Abstract
Prostate cancer is the second most prevalent solid tumor diagnosed in men in the United States and Western Europe. Conventionally fractionated external beam radiation therapy (1.8-2.0 Gy/fraction) is an established treatment modality for men in all disease risk groups. Emerging evidence from experimental and clinical studies suggests that the α/β ratio for prostate cancer may be as low as 1.5 Gy, which has prompted investigators around the world to explore moderately hypofractionated radiation therapy (2.1-3.5 Gy/fraction). We review the impetus behind moderate hypofractionation and the current clinical evidence supporting moderate hypofractionated radiation therapy for prostate cancer. Although hypofractionated radiation therapy has many theoretical advantages, there is no clear evidence from prospective, randomized, controlled trials showing that hypofractionated schedules have improved outcomes or lower toxicity than conventionally fractionated regimens. Currently, hypofractionated schedules should only be used in the context of clinical trials. High dose rate brachytherapy and stereotactic body radiation therapy (fraction size 3.5 Gy and greater) are alternative approaches to hypofractionation, but are beyond the scope of this report.
Keywords: Prostate cancer, radiotherapy, hypofractionation, toxicity, quality of life
INTRODUCTION
Prostate cancer is the second most prevalent solid tumor diagnosed in men of the United States and Western Europe.1 Given the widespread utilization of prostate-specific antigen (PSA) testing, most contemporary prostate cancer patients present with localized disease (clinical stage T1-T2). The standard non-surgical approach to aggressive treatment for localized prostate cancer is radiation therapy, and external beam radiation therapy (EBRT) is delivered frequently for this indication. Currently, most men who receive EBRT are treated with conventionally fractionated radiation therapy (CFRT, a single 1.8-2.0 Gy fraction lasting one hour per day, five days per week, for about eight weeks) to a total dose of 76-80 Gy.
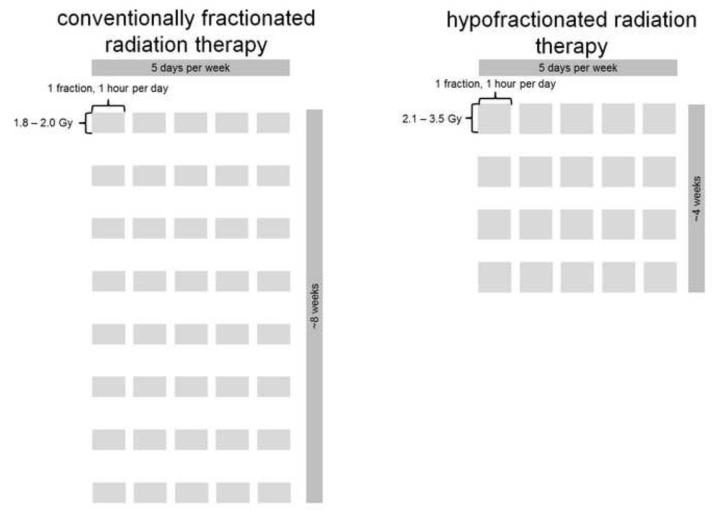
CFRT has inherent limitations as a treatment modality. Dose escalation, which has been shown in multiple phase III randomized controlled trials (RCT) to improve biochemical control,2-6 results in as many as 45 treatment sessions delivered over nine weeks.7 There has been recent interest in increasing fraction size to improve the risk/benefit ratio and reduce overall treatment time. Hypofractionated radiotherapy (HFRT, a single 2.1-3.5 Gy fraction lasting one hour per day, five days per week, for about four weeks) could be more advantageous from a radiobiological perspective, to the patient himself, and for resource allocation. The CFRT and HFRT schedules are juxtaposed in Figure 1. From a radiobiological perspective, a protracted course of RT is disadvantageous because dose escalation is necessary to offset the accelerated tumor cell repopulation,8 and there is potential for therapeutic gain with larger fraction sizes.9, 10
Figure 1.
Juxtaposed schedules of conventionally fractionated radiation therapy and hypofractionated radiation therapy.
Currently, most men who receive external beam radiation therapy for prostate cancer are treated with conventionally fractionated radiation therapy, which is typically defined as a single 1.8-2.0 Gy fraction lasting one hour per day, five days per week, for about eight weeks to a total dose of 76-80 Gy. A hypofractionated schedule differs in that it is a single 2.1-3.5 Gy fraction lasting one hour per day, five days per week, for about four weeks.
HFRT would be more appreciated by each prostate cancer patient. The long duration of therapy is inconvenient to those who live far from RT centers or are unable to travel, and it is the most frequently patient-cited disadvantage of CFRT and a major cause of patient non-adherence.11 Considering the travel time, long distance patients may have to travel to the cancer center, and the expense of parking, the shorter course of HFRT may save each man an average of $1,900 in out-of-pocket expenses.12
Finally, resource allocation could also be improved with HFRT. Calculations models have shown that wage costs outweigh the cost of machines due to the labor-intensive nature of RT planning and delivery.13-16 Moreover, although treatment planning complexity is increasing with evolving technology, the planning is only done at the beginning of therapy while cost builds with the delivery of each fraction.17 Kesteloot et al.18 estimated that staffing RT facilities accounts for 50% of their cost. Thus, changing to a hypofractionated schedule may decrease the number of work-hours and overall cost of treating each patient.
While these theoretical advantages of HFRT are important, it is crucial to first develop clinical evidence of effectiveness before a shift occurs within the field of prostate cancer to move away from CFRT. Moreover, it is necessary to define the need for image guided radiation therapy (IGRT) and prostate immobilization for HFRT. These technologies are required for stereotactic body radiation therapy (SBRT, 6.5 Gy/fraction and greater), because there is increased potential of high fraction doses delivered to normal tissues.19, 20
Herein, we review the current clinical evidence supporting HFRT. We compare the outcomes of phase III studies with HFRT and CFRT arms to phase III studies of dose-escalation with CFRT, which are the current standard of care in determination of dose schedules. High dose rate brachytherapy and SBRT are alternative approaches to hypofractionation, but are beyond the scope of this report.
SEARCH STRATEGY
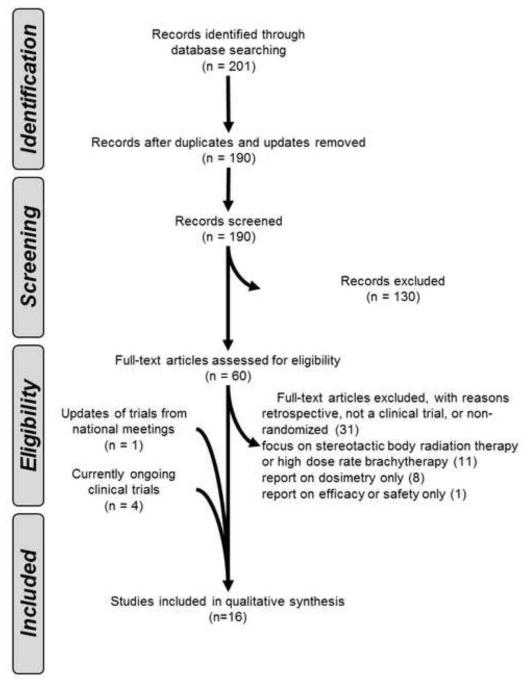
As seen in Table 1, we defined inclusion criteria for the literature search using the PICOS (Population, Intervention, Control, Outcome, Study Design) approach. We conducted a systematic search using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) literature selection process (Figure 2). We searched the published English medical literature from 1970 until 2012 in MEDLINE and PubMed using the terms “prostate cancer,” “hypofractionated,” and “radiation therapy.” The search yielded 190 articles after discarding duplicates. After identifying full-text articles, we excluded studies of SBRT, high dose rate brachytherapy, and studies that were not prospective, multi-arm, randomized clinical trials reporting outcomes and toxicities. We performed a sub-analysis of the older21-23 and modern trials24-29 based on their radiobiologically-established hypotheses. We updated results of trials from presented reports at national meetings.28 Finally, we integrated the hypotheses and methods of the ongoing phase III studies29-31 that have yet to publish their results.
Table 1.
Inclusion criteria
| Population | Men with localized (T1-T2, N0-Nx, M0) and locally advanced (T3-T4, N0- Nx, M0) prostate cancer |
|
| |
| Intervention | HFRT, defined as radiation that is delivered as a single 2.1-3.5 Gy fraction lasting about one hour per day, five days per week, for about four weeks |
|
| |
| Control | CFRT, defined as a single 1.8-2.0 Gy fraction lasting one hour per day, five days per week, for about eight weeks |
|
| |
| Outcomes | |
| Efficacy | Clinical (surrogate outcomes) for all studies: PSA kinetics; FFBF as defined by ASTRO or Phoenix definitions |
| Patient and study-specific: DM; LF; LRF; OS; post-treatment biopsy | |
| Safety | Acute and chronic RTOG GU, GI toxicities |
|
| |
| Study design | |
| Efficacy | All prospective studies, >50 patients, with two or more arms, >24 month FU |
| Safety | All prospective studies, >50 patients, with two or more arms, >24 month FU |
Abbreviations: ASTRO: American Society of Therapeutic Radiology and Oncology; CFRT: conventionally fractionated radiotherapy; DM: distant metastasis; L/R F: local / regional failure; FFBF: freedom from biochemical failure; FU: follow-up; GI: gastrointestinal; GU: genitourinary; HFRT: hypofractionated radiotherapy; OS: overall survival
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram literature selection process.
In all studies in our review, 910 patients were treated with HFRT, matched to 918 patients treated with CFRT. Of these 1828 patients, 1153 were included in the initial trials, and 675 were included in the modern trials comparing the two regimens. To further analyze the patients treated on HFRT arms, we benchmarked their outcomes and toxicities to those of patients treated on the dose-escalated arms (total n, 742) of the dose escalation studies which provide reasoning behind the current CFRT dose schedules.
RADIOBIOLOGICAL THEORY PROVIDES A CUT POINT IN RATIONALE BETWEEN INITIAL AND MODERN TRIALS OF HFRT VS. CFRT
In general, as RT dose increases, the number of surviving cancer cells decreases. However, these benefits are countered by the toxicity to surrounding normal tissues. An α/β ratio is used to estimate the effects of radiation on various tissues and compare various dose and fractionation schemes. The α/β ratio is estimated to be > 10 Gy for early-responding tissues (e.g. skin, mucosa, and most tumors); and 3 - 5 Gy for late responding tissues (e.g. connective tissue, bladder/rectal mucosa, muscles). The α/β ratio is used in the calculation of the biologically equivalent dose (BED):
where n is the number of radiation fractions and d is the fraction size.
In 2001, hypothesis-generating reports suggested that prostate cancer had a low α/β ratio of ~1.5 Gy,32 implying that those cells were more sensitive to doses delivered in larger fraction size. Calculations with an α/β ratio of 1.5 Gy showed that a protracted course of RT is disadvantageous because: (1) given the lower α/β ratio for prostate cancer than late-responding tissues, there is potential for therapeutic gain with larger fraction sizes;9, 10 and (2) dose escalation is necessary to offset the accelerated tumor cell repopulation.8
Thus, investigators of HFRT trials after 2001 (i.e. “modern” trials)24-29 tried to maintain a high BED (at an α/β ratio of 1.5) to kill prostate cancer cells while minimizing the BED (at α/β ratios of 3 - 10) for toxicities. In contrast, the “initial” (i.e. pre-2001) trials21-23 had no assumptions about the α/β ratio and used older dose regimens.
INITIAL TRIALS COMPARING CFRT AND HFRT
In the mid-1990s, the most commonly used method to deliver EBRT was a 4-field technique. At the time, there was no consistent agreement on the optimal schedule or dose used for patient treatment.33 CFRT schedules were typically administered over 6 to 7 weeks with doses of 60 to 70 Gy.7
Nonrandomized studies from the UK,34, 35 Australia,36 and Canada37 reported similar outcomes between HFRT and CFRT schedules. Lukka et al.21 published one of the first phase III prospective RCTs comparing CFRT and HFRT. Freedom from biochemical failure (FFBF) and acute toxicity were worse in the hypofractionated arm, and late toxicities were equal. Yeoh et al.22, 23 compared CFRT and HFRT. Forty-nine of 109 patients developed BF in the CFRT arm in comparison to 36 of 108 in the HFRT arm. Overall survival (OS) was not significantly different between the arms. Multivariate analyses revealed that the CFRT schedule was the only statistically significant variable for increased BF and worse GU symptoms at 4 years. These initial studies (shown in Table 2) provided the first prospective outcomes of a hypofractionated schedule.
Table 2.
Multi-arm phase III studies comparing CFRT and HFRT.
| Era | Reference | n | risk groups | BED (Gy), at α/β = |
FFBF | Other outcomes | RTOG late toxicity Grade ≥2 (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| median FU (months) |
total dose (Gy) | total fractions | Gy/ fraction | 1.5 | 10 | GU | GI | ||||||
| Initial | Lukka21 | 470 | low, intermediate, high |
64 | 66 | 33 | 2 | 154 | 79 | 5-year ASTRO: 60% | 5-year OS: 85% (NS) 2-year positive biopsy rate: 53% (NS) |
1.3 | 1.9 |
| 466 | 52.5 | 20 | 2.63 | 144 | 66 | 5-year ASTRO: 53% | 5-year OS: 87% (NS) 2-year positive biopsy rate: 51% (NS) |
1.3 | 1.9 | ||||
|
| |||||||||||||
| Yeoh22, 23 | 109 | NR | 90 | 64 | 32 | 2 | 149 | 77 | 7.5-year Phoenix: 34% (p < 0.05) 7.5-year ASTRO: 44% (NS) |
7.5-year OS: 71% (NS) | NR | NR | |
| 108 | 55 | 20 | 2.75 | 156 | 70 | 7.5-year Phoenix: 53% (p < 0.05) 7.5-year ASTRO: 44% (NS) |
7.5-year OS: 69% (NS) | NR | NR | ||||
|
| |||||||||||||
| Modern | Arcangeli24-26 | 85 | high | 35 | 80 | 40 | 2 | 187 | 96 | 5-year Phoenix: 79% (p = 0.035) | 5-year DM: 14% (NS) 5-year LF: 11% (NS) |
16 | 17 |
| 83 | 62 | 20 | 3.1 | 190 | 81 | 5-year Phoenix: 85% (p = 0.035) | 5-year DM: 10% (NS) 5-year LF: 7% (NS) |
11 | 14 | ||||
|
| |||||||||||||
| Pollack27, 28 | 152 | intermediate, | >60 | 76 | 38 | 2 | 177 | 91 | 5-year Phoenix: 86% (NS) | 5-year LRF/DM: 1.0% (NS) | 8.3 | 5 | |
| 151 | high | 70.2 | 26 | 2.7 | 197 | 89 | 5-year Phoenix: 86% (NS) | 5-year LRF/DM: 1.3% (NS) | 18.3 | 6.8 | |||
|
| |||||||||||||
| Kuban29 | 102 | mostly low, intermediate |
56 | 75.6 | 42 | 1.80 | 166 | 89 | 5-year ASTRO: 92% (NS) 5-year Phoenix: 94% (NS) |
5-year clinical failure: 0% (NS) |
19 | 6 | |
| 102 | 72 | 30 | 2.40 | 187 | 89 | 5-year ASTRO: 96% (NS) 5-year Phoenix: 97% (NS) |
5-year clinical failure: 0% (NS) |
19 | 14 | ||||
Abbreviations: ASTRO: American Society of Therapeutic Radiology and Oncology; BED: biologically equivalent dose; CFRT: conventionally fractionated radiotherapy; DM: distant metastasis; L/R F: local / regional failure; FFBF: freedom from biochemical failure; FU: follow-up; GI: gastrointestinal; GU: genitourinary; HFRT: hypofractionated radiotherapy; NS: not significant; NR: not reported; OS: overall survival
Interestingly, Lukka et al.21 and Yeoh et al.22, 23 reached opposite conclusions about HFRT compared to CFRT. Their contrary outcomes may be due to a number of factors. First, the trials were designed to compare widely used regimens in their respective countries with no specific assumptions about the α/β ratio before the trial. Second, the total dose of the CFRT arms were 66 Gy21 and 64 Gy,22, 23 (at an α/β ratio of 1.5, the BEDs are 15421 and 14422, 23) which is lower than more contemporary conventional doses of 78-80 Gy.2-4 Third, the arms were not designed to be isoeffective. Fourth, the percent of positive biopsies at 2 years was equal between the arms of the Lukka study,21 which is in contradiction to the FFBF, perhaps suggesting that local control was equivalent, but patients in the HFRT arm had developed micrometastatic disease. Finally, while the Lukka study21 used the ASTRO definition38 (i.e. 3 consecutive PSA rises) and other definitions of BF, the Yeoh study22, 23 used the ASTRO38 and Phoenix (i.e. nadir + 2 ng/mL)39, 40 definitions. Yeoh et al.22, 23 found a significant difference between their FFBF rates with the use of the Phoenix definition, but not the ASTRO definition. The relationship between ASTRO-defined BF and cancer-specific survival or OS has not been clearly demonstrated.41, 42 The Phoenix definition has since been shown to be a better predictor of distant metastasis, cancer-specific survival, and OS.39, 40 While these older studies provided valuable insight in comparing HFRT and CFRT, their outcomes did not provide conclusive evidence with regard to biochemical control or toxicity likely because their methods differed from those of more modern trials.
MODERN TRIALS COMPARING CFRT AND HFRT
Although there has been controversy in calculating the accurate α/β ratio for prostate cancer,43 prospective phase III superiority studies24-29 (also on Table 2) have been published based on the assumption that the α/β ratio for prostate cancer is 1.5 Gy. The hypotheses of the trials were designed to show one of two endpoints: (1) if the BED of the HFRT course were isoeffective to a CFRT course, then FFBF rates should be equivalent, and there should be reduced toxicity in the HFRT arm; or (2) if the HFRT course had a higher BED than the CFRT arm, then HFRT FFBF rates should be improved while having equal toxicity. Surprisingly, trials failed to reach either endpoint.
Arcangeli et al.24-26 hypothesized that the delivery of an isoeffective dose to prostate tumors using HFRT would reduce the incidence of late complications, have a sparing effect on early responding tissues, while producing the same level of tumor control in comparison to CFRT. However, this hypothesis proved incorrect as there was no reported difference in late toxicity at five years between the two schedules, and the 5-year FFBF rates were 79% and 85%, showing no benefit for either schedule. The lack of difference in observed toxicity rates was attributed to the use of a different toxicity grading methods, planning target volumes (prostate only vs. prostate and seminal vesicles), treatment setup, and the possibility of different mechanisms of radiation damage or repair for late rectal and bladder effects with HFRT in presence of concomitant androgen deprivation therapy. The BED difference between the two arms was only 3 based on their assumptions, yet a statistically significant difference in FFBF was noted. To put this in perspective, the dose escalation RCTs,2-6 which showed significant improvements of BF, had a difference of BED of approximately 20. The authors did not provide a clear reason for this observation, but noted that patients at very high risk (in particular, those with Gleason >7 and high PSA levels) were those who had improved BF rates following HFRT.
Pollack et al.27, 28 designed their RCT to have equivalent acute toxicity between the HFRT (BED at α/β of 3 = 133) and CFRT (BED at α/β of 3 = 127) with improved tumor control in the HFRT arm. In their 2011 update,28 they reported similar 5-year rates FFBF and concluded that there remain no statistically significant differences between the treatment arms in terms of BF or any failure. While, the grade ≥2 GI toxicities were similar, GU toxicities were statistically higher in the HFRT arm (18.3% vs. 8.3%, p = 0.028). Moreover, men on the HFRT schedule had a significantly higher rate of GI toxicity during weeks 2 through 4. The authors attributed this to the use of a modified RTOG toxicity scale, the inclusion of lymph nodes in the high-risk patients, the shorter course of the HFRT schedule, and the mean biological doses to the prostate (including the urethra) being >80 Gy. Surprisingly, the initial assumption of isoeffectiveness of the trial design appears to be incorrect.
Kuban et al.29 reported on the preliminary outcome and toxicity of a phase III RCT which based the treatment regimens on maintaining equivalent acute toxicities while delivering a higher BED to the prostate. They randomized 102 men to receive CFRT (BED at α/β of 3 = 121) to a dose of 75.6 Gy in 42 fractions and 102 men to receive HFRT (BED at α/β of 3 = 130) to a dose of 72 Gy in 30 fractions. The 5-year Phoenix FFBF rates were 92% and 96% (NS), respectively, and no patient had a clinical failure. GI and GU toxicity rates were similar between the two groups.
Dose-escalation studies 2-6 have helped to determine the standard of care in determining the optimal CFRT schedule (Table 3). To allow for a more thorough understanding of the outcomes in the HFRT vs. CFRT RCTs, FFBF (Figure 3) and toxicity (Figures 4, 5) were benchmarked to the phase III dose-escalation RCTs.
Table 3.
Dose escalation CFRT studies used as a benchmark for HFRT studies.
| Reference | n | risk groups | BED (Gy), at α/β = |
FFBF | RTOG late toxicity Grade ≥2 (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| median FU (months) |
total dose (Gy or GyE) |
total fractions | Gy/ fraction | 1.5 | 10 | GU | GI | ||||
| MD Anderson / Kuban2 |
151 | mostly low, intermediate |
43.5 | 78 | 39 | 2 | 182 | 94 | 8-year Phoenix: 78% (p = 0.001) | 8 | 26 |
| 150 | 70 | 35 | 2 | 163 | 84 | 8-year Phoenix: 59% (p = 0.001) | 13 | 13 | |||
|
| |||||||||||
| Dutch / Al- Mamgani3 |
333 | low, intermediate, |
70 | 78 | 39 | 2 | 182 | 94 | 7-year ASTRO: 54% (p = 0.04) 7-year Phoenix: 56% (p = 0.03) |
40 | 35 |
| 331 | high | 68 | 34 | 2 | 159 | 82 | 7-year ASTRO: 47% (p = 0.04) 7-year Phoenix: 45% (p = 0.03) |
41 | 25 | ||
|
| |||||||||||
| MGH / Zietman4, 5 |
195 | low, intermediate, |
66 | 79.2 | 44 | 1.8 | 174 | 93 | 5-year ASTRO: 80% (p < 0.001) 10-year ASTRO: 83% (p < 0.001) |
21 | 19 |
| 197 | high | 70.2 | 39 | 1.8 | 154 | 83 | 5-year ASTRO: 61% (p < 0.001) 10-year ASTRO: 68% (p < 0.001) |
20 | 9 | ||
|
| |||||||||||
| UK MRC / Dearnaley 6 |
63 | low, intermediate, |
64 | 74 | 37 | 2 | 173 | 89 | 5-year ASTRO: 71% (p = 0.1) | 18 | 23 |
| 64 | high | 64 | 32 | 2 | 149 | 77 | 5-year ASTRO: 59% (p = 0.1) | 11 | 11 | ||
Abbreviations: ASTRO: American Society of Therapeutic Radiology and Oncology; BED: biologically equivalent dose; CFRT: conventionally fractionated radiotherapy; DM: distant metastasis; FFBF: freedom from biochemical failure; FU: follow-up; GI: gastrointestinal; GU: genitourinary; HFRT: hypofractionated radiation therapy; NS: not significant; NR: not reported; OS: overall survival
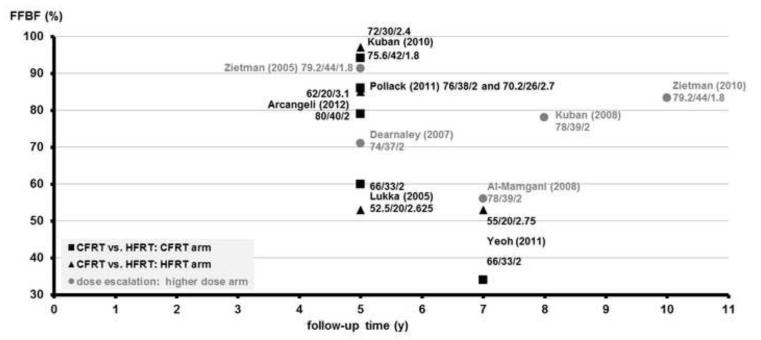
Figure 3.
FFBF vs. follow-up time of CFRT vs. HFRT RCTs benchmarked to high-dose RT arm of escalation RCTs for prostate cancer.
The y-axis shows the freedom from biochemical failure (FFBF), and the x-axis shows the follow-up time in years. Individual arms of phase III randomized controlled trials (RCTs) comparing conventionally fractionated radiotherapy (CFRT, black squares, ∎) and hypofractionated radiation therapy (HFRT, black triangles, ▴) are shown on the plot with first author and year of publication. As a benchmark for these studies, the higher-dose arms of dose escalation studies (gray circles, ●) are shown on the plot with first author and year of publication. The individual RT schedule of an arm is shown near each data point: total dose, number of fractions, and Gy/fraction.
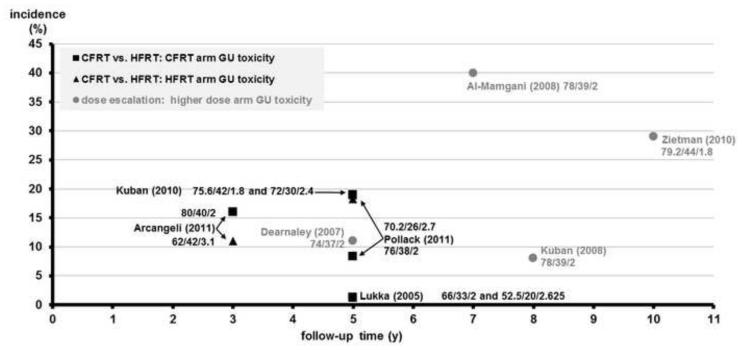
Figure 4.
RTOG Grade ≥2 GU toxicity vs. follow-up time of CFRT vs. HFRT RCTs benchmarked to high-dose RT arm of escalation RCTs for prostate cancer
The y-axis shows incidence of late RTOG Grade ≥2 toxicity (%), and the x-axis shows the follow-up time in years. Individual arms of phase III randomized controlled trials (RCTs) comparing conventionally fractionated radiotherapy (CFRT) and hypofractionated radiation therapy (HFRT) are shown on the plot with first author and year of publication. As a benchmark for these studies, the higher-dose arms of dose escalation RCTs are shown on the plot with first author and year of publication. Black squares (∎) refer to the GU toxicity rates of CFRT arms. Black triangles (▴) refer to the GU toxicity rates of HFRT arms. Gray circles (●) refer to the GU toxicity rates of the higher dose arm of dose escalation studies. The individual RT schedule of an arm is shown near each data point: total dose, number of fractions, and Gy/fraction.
Figure 5.
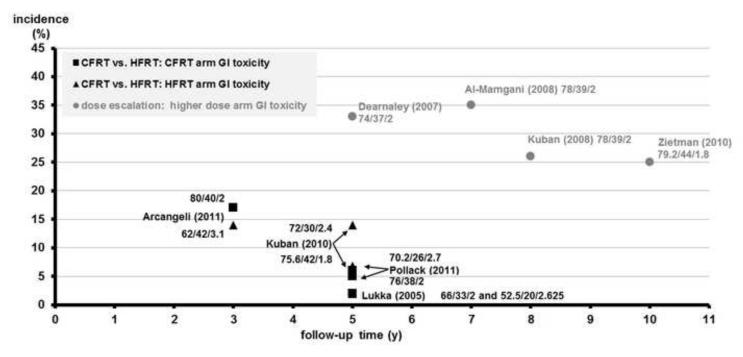
RTOG Grade ≥2 GI toxicity vs. follow-up time of CFRT vs. HFRT RCTs benchmarked to high-dose RT arm of escalation RCTs for prostate cancer
The y-axis shows incidence of late RTOG Grade ≥2 toxicity (%), and the x-axis shows the follow-up time in years. Individual arms of phase III randomized controlled trials (RCTs) comparing conventionally fractionated radiotherapy (CFRT) and hypofractionated radiation therapy (HFRT) are shown on the plot with first author and year of publication. As a benchmark for these studies, the higher-dose arms of dose escalation RCTs are shown on the plot with first author and year of publication. Black squares (∎) refer to the GI toxicity rates of CFRT arms. Black triangles (▴) refer to the GI toxicity rates of HFRT arms. Gray circles (●) refer to the GI toxicity rates of the higher dose arm of dose escalation studies. The individual RT schedule of an arm is shown near each data point: total dose, number of fractions, and Gy/fraction.
There are a number of important caveats to consider when interpreting the HFRT findings. First, all three modern studies24-29 were designed as superiority studies, and it would be incorrect to infer that the absence of a difference in any study means that the arms are equivalent. While all of the dose-escalation studies indicated that an increased dose was associated with less biochemical recurrence, the five published phase III studies of HFRT are inconsistent in their results. The methods of the older studies differed greatly from the modern studies, and the more modern studies have not shown a clear reduction in the incidence of late complications while maintaining biochemical control rates. Finally, when compared to dose-escalation studies, their follow-up of the HFRT studies is not yet as long, and the significance of the results may change.
There are several ongoing phase III non-inferiority trials underway which should provide clear evidence of HFRT in comparison to CFRT (Table 4). RTOG 0415 is a phase III RCT with fractionation schedules similar to the regimen of the phase I/II trial by Kupelian et al.30 If the α/β ratio for prostate cancer is closer to 10, the trial will demonstrate equivalence between the fractionation regimens; if it is closer to 1.5, the HFRT schedule should produce better rates of biochemical control. While the phase I and II portions of the CHHiP trial31 have estimated toxicity; the UK Medical Research Council (MRC) phase III non-inferiority study will include over 3,000 patients in a 3-arm design to extrapolate the isoeffective dose for complications and address whether HFRT is equivalent to CFRT. The NCIC trial is a noninferiority trial that compares 78 Gy in 2 Gy fractions to 60 Gy in 3 Gy fractions. Its goal is to demonstrate the safety and efficacy of HFRT and evaluate it as a replacement for CFRT. Finally, the MD Anderson trial will include over 200 patients, and analyze cancer- and patient-specific biomarkers. In summary, these phase III RCTs will provide outcomes of FFBF, toxicity, and other measures to provide a definitive answer to the question of whether HFRT is equivalent to CFRT. If this assumption holds true, then it would most likely translate into a shift from CFRT to HFRT in the management of prostate cancer.
Table 4.
Ongoing phase III studies comparing CFRT and HFRT.
| trial | hypothesis driving patient selection |
identifier | dates | status | BED (Gy), at α/β = |
outcome measures | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| estimated n | risk groups | total dose (Gy) | total fractions | Gy/ fraction | 1.5 | 10 | primary | secondary and unique |
|||||
| RTOG 0415 |
determine if α/β is closer to 10 or 1.5; determine if efficacy of either outweighs QOL30 |
NCT00331773 | 04/2006 - 05/2011 |
unknown | 1067 | low | 70 | 28 | 2.5 | 187 | 88 | DFS | FFBF; toxicity; genomic biomarkers; QOL; anxiety, depression assessment; cost- utility; tissue block serum, plasma, buffy coat cells |
| 73.8 | 41 | 1.8 | 162 | 87 | |||||||||
|
| |||||||||||||
| UK MRC / CHHiP |
3-arm design to extrapolate isoeffective dose for complications31 |
NCT00392535 | 11/2002 - 09/2012 |
accruing | 3216 | low, intermediate |
78 | 39 | 2 | 182 | 94 | GI/GU toxicity; FFBF |
DM; recommencement of ADT; CSS; OS; QOL; health economics; models of normal tissue and tumor control |
| 57 | 19 | 3 | 180 | 78 | |||||||||
| 60 | 20 | 3 | 182 | 94 | |||||||||
|
| |||||||||||||
| NCIC / Ontario / PROFIT |
demonstrate safety, efficacy of HFRT to evaluate its replacement of CFRT |
NCT00304759 | 05/2006 - 06/2020 |
ongoing, not recruiting |
1204 | intermediate | 78 | 39 | 2 | 182 | 94 | FFBF | clinical failure; CSS; toxicity; QOL |
| 60 | 20 | 3 | 180 | 78 | |||||||||
|
| |||||||||||||
| MD Anderson |
compare safety, efficacy of HFRT, CFRT (assuming α/β of 1.5); find cancer-, patient-specific markers that could promote either29 |
NCT00667888 | 05/2001 - 01/2013 |
ongoing, not recruiting |
225 | low, intermediate |
78 | 39 | 2 | 182 | 94 | FFBF | cancer-specific markers (p53, bcl-2, bax and Ki-67); patient-specific markers (testosterone, SHBG, estradiol) |
| 60 | 20 | 3 | 180 | 78 | |||||||||
Abbreviations: ADT: androgen deprivation therapy; ASTRO: American Society for Radiation Oncology; CHHiP: Conventional or Hypofractionated High Dose Intensity Modulated Radiotherapy for Prostate Cancer; CSS: cancer specific survival; CFRT: conventionally fractionated radiation therapy; DFS: disease free survival; DM: distant metastasis; FFBF: freedom from biochemical failure; HFRT: hypofractionated radiotherapy; GI: gastrointestinal; GU: genitourinary; IMRT: intensity modulated radiation therapy; MRC: Medical Research Council; NCIC: National Cancer Institute of Canada; OS: overall survival; RCT: randomized controlled trial; RTOG: radiation therapy oncology group; QOL: quality of life; SHBG: sex hormone binding globulin
LIMITATIONS AND FUTURE DIRECTION
Comparing the outcomes of studies is difficult for a number of reasons. First, there are differing treatment techniques, planning target margins, number of days RT is given per week, and dose, which may all affect outcomes.44 The specific endpoints evaluated in these studies vary. For example, FFBF rate may differ by 20% by using both ASTRO and Phoenix definitions.45 Late-effects from radiation can technically occur decades after therapy. Moreover, the RTOG toxicity score does not include the evaluation of anorectal symptoms such as urgency of defecation and fecal incontinence.46 Additionally, more quality of life metrics may have to be incorporated, including the Short-Form-36 or the International Index of Erectile Function-15.47
While Arcangeli et al.24-26 noted improved BF rates among high-risk patients, no HFRT study has looked at the effect of the optimal RT schedule among individual risk groups. Ongoing studies (listed in Table 4) are incorporating comparative dose-volume histograms, side-effect analysis for hypofractionated groups, and collection of germline DNA for radiogenomics. Cancer- and patient-specific biomarkers including Bcl-2, Bax, and the Bcl2/Bax ratio;48-51 p53;50 p21/waf1;50 P120;52 Ki-67;51, 52 and PCNA52 affect rates of cellular growth and have been shown to predict cancer aggressiveness and recurrence. These biomarkers may contribute to variations in α/β ratios among individual prostate cancer cell lines. Future models will likely combine these markers with traditional pretreatment variables (e.g. PSA, Gleason) to create personalized fractionation schedules and predict outcomes.53
Further, technology has evolved dramatically since the publication of the earliest HFRT studies. For example, IGRT and prostate immobilization have become a critical component of all SBRT because there is potential of large doses delivered to normal tissues;19, 20 however, the role of these technologies and their impact in HFRT is still under active investigation. For example, 72% of the patients in the Yeoh et al.22, 23 study were treated with 2D RT; comparatively, these patients could be now be treated with volumetric modulated arc therapy and 4D radiofrequency tracking to increase normal organ sparing54 and decrease intrafractional motion.55 Some retrospective studies show that such novel technologies may improve outcomes,56-58 but others show no effect in the context of HFRT.59 Nonetheless, since daily imaging and prostate immobilization are frequent requirements in ongoing HFRT trials, future reports should be mindful of their potential impact.
Finally, SBRT (delivered by both gantry- and robotic arm-mounted devices) and hadron therapy (specifically, protons), are gaining popularity as alternatives to CFRT and HFRT schedules. RTOG trials from previous decades included specific constraints regarding modalities that could or could not be tested. Currently, many of the current RTOG trials are stratifying by technology to determine the impact; assuming that prostate cancer has an α/β ratio of 1.5. However, SBRT and protons do not yet have equivalent efficacy when compared to HFRT, CFRT, or other standard treatment options.19, 59-61 Moreover, based on this review and a time-dependent meta-analysis of α/β ratios,62 it is unclear if the α/β ratio is truly 1.5. Thus, the results from future trials may be hypothesis generating, but not conclusive in defining the role of a particular therapy for individual patients.
CONCLUSIONS
EBRT is an established treatment modality for almost all prostate cancer patients. Determining the optimal fractionation scheme has been one of the goals of radiation oncologists. HFRT is hypothesized to improve tumor control, patient quality of life, and cost. RCTs comparing HFRT and CFRT have been inconsistent in their results: the methods of earlier studies are not comparable to modern techniques, and the modern studies have rejected their hypotheses of superiority of HFRT. While it is difficult to infer the findings of one study to any other, as they use different doses, treatment techniques, target margins, and outcome measures, non-inferiority studies with more robust databases of patient outcomes will help to determine which patients would benefit from HFRT. Currently, HFRT regimens should currently only be used in the context of a clinical trial.
Acknowledgements
None
Funding sources: This work supported in part by the Kimmel Cancer Center’s NCI Cancer Center Support Grant P30 CA56036, as well as by Young Investigator Awards from the Prostate Cancer Foundation (R.B.D. and T.N.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Approval/disclosures: All authors have read and approved the manuscript. We have no financial disclosures. We are not using any copyrighted information, patient photographs, identifiers, or other protected health information in this paper. No text, text boxes, figures, or tables in this article have been previously published or owned by another party.
Conflicts of Interest Notification: We have no conflicts of interests.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):980–8. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 4.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 5.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therpay in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 7.Zagars GK, von Eschenbach AC, Johnson DE, Oswald MJ. The role of radiation therapy in stages A2 and B adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1988;14(4):701–9. doi: 10.1016/0360-3016(88)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang JZ, Li XA. Impact of tumor repopulation on radiotherapy planning. Int J Radiat Oncol Biol Phys. 2005;61(1):220–7. doi: 10.1016/j.ijrobp.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56(4):1093–104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 11.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what’s important. J Gen Intern Med. 2000;15(10):694–701. doi: 10.1046/j.1525-1497.2000.90842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethukavalan P, Cheung P, Tang CI, et al. Patient costs associated with external beam radiotherapy treatment for localized prostate cancer: the benefits of hypofractionated over conventionally fractionated radiotherapy. Can J Urol. 2012;19(2):6165–9. [PubMed] [Google Scholar]
- 13.Parthan A, Pruttivarasin N, Taylor D, et al. CyberKnife for prostate cancer: Is it cost-effective? ASCO: Genitourinary Cancers Symposium. J Clin Onc. 2011;(suppl 7) abstr 87. [Google Scholar]
- 14.Perez CA, Kobeissi B, Smith BD, et al. Cost accounting in radiation oncology: a computer-based model for reimbursement. Int J Radiat Oncol Biol Phys. 1993;25(5):895–906. doi: 10.1016/0360-3016(93)90321-l. [DOI] [PubMed] [Google Scholar]
- 15.Lievens Y, van den Bogaert W, Kesteloot K. Activity-based costing: a practical model for cost calculation in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57(2):522–35. doi: 10.1016/s0360-3016(03)00579-0. [DOI] [PubMed] [Google Scholar]
- 16.Norlund A. Costs of radiotherapy. Acta Oncol. 2003;42(5-6):411–5. doi: 10.1080/02841860310011140. [DOI] [PubMed] [Google Scholar]
- 17.Van de Werf E, Lievens Y, Verstraete J, Pauwels K, Van den Bogaert W. Time and motion study of radiotherapy delivery: Economic burden of increased quality assurance and IMRT. Radiother Oncol. 2009;93(1):137–40. doi: 10.1016/j.radonc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Kesteloot K, Lievens Y, van der Schueren E. Improved management of radiotherapy departments through accurate cost data. Radiother Oncol. 2000;55(3):251–62. doi: 10.1016/s0167-8140(99)00034-1. [DOI] [PubMed] [Google Scholar]
- 19.Zaorsky NG, Studenski MT, Dicker AP, Gomella L, Den RB. Stereotactic body radiation therapy for prostate cancer: Is the technology ready to be the standard of care? Cancer Treat Rev. 2012 doi: 10.1016/j.ctrv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol (R Coll Radiol) 2010;22(8):698–706. doi: 10.1016/j.clon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23(25):6132–8. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh EE, Holloway RH, Fraser RJ, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: Updated results of a phase III randomized trial. Int J Radiat Oncol Biol Phys. 2006;66(4):1072–83. doi: 10.1016/j.ijrobp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81(5):1271–8. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 24.Arcangeli G, Saracino B, Gomellini S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(1):11–8. doi: 10.1016/j.ijrobp.2009.07.1691. [DOI] [PubMed] [Google Scholar]
- 25.Arcangeli G, Fowler J, Gomellini S, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79(4):1013–21. doi: 10.1016/j.ijrobp.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1172–8. doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64(2):518–26. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack A, Walker G, Buyyounouski M, et al. Five Year Results of a Randomized External Beam Radiotherapy Hypofractionation Trial for Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011;81(2):S1. [Google Scholar]
- 29.Kuban DA, Nogueras-Gonzalez GM, Hamblin L, et al. Preliminary Report of a Randomized Dose Escalation Trial for Prostate Cancer using Hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78(3):S58–S9. [Google Scholar]
- 30.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68(5):1424–30. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 31.Dearnaley D, Syndikus I, Sumo G, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13(1):43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 32.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50(4):1021–31. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 33.Hanks GE. External-beam radiation therapy for clinically localized prostate cancer: patterns of care studies in the United States. NCI Monogr. 1988;(7):75–84. [PubMed] [Google Scholar]
- 34.Duncan W, Warde P, Catton CN, et al. Carcinoma of the prostate: results of radical radiotherapy (1970-1985) Int J Radiat Oncol Biol Phys. 1993;26(2):203–10. doi: 10.1016/0360-3016(93)90198-5. [DOI] [PubMed] [Google Scholar]
- 35.Read G, Pointon RC. Retrospective study of radiotherapy in early carcinoma of the prostate. Br J Urol. 1989;63(2):191–5. doi: 10.1111/j.1464-410x.1989.tb05163.x. [DOI] [PubMed] [Google Scholar]
- 36.Kearsley JH. High-dose radiotherapy for localized prostatic cancer. An analysis of treatment results and early complications. Med J Aust. 1986;144(12):624–8. [PubMed] [Google Scholar]
- 37.Preston CI, Duncan W, Kerr GR. Radical treatment of prostatic carcinoma by megavoltage X-ray therapy. Clin Radiol. 1986;37(5):473–7. doi: 10.1016/s0009-9260(86)80058-7. [DOI] [PubMed] [Google Scholar]
- 38.Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37(5):1035–41. [PubMed] [Google Scholar]
- 39.Abramowitz MC, Li T, Buyyounouski MK, et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. 2008;112(1):55–60. doi: 10.1002/cncr.23139. [DOI] [PubMed] [Google Scholar]
- 40.Buyyounouski MK, Hanlon AL, Eisenberg DF, et al. Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63(5):1455–62. doi: 10.1016/j.ijrobp.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 41.Horwitz EM, Vicini FA, Ziaja EL, et al. The correlation between the ASTRO Consensus Panel definition of biochemical failure and clinical outcome for patients with prostate cancer treated with external beam irradiation. American Society of Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 1998;41(2):267–72. doi: 10.1016/s0360-3016(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 42.Kupelian PA, Buchsbaum JC, Patel C, et al. Impact of biochemical failure on overall survival after radiation therapy for localized prostate cancer in the PSA era. Int J Radiat Oncol Biol Phys. 2002;52(3):704–11. doi: 10.1016/s0360-3016(01)02778-x. [DOI] [PubMed] [Google Scholar]
- 43.Miles EF, Lee WR. Hypofractionation for prostate cancer: a critical review. Semin Radiat Oncol. 2008;18(1):41–7. doi: 10.1016/j.semradonc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 44.McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118(15):3681–90. doi: 10.1002/cncr.26699. [DOI] [PubMed] [Google Scholar]
- 45.Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67(4):1099–105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 46.Denham JW, O’Brien PC, Dunstan RH, et al. Is there more than one late radiation proctitis syndrome? Radiother Oncol. 1999;51(1):43–53. doi: 10.1016/s0167-8140(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 47.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Khor LY, Moughan J, Al-Saleem T, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13(12):3585–90. doi: 10.1158/1078-0432.CCR-06-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollack A, Cowen D, Troncoso P, et al. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma: Ki-67, bcl-2, bax, and bcl-x. Cancer. 2003;97(7):1630–8. doi: 10.1002/cncr.11230. [DOI] [PubMed] [Google Scholar]
- 50.Zhou JR, Yu L, Zerbini LF, Libermann TA, Blackburn GL. Progression to androgen-independent LNCaP human prostate tumors: cellular and molecular alterations. Int J Cancer. 2004;110(6):800–6. doi: 10.1002/ijc.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollack A, Cowen D, Troncoso P, et al. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma. Cancer. 2003;97(7):1630–8. doi: 10.1002/cncr.11230. [DOI] [PubMed] [Google Scholar]
- 52.Bantis A, Giannopoulos A, Gonidi M, et al. Expression of p120, Ki-67 and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic value. Cytopathology. 2004;15(1):25–31. doi: 10.1046/j.0956-5507.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 53.Roach M, 3rd, Waldman F, Pollack A. Predictive models in external beam radiotherapy for clinically localized prostate cancer. Cancer. 2009;115(13 Suppl):3112–20. doi: 10.1002/cncr.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zwahlen DR, Lang S, Hrbacek J, et al. The use of photon beams of a flattening filter-free linear accelerator for hypofractionated volumetric modulated arc therapy in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83(5):1655–60. doi: 10.1016/j.ijrobp.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Klayton T, Price R, Buyyounouski MK, et al. Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys. 2012;84(1):130–6. doi: 10.1016/j.ijrobp.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zelefsky MJ, Kollmeier M, Cox B, et al. Improved Clinical Outcomes With High-Dose Image Guided Radiotherapy Compared With Non-IGRT for the Treatment of Clinically Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):125–9. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 57.de Crevoisier R, Tucker SL, Dong L, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(4):965–73. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 58.Heemsbergen WD, Hoogeman MS, Witte MG, et al. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 GY versus 78 Gy. Int J Radiat Oncol Biol Phys. 2007;67(5):1418–24. doi: 10.1016/j.ijrobp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Song WY, Schaly B, Bauman G, Battista JJ, Van Dyk J. Evaluation of image-guided radiation therapy (IGRT) technologies and their impact on the outcomes of hypofractionated prostate cancer treatments: a radiobiologic analysis. Int J Radiat Oncol Biol Phys. 2006;64(1):289–300. doi: 10.1016/j.ijrobp.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 60.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307(15):1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coen JJ, Paly JJ, Niemierko A, et al. Long-term quality of life outcome after proton beam monotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):e201–9. doi: 10.1016/j.ijrobp.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 62.Vogelius IR, Bentzen SM. Meta-analysis of the Alpha/Beta Ratio for Prostate Cancer in the Presence of an Overall Time Factor: Bad News, Good News, or No News? Int J Radiat Oncol Biol Phys. 2013;85(1):89–94. doi: 10.1016/j.ijrobp.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]