF and DSF groups at each time point; ** p< 0.01.
3.2. Effects of the BB-DSF diet on glucose metabolism
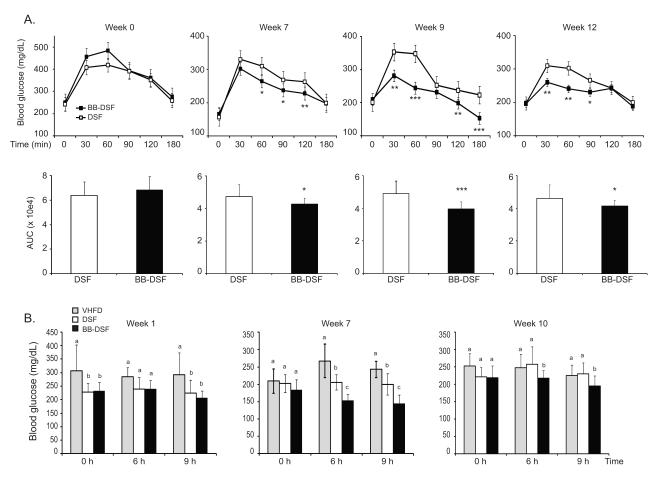
After mice consumed the VHFD for 12 weeks to induce the diabetic profile, a baseline (week 0) oral glucose tolerance test (OGTT) was performed. The baseline OGTT results were used to assign cages of mice to receive the BB-DSF diet or DSF diet, with one cage of 5 mice remaining on the VHFD. There was no difference in glucose clearance prior to introduction of the intervention diets (Fig. 2A, Week 0). Oral glucose tolerance tests were performed on mice in the BB-DSF and DSF groups at indicated weeks post-intervention. Compared to mice consuming the DSF diet, mice on the BB-DSF diet showed significantly improved oral glucose tolerance at week 7 and this effect was sustained at weeks 9 and 12 (Fig 2A). Differences in glucose tolerance between BB-DSF and DSF groups were not observed from weeks 3 to 6 (Supplementary Fig. 1A). Within group comparisons showed that compared to baseline (T= 0 weeks) oral glucose tolerance was consistently improved in the BB-DSF from 3 to 12 weeks post-intervention while the DSF group showed decreases at weeks 3, 5, 7, 9 and 12 (Supplementary Fig. 1B). These data indicate that in addition to blueberry polyphenols, DSF components also contribute to improvements in glucose metabolism.
Figure 2.
BB-DSF diet improves oral glucose tolerance and decreases fasting blood glucose. A. (Top row) Blood glucose concentrations (mg/dL) expressed as mean ± SD were measured at the indicated time points (0 - 180 min) after administration of 2 g/kg glucose to mice after consumption of BB-DSF or DSF diets for 0, 7, 9 and 12 weeks. (Bottom row) Mean area under the curves for blood glucose data obtained for BB-DSF and DSF groups at each of the above indicated weeks. Each bar represents the mean ± SD (n=15) for group. T-test (2-tailed): * p< 0.05; ** p< 0.01; *** p< 0.001. B. Blood glucose measurements (mg/dL) taken in the unfasted state (T= 0 h), and then after 6 h or 9 h of fasting at 1, 7, or 10 weeks post-intervention. Each bar represents the mean ± SD (n=15) of each group. One-way ANOVA followed by unequal N HSD post-hoc test was performed. Significant difference between groups for each week is signified by letter a, b or c; different letters indicate significant difference (p <0.05) between groups while the same letter or absence of a letter indicates no difference.
Fasting blood glucose tests were performed on mice in all three diet groups at 1, 7, and 10 weeks post-intervention. Blood glucose levels were measured prior to beginning the fast (T= 0 h) and then again after 6 h and 9 h of daytime fasting. At week 1 the fasting glucose levels were not different between mice on the BB-DSF and DSF diets, but compared to the VHFD group, the BB-DSF and DSF groups had lower blood glucose levels in the unfasted state (T= 0) and also after 9 h of fasting (Fig. 2B). At 7 and 10 weeks post intervention all mice had the same blood glucose levels in the unfasted state (T=0). At 7 weeks after mice were fasted for 6 h or 9 h the DSF diet group had significantly lower glucose levels than mice fed the VHFD, while mice fed the BB-DSF diet had lower fasting glucose levels than both the VHFD and DSF groups. At 10 weeks post-intervention mice consuming the BB-DSF diet still had lower 6 h and 9 h fasting glucose levels compared to mice on VHFD or DSF diets, which showed similar blood glucose levels (Fig. 2B).
Differences in fasting glucose were apparent when the mice were fasted during the day for 6 h or 9 h (Fig 2B), but not when mice were fasted overnight for 13 h (Fig 1, T= 0h time point). This could be due to the difference in time of day that the fast was carried out. In addition the longer fasting time used in the OGTT may have resulted in the blood glucose levels in all groups decreasing to similar levels.
3.3. Serum biochemistry
Analysis of blood serum showed that total cholesterol levels in mice receiving the BB-DSF diet were 13.2% lower (T-test, p= 0.003) than mice on the DSF diet (Table 2). There were no significant differences observed between the BB-DSF and DSF diet groups in terms of serum triglycerides, insulin, adiponectin, RBP4 protein, total antioxidant capacity, IL-1β, IL-6 or TNFα (Table 2).
Table 2.
Serum Biochemistry
| BB-DSF | DSF | |
|---|---|---|
| Cholesterol (mg/dL) | 138 ± 8 * | 159 ± 10 |
| Triglycerides (mg/dL) | 65 ± 24 | 62 ± 21 |
| Adiponectin (pg/mL) | 2618 ± 446 | 2486 ± 550 |
| Insulin (pg/mL) | 13814 ± 3135 | 12749 ± 2895 |
| RBP4 (ng/mL) | 5.9 ± 1.0 | 7.0 ± 1.2 |
| FRAP (μmol/L) | 809 ± 53 | 870 ± 190 |
| IL-1β (pg/mL) | 6.9 ± 11.4 | 35 ± 47.8 |
| IL-6 (pg/mL) | 6.4 ± 3.9 | 7.9 ± 4.3 |
| TNFα (pg/mL) | 4.4 ± 3.4 | 11.2 ± 11.7 |
p< 0.003, two-tailed T-test
3.4. In vivo hypoglycemic effect of compounds eluted from BB-DSF and DSF
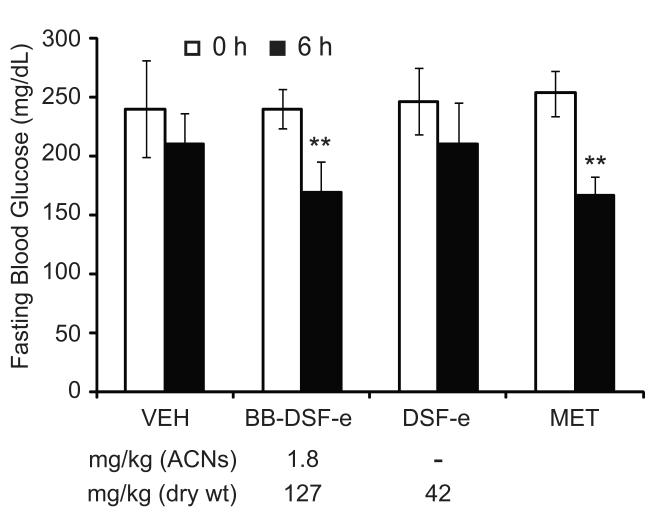
For in vitro experiments we eluted blueberry polyphenols from the BB-DSF matrix (BB-DSF-e) and control DSF matrix (DSF-e) as described in methods. Prior to in vitro experiments we performed an acute single dose experiment in C57BL/6 mice to determine whether BB-DSF-e was hypoglycemic in vivo. Mice were fasted for 4 h and blood glucose was measured prior to oral administration of BB-DSF-e delivering 1.8 mg/kg of anthocyanins, as we have previously shown that administration of a single dose of BB-DSF powder delivering 1.8 mg/kg of anthocyanins could significantly lower blood glucose in diabetic C57BL/6 mice [20]. Other groups of mice received the indicated doses of DSF-e, Metformin® or vehicle (75% Labrasol). Blood glucose levels were significantly different between groups 6 h after treatment (ANOVA, p= 0.037), but not before treatment (ANOVA, p= 0.9). Compared to before treatment, BB-DSF-e induced a significant hypoglycemic effect in the mice similar to Metformin, while DSF-e did not (Fig. 3) demonstrating that BB-DSF-e had hypoglycemic activity in vivo.
Figure 3.
BB-DSF-e is hypoglycemic in C57BL/6 mice. Blood glucose levels of mice before and 6 h after treatment with 75% Labrasol (VEH), BB-DSF-e, DSF-e or 300 mg/kg Metformin® (MET). BB-DSF-e dosed at 127 mg/kg (dry wt.) delivered 1.8 mg of anthocyanins (ACNs) per kg. This BB-DSF-e preparation was composed of 33% DSF compounds, therefore the DSF-e control was dosed at 42 mg/kg (dry wt.). Each bar represents mean ± SD (n = 5). ** p= 0.01 (Paired T-test comparing before and after treatment). As described in text, BB-DSF-e and DSF-e refer to the C-18 SPE column-purified acidic methanol eluates produced from BB-DSF or DSF; BBJ-c18 refers to C-18 SPE column-purified blueberry juice.
3.5. Compounds eluted from blueberry polyphenol-enriched DSF (BB-DSF-e) and DSF (DSF-e) repress glucose production in H4IIE hepatocytes, but do not induce glucose uptake in L6 myotubes
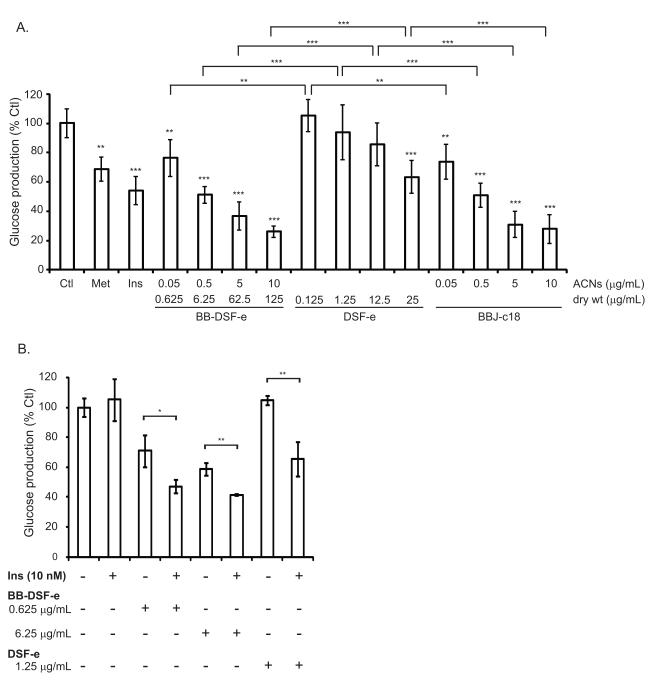
As described in methods compounds were eluted from BB-DSF powder (BB-DSF-e) or DSF powder (DSF-e) and C18 SPE column purified BBJ (BBJ-c18) was prepared. These were then tested for the ability to suppress glucose production in H4IIE hepatocytes, as described in methods. Briefly, H4IIE cells cultured in starvation medium overnight to induce glucose production then BB-DSF-e, DSF-e or BBJ-c18 treatments were applied to the cells for 6 h. To control for the proportion of soybean compounds present in the BB-DSF-e eluate, the DSF-e controls were delivered at 20% the BB-DSF-e dose, thus normalizing for the difference in dry weight of the DSF-e and BB-DSF-e eluates used for these experiments. BBJ-c18 treatments were dosed to deliver the same level of anthocyanins as BB-DSF-e. Insulin (50 nM) and metformin (2 mM) were used as positive controls and cells treated with medium alone served as the negative control (CTL) for inhibition of glucose release. After 6 h the glucose released into the cell culture medium was quantified with the Amplex® Red Glucose assay kit (Invitrogen). Doses of BB-DSF-e or BBJ-e delivering 5 or 20 ng/mL of anthocyanins did not show any significant reduction in glucose production (Supplementary Fig. 2). As shown in Fig. 4A, compared to the untreated control (100% glucose production), application of BB-DSF-e or BBJ-c18 delivering 0.05 - 10 μg/mL of anthocyanins or 25 μg/mL of DSF-e could significantly repress glucose release from H4IIE cells, as well or better than metformin or insulin (ANOVA p< 0.001, Dunnett test). Pair-wise comparisons of BB-DSF-e treatments (0.625, 6.25, 62.5 and 125 μg/mL) to their corresponding DSF-e controls (0.125, 1.25, 12.5 and 25 μg/ml) indicate that the presence of blueberry compounds in the BB-DSF-e eluate suppressed glucose release to a significantly greater extent. However, pair-wise comparison of BB-DSF-e treatments delivering the same amount of anthocyanins as BBJ-c18 treatments revealed similar levels of repression of glucose production. This similarity demonstrates that the DSF matrix effectively captures the assortment of anthocyanins and other polyphenols required to repress glucose production in this assay. Secondly, the combination of DSF and blueberry phytochemicals present in BB-DSF-e appears to have additive rather than synergistic effects on inhibition of glucose production in vitro.
Figure 4.
BB-DSF-e and DSF-e repress glucose production in H4IIE cells. (A) Release of glucose from starved control cells treated with medium alone (Ctl) was set as 100% glucose release. Triplicate wells of cells were treated with 2 mM metformin (Met), 50 nM insulin (Ins) or the indicated amounts of BB-DSF-e, DSF-e and BBJ-c18. The BB-DSF-e and BBJ-c18 samples were normalized to deliver equivalent amounts of anthocyanins (ACNs) as indicated. The dry weight amounts of DSF-e delivered was 20% of the amount of BB-DSF-e delivered. Bars represent mean ± SD (n=6) of data combined from two independent experiments performed in triplicate. Asterisks directly above each bar represent significant difference relative to Ctl as determined by ANOVA followed by Dunnett’s test (* p< 0.05; ** p< 0.01; *** p< 0.001). Asterisks above graph represent differences between indicated pairs of BB-DSF-e and DSF-e samples as well as differences between pairs of DSF-e and BBJ-c18 samples, as determined by T-test (2-tailed): * p< 0.05; ** p< 0.01; *** p< 0.001. (B) Triplicate wells of cells were treated with medium only or the indicated doses and combinations of 10 nM insulin (Ins), BB-DSF-e, or DSF-e. Bars represent mean ± SD (n=3) of data from triplicate samples. Asterisks above graph represent differences between treatment in the absence or presence of 10 nM insulin, as determined by T-test (2-tailed): * p< 0.05; ** p< 0.01. As detailed in text, BB-DSF-e and DSF-e refer to the C-18 SPE column-purified eluates produced from BB-DSF or DSF; BBJ-c18 refers to a C-18 SPE column-purified blueberry juice.
The concentration of DSF-e used as control treatment was 20% that used for a given concentration of BB-DSF-e to take into account the proportion of compounds that DSF contributed to the BB-DSF-e material. Calcein-AM cytotoxicity assays indicated that the concentrations of eluates tested did not decrease cell viability (> 90% cells were viable); however, it was conceivable that the higher repression of glucose production in BB-DSF-e treated cells compared to DSF-e treated cells could be a side-effect of applying greater amounts of the of BB-DSF-e material. To determine whether this was the case cells were treated for 6 h with equal concentrations (wt/vol) of DSF-e and BB-DSF-e, therefore cells were exposed to 5× the amount of DSF compounds present in the BB-DSF-e. Glucose production was measured as described. BB-DSF-e treatment still had a significantly greater repressive effect on glucose production in H4IIE cells than an equal concentration of DSF-e (Supplementary Fig. 3); therefore the decrease in glucose production appears to be specific to blueberry-derived compounds.
BB-DSF-e and DSF-e were tested for ability to induce glucose uptake in L6 myotubes. L6 cells were cultured and differentiated as described in methods. Triplicate wells of cells were incubated for 6 h with BB-DSF-e delivering anthocyanins at a concentration of 0.05, 0.5, 5 or 10 μg/mL. Insulin (50 nM) was used as a positive control for glucose uptake and cells treated with medium alone served as a negative control for inhibition of glucose release. Compared to the control, insulin was able to induce glucose uptake by 31%, but BB-DSF-e and DSF-e did not induce significant glucose uptake in L6 myotubes (Supplementary Fig. 4), suggesting that blueberry and DSF compounds do not use this mechanism to lower blood sugar levels, at least in vitro.
3.6. Compounds eluted from blueberry polyphenol-enriched DSF (BB-DSF-e) and DSF (DSF-e) sensitized H4IIE cells to insulin-induced repression of glucose production
A sub-optimal 10 nM dose of insulin was ineffective at repressing glucose production in H4IIE cells (Fig 4B). We investigated whether BB-DSF-e or DSF-e could sensitize H4IIE cells to this sub-optimal dose of insulin, as measured by increased repression of glucose production. H4IIE cells were starved overnight to induce glucose production and then incubated for 6 h with medium alone, 10 nM insulin, BB-DSF-e (0.625 μg/mL or 6.25 μg/mL), DSF-e (1.25 μg/mL) or 10 nM insulin in combination with indicated doses of BB-DSF-e or DSF-e. Glucose production was measured as described. Compared to untreated cells (100% glucose production), cells treated with insulin (ANOVA, Dunnett’s test, p= 0.94) or DSF-e (ANOVA, Dunnett’s test, p= 0.96) alone did not show any inhibition of glucose production; however, the combination of these suboptimal doses of insulin and DSF-e significantly repressed glucose production (ANOVA, Dunnet’s test p= 5 × 10−4) by 35%. Compared to untreated cells, cells treated with BB-DSF-e at a dose of 0.625 μg/mL delivering 0.05 μg/mL anthocyanins (ANOVA, Dunnet’s test p= 1 × 10−5) or a dose of 6.25 μg/mL delivering 0.5 μg/mL anthocyanins (ANOVA, Dunnet’s test p= 7.5 × 10−6) significantly decreased glucose production; however the decrease was enhanced when BB-DSF-e treatment was combined with 10 nM insulin (Fig. 4B).
4.0. Discussion
Current laboratory methods require the use of organic solvents and costly affinity columns to concentrate and partially purify nutritionally valuable polyphenols from the carbohydrate and lipid components of edible plants. While such standard methods are effective for obtaining polyphenol-enriched extracts, they are not compatible with food applications. By leveraging the natural affinity of polyphenols for proteins we have used protein-rich DSF to effectively and selectively sorb polyphenols from blueberry, cranberry and grape juices [20-21]. The process provides a natural means of substituting nutritionally-poor sugars and excess water in blueberry juice with nutritionally complete soy protein and enables delivery of anti-diabetic blueberry anthocyanins in the context of a low-sugar, shelf-stable food ingredient.
We have previously demonstrated that DSF does not sorb or concentrate sugars from fruit juices [20-21]; however, in this study nutritional analysis of freeze-dried BB-DSF powder showed that it contained 11.9% fructose, 10.4% glucose and 0.8% sucrose. In preparing the BB-DSF matrix we used a standard swinging bucket centrifuge and the sugar-laden juice remains on top of the solids until decanted. The moist BB-DSF pellet is therefore associated with residual blueberry juice, which is dried together with the matrix. We expect that sugar levels in the BB-DSF matrix will be further decreased by using a filtering centrifuge system, more commonly found in food manufacturing facilities, where the juice supernatant will be forced out through a filter. Nevertheless, the resulting BB-DSF matrix contains significantly less sugars than blueberry fruit when normalized for the level of anthocyanins delivered. For example, highbush blueberries (Vaccinium corymbosum) contain on average 1 mg of anthocyanins per gram of fresh weight [11] and a half-cup (73 g) serving of blueberries contains 73 mg of anthocyanins and 7 g of sugars http://www.fruitsandveggiesmatter.gov/month/berries.html. The preparation of BB-DSF used in this study contained 5 mg/g of anthocyanins therefore 15 g of the BB-DSF ingredient would deliver the amount of anthocyanins contained in one serving of blueberries along with 3.4 g of sugars, or 51% less sugars than the fresh blueberry fruit. This approach of capturing and stabilizing polyphenols from blueberries in a high-protein food matrix while minimizing sugars can be applied to other perishable and/or seasonal produce.
A previous study reported that C57BL/6 mice fed a high fat diet supplemented with 10% whole blueberry powder (delivering 2.8 mg of anthocyanins per gram of diet) had more body weight gain and adiposity than mice fed a high fat diet [19]. However, decreased body weight and improved lipid profiles were observed when mice were fed a high fat diet supplemented with an anthocyanin-enriched blueberry extract, indicating that sugars and possibly other components were masking the benefits of the anthocyanins and other polyphenols [18-19,26]. While BB-DSF contained blueberry sugars, the amounts present did not hinder the beneficial effects of the blueberry anthocyanins and other polyphenols on weight gain, serum cholesterol and blood glucose endpoints. In the BB-DSF diet group improvements in fasting blood glucose levels were observed in the mice after one week and up to ten weeks on the diet, as compared to DSF or VHFD control groups. The DSF control diet group also showed decreased fasting glucose levels compared to the VHFD group at week-1 and week-7, although this difference disappeared at week-10 (Fig. 1B). While BB-DSF groups showed significant improvements in OGTT over the DSF group, within group comparisons showed that both groups had improved glucose tolerance compared to week 0 baseline measurements (Fig. 2A and Supplementary Fig 1B). In contrast DSF-e did not show any significant effect in the acute hypoglycemic model (Fig. 3) perhaps due to the fact that only a single dose is administered. These positive effects of the DSF diet on glucose metabolism are consistent with our in vitro data showing that DSF-e was effective in repressing glucose production in H4IIE cells. The effects of soybean products on glycemia have been reported in several studies with inconsistent results; however, positive outcomes seem to be associated with the consumption of whole soy foods [27-29]. The mechanism(s) behind the observed reduction in weight gain in the BB-DSF group remains to be determined; however, decreased digestive enzyme activity via polyphenol-protein interactions [30] and/or polyphenol-mediated modulation of metabolic pathways involved in adiposity and/or energy expenditure [31] are possibilities.
Our supportive in vitro data suggest that anthocyanins and other polyphenols eluted from BB-DSF may improve glucose metabolism by repressing glucose production in hepatocytes (Figs. 4 and S2-4). While most glycosylated polyphenols are poorly bioavailable in vivo, intact anthocyanins can be absorbed and detected in circulation and urine; albeit at very low concentrations [32-33]. Tissue accumulation of intact anthocyanins is higher than absorption into circulation indicating that tissues such as liver, adipose and intestine are potential sites of action for these compounds [34-36]. Unabsorbed polyphenols are metabolized by colonic microflora to simple phenolic acids [32,37]. The sorption of polyphenols to DSF may have a protective effect in the digestive tract allowing greater amounts of polyphenols to be absorbed in the small intestine and/or permitting delivery of higher levels to the colon for further metabolism prior to absorption. Further studies on the bioavailability of polyphenols sorbed to the DSF matrix are needed to explore these hypotheses. In conclusion our present findings demonstrate that BB-DSF has beneficial effects on blood glucose, body weight and serum cholesterol and may be useful for the prevention and/or management of metabolic syndrome and diabetes.
Supplementary Material
Acknowledgements
We thank Andrew Oren (Rutgers University) and Kristen Moskal (Rutgers University) for technical assistance, Jennifer Rood (Pennington Biomedical Research Center) for performing blood chemistry analysis and Michael Pellizzon (Research Diets) for assistance in formulating experimental diets. This work was supported in part by the NIH training grants T32 AT004094 (supporting DER) and P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements.
Abbreviations
- DSF
defatted soybean flour
- BB-DSF
blueberry polyphenol-enriched defatted soybean flour
- VHFD
very high fat diet
- OGTT
oral glucose tolerance test
Footnotes
Author disclosures: DER, MAL and IR have equity in Nutrasorb LLC, which has interest in developing polyphenol sorption technology
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United states, 2003–2006. In: Statistics NCfH, editor. National Health Statistics Report; Hyattsville, MD: 2009. [PubMed] [Google Scholar]
- 3.WHO World health statistics. 2012 [Google Scholar]
- 4.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 5.Cherniack EP. Polyphenols: Planting the seeds of treatment for the metabolic syndrome. Nutrition. 2011;27:617–623. doi: 10.1016/j.nut.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Visioli F, De La Lastra CA, Andres-Lacueva C, Aviram M, Calhau C, Cassano A, et al. Polyphenols and human health: A prospectus. Crit Rev Food Sci Nutr. 2011;51:524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 7.Jellin JM, Gregory P, Batz F, Hitchens K. Pharmacist’s letter/prescriber’s letter natural medicines comprehensive database. Therapeutic Research Faculty; Stockton, CA: 2005. [Google Scholar]
- 8.Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, et al. Anti-diabetic properties of the canadian lowbush blueberry vaccinium angustifolium ait. Phytomedicine. 2006;13:612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu XL, Prior RL. Systematic identification and characterization of anthocyanins by hplc-esims/ms in common foods in the united states: Fruits and berries. J Agr Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Wang CY, Wang SY, Zheng W. Effect of high-oxygen atmospheres on blueberry phenolics, anthocyanins, and antioxidant capacity. J Agric Food Chem. 2003;51:7162–7169. doi: 10.1021/jf030440k. [DOI] [PubMed] [Google Scholar]
- 11.Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (orac) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J Agr Food Chem. 2001;49:2222–2227. doi: 10.1021/jf0013656. [DOI] [PubMed] [Google Scholar]
- 12.Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140:1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaughnessy KS, Boswall IA, Scanlan AP, Gottschall-Pass KT, Sweeney MI. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr Res. 2009;29:130–138. doi: 10.1016/j.nutres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Kristo AS, Kalea AZ, Schuschke DA, Klimis-Zacas DJ. A wild blueberry-enriched diet (vaccinium angustifolium) improves vascular tone in the adult spontaneously hypertensive rat. J Agric Food Chem. 2010;58:11600–11605. doi: 10.1021/jf101839u. [DOI] [PubMed] [Google Scholar]
- 16.Wiseman W, Egan JM, Slemmer JE, Shaughnessy KS, Ballem K, Gottschall-Pass KT, et al. Feeding blueberry diets inhibits angiotensin ii-converting enzyme (ace) activity in spontaneously hypertensive stroke-prone rats. Can J Physiol Pharmacol. 2011;89:67–71. doi: 10.1139/y10-101. [DOI] [PubMed] [Google Scholar]
- 17.Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, vaccinium angustifolium aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prior RL, S EW, T RR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 19.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the c57bl/6j mouse model of obesity. J Agric Food Chem. 2008;56:647–653. doi: 10.1021/jf071993o. [DOI] [PubMed] [Google Scholar]
- 20.Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Pouleva A, et al. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roopchand DE, Kuhn P, Poulev A, Oren A, Lila MA, Fridlender B, et al. Biochemical analysis and in vivo hypoglycemic activity of a grape polyphenol-soybean flour complex. J Agric Food Chem. 2012;60:8860–8865. doi: 10.1021/jf300232h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- 23.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 24.Loike JD, Zalutsky DL, Kaback E, Miranda AF, Silverstein SC. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci U S A. 1988;85:807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, et al. In vitro and in vivo anti-diabetic effects of anthocyanins from maqui berry (aristotelia chilensis) 2012;151:387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prior RL, Wu X, Gu L, Hager T, Hager A, Wilkes S, et al. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol Nutr Food Res. 2009;53:1406–1418. doi: 10.1002/mnfr.200900026. [DOI] [PubMed] [Google Scholar]
- 27.Chang JH, Kim MS, Kim TW, Lee SS. Effects of soybean supplementation on blood glucose, plasma lipid levels, and erythrocyte antioxidant enzyme activity in type 2 diabetes mellitus patients. Nutr Res Pract. 2008;2:152–157. doi: 10.4162/nrp.2008.2.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: A 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr. 2010;91:1394–1401. doi: 10.3945/ajcn.2009.28813. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93:1092–1101. doi: 10.3945/ajcn.110.007187. [DOI] [PubMed] [Google Scholar]
- 30.Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol-protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3:592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- 31.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: Towards a better understanding. Molecular Nutrition & Food Research. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 33.Nurmi T, Mursu J, Heinonen M, Nurmi A, Hiltunen R, Voutilainen S. Metabolism of berry anthocyanins to phenolic acids in humans. J Agric Food Chem. 2009;57:2274–2281. doi: 10.1021/jf8035116. [DOI] [PubMed] [Google Scholar]
- 34.Felgines C, Krisa S, Mauray A, Besson C, Lamaison JL, Scalbert A, et al. Radiolabelled cyanidin 3-o-glucoside is poorly absorbed in the mouse. Br J Nutr. 2010;103:1738–1745. doi: 10.1017/S0007114510000061. [DOI] [PubMed] [Google Scholar]
- 35.Felgines C, Texier O, Garcin P, Besson C, Lamaison JL, Scalbert A. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet. Mol Nutr Food Res. 2009;53:1098–1103. doi: 10.1002/mnfr.200800323. [DOI] [PubMed] [Google Scholar]
- 36.Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA, Graf BA, et al. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 37.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Brit J Nutr. 2010;104:S67–S90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.