Abstract
Levels of immunoreactive β-amyloid precursor protein and interleukin-1α were found to be elevated in surgically resected human temporal lobe tissue from patients with intractable epilepsy compared with postmortem tissue from neurologically unaffected patients (controls). In tissue from epileptics, the levels of the 135-kDa β-amyloid precursor protein isoform were elevated to fourfold (p < 0.05) those of controls and those of the 130-kDa isoform to threefold (p < 0.05), whereas those of the 120-kDa isoform (p > 0.05) were not different from control values. β-Amyloid precursor protein-immunoreactive neurons were 16 times more numerous, and their cytoplasm and proximal processes were more intensely immunoreactive in tissue sections from epileptics than controls (133 ± 12 vs. 8 ± 3/mm2; p < 0.001). However, neither β-amyloid precursor protein-immunoreactive dystrophic neurites nor β-amyloid deposits were found in this tissue. Interleukin-1α-immunoreactive cells (microglia) were three times more numerous in epileptics than in controls (80 ± 8 vs. 25 ± 5/mm2; p < 0.001), and these cells were often found adjacent to β-amyloid precursor protein-immunoreactive neuronal cell bodies. Our findings, together with functions established in vitro for interleukin-1, suggest that increased expression of this protein contributes to the increased levels of β-amyloid precursor protein in epileptics, thus indicating a potential role for both of these proteins in the neuronal dysfunctions, e.g., hyperexcitability, characteristic of epilepsy.
Keywords: β-Amyloid precursor protein, Epilepsy, Interleukin-1
Epilepsy is a condition characterized by neuronal hyperexcitability, but the pathogenesis of this neuronal membrane dysfunction is unclear. The β-amyloid precursor proteins (β-APPs) are a group of neuronal membrane proteins that have important developmental functions (Selkoe et al., 1988; Löffler and Huber, 1992), but the function of these molecules in adult brain is less clear. Of the several β-APP isoforms, three have been studied extensively (β-APP 770, 751, and 695), are abundant, and can be detected by western immunoblot analysis at 135, 130, and 120 kDa (Arai et al., 1990; Dooley et al., 1990). Altered expression of β-APP is widely accepted as seminal in the pathogenesis of neurodegenerative conditions such as Alzheimer’s disease (Joachim and Selkoe, 1992), Down’s syndrome (Rumble et al., 1989), and head injury (Roberts et al., 1992), with the latter an established risk factor for later development of Alzheimer’s disease (Gentleman and Roberts, 1991) and epilepsy (Armstrong, 1993). β-APP 770 and 751 contain a Kunitz-type protease inhibitor sequence that is not present in the β-APP 695 isoform (Oltersdorf et al., 1989), and secreted fragments containing this region have been shown to regulate neuronal growth (Kitaguchi et al., 1988), to enhance neuronal survival, to regulate the effects of nerve growth factor on neurite outgrowth (Whitson et al., 1989; Araki et al., 1991; Milward et al., 1992), and to modulate cell adhesion (Schubert et al., 1989). Although the potential benefits of increased expression of β-APP during neuronal development or injury are readily apparent, continued excessive expression of β-APP may cease to be beneficial, as such excessive expression has been observed in various pathological conditions (Cochran et al., 1991).
β-APP synthesis (Goldgaber et al., 1989; Donnelly et al., 1990) and processing (Buxbaum et al., 1992) are promoted by interleukin-1 (IL-1), a monokine that exists as two isoforms (IL-1α and IL-1β) (Dinarello and Wolff, 1993), both of which are synthesized and released from activated microglia (Hetier et al., 1988; Righi et al., 1989). In the periphery, IL-1 functions as an acute phase-response protein (Dinarello and Wolff, 1993), and its levels were recently shown to be elevated in brain within 12 h following head injury (Griffin et al., 1994). Although the reason for increased IL-1 levels in response to injury in brain is unknown, it may be related to the ability of IL-1 to promote neuronal survival (Brenneman et al., 1992). However, as in the periphery (Dinarello and Wolff, 1993), increases in IL-1 levels above some threshold may be injurious, as high levels of IL-1α have been shown to be toxic to neurons (Brenneman et al., 1993). In addition, IL-1 induces gliosis (an increased number of activated glia) (Giulian et al., 1988), a common neuropathological finding in epilepsy of long standing.
Based on the functions of IL-1 and β-APP established in vitro and the increased expression of these two proteins following head injury (Griffin et al., 1994), we sought to determine the levels of expression and the spatial relationship between β-APP in neurons and IL-1α in microglia in temporal lobe epilepsy. Our findings suggest a link between the expression of these two proteins and epilepsy.
MATERIALS AND METHODS
Patients and specimens
Surgically resected temporal lobe tissue was obtained fresh from eight patients (five males and three females) ranging in age from 10 to 45 years (mean, 30 years). All underwent anterior temporal lobectomy for treatment of intractable complex partial seizures that were poorly controlled despite treatment with appropriate antiepileptic drugs. Routine hematoxylin and eosin-stained sections of these resected specimens (Table 1) showed normal temporal lobe histology in seven cases and porencephalic cyst in one. The hippocampal histology was normal or showed gliotic or sclerotic changes (Table 1).
TABLE 1.
Patient information
| Case no. | Sex/age (years) | H&E histological findings (temporal lobe/hippocampus) | Postmortem interval (h) | Postoperative outcome Engle class (month) or cause of death in Ca |
|---|---|---|---|---|
| EP 1 | M/21 | Normal/normal | I (24) | |
| EP 2 | M/43 | Normal/normal | II (30) | |
| EP 3 | M/14 | Normal/minimal gliosis | I (29) | |
| EP 4 | F/17 | Normal/minimal gliosis | II (29) | |
| EP 5 | F/41 | Normal/mild gliosis | II (34) | |
| EP 6 | M/32 | Normal/Ammon’s horn sclerosis | I (28) | |
| EP 7 | M/10 | Normal/Ammon’s horn sclerosis | I (27) | |
| EP 8 | F/14 | Porencephalic cyst | I (23) | |
| C 1 | M/66 | Normal/normal | 3.5 | Pneumonia, pulmonary emboli |
| C 2 | F/59 | Normal/normal | 14.0 | Cervical carcinoma, pyelonephritis, sepsis |
| C 3 | M/47 | Normal/normal | 23.5 | Myocardial infarction |
| C 4 | M/68 | Normal/normal | 1.5 | Gastric carcinoma |
| C 5 | F/10 | Normal/normal | 1.2 | Rhabdomyosarcoma |
| C 6 | F/36 | Normal/normal | 15.0 | Adenocarcinoma, peritonitis |
| C 7 | M/50 | Normal/normal | 18.0 | Malignant melanoma |
| C 8 | M/9 | Normal/normal | 14.0 | Pulmonary emboli |
EP, epilepsy; C, control; H&E, routine hematoxylin and eosin staining.
Class I, free of disabling seizures; Class II, rare disabling seizures (Engle et al., 1993).
Control temporal lobe tissue samples were obtained postmortem from eight patients (five males and three females) who had no known neurological disease (controls), ranging in age from 9 to 66 years (mean, 43 years; Table 1). The average postmortem interval was 11.3 h (Table 1).
For histological study, temporal lobe tissues were fixed in 10% formalin, embedded in paraffin, sectioned at 10 μm, and prepared for immunohistochemical analysis and routine neuropathological examination. For western immunoblot analysis and protein assays, tissue samples (~250 mg) were diluted in 4 volumes of ice-cold phosphate-buffered saline, containing 1 mM phenylmethylsulfonyl fluoride, and homogenized by applying 25 strokes with a size AA tissue grinder (Thomas Scientific, Swedsboro, NJ, U.S.A.). Following centrifugation at 10,000 g for 15 min at 4°C, the supernatant was aliquoted and stored at −85°C.
Western immunoblot
The relative levels of β-APP in samples of temporal lobe from controls and patients with epilepsy were determined by western immunoblot analysis as previously described (Griffin et al., 1993; Sheng et al., 1993). In brief, 10 μg of protein was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis at 150 V for 1 h and transferred to polyvinylidene difluoride filters (Millipore Corp.) at 30 V overnight. Filters were incubated in blocking buffer (Tropix, Bedford, MA, U.S.A.) for 1 h and then incubated with monoclonal (clone 22C11) anti-β-APP (anti-Alzheimer precursor protein A4; Boehringer-Mannheim Biochemica), diluted 1:100 in blocking buffer, for 1 h; goat anti-mouse alkaline phosphatase conjugate (Tropix; diluted 1:25,000) for 15 min; and substrate, disodium 3-[4-methoxyspiro(1,2-dioxetane-3,2′-tricyclo[3.3.1.1]decan)-4-yl] phenyl phosphate, for 5 min. Chemiluminescence was detected by exposing immunoblots to x-ray film (XAR; Kodak) for 5 min. The relative levels of β-APP were determined by scanning the film using a Beckman DU-62 spectro-photometric analysis program.
Immunohistochemical study
For immunohistochemical reactions, 10-μm-thick paraffin sections were deparaffinized in xylene, rehydrated in graduated ethanol solutions, and then permeabilized in 0.5% Triton X-100 (10 min) and 0.2 M HCl (20 min). Endogenous peroxidase was blocked with 3% H2O2 in 97% methanol for 30 min. Primary antibodies—mouse anti-human β-APP (1:10), rabbit anti-human β-amyloid (1:10), or rabbit anti-human IL-1α (1:20)—were diluted in 2% normal goat serum in Tris-buffered saline and incubated on the sections overnight at room temperature. The link antibodies—anti-mouse or anti-rabbit IgG (Cappel)—were diluted in 2% nonimmune goat serum in Tris-buffered saline and incubated on the section for 30 min. The secondary antibodies—goat anti-mouse or goat anti-rabbit peroxidase–antiperoxidase (DAKO) —were incubated for 30 min on each section. The sections were then washed in three changes, 5 min each, of Tris-buffered saline and developed in 3,3-diaminobenzidine tetrahydrochloride (Sigma) solution to yield a brown reaction product. The sections were counterstained with Mayer’s hematoxylin. β-APP-positive neurons and IL-1α-positive cells were counted at a magnification of 250 diameters in each of five microscopic fields of gray matter of each case.
Statistical analysis
Average tissue levels of the three isoforms of β-APP and the mean numbers of β-APP-positive neurons and IL-1α-positive microglia were compared in samples from epileptics and controls. The statistical significance of differences between epileptics and controls was determined using Student’s t test for unpaired data.
RESULTS
Tissue levels of β-APP
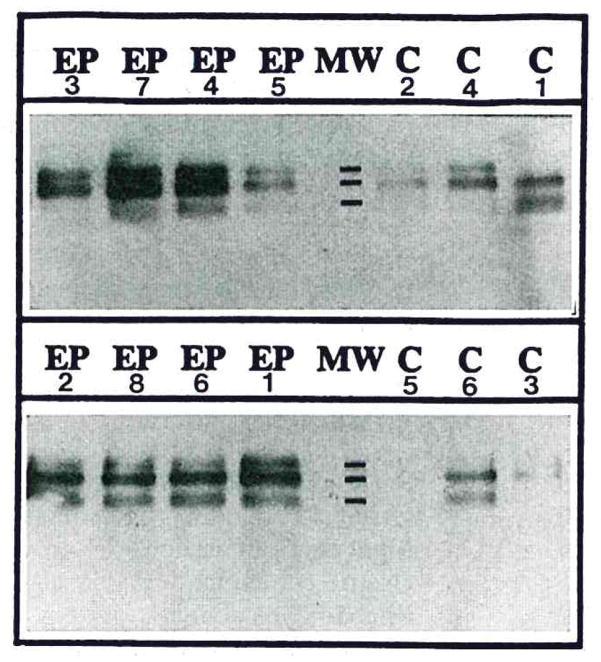
β-APP isoforms were visualized at 135, 130, and 120 kDa on x-ray films using western immunoblot transfer chemiluminescence labeling (Fig. 1). These molecular mass bands correspond well with those previously reported for β-APP 770, 751, and 695 (Arai et al., 1990). The immunoreactive bands detected here by chemiluminescence were at similar molecular weights to those previously reported using chromogen labeling (Dooley et al., 1990).
FIG. 1.
Western immunoblots depict β-APP-immunoreactive products in samples of resected temporal lobes from eight epileptic patients (EP) and in samples from analogous regions of temporal lobe collected postmortem from six controls (C). Monoclonal anti-β-APP antibody (clone 22C11) sensitively labeled three bands on western immunoblots at molecular weight (MW) marks shown at 135K, 130K, and 120K.
The average level of the 130-kDa β-APP isoform in homogenates from epilepsy was threefold that of controls, and the level of the 135-kDa β-APP isoform was fourfold that of controls (Table 2; p < 0.05). However, the level of the 120-kDa isoform was not significantly different from that of controls (p > 0.05). These results indicate that epilepsy is characterized by elevated levels of β-APP 770 and 751, the same two isoforms that appear to be elevated in level in Alzheimer’s disease (Tanaka et al., 1989).
TABLE 2.
Levels of three β-APP isoforms in temporal lobe from epileptics and controls
| Patients (no.) | β-APP 770 (135 kDa) | β-APP 751 (130 kDa) | β-APP 695 (120 kDa) |
|---|---|---|---|
| Control (6) | 0.08 ± 0.03 | 0.20 ± 0.05 | 0.22 ± 0.04 |
| Epileptic (8) | 0.37 ± 0.11a | 0.61 ± 0.12a | 0.20 ± 0.03b |
Data are mean ± SEM values of optical density units derived from densitometric scanning of β-APP-immunoreactive bands visualized on x-ray films of western immunoblots, prepared as described in Materials and Methods.
p < 0.05,
p > 0.05 when compared with the respective control values.
Immunoreactivity of β-APP and IL-1α in cells
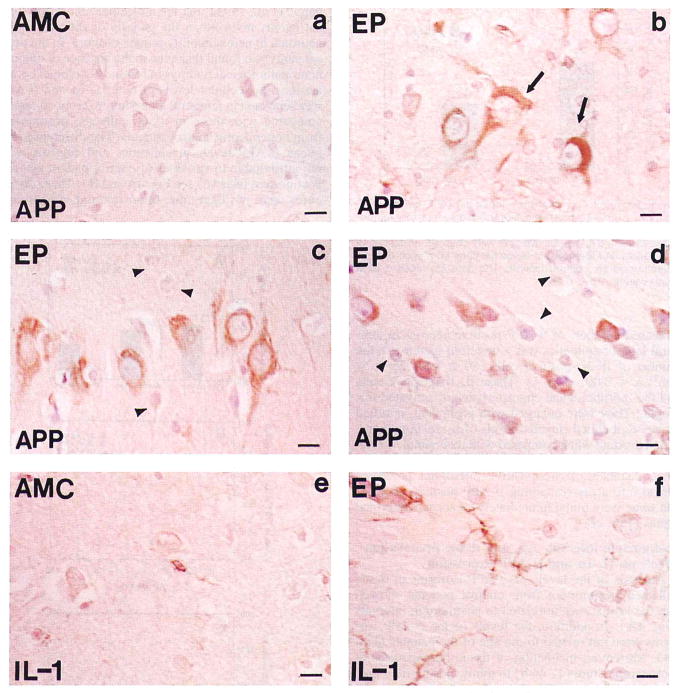
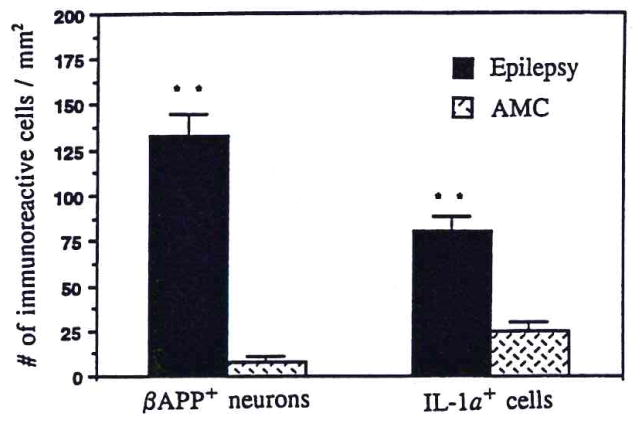
There were obvious increases in the numbers of β-APP-positive neurons in tissue sections from epileptics (Fig. 2). β-APP-positive neurons were most notable in hippocampus and in temporal lobe cortical layers III, IV, and V, where most of the large pyramidal neurons contained high levels of β-APP (Fig. 2b and c). In contrast, there was less β-APP immunoreactivity in the small and medium neurons of these layers (Fig. 2c). However, in layer VI some of the smaller pyramidal neurons were also intensely β-APP immunoreactive (Fig. 2d). The β-APP immunoreaction product appeared in these cells as dense particles or granules within the neuronal cell cytoplasm and prominent neuronal processes. In some cases, β-APP appeared tangle-like, filling the perikaryal cytoplasm and dislocating the nucleus (Fig. 2b) as neurofibrillary tangles do in Alzheimer’s disease (Bancher et al., 1989; Yamaguchi et al., 1990). In none of the eight epileptics was extracellular deposition of β-amyloid detectable by either Congo red staining or immunohistochemical reaction. In contrast to their appearance in tissue sections from cortical regions of epileptics, neurons in identically treated tissue sections from controls were non-or only weakly β-APP immunoreactive (Fig. 2a vs. b and c). The numbers of β-APP-positive neurons were 133 ± 12/mm2 in epilepsy and 8 ± 3/mm2 in controls (mean ± SEM; p < 0.001; Fig. 3).
FIG. 2.
Photomicrographs of β-APP (APP; a–d) and IL-1α (IL-1; e and f) immunoreaction product in cells in tissue sections of temporal lobe from epileptics (EP; b–d and f) and controls (AMC; a and e). Simultaneous immunohistochemical reaction with anti-β-APP or anti-IL-1α antibodies was performed on 10-μm-thick paraffin-embedded temporal lobe tissue sections from epileptics and controls, as described in Materials and Methods, a: β-APP-immunoreacted tissue sections of temporal lobe gray matter from control (AMC) illustrate the paucity of immunoreaction product in neurons (layer V). b: β-APP-positive neurons in analogous sections of temporal lobe (layer V) from an epileptic (EP 7) demonstrate the relatively high levels of immunoreaction product (brown); β-APP-positive intracellular tangle-like structures were observed in some neurons (arrow), c and d: β-APP-positive neurons in tissue sections from case EP 3 show large pyramidal neurons (c) in temporal lobe cortical layer V, containing high levels of β-APP immunoreaction product (brown) relative to the levels in small and medium neurons (c and d; arrowheads). Some small neurons in layer VI in case EP 3 (d) also contained high levels of β-APP immunoreaction product (brown), whereas others did not (arrowhead), e: Tissue section shows the very low IL-1α immunoreactivity characteristic of cells in gray matter of controls (AMC). f: Tissue section from an epileptic (EP 7) illustrates the increase in the number, size, and immunoreactive intensity of IL-1α-positive cells (brown) in epilepsy; many were found immediately adjacent to neurons in layer V of temporal lobe. Bar = 15 μm.
FIG. 3.
Bar graph compares the number of β-APP-positive neurons/mm2 and IL-1α-positive cells/mm2 in gray matter of temporal lobe from epileptic patients and controls (AMC). β-APP-positive neurons and IL-1α-positive cells were counted, as described in Materials and Methods, in tissue sections from each of eight epileptic and six control patients. The data are mean ± SEM (bars) values. **p < 0.001.
Accompanying the elevation of β-APP levels and increased numbers of β-APP-positive neurons in temporal lobe of epileptics was a threefold increase in the number of IL-1α-positive cells (80 ± 8 vs. 25 ± 5/mm2; p < 0.001; Fig. 3). These IL-1α-positive cells had the morphological characteristics of activated microglia: They were enlarged with prominent, ramified processes and had elevated levels of IL-1α immunoreaction product when compared with their small, nonactivated counterparts in controls (Fig. 2e and f). In some instances, IL-1α-positive microglial processes spread into areas containing β-APP-positive neurons, and some were found immediately adjacent to neuronal somas (Fig. 2f).
Postmortem interval, age, and tissue preparation effects on IL-1α and β-APP expression
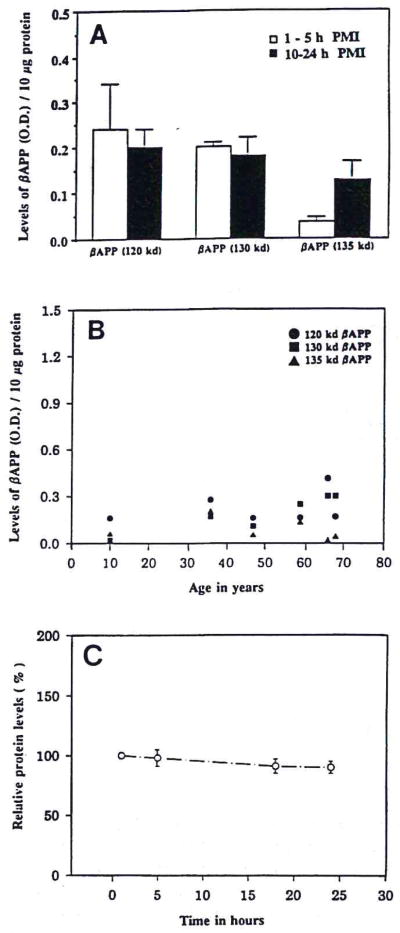
Analysis of the levels of β-APP isoforms in tissue collected postmortem from control patients showed that the levels were unrelated to postmortem intervals (Fig. 4A). In addition, the levels of the β-APP isoforms were not related to the age of the patients (Fig. 4B). Moreover, incubation of tissue homogenates at room temperature (1–24 h) to mimic postmortem protein loss did not significantly effect the level of total protein in the samples (Fig. 4C). Similarly, IL-1α levels in activated microglia were not affected by either agonal state or postmortem interval, as we have previously shown (Stanley et al., 1994).
FIG. 4.
A: Bar graph correlates the levels of three β-APP isoforms in temporal lobe samples with postmortem intervals (PMIs) of control patients. Data are mean ± SEM (bars) values for three patients in each group. B: Dot graph correlates the levels of three β-APP isoforms in temporal lobe samples with ages of control patients. C: Loss of measurable protein in homogenates of surgical temporal lobe samples of epileptic patients incubated at room temperature between 1 and 24 h. Data are mean ± SEM (bars) values for six samples relative to the initial (1-h) value.
DISCUSSION
Temporal lobe epilepsy is a common and often refractory syndrome characterized by complex partial seizures and associated electrophysiological abnormalities in hippocampus and other limbic structures of temporal lobe. Sometimes nonspecific neuropathological findings such as neuronal loss and gliosis are noted in epilepsy (Pollen and Trachtenberg, 1970; Castiglioni and Castiglioni, 1986; Lanerolle et al., 1989; Brigande et al., 1992), but the molecular neuropathological events involved in the genesis of the underlying neuronal hyperexcitability remain obscure. In the present study, we found that even in the absence of obvious neuropathological findings in routine histological preparations, the cellular levels of both IL-1α and β-APP were elevated in temporal lobe samples from epileptics compared with those in tissue collected postmortem from nonepileptic control patients. The differences between β-APP levels in epileptics and controls were not attributable to variables of control patient age and postmortem interval (see Fig. 4A and B). These differences were not likely due to postmortem protein loss as homogenates incubated at room temperature showed <0.5% loss of total protein/h in the first 24 h (Fig. 4C). Such decreases are insufficient to account for the profound differences between epileptic tissue and control tissue reported here. Another possible confounding factor is agonal states of the control patients. Three of the six control patients used here for western immunoblot analysis of β-APP died with infectious diseases: pneumonia, pyelonephritis with sepsis, and peritonitis. Tissue levels of β-APP in these three patients (Fig. 1C1, C2, and C6) were not lower than in those dying without systemic infections, suggesting that metabolic alterations associated with terminal infections were not a confounding factor in this study. Elevated β-APP levels in temporal lobe neurons have been demonstrated in postmortem tissue from patients with early Alzheimer’s disease (Roberts et al., 1993), and we previously showed that irrespective of postmortem interval and agonal state, IL-1α levels are elevated in activated microglia in HIV-positive individuals (Stanley et al., 1994). Additional evidence against a confounding effect of postmortem changes on our results may be found in the similarity in the levels of the 120-kDa β-APP isoform in epileptics and controls.
Increased β-APP expression has been shown in several neuropathological conditions (Cochran et al., 1991). Our findings of high levels of β-APP 770 and 751 (but not β-APP 695) in epileptics suggest that these two β-APP isoforms perform unique functions. In addition, our finding of differential expression of β-APP according to neuron size in temporal lobe suggests differential susceptibility of neuronal subclasses in epilepsy. The increased levels of the β-APP 751 and 770 suggest that increased expression of these two isoforms is a component of the response of dysfunctional neurons to epilepsy. β-APP has been localized to synaptic sites in normal brain and is known to interact with cell surface receptors, to activate second messenger systems, to inhibit proteases, to promote cell adhesion, and to regulate cell growth (Kang et al., 1987; Saitoh et al., 1989; Schubert et al., 1991). Thus, alterations in β-APP expression in neurons might have adverse effects on synaptic transmission or on synaptic activity or could alter cell–cell or cell–matrix interactions that may be important in the pathophysiology of temporal lobe epilepsy. Elevated levels of β-APP 770 and 751 could also contribute to neuronal dysfunction by stimulating aberrant growth of processes, thus perhaps explaining the neuritic sprouting characteristic of temporal lobe epilepsy (Represa et al., 1989). In either case, increased expression of β-APP may be a useful marker for seizure-induced neuronal damage in epilepsy.
Findings in Alzheimer’s disease, where excessive expression of β-APP is associated with deposition of β-amyloid (Ohgami et al., 1992; Roberts et al., 1993), might be interpreted to suggest that excessive expression of β-APP in neurons in epilepsy would lead to deposition of β-amyloid. However, we did not detect deposits of β-amyloid in tissue from epileptics by either Congo red staining or β-amyloid immunoreactivity. An immunohistochemical pattern of elevated β-APP expression without β-amyloid accumulation has been reported following excitotoxic lesioning in rat brain (Nakamura et al., 1992) and in several pathological conditions in humans (Cochran et al., 1991). At present, we cannot rule out the possibility that prolonged excessive expression of neuronal β-APP in epilepsy may promote deposition of β-amyloid in affected regions later in the course of this disorder. Such is postulated to occur following head injury (Roberts et al., 1992), a known risk factor for later development of epilepsy (Armstrong, 1993). The increased expression of IL-1α in microglia in epilepsy, shown here, may be the result of neuronal injury or loss, as has been postulated in other diseases where neuronal injury and cell death are accompanied by elevated IL-1 expression (Griffin et al., 1989, 1994; Griffin and Stanley, 1993). IL-1 in epilepsy may contribute to neuronal dysfunction by upregulating β-APP expression (Goldgaber et al., 1989) or by inducing astrogliosis (Giulian et al., 1988), perhaps via IL-1 receptors recently discovered on the astrocyte cell surface (Ban et al., 1993). Our finding of increased levels of β-APP and IL-1α in epilepsy, together with known functions of IL-1, suggests that excessive expression of IL-1 may be causally related to the increased expression of β-APP. In addition, the intimate spatial relationship between neurons and activated IL-1α-positive microglia suggests that high levels of IL-1α in the local environs of neurons may contribute directly to the neuronal dysfunction; IL-1α was recently shown to be neurotoxic at high levels in vitro (Brenneman et al., 1993).
In conclusion, we propose that our findings of increased expression of β-APP and IL-1α in resected temporal lobe of patients with refractory complex partial seizures are related to the neuropathophysiological manifestations of epilepsy. Our findings also allow us to conclude that the functions of β-APP 751 and 770 are more importantly related to events in epilepsy than are other isoforms of this abundant membrane protein. These results suggest new directions for research in epileptogenesis.
Acknowledgments
We would like to thank C. R. Rovnaghi, S. Woodward, B. Barnett, and A. Vondran for skillful technical assistance, P. Free for secretarial support, and Drs. S. Al-Mefty and S. Flanigan for other support. This work was supported in part by grants AG 10208 and NS 27414 from the National Institutes of Health.
Abbreviations used
- β-APP
β-amyloid precursor protein
- IL-1
interleukin-1
References
- Arai H, Lee VM, Otvos L, Jr, Greenberg BD, Lowery DE, Sharma SK, Schmidt ML, Trojanowski JQ. Defined neurofilament, tau and β-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaque. Proc Natl Acad Sci USA. 1990;87:2249–2253. doi: 10.1073/pnas.87.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52:433–443. doi: 10.1097/00005072-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Ban EM, Sarlieve LL, Haour FG. Interleukin-1 binding sites on astrocytes. Neuroscience. 1993;52:725–733. doi: 10.1016/0306-4522(93)90421-b. [DOI] [PubMed] [Google Scholar]
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Page SW, Schultzberg M, Thomas FS, Zelazowski P, Burnet P, Avidor R, Sternberg EM. A decomposition product of a contaminant implicated in L-tryptophan eosinophilia myalgia syndrome affects spinal cord neuronal cell death and survival through stereospecific, maturation and partly interleukin-1-dependent mechanisms. J Pharmacol Exp Ther. 1993;266:1029–1035. [PubMed] [Google Scholar]
- Brigande JV, Wieraszko A, Albert MD, Balkema GW, Seyfried TN. Biochemical correlates of epilepsy in the E1 mouse: analysis of glial fibrillary acidic protein and gangliosides. J Neurochem. 1992;58:752–760. doi: 10.1111/j.1471-4159.1992.tb09782.x. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni ET, Castiglioni AJ., Jr . Astrocytes in epilepsy. In: Fedoroff S, Vernadakis A, editors. Astrocytes. Vol. 3. Academic Press; London: 1986. pp. 401–424. [Google Scholar]
- Cochran E, Bacci B, Chen Y, Patton A, Gambetti P, Autilio-Gambetti L. Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer’s disease. Am J Pathol. 1991;139:485–489. [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Donnelly RJ, Friedhoff AJ, Beer B, Blume AJ, Vitek MP. Interleukin-1 stimulates the beta-amyloid precursor protein promoter. Cell Mol Neurobiol. 1990;10:485–495. doi: 10.1007/BF00712843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley NP, Gauthier S, Durham HD. Antibody to beta-amyloid precursor protein recognizes an intermediate filament-associated protein in Alzheimer’s and control fibroblasts. J Neurosci Res. 1990;33:60–67. doi: 10.1002/jnr.490330108. [DOI] [PubMed] [Google Scholar]
- Engle J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engle J, editor. Surgical Treatment of the Epilepsies. Raven Press; New York: 1993. pp. 609–622. [Google Scholar]
- Gentleman SM, Roberts GW. Risk factors in Alzheimer’s disease. Br Med J. 1991;304:118–119. doi: 10.1136/bmj.304.6819.118-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC. Glial activation as a common denominator in neurodegenerative disease: a hypothesis in neuropathophysiology. In: Fedoroff S, Juurlink BHJ, Doucette R, Burkholder G, editors. Biology and Pathology of Astrocyte–Neuron Interactions. Plenum Press; New York: 1993. pp. 359–382. [Google Scholar]
- Griffin WST, Stanley LC, Ling C, White L, Macleod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S100β immunoreactivity are elevated in Down’s syndrome and Alzheimer’s disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC, Yeralan O, Rovnaghi CR, Marshak DR. Method for the study of cytokines in human neurodegenerative disease. Methods Neurosci. 1993;17:268–287. [Google Scholar]
- Griffin WST, Sheng JG, Gentleman SM, Graham DI, Mrak RE, Roberts GW. Microglial interleukin-1α expression in human head injury: Correlations with neuronal and neuritic β-amyloid precursor protein expression. Neurosci Lett. 1994 doi: 10.1016/0304-3940(94)90066-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetier E, Ayala J, Denefle P, Bousseau A, Rouget P, Mallat M, Prochiantz A. Brain macrophages synthesize interleukin-1 and interleukin-1 mRNAs in vitro. J Neurosci Res. 1988;21:391–397. doi: 10.1002/jnr.490210230. [DOI] [PubMed] [Google Scholar]
- Joachim CL, Selkoe DJ. The seminal role of β-amyloid in the pathogenesis of Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1992;6:7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Crzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H. Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitor activity. Nature. 1988;331:530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Koh JY, Yang LL, Cotman CW. Amyloid protein increased the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990;533:315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer’s disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Löffler J, Huber G. β-Amyloid precursor protein isoforms in various rat brain regions and during brain development. J Neurochem. 1992;59:1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Takeda M, Niigawa H, Hariguchi S, Nishimura T. Amyloid β-protein precursor deposition in rat hippocampus lesioned by ibotenic acid injection. Neurosci Lett. 1992;136:95–98. doi: 10.1016/0304-3940(92)90656-r. [DOI] [PubMed] [Google Scholar]
- Ohgami T, Kitamoto T, Tateishi J. Alzheimer’s amyloid precursor protein accumulated within axonal swellings in human brain lesions. Neurosci Lett. 1992;136:75–78. doi: 10.1016/0304-3940(92)90651-m. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Fritz LC, Schenk DB, Lieberburg I, Johnson-Wood KL, Beattie EC, Ward PJ, Blacher RW, Dovey HF, Sinha S. The secreted form of the Alzheimer’s amyloid precursor protein with Kunitz domain is protease nexin-II. Nature. 1989;341:144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Pollen DA, Trachtenberg MC. Neuroglia: gliosis and focal epilepsy. Science. 1970;167:1252–1253. doi: 10.1126/science.167.3922.1252. [DOI] [PubMed] [Google Scholar]
- Represa A, Robain O, Tremblay E, Ben-Ari Y. Hippocampal plasticity in childhood epilepsy. Neurosci Lett. 1989;99:351–355. doi: 10.1016/0304-3940(89)90472-2. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19:1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI. βA4 amyloid protein deposition in the brain after head trauma. Lancet. 1992;338:131–133. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Nash M, Ince PG, Royston MC, Gentleman SM. On the origin of Alzheimer’s disease: a hypothesis. Neuroreport. 1993;4:709–710. doi: 10.1097/00001756-199301000-00001. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol Sci. 1991;12:430–435. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup R, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer disease. N Engl J Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Sundsmo M, Roch JM, Kimura N, Cole G, Schubert D, Oltersdorf T, Schenk DB. Secreted form of amyloid β-protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;56:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Schubert D, Jin LW, Saitoh T, Cole G. The regulation of amyloid β protein precursor secretion and its modulatory role in cell adhesion. Nature. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer’s βA4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563:184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. β-Amyloid precursor protein of Alzheimer’s disease occurs as 110 to 135 kilodalton membrane-associated proteins in neuronal and non-neuronal tissue. Proc Natl Acad Sci USA. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Shirabe S, Nishiyama N, Schwartz JP. Alterations in striatal glial fibrillary acidic protein (GFAP) expression in response to 6-hydroxydopamine-induced denervation. Exp Brain Res. 1993;95:450–456. doi: 10.1007/BF00227138. [DOI] [PubMed] [Google Scholar]
- Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang SX, Marshak DR, Nelson SJ, Griffin WST. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53:231–238. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Shiojiri S, Takahashi Y, Kitaguchi N, Ito H, Kameyama M, Kimura J, Nakamura S, Ueda K. Tissue-specific expression of three types of β-protein precursor mRNA: enhancement of protease inhibitor-harboring type in Alzheimer’s disease brain. Biochem Biophys Res Commun. 1989;165:1406–1414. doi: 10.1016/0006-291x(89)92760-5. [DOI] [PubMed] [Google Scholar]
- Whitson JS, Selkoe DJ, Cotman CW. Amyloid β protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Ishiguro K, Shoji M, Yamazaki T, Nakazato Y, Ihara Y, Hirai S. Amyloid β/A4 protein precursor is bound to neurofibrillary tangles in Alzheimer-type dementia. Brain Res. 1990;537:318–322. doi: 10.1016/0006-8993(90)90377-n. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neuronotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]