Abstract
Neuronal injury associated with amyloid plaque progression in Alzheimer disease was examined using TUNEL combined with β-amyloid immunolabeling. There was a progressive increase in the frequency of TUNEL-positive neurons associated with plaque types representing a hypothesized sequence of plaque evolution, from 20% of neurons not associated with plaques to 40%, 70–80%, and 100% of neurons in diffuse, neuritic, and dense-core non-neuritic plaques, respectively. The total number of neurons associated with end-stage, dense-core, non-neuritic plaques declined by 70% (per unit plaque area) compared with neuritic plaque forms. This decline, together with the fact that virtually all of those remaining were TUNEL-positive, suggests that neuronal cell damage increases as plaques evolve from diffuse to more complex forms and that eventually all plaque-associated neurons are lost. This novel demonstration of neurotoxicity associated with amyloid plaque formation and progression suggests that plaque-associated neuronal injury is a major cause of neuronal loss in Alzheimer disease.
Keywords: Alzheimer disease, β-amyloid plaque progression, Neuronal cell injury and loss, TUNEL
INTRODUCTION
Neuronal cell injury and loss are major neuropathological features of Alzheimer disease. β-Amyloid, the principal component of the diagnostic plaques of Alzheimer disease, has been shown to be neurotoxic to hippocampal neurons in vitro (1), suggesting that the β-amyloid in Alzheimer plaques may contribute to neuronal loss in Alzheimer disease.
The TUNEL technique allows identification of cellular genomic damage in situ in paraffin sections, and is applicable to investigations using human postmortem material. This technique identifies free 3′-OH DNA ends in a template- independent manner to label DNA strand breaks (2), thus identifying injured cells (3). TUNEL+ neurons are prominent in postmortem brain tissue sections from Alzheimer patients (4–10).
We sought to define the relationship between the formation and progression of β-amyloid plaques and neuronal cell injury and death in Alzheimer disease, using β-amyloid immunohistochemistry combined with TUNEL. β-Amyloid plaques were classified, according to a hypothesized sequence of plaque evolution, as diffuse amyloid deposits (the putative initial stage of amyloid plaque formation; 11), as diffuse neuritic and dense-core neuritic plaque forms (2 intermediate and more complex plaque stages), and as dense-core, non-neuritic plaques (end-stage or burned out plaques), based on morphological criteria. Using these approaches, we find a significant correlation between plaque stage and incidence of TUNEL positivity in associated neurons.
MATERIALS AND METHODS
Patients
Tissue sections from hippocampus and adjacent parahippocampal cortex at the level of the lateral geniculate nucleus were obtained postmortem from 9 clinically demented patients (4 females, 5 males; age range 65–83 years; postmortem interval 8 ± 3 h) with neuropathological confirmation of Alzheimer disease according to CERAD criteria (12). Control tissue from the same region was obtained postmortem from 5 neurologically normal patients (4 males, 1 female; age range 61–93 years; postmortem interval 7 ± 2 h). Tissue was obtained from brains fixed in phosphate-buffered 20% formalin for 2 weeks. Tissue blocks were embedded in paraffin and sections were cut at 10 µm thickness.
For all patients, paraffin sections were prepared for tissue blocks from (a) the middle frontal gyrus just rostral to the temporal tip, (b) the superior and middle temporal gyri at the level of mammillary body, (c) the inferior parietal lobule, (d) the occipital lobe to include calcarine cortex, parastriate field, and peristriate regions (usually two blocks); (e) the amygdala, (f) the entorhinal/transentorhinal cortex at the level of the uncus; and (g) hippocampus and adjacent parahippocampal gyrus at the level of the lateral geniculate body. These were stained with either hematoxylin and eosin or with the Sevier-Munger modification of the Bielschowsky technique. Alzheimer disease was confirmed according to CERAD criteria for all Alzheimer patients included in this study. Sections from control patients revealed small numbers of neuritic plaques in 3 of the 5 control patients. One patient, age 69, had “moderate” numbers (CERAD criteria) of neuritic plaques focally in the hippocampus and in the amygdaloid nucleus, and rare, isolated neuritic plaques in mesial temporal cortical areas. A second patient, also age 69, had sparse neuritic plaques in the amygdaloid nucleus and parahippocampal gyrus. A third patient, age 93, had 3 neuritic plaques in the hippocampal formation and none in the parahippocampal gyrus. The 2 remaining control patients, ages 61 and 67, had no neuritic plaques in any sections examined. The incidence of neurofibrillary tangles within the hippocampal formation and the parahippocampal gyrus was assessed for all patients. Within the hippocampus, neurofibrillary tangles were “frequent” (CERAD criteria) in 8 of the 9 Alzheimer patients, and were not found in 3 of the 5 control patients. Two of the control patients (aged 67 and 93) and 1 Alzheimer patient (aged 73) had rare, isolated neurofibrillary tangles in the hippocampal formation. Within the parahippocampal gyrus, 5 of the 9 Alzheimer patients had “frequent” neurofibrillary tangles, 1 had “moderate” numbers of tangles, 1 had “sparse” tangles, 1 had rare, isolated tangles; and 1 had no tangles. Three of the 5 control patients had no neurofibrillary tangles within the parahippocampal gyrus. One control patient (aged 69) had rare, isolated tangles, and 1 control patient (aged 93) had “moderate” numbers of neurofibrillary tangles in the parahippocampal gyrus.
Immunohistochemistry
Immunohistochemical labeling was performed as previously described (4, 13). The primary antibodies were either a monoclonal mouse anti-human β-amyloid antibody (a gift from Dr G.W. Roberts, Opine Consultancy, Cambridge, UK) or a polyclonal rabbit anti-bovine glial fibrillary acidic protein antibody (GFAP; DAKO, Carpinteria, Calif). These were diluted 1:50 or 1:100, respectively, with 2% normal goat serum in tris-buffered saline, and were incubated on the sections overnight at room temperature. Mouse peroxidase-anti-peroxidase labeled with fast red was used to visualize β-amyloid-immunoreactive structures.
TUNEL
TUNEL was performed on β-amyloid-immunoreacted tissue sections using an In Situ Cell Death Detection Kit-POD (Boeh-ringer-Mannheim Biochemica, Indianapolis, In) according to manufacturer’s protocol, except that converter-POD was applied to the section at a 1:2 dilution (in 100 mM Tris-HCl, 150 mM NaCl, pH 7.5) and sections were incubated in the humidified chamber for 20 rather than 30 minutes (min), at 37°C. TUNEL positive (TUNEL+) neurons were visualized after incubation with metal-enhanced diaminobenzidine substrate (Boehringer Mannheim Biochemica). Sections were then lightly counterstained with hematoxylin. We have previously found no obvious correlation between numbers of TUNEL+ neurons and postmortem interval, for either Alzheimer patients or controls, over a postmortem interval range of 1.5–15 hours (4).
Quantification of TUNEL+ and TUNEL− Neurons
Microscopical images of plaque-rich areas of parahippocampal gyrus were captured using a CCD video camera attached to a Macintosh computer. β-Amyloid plaques were classified as diffuse, diffuse neuritic, dense-core neuritic, and dense-core non-neuritic plaques as previously described (14) (Fig. 1). A neuron was defined as plaque-associated if the cell cytoplasm lay within or directly abutted upon the β-amyloid deposit. Plaque-associated TUNEL+ and TUNEL− neurons were counted in 10 plaques of each defined plaque type in tissue sections from each Alzheimer patient. Non-plaque-associated TUNEL+ and TUNEL− neurons were counted in the same fields (a total of 803 neurons). β-Amyloid plaques were identified in parahippocampal gyrus of only 2 of the 5 control patients, and these were sparse. A total of 60 β-amyloid plaques from control patients were analyzed.
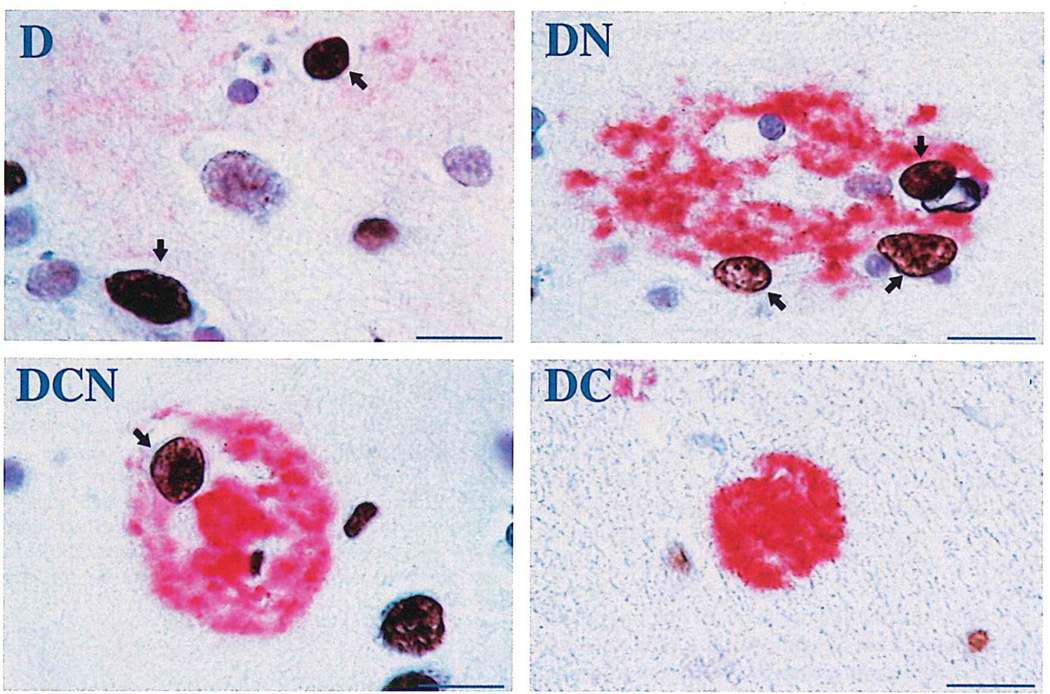
Fig. 1.
Illustrations of TUNEL+ neurons (brown color, open arrows) associated with each of 4 defined β-amyloid (red) plaque types in Alzheimer disease. D = diffuse (non-neuritic) amyloid plaque. DN = diffuse, neuritic β-amyloid plaque. DCN = dense-core, neuritic β-amyloid plaque. DC = dense-core, non-neuritic β-amyloid plaque. Note the non-plaque-associated TUNEL+ neuron next to the DCN plaque. Bars = 15 µm.
To distinguish TUNEL+ neurons from TUNEL+ astrocytes, we performed a preliminary analysis of 200 TUNEL+ cells in sections double-labeled using TUNEL and immunohistochemistry for GFAP. NIH image software (version 1.62) was used to determine nuclear diameters for TUNEL+ cells, and astrocytes were determined to have a nuclear diameter of 8 µm or less. Based on this, TUNEL+ and TUNEL− cells were accepted as neurons only if their nuclear diameter exceeded 8 µm.
Statistical Analysis
Student’s t-test (unpaired data) was used to assess statistical significance of differences between numbers of TUNEL+ and TUNEL− neurons associated with different plaque types, representing consecutive steps in the hypothesized sequence of plaque evolution, e.g. the number of TUNEL+ neurons in diffuse non-neuritic plaques was compared with the number in diffuse neuritic plaques and these latter were, in turn, compared with the number in dense-core neuritic plaques, and so forth. Student’s t-test (unpaired data) was also used to assess statistical significance of differences between numbers of TUNEL+ and TUNEL− neurons associated with diffuse β-amyloid plaques and those not associated with β-amyloid plaques.
RESULTS
TUNEL positivity was present in 23 ± 5% (mean ± SD) of non—plaque-associated neurons in plaque-rich fields of mesial temporal cortex from Alzheimer patients. In the plaque-containing fields of the 2 control patients in which parahippocampal amyloid plaques were found, the incidence of TUNEL positivity among non-plaque-associated neurons (19 ± 1%) was similar to that seen in Alzheimer tissue. Small numbers of TUNEL+ neurons were also found in corresponding areas of those control patients without parahippocampal amyloid plaques. In contrast, plaque-associated TUNEL+ neurons were twice as frequent (expressed per unit plaque area) in tissues from Alzheimer patients as those from control patients (4.6 ± 0.6 vs 1.9 ± 0.5 TUNEL+ neurons/1,000 µm2 plaque area, p = 0.03). In both patient populations, TUNEL+ neurons showed peripheral nuclear chromatin condensation with apparent preservation of nuclear membrane integrity (see Fig. 1).
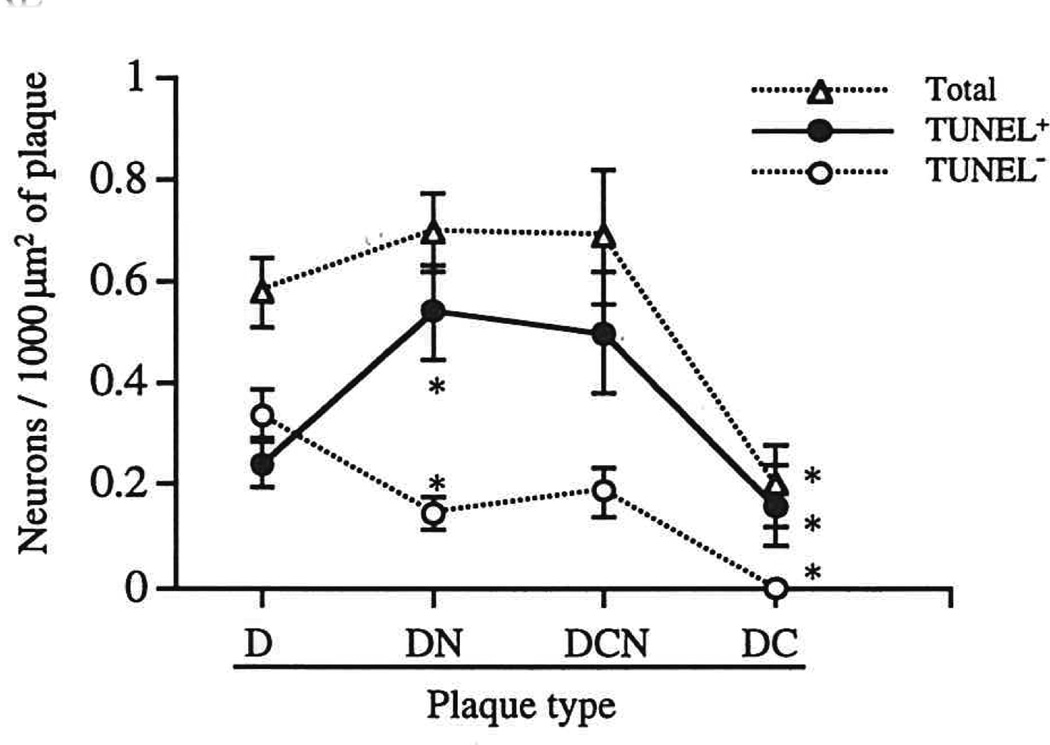
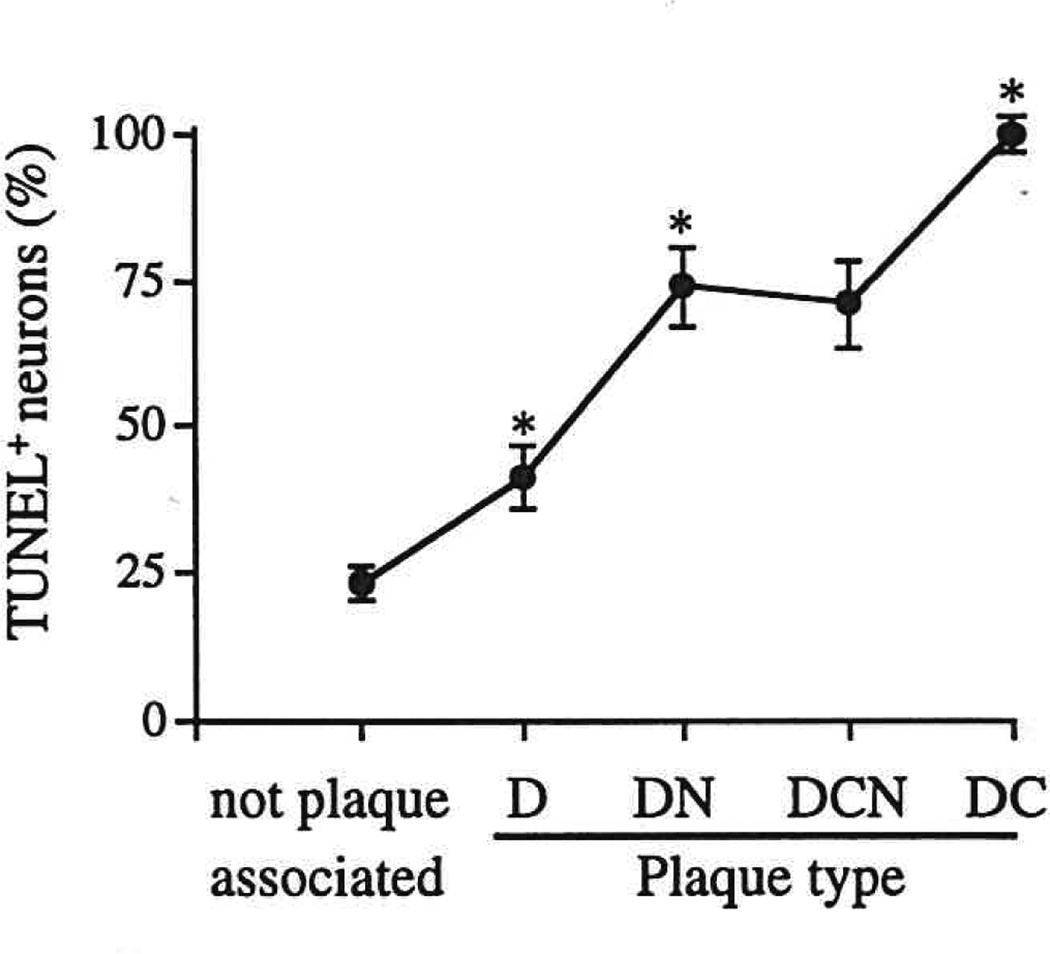
TUNEL+ and TUNEL− neurons were differentially distributed among different plaque types in Alzheimer tissue (Figs. 2, 3). In diffuse non-neuritic amyloid deposits, thought to represent the initial stage of plaque development (11), only a minority (41%) of neurons were TUNEL+. In contrast, for all other plaque types, the majority of associated neurons were TUNEL+: 70% to 80% of neurons associated with neuritic plaques and 100% of neurons associated with dense-core non-neuritic plaques. The total (TUNEL+ plus TUNEL−) number of plaque-associated neurons (expressed per unit plaque area) was constant across the first 3 stages of plaque evolution (diffuse non-neuritic, diffuse neuritic, and dense-core neuritic plaques), but declined significantly (a 70% to 80% decrease) in end-stage, dense-core, non-neuritic plaques (p < 0.05).
Fig. 2.
Numbers of plaque-associated neurons, and of TUNEL+ and TUNEL− neurons, per 1,000 µm2 of plaque area, in each of the 4 defined amyloid plaque types in Alzheimer disease. * = value is significantly different from corresponding value for preceding data point (i.e. the data point to the left), p < 0.05 or better. D = diffuse (non-neuritic) amyloid plaque. DN = diffuse, neuritic β-amyloid plaque. DCN = dense-core, neuritic β-amyloid plaque. DC = dense-core, non-neuritic β-amyloid plaque.
Fig. 3.
TUNEL+ neurons, expressed as percent of all neurons, not associated with amyloid plaques, and associated with each of the 4 defined amyloid plaque types in Alzheimer disease. * = value is significantly different from corresponding value for preceding data point (i.e. the data point to the left), p < 0.05 or better. D = diffuse (non-neuritic) amyloid plaque. DN = diffuse, neuritic β-amyloid plaque. DCN = dense-core, neuritic β-amyloid plaque. DC = dense-core, non-neuritic β-amyloid plaque.
DISCUSSION
Neuronal injury, assessed by TUNEL, is prominent in Alzheimer disease (4–10). We have previously shown that progressive stages of neurofibrillary tangle formation in Alzheimer disease are accompanied by increasing frequency of TUNEL positivity in affected neurons, confirming that tangle formation is responsible in part for neuronal injury and DNA damage (4). However, the extent of neuronal damage and loss in Alzheimer disease greatly exceeds the extent of neurofibrillary tangle formation (9), suggesting that there are additional mechanisms of neuronal injury. Our present results, showing that plaque-associated neurons in Alzheimer disease are more frequently TUNEL-positive than non-plaque-associated neurons, together with our finding that non—plaque-associated neurons show similar frequencies of TUNEL positivity in Alzheimer and control tissue, suggest that much of the neuronal injury and DNA damage occurring in Alzheimer disease occurs in neurons closely associated with amyloid plaques.
Neurons associated with diffuse non-neuritic plaques, the putative initial stage of plaque formation in Alzheimer disease (11), showed a frequency of TUNEL positivity nearly twice that found in non-plaque-associated neurons (41% vs 23%). This suggests that deposition of diffuse amyloid in Alzheimer disease is accompanied by injury to neurons in the vicinity of the deposit. Diffuse amyloid plaques are thought to evolve into neuritic β-amyloid plaque forms, the diagnostic histopathologic lesions of Alzheimer disease. These neuritic plaque forms show an even greater frequency of TUNEL positivity in associated neurons (70—80%), further supporting the idea that plaque progression is accompanied by increasing neurotoxicity. Our finding that dense-core, non-neuritic plaques (thought to represent a “burned out” end stage of plaque progression) show decreased numbers of associated neurons, all of which were TUNEL+, suggests that plaque progression is accompanied by death and disappearance of plaque- associated neurons.
These results are the first demonstration of a progressive association between stage of β-amyloid plaque formation and prevalence of neuronal cell injury. Thus, a significant proportion of the neuronal cell injury and loss in Alzheimer disease may be attributable to neurotoxicity associated with evolution of amyloid plaques. Such neurotoxicity might result from the evolving physical characteristic of amyloid itself, from the actions of other proteins codeposited with amyloid, or from soluble factors elaborated by associated activated glial cells (15).
ACKNOWLEDGMENTS
Supported by NIH Grants AG10208 and AG12411.
The authors thank S. Woodward for skilled technical assistance.
REFERENCES
- 1.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neuro-toxic effects of amyloid β protein: Reversal by tachykinin neuro-peptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 2.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tominaga T, Kure S, Narisawa K, Yoshimoto T. Endonuclease activation following focal ischemic injury in the rat brain. Brain Res. 1993;608:21–26. doi: 10.1016/0006-8993(93)90768-i. [DOI] [PubMed] [Google Scholar]
- 4.Sheng JG, Mrak RE, Griffin WST. Progressive neuronal DNA damage associated with neurofibrillary tangle formation in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:323–328. doi: 10.1097/00005072-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Anderson A, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer’s disease colocalization with c-jun immunoreactivity, relationship to brain area and effect of postmortem delay. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer’s disease patients. Neurobiol Aging. 1990;11:169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 7.Troncoso JC, Sukhov RR, Kawas CH, Koliatsos VE. In situ labeling of dying cortical neurons in normal aging and in Alzheimer’s disease: Correlations with senile plaques and disease progression. J Neuropath Exp Neurol. 1996;55:1134–1143. doi: 10.1097/00005072-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuro-Report. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Lassmann H, Bancher C, Breitschopf H, et al. Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89:35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- 10.Smale G, Nichols NY, Brady DR, Finch CE, Horton WE. Evidence for apoptotic cell death in Alzheimer’s disease. Exp Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 11.Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL. A4 protein in Alzheimer’s disease: Primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989;48:674–691. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 13.Sheng JG, Mrak RE, Griffin WST. S100β protein expression in Alzheimer disease: Potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 14.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease, significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Griffin WST, Sheng JG, Royston MC, et al. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]