Abstract
Interleukin-1 (IL-1) has been implicated as a key molecule in Alzheimer pathogenesis based on findings of an IL-1 overexpression in Alzheimer brain that is directly related to plaque progression and tangle formation, and on findings that IL-1 induces excessive synthesis, translation, and processing of neuronal β-amyloid precursor protein (βAPP) as well as synthesis of most known plaque-associated proteins. In addition, IL-1 activates astrocytes, with the important consequence of over-expression of the neuritogenic cytokine S100β and overgrowth of dystrophic neurites in neuritic plaques. As further evidence of the importance of IL-1 in Alzheimer pathogenesis, two new genetic studies of inheritance of specific polymorphisms in IL-1 genes in Alzheimer and control patients show that homozygosity for a specific IL-1A gene polymorphism at least triples risk for development of Alzheimer’s disease. This increase is associated with earlier age of onset. Homozygosity for this polymorphism plus another in the IL-1B gene further increases risk.
Keywords: Alzheimer’s disease, Interleukin-1, Genetic risk, Immunogenetics
An understanding of Alzheimer’s disease must necessarily commence with an understanding of the pathogenesis of the neuritic β-amyloid plaques that are diagnostic of the disease. Similarly, an understanding of the origins of Alzheimer’s disease must commence with identification of pathological changes that precede the appearance of these plaques, and identification of candidate molecules that orchestrate the pathogenic mechanisms culminating in Alzheimer pathogenesis. Our early consideration of a potential role for immune-response generating cytokines as driving forces in pathogenic processes in brain was a departure from more conventional ideas regarding Alzheimer pathogenesis, and our early demonstration of interleukin-1 (IL-1) overexpression in Alzheimer brain (Griffin et al., 1989) was the first evidence supporting this controversial new idea. This overexpression is principally a manifestation of activated (enlarged) microglia that are found both associated with β-amyloid plaques and located immediately adjacent to neurons. The central and important role of IL-1 in Alzheimer pathogenesis has been supported by this and numerous subsequent findings, including the observations that IL-1: (i) is excessively expressed in Alzheimer brain tissue; (ii) induces excessive synthesis of the β-amyloid precursor protein (βAPP) in neurons (Forloni et al., 1992); and (iii) activates astrocytes (Giulian et al., 1988) with the important consequence of astrocytic overexpression (Griffin et al. 1995) of the neuritogenic (Kligman and Marshak, 1985) cytokine S100β. These findings were the basis for genetic studies showing that specific polymorphisms in IL-1 genes increase risk for Alzheimer’s disease (Nicoll et al., 2000; Grimaldi et al., 2000), lending importance to what we propose as a new area for exploration: the immunogenetics of Alzheimer’s disease. These IL-1 mediated influences in Alzheimer pathogenesis are depicted in Fig. 1.
Fig. 1.
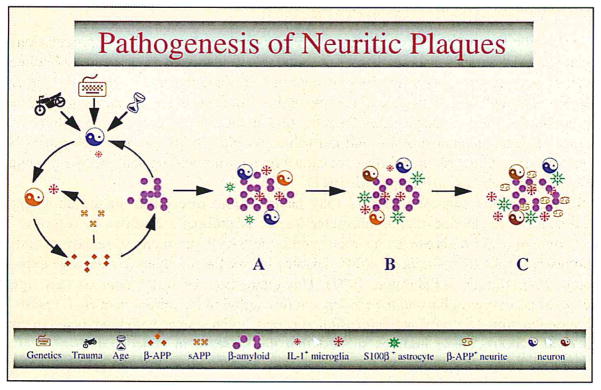
This is a schematic diagram demonstrating pathogenesis of neuritic plaque as a consequence of neuronal injury, activation of microglia with overexpression of IL-1, and subsequent initiation of IL-1 driven cascades. (A), (B), and (C) represent progressive stages of β-amyloid plaque evolution.
This review focuses on two lines of investigation delineating the contribution of IL-1 to Alzheimer pathogenesis. The first is the definition of the molecular events involved in IL-1-driven cascades, and their potential to initiate and propagate the neuropathological changes of Alzheimer’s disease. The second is the identification of Alzheimer disease risk associated with polymorphisms within the two genes (IL-1A and IL-1B) encoding the two isoforms of IL-1, IL-1α and IL-1β, respectively.
1. Early evidence for IL-1 cascades in Alzheimer pathogenesis
An early observation supporting a role for IL-1 in the pathogenesis of Alzheimer’s disease was the overexpression of IL-1 in Down’s syndrome. Florid Alzheimer-type neuropathological changes are virtually inevitable consequences of Down’s syndrome (Wisniewski et al., 1985), making this condition a natural model of Alzheimer pathogenesis. IL-1 is dramatically overexpressed in activated microglia in Down’s fetuses and neonates, decades before the inevitable appearance of Alzheimer-like changes (Griffin et al., 1989). This observation suggested that the excessive expression of IL-1 in Alzheimer brain might be a seminal and continuing driving force, responsible for a cascade of neurodegenerative events that culminate in the neuropathological changes characteristic of Alzheimer disease. By analogy with immune cascades in the periphery (Dinarello and Wolff, 1993), it was logical to conclude that neural cascades include IL-1, which is known to activate astrocytes (Giulian et al., 1988), and thus would induce expression of one or more astrocyte-derived secreted neuronotrophic proteins. We proposed that the neuritogenic cytokine S100β (Kligman and Marshak, 1985) would provide a link whereby IL-1 could contribute to the proliferation of dystrophic neurites and thus to the progression of amyloid deposits into the neuritic β-amyloid plaques diagnostic of Alzheimer’s disease. Our subsequent work has confirmed this possibility, as we have shown that IL-1 does induce astrocytic overexpression of S100β and S100β mRNA (Sheng et al., 1996); that the tissue levels of S100β in cerebral cortex in Alzheimer’s disease are directly proportional to the numbers of neuritic β-amyloid plaques (Sheng et al., 1994); and that the numbers of plaque-associated, S100β-expressing astrocytes correlates with the abundance of dystrophic neurites within individual plaques (Mrak et al., 1996).
The early landmark study (Goldgaber et al., 1989), showing that IL-1 induces excessive expression of the β-amyloid precursor protein (βAPP), confirmed a link between the overexpression of IL-1 in Alzheimer’s disease and the deposition of β-amyloid that is so characteristic of the disease. The importance of IL-1 regulation of βAPP expression was subsequently expanded to include regulation of its translation (Rogers et al., 1999) and processing into secreted fragments (sAPP) and β-amyloidogenic fragments (Buxbaum et al., 1992). These results suggest that IL-1 overexpression is an important factor in driving β-amyloid production, deposition, and thus plaque formation.
1.1. Interleukin-1 and neuronal dysfunction
In addition to putative effects of IL-1 overexpression on the genesis and progression of plaques in Alzheimer’s disease, there is considerable evidence of direct neurotoxic effects of IL-1, with potential contributions to neuronal dysfunction and loss in Alzheimer’s disease. Although IL-1 is trophic to neurons at low concentrations, higher concentrations are neurotoxic (Brenneman et al., 1992a,b). IL-1 induces overexpression and phosphorylation of neurofilament proteins and tau (Sheng et al., 2000), and activated microglia, overexpressing IL-1, are intimately associated with neurons bearing neurofibrillary tangles in Alzheimer’s disease (Sheng et al., 1997).
Recent findings suggest that IL-1 may have a more direct role in the cholinergic dysfunction and decline that is characteristic of Alzheimer’s disease. In response to both toxic and sub-toxic stress, neurons synthesize βAPP and increase release of sAPP, and this neuronal stress-induced sAPP activates microglia and induces excessive expression of IL-1 (Barger and Harmon, 1997). This interaction, by which neurons may signal their distress to microglia, and the consequent microglial overexpression of IL-1, results in increased neuronal activity and expression of acetylcholinesterase both in vivo and in vitro (Li et al., 2000). These findings suggest that the increase in concentration of IL-1 in Alzheimer brain contributes to the decrease in acetylcholine by upregulating acetylcholinesterase.
Recent work has elucidated, in part, the intracellular regulation of IL-1 synthesis and activation as well. IL-1β converting enzyme (ICE) is responsible for converting pro-IL-1β to active IL-1β, and the activity and expression of ICE is increased in Alzheimer’s disease (Chan et al., 1999; Zhu et al., 1999). In addition to its role in activating IL-1β, ICE may also contribute directly to neuronal toxicity and death. ICE is the first described member of a caspase family (caspase I), and its activation is associated with, and necessary for, neuronal and astrocyte apoptosis (Keane et al., 1997). This suggests that the overexpression of ICE by plaque-associated and neuron-associated microglia might contribute to the DNA damage observed in neurons within and adjacent to neuritic β-amyloid plaques (Sheng et al., 1998a) and in non-plaque-associated neurons bearing neurofibrillary tangles in Alzheimer’s disease (Sheng et al., 1998c).
1.2. IL-1 and β-amyloid plaque formation and progression
Microglia show a characteristic laminar distribution pattern in normal brain, and the laminar distribution of β-amyloid plaques in Alzheimer brain mirrors that pattern (Sheng et al., 1998b). There is also a correlation between the number of activated microglia overexpressing IL-1 and the number of neuritic β-amyloid plaques across brain regions (Sheng et al., 1995). These findings suggest that IL-1 is a principle driving force in the initiation and spread of β-amyloid plaque pathology in Alzheimer’s disease.
IL-1 promotes astrocytic expression of a number of important, Alzheimer-related proteins, in addition to S100β. These include IL-6 (Bauer et al., 1991), α1-antichymotrypsin and apolipoprotein E (Das and Potter, 1995), and some complement proteins (Barnum and Jones, 1995). All of these proteins are found co-deposited with β-amyloid in the plaques of Alzheimer’s disease. This, together with the effects of IL-1 on synthesis and processing of βAPP, suggests a powerful role for IL-1 in driving deposition of protein within β-amyloid plaques. IL-1 may also contribute to the formation of dystrophic neurites within these plaques—and thus to the conversion of diffuse amyloid deposits into neuritic β-amyloid plaques—through regulation of astrocytic S100β expression (Sheng et al., 1996) and through the consequent neuritogenic actions of S100β (Marshak et al., 1991).
2. IL-1 polymorphisms in Alzheimer pathogenesis
The postulated role for IL-1 as a key orchestrating cytokine in Alzheimer pathogenesis suggested that variations in the genes for IL-1 might confer differential risk for development of Alzheimer’s disease. One common polymorphism in the promoter region of the IL-1A gene (allele 2) has already been shown to confer increased risk for juvenile rheumatoid arthritis (McDowell et al., 1995). Another common polymorphism (also known as allele 2) in the coding region of the IL-1B gene has been associated with increased production of IL-1β (Pociot et al., 1992). In a study of a group of neuropathologically confirmed Alzheimer patients from four centers in the US and UK (Nicoll et al., 2000), the prevalence of the IL-1A 2,2 genotype was 12.9% in Alzheimer patients, compared to 6.6% in control patients. Patients homozygous for the IL-1A 2 allele thus carry three times the risk of developing Alzheimer’s disease. Furthermore, homozygosity for both IL-1A allele 2 and IL-1B allele 2, was associated with a 10-fold increased risk of developing Alzheimer’s disease in this study. Another study of a large cohort of clinically assessed patients in the Italian Longitudinal Study was directed toward the possible effects of IL-1 polymorphisms on age at onset of Alzheimer’s disease (Grimaldi et al., 2000). Homozygosity for the IL-1A allele 2 strongly influenced age at onset of Alzheimer’s disease (yielding an odds ratio of 4.74): the age at onset of IL-1A 2 homozygous patients was, on average, 61 years, compared to 68 years for hemizygous patients, and 70 years for IL-1A 1,1 patients. In this generally younger population of clinically assessed Alzheimer patients, there was an IL-1A genotype dose effect: an odds ratio of 6.33 for IL-1A 2,2 and 1.84 for IL-1A 1,2. Another polymorphism located in the promoter region of the IL-1B gene also increases risk for developing Alzheimer’s disease. Homozygosity for this polymorphism confers twice the risk for developing Alzheimer’s disease, and the risk is for late-onset, rather than early-onset, disease. In both of these studies, the increased risk for development of Alzheimer’s disease with specific polymorphisms in IL-1 genes was independent of ApoE genotype.
We have proposed (Nicoll et al., 2000) that these IL-1 gene polymorphisms act by increasing the gain of an IL-1 driven cycle, referred to as the cytokine cycle (Griffin et al., 1998), that acts through the IL-1 mediated cascades discussed here to favor plaque formation and progression, dystrophic neurite proliferation, and neuronal dysfunction and loss in Alzheimer’s disease. These neurodegenerative consequences of IL-1 overexpression, in turn, engender further activation of microglia, through stress-induced overexpression of neuronal βAPP and β-amyloid deposition, and further amplification of IL-1 overexpression. In this way, the cytokine cycle becomes self-propagating, a requisite for cycles that give rise to and maintain the progression of degenerative diseases.
Acknowledgments
This work was supported in part by National Institutes of Health grants AG10208 and AG12411, and by the Medical Research Council.
References
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;28:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Barnum SR, Jones JL. Differential regulation of C3 gene expression in human astroglioma cells by interferon-gamma and interleukin-1 beta. Neurosci Lett. 1995;197:121–124. doi: 10.1016/0304-3940(95)11923-k. [DOI] [PubMed] [Google Scholar]
- Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Volk B, Berger M. Interleukin-6 and alpha2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- Brenneman RE, Page SW, Schultzberg M, Thomas FH, Zelazowski P, Burnet P, Avidor R, Sternberg EM. A decomposition product of a contaminant implicated in L-tryptophan eosinophilia myalgia syndrome affects spinal cord neuronal cell death and survival through stereospecific, maturation and partly interleukin-1-dependent mechanisms. J Pharmacol Exp Ther. 1992;266:1029–1035. [PubMed] [Google Scholar]
- Brenneman DR, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Griffin WST, Mattson MP. Evidence for Caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer’s disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Das S, Potter H. Expression of the Alzheimer amyloid-promoting factors α1-antichymotrypsin and apolipoprotein E is induced in astrocytes by IL-1. Neuron. 1995;14:447–456. doi: 10.1016/0896-6273(95)90300-3. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res Mol Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RG, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC, Ling C, White L, Macleod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease. J Neuropath Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘Cytokine Cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi LME, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, Biunno I, De Bellis G, Sorbi S, Mariani C, Griffin WST, Franceschi M. Association of early onset Alzheimer’s disease with an Interleukin-1α gene polymorphism. Ann Neurol. 2000;47:361–365. [PubMed] [Google Scholar]
- Keane RW, Srinivasan A, Foster LM, et al. Activation of CPP32 during apoptosis of neurons and astrocytes. Cell. 1997;48:168–180. doi: 10.1002/(sici)1097-4547(19970415)48:2<168::aid-jnr9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci USA. 1985;82:7136–7139. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WST. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak DR, Pesce SA, Stanley LC, Griffin WST. Increased S100β neurotrophic activity in Alzheimer disease temporal lobe. Neurobiol Aging. 1991;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- McDowell TL, Symons JA, Ploski R, Forre O, Duff GW. A genetic association between juvenile rheumatoid arthritis and a novel interleukin-1 alpha polymorphism. Arthritis Rheum. 1995;38:221–228. doi: 10.1002/art.1780380210. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WST. Correlation of astrocytic S100β expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:273–279. doi: 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JAR, Mrak RE, Graham DI, Stewart J, Wilcock G, MacGowan S, Esiri MM, Murray LS, Dewar D, Love S, Moss T, Griffin WST. Association of interleukin-1 gene polymorphisms with Alzheimer’s disease. Ann Neurol. 2000;47:365–368. [PMC free article] [PubMed] [Google Scholar]
- Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup JA. TaqI polymorphism in the human interleukin-1β (IL-1β) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Leiter LM, McPhee J, Cahill CM, Zhan SS, Potter H, Nilsson LN. Translation of the Alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J Biol Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. S100β protein expression in Alzheimer’s disease: potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. Interleukin-1 expression in brain regions in Alzheimer’s disease: correlation with neuritic plaque distribution. Neuropathol Appl Neurobiol. 1995;21:290–301. doi: 10.1111/j.1365-2990.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, Griffin WST. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. Glial-neuronal interactions in Alzheimer’s disease: progressive association of IL-1α+ microglia and S100β+ astrocytes with neurofibrillary tangle stages. J Neuropathol Exp Neurol. 1997;56:285–290. [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. Progressive neuronal injury associated with amyloid plaque formation in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:714–717. doi: 10.1097/00005072-199807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. Distribution of interleukin-1-immunoreactive microglia in cerebral cortical layers: implications for neuritic plaque formation in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1998;24:278–283. doi: 10.1046/j.1365-2990.1998.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Zhou XQ, Mrak RE, Griffin WST. Progressive neuronal injury associated with neurofibrillary tangle formation in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:323–328. doi: 10.1097/00005072-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Zhu SG, Griffin WST, Mrak RE. Interleukin-1 promotes expression and phosphorylation and tau protein in vivo. Exp Neurol. 2000 doi: 10.1006/exnr.2000.7393. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Sheng JG, Jones RA, Brewer MM, Zhou XQ, Mrak RE, Griffin WST. Increased interleukin-1β converting enzyme expression and activity in Alzheimer disease. J Neuropathol Exp Neurobiol. 1999;58:582–587. doi: 10.1097/00005072-199906000-00002. [DOI] [PubMed] [Google Scholar]


