Abstract
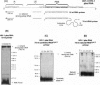
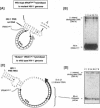
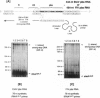
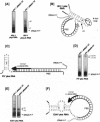
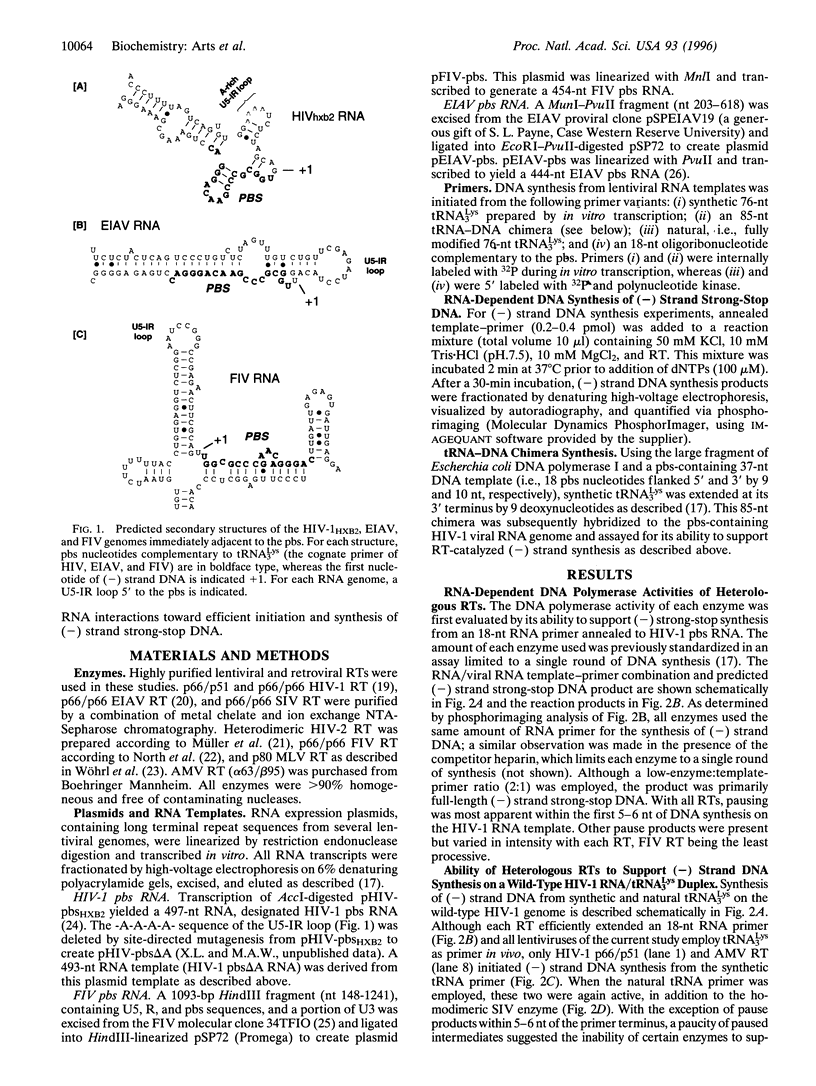
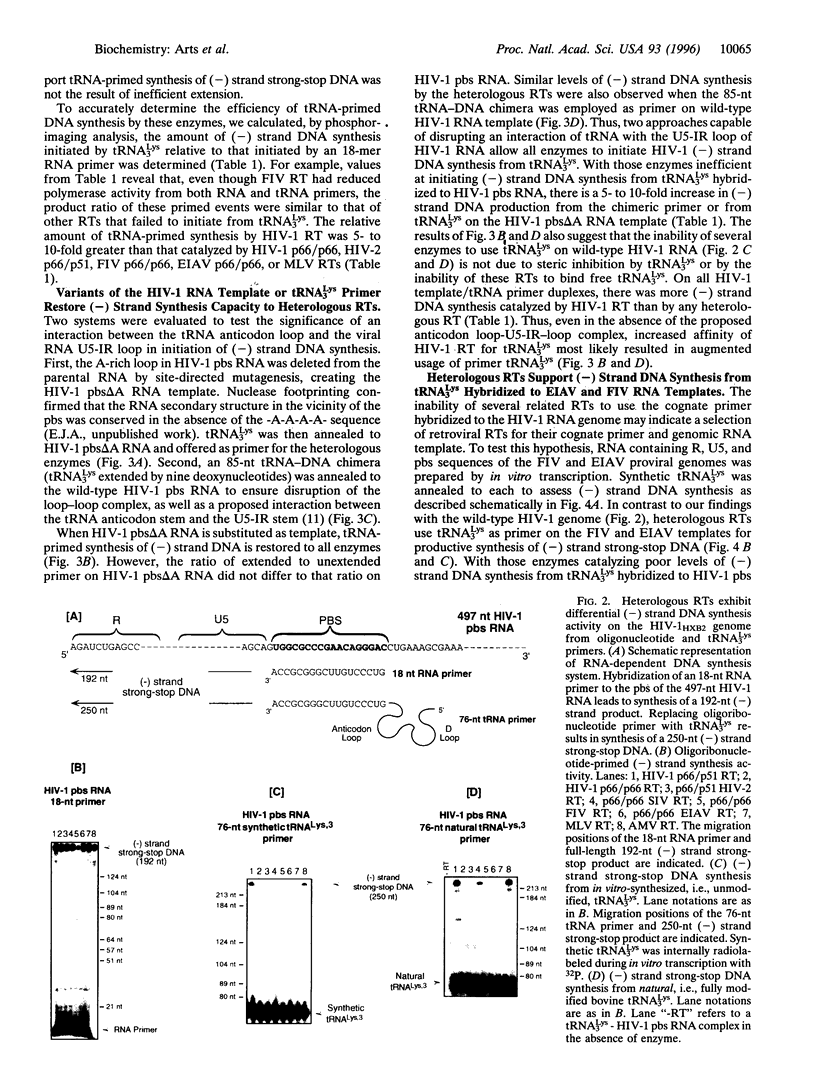
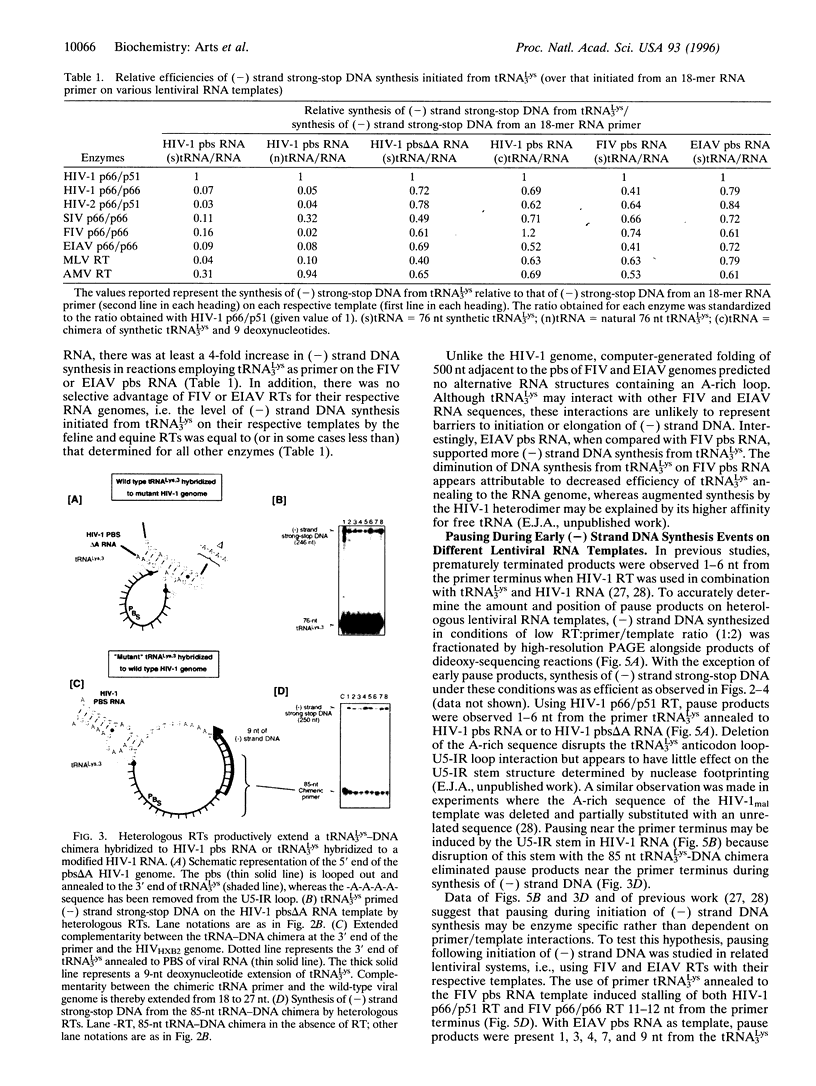
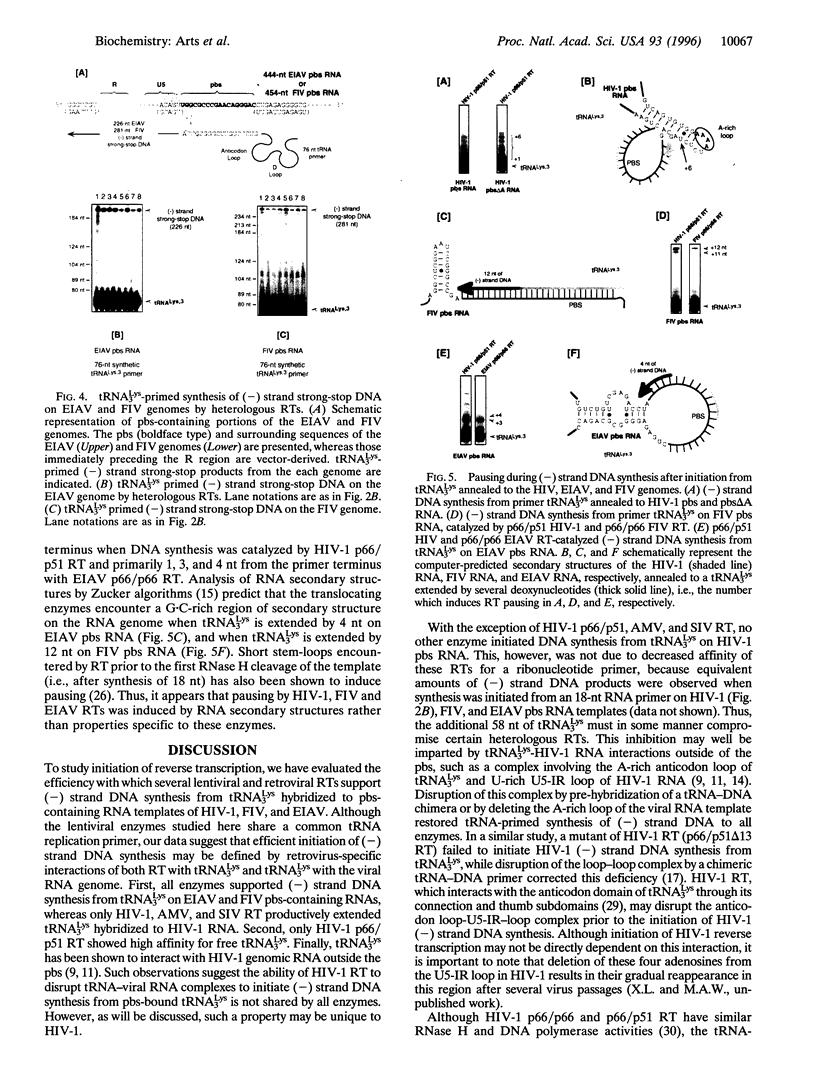
Initiation of minus (-) strand DNA synthesis was examined on templates containing R, U5, and primer-binding site regions of the human immunodeficiency virus type 1 (HIV-1), feline immunodeficiency virus (FIV), and equine infectious anemia virus (EIAV) genomic RNA. DNA synthesis was initiated from (i) an oligoribonucleotide complementary to the primer-binding sites, (ii) synthetic tRNA(3Lys), and (iii) natural tRNA(3Lys), by the reverse transcriptases of HIV-1, FIV, EIAV, simian immunodeficiency virus, HIV type 2 (HIV-2), Moloney murine leukemia virus, and avian myeloblastosis virus. All enzymes used an oligonucleotide on wild-type HIV-1 RNA, whereas only a limited number initiated (-) strand DNA synthesis from either tRNA(3Lys). In contrast, all enzymes supported efficient tRNA(3Lys)-primed (-) strand DNA synthesis on the genomes of FIV and EIAV. This may be in part attributable to the observation that the U5-inverted repeat stem-loop of the EIAV and FIV genomes lacks an A-rich loop shown with HIV-1 to interact with the U-rich tRNA anticodon loop. Deletion of this loop in HIV-1 RNA, or disrupting a critical loop-loop complex by tRNA(3Lys) extended by 9 nt, restored synthesis of HIV-1 (-) strand DNA from primer tRNA(3Lys) by all enzymes. Thus, divergent evolution of lentiviruses may have resulted in different mechanisms to use the same host tRNA for initiation of reverse transcription.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar A., Ge Z., Leis J. A specific orientation of RNA secondary structures is required for initiation of reverse transcription. J Virol. 1994 Feb;68(2):611–618. doi: 10.1128/jvi.68.2.611-618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts E. J., Ghosh M., Jacques P. S., Ehresmann B., Le Grice S. F. Restoration of tRNA3Lys-primed(-)-strand DNA synthesis to an HIV-1 reverse transcriptase mutant with extended tRNAs. Implications for retroviral replication. J Biol Chem. 1996 Apr 12;271(15):9054–9061. doi: 10.1074/jbc.271.15.9054. [DOI] [PubMed] [Google Scholar]
- Arts E. J., Marois J. P., Gu Z., Le Grice S. F., Wainberg M. A. Effects of 3'-deoxynucleoside 5'-triphosphate concentrations on chain termination by nucleoside analogs during human immunodeficiency virus type 1 reverse transcription of minus-strand strong-stop DNA. J Virol. 1996 Feb;70(2):712–720. doi: 10.1128/jvi.70.2.712-720.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts E.J., Li Z., Wainberg M.A. Analysis of Primer Extension and the First Template Switch during Human Immunodeficiency Virus Reverse Transcription. J Biomed Sci. 1995 Oct;2(4):314–321. doi: 10.1007/BF02255218. [DOI] [PubMed] [Google Scholar]
- Barat C., Lullien V., Schatz O., Keith G., Nugeyre M. T., Grüninger-Leitch F., Barré-Sinoussi F., LeGrice S. F., Darlix J. L. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989 Nov;8(11):3279–3285. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin F., Marquet R., Isel C., Darlix J. L., Ehresmann B., Ehresmann C. Functional sites in the 5' region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol. 1993 Jan 20;229(2):382–397. doi: 10.1006/jmbi.1993.1041. [DOI] [PubMed] [Google Scholar]
- Davies J. F., 2nd, Hostomska Z., Hostomsky Z., Jordan S. R., Matthews D. A. Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase. Science. 1991 Apr 5;252(5002):88–95. doi: 10.1126/science.1707186. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N., Rank K. B., Leone J. W., Heinrikson R. L., Bannow C. A., Smith C. W., Evans D. B., Poppe S. M., Tarpley W. G., Rothrock D. J. The differential processing of homodimers of reverse transcriptases from human immunodeficiency viruses type 1 and 2 is a consequence of the distinct specificities of the viral proteases. J Biol Chem. 1995 Jun 2;270(22):13573–13579. [PubMed] [Google Scholar]
- Isel C., Ehresmann C., Keith G., Ehresmann B., Marquet R. Initiation of reverse transcription of HIV-1: secondary structure of the HIV-1 RNA/tRNA(3Lys) (template/primer). J Mol Biol. 1995 Mar 24;247(2):236–250. doi: 10.1006/jmbi.1994.0136. [DOI] [PubMed] [Google Scholar]
- Isel C., Lanchy J. M., Le Grice S. F., Ehresmann C., Ehresmann B., Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNA3Lys. EMBO J. 1996 Feb 15;15(4):917–924. [PMC free article] [PubMed] [Google Scholar]
- Isel C., Marquet R., Keith G., Ehresmann C., Ehresmann B. Modified nucleotides of tRNA(3Lys) modulate primer/template loop-loop interaction in the initiation complex of HIV-1 reverse transcription. J Biol Chem. 1993 Dec 5;268(34):25269–25272. [PubMed] [Google Scholar]
- Jiang M., Mak J., Ladha A., Cohen E., Klein M., Rovinski B., Kleiman L. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol. 1993 Jun;67(6):3246–3253. doi: 10.1128/jvi.67.6.3246-3253.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Steitz T. A. Reverse transcriptase of human immunodeficiency virus can use either human tRNA(3Lys) or Escherichia coli tRNA(2Gln) as a primer in an in vitro primer-utilization assay. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9652–9656. doi: 10.1073/pnas.89.20.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Grüninger-Leitch F. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur J Biochem. 1990 Jan 26;187(2):307–314. doi: 10.1111/j.1432-1033.1990.tb15306.x. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Panin M., Kalayjian R. C., Richter N. J., Keith G., Darlix J. L., Payne S. L. Purification and characterization of recombinant equine infectious anemia virus reverse transcriptase. J Virol. 1991 Dec;65(12):7004–7007. doi: 10.1128/jvi.65.12.7004-7007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Zehnle R., Mous J. A single 66-kilodalton polypeptide processed from the human immunodeficiency virus type 2 pol polyprotein in Escherichia coli displays reverse transcriptase activity. J Virol. 1988 Jul;62(7):2525–2529. doi: 10.1128/jvi.62.7.2525-2529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J., Jiang M., Wainberg M. A., Hammarskjöld M. L., Rekosh D., Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNA(Lys) into human immunodeficiency virus type 1 particles. J Virol. 1994 Apr;68(4):2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Steitz J. A. Site-specific crosslinking of 4-thiouridine-modified human tRNA(3Lys) to reverse transcriptase from human immunodeficiency virus type I. EMBO J. 1995 Jun 1;14(11):2679–2687. doi: 10.1002/j.1460-2075.1995.tb07266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Restle T., Kühnel H., Goody R. S. Expression of the heterodimeric form of human immunodeficiency virus type 2 reverse transcriptase in Escherichia coli and characterization of the enzyme. J Biol Chem. 1991 Aug 5;266(22):14709–14713. [PubMed] [Google Scholar]
- Müller B., Restle T., Weiss S., Gautel M., Sczakiel G., Goody R. S. Co-expression of the subunits of the heterodimer of HIV-1 reverse transcriptase in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):13975–13978. [PubMed] [Google Scholar]
- North T. W., Cronn R. C., Remington K. M., Tandberg R. T., Judd R. C. Characterization of reverse transcriptase from feline immunodeficiency virus. J Biol Chem. 1990 Mar 25;265(9):5121–5128. [PubMed] [Google Scholar]
- Peters G. G., Hu J. Reverse transcriptase as the major determinant for selective packaging of tRNA's into Avian sarcoma virus particles. J Virol. 1980 Dec;36(3):692–700. doi: 10.1128/jvi.36.3.692-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch J. W., Arts E. J., Wöhrl B. M., Le Grice S. F. Involvement of C-terminal structural elements of equine infectious anemia virus reverse transcriptase in DNA polymerase and ribonuclease H activities. J Mol Biol. 1996 Apr 5;257(3):500–511. doi: 10.1006/jmbi.1996.0181. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M., Wilhelm F. X., Keith G., Agoutin B., Heyman T. Yeast Ty1 retrotransposon: the minus-strand primer binding site and a cis-acting domain of the Ty1 RNA are both important for packaging of primer tRNA inside virus-like particles. Nucleic Acids Res. 1994 Nov 11;22(22):4560–4565. doi: 10.1093/nar/22.22.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrl B. M., Georgiadis M. M., Telesnitsky A., Hendrickson W. A., Le Grice S. F. Footprint analysis of replicating murine leukemia virus reverse transcriptase. Science. 1995 Jan 6;267(5194):96–99. doi: 10.1126/science.7528942. [DOI] [PubMed] [Google Scholar]