Abstract
The neurite extension factor S100β is overexpressed by activated astrocytes associated with amyloid-containing plaques in Alzheimer’s disease, and has been implicated in dystrophic neurite formation in these plaques. This predicts (a) that the appearance of S100β-immunoreactive (S100β+) astrocytes precedes that of dystrophic neurites in diffuse amyloid deposits and (b) that the number of these astrocytes correlates with the degree of dystrophic neurite proliferation in neuritic plaques. As a test of the first prediction, we determined the number of S100β+ astrocytes associated with different plaque types: diffuse non-neuritic, diffuse neuritic, dense-core neuritic, and dense-core non-neuritic. Diffuse non-neuritic plaques had small numbers of associated S100β+ astrocytes (1.3 ± 0.1 S100β+ astrocytes per plaque [mean ± SEM]; 80% of plaques had one or more). These astrocytes were most abundant in diffuse neuritic plaques (4.2 ± 0.2; 100%), were somewhat less numerous in dense-core neuritic plaques (1.6 ± 0.2; 90%), and were only rarely associated with dense-core non-neuritic plaques (0.15 ± 0.05; 12%). As a test of the second prediction, we correlated the number of S100β+ astrocytes per plaque with the area of β-amyloid precursor protein (β-APP) immunoreactivity per plaque (an index of the size of the plaques’ dystrophic neurite shells) and found a significant positive correlation (r = 0.74, p < 0.001). This correlation was also evident at the tissue level: the numbers of S100β+ astrocytes per plaque-rich field correlated with the total area of β-APP immunoreactivity in these fields (r = 0.66, p < 0.05). These correlations support the idea that astrocytic activation and S100β overexpression are involved in the induction and maintenance of dystrophic neurites in amyloid deposits, and support the concept of a glial cytokine-mediated cascade underlying the progression of neuropathological changes in Alzheimer’s disease.
Keywords: Alzheimer’s disease, β-amyloid plaque evolution, β-amyloid precursor protein, S100β protein
INTRODUCTION
β-Amyloid-containing neuritic plaques are the histological hallmark of Alzheimer’s disease (1, 2). These complex structures are thought to evolve from simpler diffuse amyloid deposits that lack neuritic components (3). We have previously proposed that a chronic inflammatory response involving microglia and astrocytes drives the progression of simple diffuse amyloid deposits to complex neuritic forms (4, 5). Activated microglia are present in diffuse amyloid deposits devoid of dystrophic neurites (6), and these activated cells overexpress interleukin-1 (IL-1) (4), a potent immune cytokine that activates astrocytes (7). This implies that IL-1-induced astrocyte activation is an early event in the inflammatory response that drives the evolution of diffuse amyloid deposits into the diagnostic neuritic β-amyloid plaques of Alzheimer’s disease.
Activated astrocytes, like activated microglia, are frequent components of neuritic plaques in Alzheimer’s disease (4, 8–11) and these plaque-associated astrocytes overexpress the biologically active homodimer of S100β (12, 13), an IL-1-regulated (14), astrocyte-derived cytokine. The significance of S100β overexpression in activated astrocytes in Alzheimer’s disease rests in the fact that this cytokine stimulates neurite growth in vitro (15) and in vivo (16). This implicates S100β as a pathogenic link between microglial IL-1 overexpression and dystrophic neurite formation in amyloid deposits, and thus in the evolution of diffuse amyloid deposits into neuritic β-amyloid plaques. Such a role is supported by the finding that tissue levels of S100β correlate both with the density of neuritic plaques in individual Alzheimer patients (13) and with the distribution of neuritic plaques across brain regions (17).
The concept of astrocyte activation and S100β over-expression as a key pathogenic link in plaque evolution predicts (a) that appearance of astrocytes overexpressing S100β should precede the appearance of dystrophic neurites in diffuse amyloid deposits and (b) that expression of S100β should correlate with the profusion of dystrophic neurites in neuritic plaques. To directly address these two predictions, we (a) quantified the number of S100β-immunoreactive (S100β+) astrocytes in 4 different plaque types (thought to represent different stages in plaque evolution) and (b) correlated the number of these astrocytes with cross-sectional area of β-amyloid precursor protein-immunoreactive (β-APP+) dystrophic neurites both within individual plaques and in temporal lobe cortical tissue sections.
MATERIALS AND METHODS
Patient Information and Tissue Preparation
Temporal lobe tissue was obtained postmortem from 12 clinically demented patients (age 65 to 88), with neuropathological confirmation of Alzheimer’s disease according to CERAD criteria (2). Postmortem intervals ranged from 1.5 to 29 hours (12 ± 9; mean ± SD). Following fixation of the right hemisphere in 20% formalin for 1 to 2 weeks, coronal sections of hippocampus with adjacent parahippocampal gyrus and lateral geniculate nucleus were embedded in paraffin and sectioned at 10 μm thickness.
S100β Antibodies
Rabbit polyclonal antibodies selective for the S100β polypeptide were a gift from Dr Linda Van Eldik (17). These antibodies react selectively with S100β as ascertained by multiple criteria (Western immunoblots, ELISA, radioimmunoassay, and immunohistochemistry) and are now available commercially (RaS100β; East Acres Biologicals, Inc., Southbridge, MA; and SWant Antibodies, Bellinzona, Switzerland).
Immunohistochemistry
To correlate numbers of S100β+ astrocytes with specific plaque types, we used dual immunolabeling. Tissue sections were pretreated with 99% formic acid for 5 minutes (min) (18) and then processed as previously described (5, 13, 19). These sections were either co-immunoreacted with polyclonal anti-S100β, diluted 1:2,000, and monoclonal anti-β-amyloid (a gift from Dr Gareth Roberts) (18), diluted 1:1,000; or with polyclonal anti-β-amyloid (Boehringer-Mannheim Biochemica, Indianapolis, IN), diluted 1:10, and monoclonal anti-β-APP (clone 22C11; Boehringer-Mannheim Biochemica), diluted 1:10. This S100β/β-amyloid and β-amyloid/β-APP labeling was performed on adjacent sections. To determine if S100β expression by plaque-associated astrocytes wanes in the presumed endstage of plaque development (the dense-core non-neuritic plaque), we counted the number of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes associated with these plaques, using tissue sections co-immunoreacted with polyclonal anti-GFAP (Dakopatts, Glostrup, Denmark), diluted 1:300, and monoclonal anti-β-amyloid.
To quantify the relationship between plaque-associated S100β+ astrocytes and β-APP+ dystrophic-neurite shell size, we co-immunoreacted polyclonal S100β antibody with monoclonal β-APP antibody. To quantify the relationship between total numbers of S100β+ astrocytes and total β-APP+ area in tissue sections, we used single-immunolabeling of consecutive tissue sections with either polyclonal S100β or monoclonal β-amyloid antibodies.
Plaque Classification
Plaques were classified into 4 types on the basis of amyloid state (diffuse vs dense core) and presence or absence of dystrophic neurites (neuritic vs non-neuritic), as previously described (6). Briefly, diffuse non-neuritic plaques had the least dense and most widely dispersed β-amyloid and did not exhibit β-APP+ dystrophic neurites. Diffuse neuritic plaques had more dense, but still widely dispersed β-amyloid and displayed a profusion of β-APP+ dystrophic neurites. Plaques containing round dense deposits (cores) of β-amyloid immunoreaction product as well as diffuse β-amyloid and β-APP+ neurites were classified as dense-core neuritic plaques. Round dense deposits of β-amyloid immunoreaction product that had neither associated diffuse β-amyloid nor β-APP+ neurites were classified as dense-core, non-neuritic plaques.
Quantification of S100β+ Astrocytes per Plaque Type
The numbers of S100β+ astrocytes associated with particular plaque types were counted in plaque-rich fields of tissue sections dual immunoreacted for S100β and β-amyloid as described above. Plaque-associated S100β+ astrocytes were counted at a magnification of 250×. A total of 5 plaques of each type were counted in each of 12 patients, for a total of 60 plaques of each type. At this magnification, a typical field contained approximately 20 β-amyloid plaques. Initial review of immunoreacted sections indicated that S100β+ astrocytes associated with plaques lay within a circle twice the diameter of the β-amyloid deposit. Therefore, for this study, S100β+ astrocytes were considered to be plaque-associated if they lay within such a circle. Regions rich in plaques were chosen by examination at low magnification (100×). At this low magnification, relative numbers and astrocytic content of different plaque types were not readily apparent on initial cursory examination, thus precluding selection bias regarding plaque types or numbers of astrocytes.
Student’s t-test (unpaired data) was used to assess statistical significance of differences between numbers of S100β+ astrocytes in different plaque types, representing consecutive steps in the hypothesized sequence of plaque evolution, e.g. the number of S100β+ astrocytes in diffuse non-neuritic plaques was compared to the number in diffuse neuritic plaques and the latter were, in turn, compared to the number in dense-core neuritic plaques and so forth.
Correlation of Plaque β-APP+ Dystrophic-neurite Area with Number of Plaque-associated S100β+ Astrocytes
To correlate the number of S100β+ astrocytes with the β-APP+ area (an index of the size of the dystrophic neurite shell) of individual neuritic plaques, S100β/β-APP dual-immuno-reacted sections were analyzed, using an NIH Image computer program (version 1.55). Microscopical images of immunoreacted tissue sections were captured using a CCD video camera attached to a Macintosh computer. A total of 50 neuritic plaques identified in 7 Alzheimer patients were analyzed. For each plaque, the number of associated S100β+ astrocytes was counted manually, and the area of plaque β-APP immunoreactivity was quantified using the NIH Image program.
In addition to analyzing correlations between S100β+ astrocytes and β-APP+ area within individual plaques, we analyzed the tissue frequency of all S100β+ astrocytes relative to the total tissue area of β-APP+ plaques in adjacent, single-immunolabeled sections of parahippocampal cortex from each of 9 Alzheimer patients. Analyses were performed using five 250× plaque-rich rectangular fields (0.1 mm2/field) of S100β-immunoreacted and of β-APP-immunoreacted sections. At this magnification, there were approximately 5 to 6 plaques per field. In these plaque-rich fields, the vast majority of the S100β+ astrocytes were associated with plaques. A detection threshold was set, the background was subtracted, and the image was edited to remove all labeled elements other than S100β+ astrocytes or β-APP+ plaques (e.g. β-APP+ neurons and artifactual labeling of blood vessels). The analyses were performed to quantify the number of S100β+ astrocytes and the total area of β-APP+ plaques. Results are expressed as number of S100β+ astrocytes or area of β-APP+ plaques per mm2 tissue.
RESULTS
The 4 defined plaque types accounted for all the β-amyloid+ plaques in the sections examined. Examples of these are shown in Figure 1. S100β+ astrocytes were apparent in 80% of the diffuse non-neuritic plaques examined, with a mean of 1.3 ± 0.1 (mean ± SEM) astrocytes/plaque (Fig. 2, Table 1). The astrocytes in these non-neuritic plaques showed evidence of activation, i.e. they were enlarged (20), but they were not as large as those in neuritic plaque types. Among the 4 plaque types examined, diffuse neuritic plaques had the greatest number of associated S100β+ astrocytes (4.2 ± 0.2). Indeed, all diffuse neuritic plaques observed contained activated S100β+ astrocytes in contrast to the observation that some diffuse non-neuritic plaques (20%) did not have S100β+ astrocytes associated with them. Dense-core neuritic plaques also almost invariably (90%) contained activated S100β+ astrocytes, but there were fewer of these cells (1.6 ± 0.2) per plaque than in diffuse neuritic plaques. These plaques also appeared to have fewer dystrophic neurites associated with them than did diffuse neuritic plaques. Rarely were activated S100β+ astrocytes observed within or adjacent to any dense-core, non-neuritic plaques in our patients: only 12% of these plaques had associated S100β+ astrocytes, with an average of 0.15 ± 0.05 astrocytes/plaque. GFAP/β-amyloid dual-immunoreacted sections showed that only rarely were GFAP+ astrocytes associated with dense-core non-neuritic plaques (0.2 ±0.1 GFAP+ astrocytes per dense-core non-neuritic plaque).
Fig. 1.
Examples of plaque types dual-immunolabeled for β-amyloid (β-AP, brown) and β-amyloid precursor protein (β-APP, red) (left column, a–d) or for S100β (S100β, brown) and β-amyloid (β-AP, red) (right column, e–h). (a, e) Diffuse non-neuritic plaques devoid of condensed amyloid and β-APP+ neurites, and with several associated S100β+ activated astrocytes, (b, f) Diffuse neuritic plaques with both diffuse and condensed amyloid as well as dystrophic β-APP+ neurites, but without a dense β-amyloid core, and with an abundance of associated activated S100β+ astrocytes, (c, g) Dense-core neuritic plaques with compact round core deposits, halos of diffuse amyloid, β-APP+ neurites, and several associated S100β+ activated astrocytes, (d, h) Dense-core, non-neuritic plaques devoid of diffuse amyloid, β-APP+ neurites, and S100β+ astrocytes. Arrowheads denote examples of S100β+ astrocytes. Bars = 15 μm.
Fig. 2.
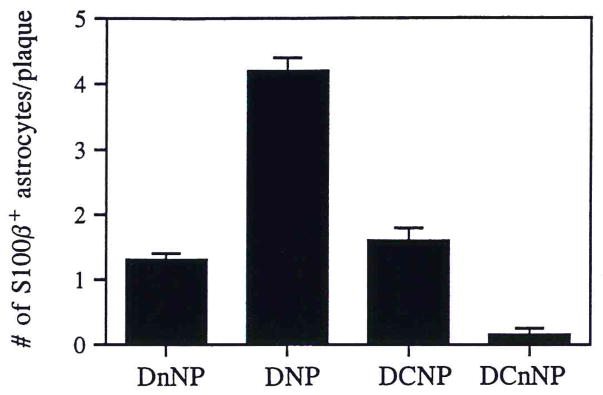
Number of S100β+ astrocytes associated with each of the 4 defined plaque types. DnNP = diffuse non-neuritic plaques; DNP = diffuse neuritic plaques; DCNP = dense-core neuritic plaques; DCnNP = dense-core, non-neuritic plaques. Data expressed as mean ± SEM for 60 plaques of each type (5 in each of 12 patients). In each case, the number of S100β+ astrocytes associated with plaque types was significantly different from that of the postulated predecessor plaque type (i.e. the plaque type to the left in the figure); p < 0.001 in each case.
TABLE 1.
Immunohistochemical Characteristics of Elements within Plaque Types in Alzheimer’s Disease
| Plaque type | Diffuse amyloid | Condensed β-amyloid | β-APP+ neurites | Number of Plaque-associated S100β+ astrocytes | Percent of plaques with associated S100β+ astrocytes |
|---|---|---|---|---|---|
| Diffuse non-neuritic | +* | none | none | 1.3 ± 0.1** | 80% |
| Diffuse neuritic | + | none | abundant | 4.2 ± 0.2 | 100% |
| Dense-core neuritic | + | + | abundant | 1.6 ± 0.2 | 90% |
| Dense-core non-neuritic | none | + | none | 0.15 ± 0.05 | 12% |
+ = present;
Values expressed as mean ± SEM.
Among neuritic plaques, those with larger β-APP+ dystrophic neurite shells had greater numbers of associated S100β+ astrocytes (Fig. 3): quantification of these parameters showed that the number of plaque-associated S100β+ astrocytes was positively correlated with the average cross-sectional area of β-APP immunoreactivity within individual plaques (r = 0.74; p = 0.001; Fig. 4). In addition, there was a positive correlation between the frequency of S100β+ astrocytes and the total tissue β-APP+ area in corresponding fields in adjacent tissue sections from 9 Alzheimer patients (r = 0.66; p < 0.05; Fig. 5).
Fig. 3.

Photomicrograph of S100β/β-APP dual-immunolabeled tissue sections showing β-amyloid precursor protein-immunoreactive dystrophic neurites (red) and associated S100β immunoreactive astrocytes (brown; arrowheads denote examples of S100β+ astrocytes) in neuritic plaques of 3 sizes. Note the greater number of S100β+ astrocytes associated with the larger plaques (b and c compared with a). Bars = 15 μm.
Fig. 4.
Positive correlation between β-APP+ dystrophic neurite cross-sectional area and the number of associated S100β+ astrocytes for 50 neuritic plaques from 7 Alzheimer patients (r = 0.74; p = 0.001).
Fig. 5.
Positive correlation between β-APP+ dystrophic neurite cross sectional area and the number of S100β+ astrocytes within plaque-rich fields in adjacent sections of parahippocampal cortex from each of 9 Alzheimer patients (r = 0.86; p < 0.05).
DISCUSSION
We have previously proposed a sequence of plaque progression in Alzheimer’s disease (6) wherein diffuse amyloid plaques are immunogenic, attracting and activating microglia that consequently overexpress IL-1. This IL-1 then activates astrocytes with consequent elaboration of the astrocytic neurite-extension-promoting cytokine, S100β, that, in turn, promotes dystrophic neurite formation in diffuse amyloid deposits. The pattern of S100β+ astrocyte distribution among different plaque types shown here parallels the distribution of IL-1+ microglia (6), supporting the idea that microglial IL-1 expression promotes astrocytic activation and S100β overexpression in amyloid deposits in Alzheimer’s disease. The present demonstration that most diffuse (non-neuritic) amyloid deposits (80%) have associated S100β+ astrocytes establishes that S100β overexpression precedes dystrophic neurite formation in these deposits. This is consistent with the idea (13) that S100β, with its neuritogenic activity, is an important early contributing factor in dystrophic neurite formation in amyloid deposits. Our demonstration of correlations between the number of plaque-associated S100β+ astrocytes and the cross-sectional area of β-APP+ dystrophic neurites, both within individual neuritic plaques and within fields of plaques in tissue sections, supports the idea that S100β is important not only in induction but also in maintenance of dystrophic β-APP+ neurites (13).
Our observation that dense-core neuritic plaques contain fewer activated S100β+ astrocytes than do diffuse neuritic plaques, together with our previous demonstration that there are fewer IL-1+ microglia in dense-core neuritic plaques than in diffuse neuritic plaques (6), suggests that the driving inflammatory response wanes as the β-amyloid condenses into dense cores. This suggests that amyloid must be present in a diffuse, non-condensed form to initiate and maintain the immunological responses that drive plaque progression. Plaque progression may terminate in the dense-core, non-neuritic plaque, a non-immunogenic structure that is devoid of diffuse amyloid, IL-1+ microglia (6), S100β+ astrocytes, and dystrophic β-APP+ neurites. These plaques are also essentially devoid of GFAP+ astrocytes, ruling out the possibility that plaque-associated astrocytes persist but no longer express detectable amounts of S100β.
The neurotrophic properties of S100β have been implicated in the development of the nervous system (16, 21). In adults, astrocytic expression of S100β is increased in conditions involving neuronal damage or dysfunction, including intractable epileptic states (22), HIV infection (23), and head trauma (24). S100β expression also increases progressively during normal aging (25). These findings suggest an important role for S100β in neuronal maintenance and repair, e.g. following injury and with the wear and tear of time. In Alzheimer’s disease this response is exaggerated, thus eliciting a pathological overgrowth of dystrophic neurites that leads to formation of neuritic plaques.
In summary, our findings are consistent with the idea that S100β is a critical pathogenic link between microglial IL-1 overexpression and the growth of dystrophic neurites in amyloid deposits in Alzheimer’s disease. Moreover, they are consistent with our hypothesis (5, 26, 27) that the critical pathological process in Alzheimer’s disease—the formation of neuritic plaques—is driven by IL-1 and the resulting cascade of acute phase responses, including astrocyte activation with overexpression of S100β.
Acknowledgments
The authors wish to thank S. Woodward and X.Q. Zhou for skilled technical assistance, and P. Free for secretarial support.
This research was supported in part by NIH AG10208, NIH AG12411, and NTH NS27414.
References
- 1.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 2.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 3.Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL. A4 protein in Alzheimer’s disease: Primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989;48:674–91. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Griffin WST, Stanley LC, Ling C, et al. Brain interleukin-1 and S100 immunoreactivity elevated in Down’s syndrome and Alzheimer’s disease. Proc Natl Acad Sci USA. 1989;86:7611–15. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin WST, Sheng JG, Gentleman SM, Graham DI, Mrak RE, Roberts GW. Microglial interleukin-1α expression in human head injury: Correlations with neuronal and neuritic β-amyloid precursor protein expression. Neurosci Letts. 1994;176:133–36. doi: 10.1016/0304-3940(94)90066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease. J Neuropathol Exp Neurol. 1995;54:276–81. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–90. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy PE, Rapapport M, Graf L. Glial fibrillary acidic protein and Alzheimer-type senile dementia. Neurology. 1980;30:778–82. doi: 10.1212/wnl.30.7.778. [DOI] [PubMed] [Google Scholar]
- 9.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–82. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 10.Mandybur TI, Chuirazzi BA. Astrocytes and the plaques of Alzheimer’s disease. Neurology. 1990;40:635–39. doi: 10.1212/wnl.40.4.635. [DOI] [PubMed] [Google Scholar]
- 11.Delacourte A. General and dramatic glial reaction in Alzheimer brains. Neurology. 1990;40:33–37. doi: 10.1212/wnl.40.1.33. [DOI] [PubMed] [Google Scholar]
- 12.Marshak DR, Pesce SA, Stanley LC, Griffin WST. Increased S100β neurotrophic activity in Alzheimer’s disease temporal lobe. Neurobiol Aging. 1991;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- 13.Sheng JG, Mrak RE, Griffin WST. S100β protein expression in Alzheimer’s disease: Potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 14.Wu JH, Sheng JG, Skinner RA, Stanley LC, Wall T, Griffin WST. Interleukin-1-(IL-1) induced expression of Alzheimer disease-related proteins in rat brain. Soc Neurosci Abs. 1993;19(Part 2):1037. [Google Scholar]
- 15.Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci USA. 1985;82:7136–39. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya A, Oppenheim RW, Prevetta D, Moore BW, Brackenbury R, Ratner N. S100 is present in developing chicken neurons and Schwann cells and promotes neuron survival in vivo. J Neurobiol. 1992;23:451–66. doi: 10.1002/neu.480230410. [DOI] [PubMed] [Google Scholar]
- 17.Van Eldik LJ, Griffin WST. S100β expression in Alzheimer’s disease: Relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223:398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 18.Roberts GW, Lofthouse R, Allsop D, et al. CNS amyloid proteins in neurodegenerative diseases. Neurology. 1988;38:1534–40. doi: 10.1212/wnl.38.10.1534. [DOI] [PubMed] [Google Scholar]
- 19.Griffin WST, Stanley LC, Yeralan O, Rovnaghi CR, Marshak DR. Methods for the study of cytokines in human neurodegenerative disease. Met Neurosci. 1993;17:268–87. [Google Scholar]
- 20.da Cunha A, Jefferson JJ, Tyor WR, Glass JD, Jannotta FS, Vitkovic L. Gliosis in human brain: Relationship to size but not other properties of astrocytes. Brain Res. 1993;600:161–65. doi: 10.1016/0006-8993(93)90415-j. [DOI] [PubMed] [Google Scholar]
- 21.Sarnat HB. Regional differentiation of the human fetal ependyma: Immunocytochemical markers. J Neuropathol Exp Neurol. 1992;51:58–75. doi: 10.1097/00005072-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Griffin WST, Yeralan O, Sheng JG, Boop FA, Mrak RE, Rovnaghi CR, Feoktistova A, Burnett BA, Van Eldik LJ. Overexpression of the neurotrophic cytokine S100β in human temporal lobe epilepsy. J Neurochem. 1995;65:228–33. doi: 10.1046/j.1471-4159.1995.65010228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanley LC, Mrak RE, Woody RC, et al. Glial cytokines as neuropathogenic factors in HIV infection: Pathogenic similarities to Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53:231–38. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kato K, Suzuki F, Morishita R, Asano T, Sato T. Selective increase in S-100β protein by aging in rat cerebral cortex. J Neurochem. 1990;54:1269–74. doi: 10.1111/j.1471-4159.1990.tb01958.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, Van Eldik LJ, Griffin WST. Human brain S100β and S100β mRNA expression increases with age: Pathogenic implications for Alzheimer’s disease. Neurobiol Aging. doi: 10.1016/0197-4580(96)00037-1. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Griffin WST, Stanley LC. Glial activation as a common denominator in neurodegenerative disease: A hypothesis in neuropathology. In: Fedoroff S, Juurlink BHJ, Doucette R, Burkholder G, editors. Biology and pathology of astrocyte-neuron interactions. Vol. 2. New York: Plenum Press; 1993. pp. 359–82. (Altschul Symposium Series). [Google Scholar]
- 27.Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s disease: Review and pathogenic implications. Human Pathol. 1995;26:816–23. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]





