Abstract
Activated microglia containing IL-1α-immunoreactive (IL-1α+) product were increased 3-fold in number in the acute phase following head injury, a risk factor for later development of Alzheimer's disease, and this increase was correlated with a 7-fold increase in the number of neurons with elevated β-amyloid precursor protein (β-APP) levels (R = 0.78; P < 0.05). Furthermore, clusters of β-APP+ dystrophic neurites present in these patients were invariably associated with activated IL-1α+ microglia. These findings suggest that early overexpression of IL-1α: and β-APP is a priming event for later neuropathological changes evident at end stages of Alzheimer's disease.
Keywords: Interleukin-1α, β-Amyloid precursor protein, Head injury, Alzheimer's disease
Head injury is an identified risk factor for later development of Alzheimer's disease [6,7,9,29]. Neuropathological changes similar to those seen in Alzheimer's disease, e.g., β-amyloid-containing plaques, dystrophic neurites and intracellular neurofibrillary tangles, may be seen following a single severe head injury [3,20] or following multiple injuries such as those sustained by boxers [19] or victims of domestic violence [22]. Recent studies have shown an increase in β-amyloid precursor protein (β-APP) immunoreactivity in neurons [8] and the appearance of numerous β-amyloid protein-immunopositive diffuse plaques within days following a severe head injury in patients as young as 10 years of age [20]. These alterations are not confined to areas of primary contusion or secondary damage, but are found throughout the cortical ribbon. In addition, increased β-APP immunoreactivity and mRNA (specifically the β-APP 751 transcript) has been reported in animal models of brain injury [7]. These findings not only suggest a molecular link between head injury and Alzheimer's disease, but also support a role for β-APP expression in the brain's acute phase response to injury [9,20].
Interleukin-1 (IL-1) is a monocyte-derived cytokine long recognized as an initiator of acute phase responses in injured non-neural tissue, where chronic overexpression of IL-1 can produce pathologic reactions [5]. In the central nervous system, IL-1 is synthesized and secreted by microglia, and excessive microglial expression ofIL-1 has been implicated in the neuropathogenesis of Alzheimer's disease [12,13,18,23,25,27,30]. Of potential relevance to this neuropathogenesis is the fact that IL-1 in vitro can induce expression of β-APP [11] and can stimulate the processing of β-APP via the secretory cleavage pathway [2]. In this study, we establish a correlation between activated IL-1-immunoreactive (IL-1+) microglia and β-APP+ neurons in the early, reactive phase following head injury in humans.
We evaluated formalin-fixed, paraffin-embedded sections of mesial temporal lobe, including hippocampus from seven patients (4 males, 3 females), 23–65 years of age, dying between 12 hours and 10 days following head injury; these patients were previously described by us [7,20]. Control tissue from six patients (5 males, 1 female), of similar ages (47–78 years), without significant neurological or neuropathological alterations, was analyzed concurrently. Positive controls were analogous tissue sections from 4 Alzheimer patients (59–73 years). Glial fibrillary acidic protein (GFAP) immunoreactivity was used to differentiate astrocytic populations from those expressing IL-1α+ cells. We used a monoclonal anti-β-APP antibody (Clone 22C11, Boehringer-Mannheim, Indianapolis, IN) that recognizes β-APP but not β-amyloid deposits (results not shown). A polyclonal antibody specific for the α isoform of IL-1 (Cistron, Pine Brook, NH) was chosen based on our previous studies [27] showing cellular localization of this uncleaved isoform. Antibodies were diluted in Tris-buffered saline containing 2% normal goat serum: rabbit anti-human IL-1α (Cistron, Pine Brook, NH), 1:20; rabbit anti-bovine GFAP (DAKO, Carpenteria, CA), 1:300; and mouse anti-β-APP, 1:10, and tissue sections were processed as previously described, for single immunohisto-chemical labeling [14] and for dual labeling, according to manufacturer's protocol in DAKO's double immunolabeling kit (K-665).
Data extracted from immunopositive glia, neurons, and neurites in five 250 diameter fields per tissue section from each case were expressed as the mean number of immunopositive entities per mm2 per case. Correlation coefficients were calculated using the non-parametric test of Spearman. Significance was calculated using Student's t-test.
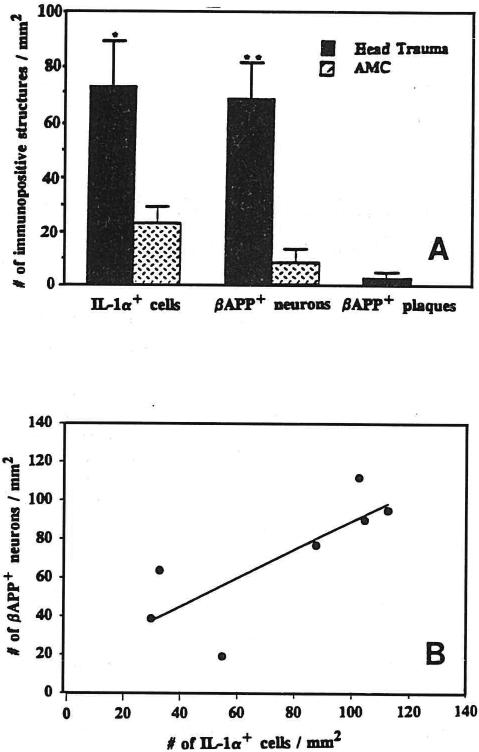
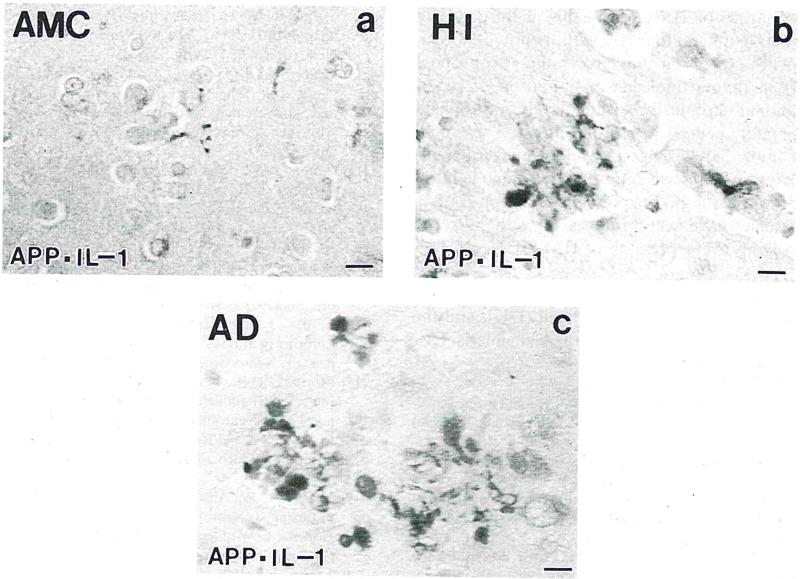
IL-1α+ cells were found throughout the sampled temporal cortex in brains from head-injured patients, and their numbers were increased 3-fold relative to those in control samples (P < 0.05; Fig. 1A). In contrast to their counterparts in analogous samples from controls, IL-1α+ cells in head-injured patients were activated, being enlarged with euchromatic nuclei and prominent processes [4] (Fig. 2A–C). Cells recognized by the IL-1α antibody used here also showed lectin binding but seldom co-express GFAP [27], or neuron or oligodendrocyte markers (results not shown), suggesting that they are microglia. As we previously reported [8], and under the conditions used here, neuronal β-APP immunoreactivity is barely detectable in neurons in tissue sections from control patients. In contrast, the number of β-APP+ neurons in tissue from head-injured patients was increased by 7 fold over controls (P < 0.01, Fig. 1A). These cells were diffusely distributed throughout the sampled temporal cortex. In addition to the widespread cortical distribution of β-APP+ neurons, there were clusters of β-APP+ structures (Fig. 2B) that were similar in appearance to those of neuritic plaques in Alzheimer's disease (Fig. 2C) and middle-aged Down's patients (not shown). In head-injured patients these structures were invariably associated with substantial numbers of activated IL-1α+ microglia (Fig. 2B,C).
Fig. 1.
A: numbers (mean ± S.E.M.) of IL-1α+ microglia and β-APP+ neurons in mesial temporal lobe tissue sections from 7 head-injured and 6 control patients.*P < 0.05; **P < 0.01. B: correlation between numbers of activated IL-1α+ microglia and β-APP+ neurons in 7 head-injured patients (R = 0.78; P < 0.05).
Fig. 2.
β-APP+ (red) neurites and IL-1α+ (brown) microglia in dual-labeled immunohistochemically reacted tissue sections from mesial temporal lobe of control (A), head-injured (B), and Alzheimer's (C) patients. Bar = 15 μm.
The numbers of IL-1α+ microglia and β-APP+ neurons and neurites in head-injured patients did not correlate with either patient age or survival time. However, the number of IL-1α+ microglia did correlate with the number of β-APP+ neurons and neurites in these patients (Fig. 1B, R = 0.78; P < 0.05). These results are consistent with the idea that elevated levels of IL-1α in vivo may be instrumental in effecting increases in β-APP expression in neurons.
The elevation in IL-1 expression reported here may be related to the brain's attempt to ameliorate damage through induction of molecular processes leading to repair or regeneration, as IL-1 is known to promote neuron survival in vitro [1] and may be involved in restoration of synaptic connectivity [28]. Similarly, a reparative function for β-APP after brain injury is suggested by its purported role in neural cell development [16] and its presence at synaptic sites [26]. Although the exact temporal and functional relationship between the increase in IL-1α expression inactivated microglia and the appearance of β-APP+ neurons and dystrophic neurites is not resolved in our study, correlative increases in the expression of these two proteins in adjacent cellular elements so soon after injury suggest that these are early and key events in the process of attempted repair and regeneration following head injury. The ability of IL-1 to induce excessive expression [11] and stimulate processing of β-APP [2] suggests that the elevated levels of IL-1a shown here may be directly responsible for the increased amounts of β-APP. Alternatively, or in addition, IL-1 may contribute indirectly to β-APP expression through astrocyte activation [10] with consequent synthesis and release of neurotrophic factors such as the astrocytic cytokine S100β, thus favoring neuron survival and neurite outgrowth [17], with concomitant membrane synthesis and β-APP expression.
These molecular and cellular events may be acutely necessary to promote neuronal survival and regeneration of synaptic connections following head injury. They may also, however, form the basis for the increased risk of later neurodegeneration in these patients. For instance, microglial activation in response to an injury may prime them and their target cells such that the post-traumatic brain is overly susceptible to additional physical, metabolic, or aging-associated insults. Further studies of longer-lived survivors of head trauma may elucidate the nature and extent to which such priming together with other factors contribute to development of Alzheimer's disease subsequent to head injury.
Similarities found between the topographical alliance of activated IL-1α+ microglia and β-APP+ neurites in plaque-like structures in head injury and in Alzheimer's disease suggest that the development of neuritic plaques and of Alzheimer's disease in long-term survivors of head injury may be related to microglial IL-1α promotion of neurite growth and neuritic β-APP expression after head injury. This alliance is reflected in the correlation found between the numbers of IL-1α+ microglia and β-APP+ neurons. These similarities and correlations in head-injured patients are consistent with the idea that chronic, above-threshold expression and processing of β-APP, at the behest of IL-1 and possibly other priming factors, could favor β-amyloid deposition, a seminal event in Alzheimer pathogenesis [15). Based on the swift and robust nature of IL-1α expression in response to head injury shown here, together with its potential relation to β-APP expression and processing, we propose that IL-1 is a pivotal molecule in initiation of repair functions and, at chronically elevated levels, in propagation of ultimately neurodegenerative effects. These considerations immediately suggest novel avenues for treatment to prevent progressive neurodegeneration via intervention at the level of IL-1 expression or action [21,24]. Similar strategies have been proposed for systemic conditions involving overexpression of IL-1 [5].
Acknowledgments
Supported in part by USPHS NIH AG10208 and NS27414, the Medical Research Council (UK), and the Haberdashers Company. SMG is an Alzheimer's Disease Society Research Fellow.
References
- 1.Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J. Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 2.Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer α/A4 amyloid protein precursor. Proc. Natl Acad. Sci. USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinton J, Ambler MW, Roberts GW. Post traumatic Alzheimer's disease: Predominance of a single plaque type, Neuropathol. Appl. Neurobiol. 1991;17:69–74. doi: 10.1111/j.1365-2990.1991.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 4.da Cunha A, Jefferson JJ, Tyor WR, Glass JD, Jannotta FS, Vitkovic L. Gliosis in human brain: relationship to size but not other properties of astrocytes. Brain Res. 1993;600:161–165. doi: 10.1016/0006-8993(93)90415-j. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA, Wolff SM. The role of Interleukin-1 in disease. New Engl. J. Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 6.Gautrin D, Gautheier S. Aizheimer's disease: environmental factors and etiologic hypotheses. Can. J. Neural. Sci. 1989;16:375–387. doi: 10.1017/s0317167100029425. [DOI] [PubMed] [Google Scholar]
- 7.Gentleman SM, Graham DI, Roberts GW. Molecular pathology of head injury: altered β-APP metabolism and the aetiology of Alzheimer's disease. The Neurobiology of Ischemic Brain Damage. In: Kogure K, Siesjö BK, editors. Progress in Brain Research. Vol. 96. Elsevier; Amsterdam: 1993. pp. 237–246. [DOI] [PubMed] [Google Scholar]
- 8.Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. β-Amyloid precursor protein (β-APP) as a marker for axonal injury after head injury. Neurosci. Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- 9.Gentleman SM, Roberts GW. Risk factors in Alzheimer's disease. Br. Med. J. 1991;304:118–119. doi: 10.1136/bmj.304.6819.118-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giulian D, Woodward J, Young DB, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J. Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RG, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endoihelial cells. Proc. Natl. Acad. Sci. USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin WST, Stanley LC. Glial activation as a common denominator in neuro-degenerative disease: a hypothesis in neuropathophysiology. In: Fedoroff S, Juurlink BHJ, Doucette R, Burkholder G, editors. Biology and Pathology of Astrocyte-Neuron Interactions, Altschul Symposium Series. Vol. 2. Plenum; New York, NY: 1993. pp. 359–382. [Google Scholar]
- 13.Griffin WST, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin-1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl Acad. Sci. USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin WST, Stanley LC, Yeralan O, Rovnaghi CR, Marshak DR. Methods for the study of cytokines in human neurodegenerative disease. In: De Souza EB, editor. Methods in Neuroscience. Vol. 17. Academic Press; Orlando: 1993. pp. 268–287. [Google Scholar]
- 15.Joachim CL, Selkoe DJ. The seminal role of β-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1992;6:7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Loffler J, Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J. Neurochem. 1992;59:1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- 17.Marshak DR, Pesce SA, Stanley LC, Griffin WST. Increased S100β neuro-trophic activity in Alzheimer disease temporal lobe. Neurobiol. Aging. 1991;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- 18.McGeer PL, Rogers J. Anti-inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology. 1992;42:447–449. doi: 10.1212/wnl.42.2.447. [DOI] [PubMed] [Google Scholar]
- 19.Roberts GW, Allsop D, Bruton CJ. The occult aftermath of boxing. J. Neural. Neurosurg. Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts GW, Gentleman SM, Lynch A, Graham DI. βA-4 amyloid protein deposition in the brain after head injury. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 21.Roberts GW, Gentleman SM, Stefan MD, Royston MC. Alzheimer's disease: prospects for treatment. Lancet. 1993;341:432. doi: 10.1016/0140-6736(93)93022-s. [DOI] [PubMed] [Google Scholar]
- 22.Roberts GW, Whitwell HL, Ackland PR, Bruton CJ. Dementia in a punch drunk wife. Lancet. 1990;i:918–919. doi: 10.1016/0140-6736(90)90520-f. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P. Complement activation by β-amyloid in Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royston MC, Rothwell NJ, Roberts GW. Alzheimer's disease: Pathology to potential treatment? TiPS. 1992;13:131–133. doi: 10.1016/0165-6147(92)90047-a. [DOI] [PubMed] [Google Scholar]
- 25.Rozemuller JM, Eikelenboom P, Pals ST, Starn FC. Microglial cells around amyloid plaques in Alzheimer's disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci. Lett. 1989;101:288–292. doi: 10.1016/0304-3940(89)90547-8. [DOI] [PubMed] [Google Scholar]
- 26.Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563:184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- 27.Stanley LC, Griffin WST. Localization of IL-1α and IL-1β in diseases with gliosis, dementia, and immune suppression. Soc. Neurosci. Abstr. 1990;16:1345. [Google Scholar]
- 28.Steward O, Tomasulo R, Torre E, Trimmer P. Reorganization of neural connections following CNS injury: is synaptic reorganization initiated by the changes in neuronal activity which occur following injury? Brain Dysfunct. 1992;5:27–49. [Google Scholar]
- 29.Tomlinson BE. Ageing and the dementias. In: Adams JH, Duchen LW, Arnold E, editors. Greenfield's Neuropathology. 5th edn. Oxford University Press; New York: 1992. pp. 1284–1410. [Google Scholar]
- 30.Vandenabeele P, Fiers W. Is amyloidogenesis during Alzheimer's disease due to an IL-1-/IL-6-mediated ‘acute phase response’ in the brain? Immunol. Today. 1991;12:217–219. doi: 10.1016/0167-5699(91)90032-O. [DOI] [PubMed] [Google Scholar]