Abstract
Brain injury due to birth asphyxia is the major cause of death and long-term disabilities in newborns. We determined whether intranasal pyrrolidine dithiocarbamate (PDTC) could provide neuroprotection in neonatal rats after brain hypoxia-ischemia (HI). Seven-day old male and female Sprague-Dawley rats were subjected to brain HI. They were then treated by intranasal PDTC. Neurological outcome were evaluated 7 or 30 days after the brain HI. Brain tissues were harvested 6 or 24 h after the brain HI for biochemical analysis. Here, PDTC dose-dependently reduced brain HI-induced brain tissue loss with an effective dose (ED)50 at 27 mg/kg. PDTC needed to be applied within 45 min after the brain HI for this neuroprotection. This treatment reduced brain tissue loss and improved neurological and cognitive functions assessed 30 days after the HI. PDTC attenuated brain HI-induced lipid oxidative stress, nuclear translocation of nuclear factor κ-light-chain-enhancer of activated B cells, and various inflammatory mediators in the brain tissues. Inhibition of inducible nitric oxide synthase after brain HI reduced brain tissue loss. Our results suggest that intranasal PDTC provides neuroprotection possibly via reducing inflammation and oxidative stress. Intranasal PDTC may have a potential to provide neuroprotection to human neonates after birth asphyxia.
Keywords: brain hypoxia-ischemia, inflammation, neonates, neuroprotection, pyrrolidine dithiocarbamate
Introduction
The World Health Organization estimates that 4 to 9 million neonates suffer from birth asphyxia each year in the world (World-Health, 2003). This leads to about 1.2 million deaths and the same number of infants with severe disability (Bang et al., 2005; Minino et al., 2007). These deaths and disabilities are mostly due to hypoxic-ischemic (HI) brain injury. The long-term neurological or cognitive disabilities include cerebral palsy, epilepsy and mental retardation (Lynch and Nelson, 2001; Sran and Baumann, 1988; Sreenan et al., 2000). Thus, it is very important to identify methods to reduce HI brain injury in neonates. However, clinically practical methods to reduce this brain injury, especially in low-income countries, have not been established yet.
There are at least two types of insults that contribute to HI brain injury. Hypoxia and ischemia, the primary insults, interrupt energy supply to cells and cause cell injury. Hypoxia-ischemia and the subsequent reoxygenation/reperfusion induce production of a broad range of “toxic chemicals”, such as free oxygen species and inflammatory cytokines. These “toxic chemicals” can cause additional cell injury (Allen and Bayraktutan, 2009; Lakhan et al., 2009; Lipton, 1999). Studies have shown that anti-oxidants and anti-inflammatory reagents are neuroprotective (Allen and Bayraktutan, 2009; Lakhan et al., 2009; Lampl et al., 2007; Lipton, 1999; Yamaguchi et al., 1998).
Pyrrolidine dithiocarbamate (PDTC) is an anti-oxidant and anti-inflammatory agent (Liu et al., 1999). It is a small molecule and relatively cheap. PDTC has been evaluated as an antiviral agent for humans (Si et al., 2005). A pre-clinical toxicity study has shown that PDTC is a safe drug (Chabicovsky et al., 2010). It can reduce focal brain ischemic injury in young adult rats (Nurmi et al., 2004a; Nurmi et al., 2004b). This protective effect occurs even if the application of PDTC is at 6 h after the onset of transient focal brain ischemia in those rats (Nurmi et al., 2004b). PDTC also reduces brain injury after HI in neonatal rats (Nurmi et al., 2006). However, the previous studies only assessed the neuroprotective effect within 7 days after brain ischemia and the drug was given intraperitoneally. Our recent data showed that oral PDTC started after transient focal brain ischemia improved neurological outcome assessed 1 or 2 months later in young adult rats (Li et al., 2012). However, the PDTC application routes used in the previous studies are difficult to apply in neonates immediately after birth asphyxia.
Intranasal application (in the form of nasal drop or spray) of a drug can be performed immediately and easily by virtually any care provider without the need of any special equipment in the setting of birth asphyxia. The absorption is quick. Intranasal application of PDTC reaches its maximal concentration in the blood within 5 min (Chabicovsky et al., 2010). The amount of drugs that get into the brain after nasal application is similar to that for the drugs to be given intravenously (Bagger and Bechgaard, 2004). A significant amount of PDTC is found in the brain after nasal application (Chabicovsky et al., 2010). Thus, we hypothesize that intranasal PDTC reduces brain injury after neonatal brain HI. To test this hypothesis, we used a well-established rat model of neonatal brain HI. Inflammatory mediators and oxidative stress indices in the brain were measured to determine the possible mechanisms for the neuroprotection induced by intranasal PDTC.
Materials and Methods
All experimental protocols were approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All surgical and experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996. Efforts were made to minimize the number of animals used and their suffering. Our manuscript was written up in accordance with the Animal Research: Reporting in vivo Experiments. Total 290 animals completed the intended study in this project.
Neonatal Cerebral Hypoxia/Ischemia Model
Cerebral HI was performed as we described before (Zhao et al., 2007). Briefly, 7-day-old male and female Sprague-Dawley rats were anesthetized by isoflurane in 30% O2–70% N2. Their right common carotid arteries were permanently ligated with a double 7–0 surgical silk. The neonates were returned to their cages with the mothers for 3 h and then placed in a chamber containing humidified 8% O2–92% N2 for 2 h at 37°C.
Drug Treatment
Sprague–Dawley rats were randomly assigned to receive intranasal PDTC (Sigma-Aldrich, St. Louis, MO, USA) or normal saline at a preset time-point. PDTC was dissolved in normal saline just before the application. The volume applied was 400 μl/kg body weight and was applied as drops to nostrils.
Some rats received intraperitoneal 1 mg/kg N-(3-(aminomethyl)benzyl) acetamidine (1400W, Enzo Life Sciences Inc., Farmingdale, NY, USA), a specific inducible nitric oxide synthase (iNOS) inhibitor, or saline at 30 min before the brain HI, 30 min before the hypoxia or 15 min after the brain HI. 1400W was dissolved in normal saline. The injected volume was from 0.15 to 0.2 ml per rat.
Brain Injury/Loss Quantification
As we described before (Zhao and Zuo, 2004), rat brains were harvested 7 days after the brain HI. The hindbrain was removed from the cerebral hemispheres and the two hemispheres were separated and weighed. The weight ratio of right to left hemispheres was calculated.
Brain Histopathology
This was performed as we described previously (Zhao et al., 2007). One month after the brain HI, rats were sacrificed under deep isoflurane anesthesia and transcardially perfused with cold normal saline. Their brains were fixed with 10% neutral buffered formalin overnight and then paraffin embedded. Five-micrometer-thick coronal sections at approximately 3.3 mm caudal to bregma were obtained and subjected to Nissl staining. These sections were examined by an observer blinded to the group assignment of the sections. The cerebral areas in each of the hemispheres were quantified by using National Institutes of Health Image 1.60 (NIH, Bethesda, MD, USA). The cerebral area ratio of the right hemisphere to the left hemisphere was calculated and used to reflect brain tissue loss in the right hemisphere after brain HI. The number of Nissl staining positive cells (neurons) in a high magnification field (×400, ~0.2 mm2) in the CA1 and CA3 regions was counted. Three determinations, each on different locations in these two brain regions, were performed and averaged to yield a single number (Neuronal density) for each brain region of individual rat. The ratio of neuronal density in the right hippocampus to that in the left hippocampus of the same animal was then calculated.
Barnes Maze
Barnes maze was used to test animals’ spatial learning and memory. Eight days before the rats were euthanized for brain histopathology, they started to be tested in a Barnes maze equipped with ANY-Maze video tracking system (San Diego Instruments, San Diego, CA) as we described previously (Li et al., 2012) with minor modifications. The test was administered and evaluated by a person blinded to the group assignment of rats. Rats were placed in the middle of a circular platform with 20 equally spaced holes. One of the holes was connected to a dark chamber that was called target box. Rats were encouraged to find this box by aversive noise (85 dB) and bright light (200W) shed on the platform. The protocol involved training sessions on 4 consecutive days that consisted of four training sessions on each day with a 15-min inter-session interval. Each session ended when the rat entered the target hole or after 3 min had elapsed. On fifth day, one trial was performed to test the animal’s retention. All trials were recorded and analyzed by using the ANY-Maze tracking system to calculate the latency for the rat to enter the target hole.
Motor Coordination Evaluation
Evaluation of motor coordination was started at 4 days before the rats were euthanized for brain histopathology as we described before (Li and Zuo, 2009). Rats were placed on a rotarod apparatus whose speed increased from 4 to 40 rpm in 5 min. All rats were trained for two consecutive days, three times per day, before the formal tests. The latency and speed of rat’s falling off the rotarod apparatus were recorded. The speed–latency index (latency in seconds x speed in rpm) of each test was calculated. Each rat was tested for three times in the formal test. The mean index value of the three trials was used to reflect the motor coordination functions of each rat.
Fear conditioning
Fear conditioning was used to determine hippocampus-dependent and hippocampus-independent learning and memory. After motor coordination evaluation was completed, fear conditioning test was performed as we described before (Lin and Zuo, 2011) and administered and evaluated by a person blinded to the group assignment of rats. Briefly, each rat was placed in a Plexiglas conditioning training chamber wiped with 70% alcohol. After a 3-min baseline exploratory period in the chamber, rats received 3 tone (2000 Hz, 90 db)-shock (1 mA, 2 s) pairings separated by 1 min between each pairing in a relatively dark room. Twenty-four hours after the training session, each rat was placed again in the training chamber for a period of 8 min in the absence of tone and foot shock to test its contextual fear conditioning. The amount of time with freezing behavior was recorded in an 8-s interval. Two hour later, the rat was placed in a test chamber that had different context and smell from the first test chamber (this second chamber was wiped with 1% acetic acid) in a relatively light room. After a 3-min exploratory period in this new chamber, a 30-s tone (2000 Hz, 90 db) was applied. Freezing behavior also was scored during this tone-related test period.
Western Blot Analysis
The right frontal cerebrum (anterior to bregma −1.5 mm) was harvested 6 h (for the nuclear expression of p65) or 24 h (for the expression of all other proteins) after the brain HI or the right hippocampal dentate gyrus was harvested at 24 h after the brain HI. For whole cell protein extracts, the cerebral tissues were homogenated in RIPA buffer (Cat. No. 89901; Thermo Scientific, Worcester, MA, USA) containing protease inhibitor cocktail (Cat. No. P2714; Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 13,000×g at 4°C for 15 min. The supernatant was saved for use. For the nuclear protein extracts, cerebral tissues were suspended in ice-cold buffer A (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.05% NP-40, 0.1 mM EDTA, 0.5 mM dithiothreitol, pH 7.9) containing protease inhibitor cocktail and lysed for 10 min on ice. The lysates were centrifuged for 10 min at 3000 rpm at 4 °C. The supernatants were removed. The nuclear pellet was resuspended in the buffer B (5 mM HEPES, 1.5 mM MgCl2, 300 mM NaCl, 0.2 mM EDTA, 26% glycerol (v/v), 0.5 mM dithiothreitol, pH 7.9) containing protease inhibitor cocktail, homogenized with 20 full strokes in glass homogenizer, and incubated at 4°C for 30 min. The homogenates were centrifuged at 24,000 g for 20 min at 4°C. The supernatants were reserved as nuclear extracts.
Equal protein samples (50 μg per lane) were separated by 10% sodium dodecyl sulfate–polyacrylamide gels and then electrotransferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were incubated with the following primary antibodies: anti-p65 antibody (1:500 dilution; Cat. No. 8242S; Cell Signaling Technology, Beverly, MA, USA), anti-histone H3 antibody (1:2,000 dilution; Cat. No. 9715L; Cell Signaling Technology), anti-iNOS antibody (1:1000 dilution; Cat. No. B2812; Santa Cruz Biotechnology, Santa Cruz, CA), anti-cyclooxygenase (COX)-2 antibody (1:1000 dilution; Cat. No. B0912; Santa Cruz Biotechnology), anti-interleukin (IL)-1β antibody (1:1000 dilution; Cat. No. k1011; Santa Cruz Biotechnology), anti-tumor necrosis factor (TNF)α antibody (1:1000 dilution; Cat. No. k2911, Santa Cruz Biotechnology) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:2000 dilution; Cat. No. G9545; Sigma). Protein bands were visualized and quantified using G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD, USA). The densities of protein bands were normalized to those of Histone H3 or GAPDH to control for errors in protein sample loading and transferring during western blotting. The results in the groups with brain HI were then normalized to those of control rats.
Measurement of Nitrotyrosine-Containing Proteins and 4-Hydroxynonenal (4-HNE)
Rats with or without brain HI were transcardially perfused with normal saline. The whole right brain hemisphere or the right hippocampal dentate gyrus was harvested and homogenized on ice in phosphate buffered saline containing protease inhibitor cocktail and 0.005% butylated hydroxytoluene (BHT, Cat. No. MKBF5218V; Sigma). Homogenates were centrifuged at 12,000×g for 10 min at 4°C. The contents of nitrotyrosine-containing proteins and 4-HNE in the brain tissues were assessed using the OxiSelect Nitrotyrosine ELISA kit (Cell Biolabs Inc., San Diego, CA, USA) and the OxiSelect HNE-His Adduct ELISA kit (Cell Biolabs Inc.), respectively, according to the manufacture’s protocols.
Toluidine Blue Staining of Sciatic Nerve
Rats received or not received intranasal 50 mg/kg PDTC were transcardially perfused with normal saline at 7 or 30 days after the PDTC application. Sciatic nerve blocks at ~1 cm length were fixed for 3 h in 4% paraformaldehyde in phosphate buffered saline, washed in phosphate buffered saline and submerged for 2 h in 2% osmium tetroxide (Sigma) solution. The nerves were then dehydrated with a series of alcohol solutions (from 50% to absolute ethanol and xylene) and embedded in paraffin. Sections at 3-μm thickness were cut and stained with 1% Toluidine blue (Sigma) for 30 – 50 s. They were observed under a 100x oil-immersion objective with an OLYMPUS BX51 light microscope equipped with OLYMPUS DP70 digital camera and an OLYMPUS image controller.
Statistical Analysis
All animals that had completed this study are reported. Parametric results are presented as means ± S.D. (n ≥ 4) and were analyzed by Student’s t test or one way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data. The results were analyzed by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed. Comparison of the Barnes maze results during training sessions in the same group and among different groups were performed by one-way and two-way repeated measures analysis of variance, respectively. A P ≤ 0.05 was accepted as significant.
A preliminary sample size estimate was performed. We anticipated a difference in means of 25% between control group and PDTC treatment group with an expected standard deviation of 10%. It was estimated that minimal 4 animals were needed in each group to achieve a desired power of 0.8 at an α level of 0.05 by t-test.
Results
Intranasal PDTC dose- and time-dependently reduced brain injury after neonatal brain HI
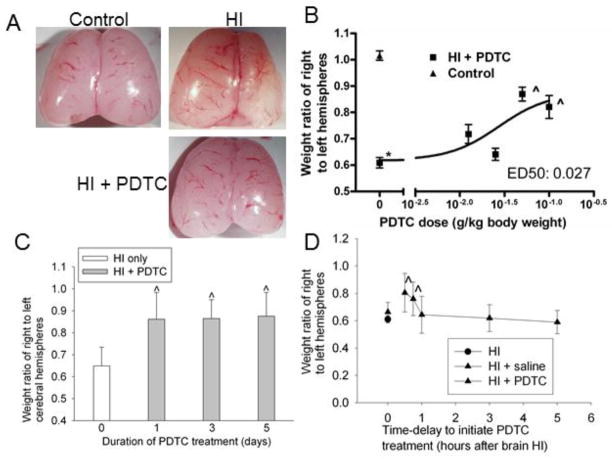
The left and right cerebral hemispheres of 14-day old control rats were similar in weight. Significant brain mass loss in the right cerebral hemisphere occurred in neonatal rats that had a right brain HI when they were 7 days old (Fig. 1A). PDTC applied intranasally daily for 3 days with the first dose at 15 min after the brain HI dose-dependently reduced this brain tissue loss. The effective dose (ED)50 for this neuroprotective effect was 27 mg/kg (Fig. 2B). A similar neuroprotective effect was observed when 50 mg/kg PDTC was given for 1, 3 or 5 days with the first dose at 15 min after the brain HI (Fig. 1C), suggesting that one dose may have maximized its neuroprotective effect. Thus, application of 50 mg/kg PDTC only once was used in the rest of experiments. The neuroprotective effect still existed after one dose of PDTC applied as late as 45 min after the brain HI. The brain tissue saving effect disappeared if PDTC was applied at a time point that was longer than 1 h after the brain HI (Fig. 1D). These results suggest that the effective time-window for PDTC-induced neuroprotection is within 45 min after the brain HI.
Fig. 1.
Neuroprotective effects of PDTC. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen). Brain was harvested at 7 days after the brain HI. A: representative brain images of a control rat, rat with the HI only and rat with HI and then treated with 50 mg/Kg PDTC intranasally once a day for 3 days with the first dose at 15 min after the brain HI. B: dose-response. Various doses of PDTC were applied intranasally once a day for 3 days with the first dose at 15 min after the HI. Results are means ± S.D. (n = 5–7). * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only. C: Duration of treatment. Seven-day old rats were subjected to the brain HI and intranasal application of PDTC (50 mg/kg once a day) for various durations with the first dose at 15 min after the HI. Results are means ± S.D. (n = 4–7). ^ P < 0.05 compared with rats that had brain HI only. D: Time-window of treatment. Seven-day old rats were subjected to the brain HI and intranasal application of PDTC (50 mg/kg) at various times after the HI. One dose of PDTC was applied to the rats. Results are means ± S.D. (n = 4–12). ^ P < 0.05 compared with rats that had brain HI only.
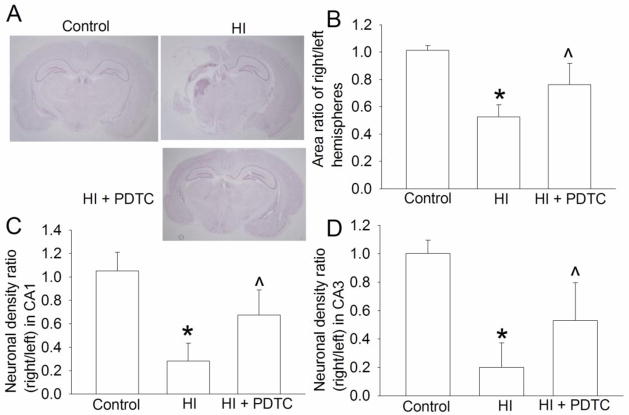
Fig. 2.
Improvement of long-term brain structures by PDTC. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Brain was harvested at one month after the brain HI. A: representative images of brain section at bregma −3.3 mm. B: area ratio of right/left hemispheres. C and D: neuronal density ratio in the CA1 and CA3. Results are means ± S.D. (n = 6–11). * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only.
Intranasal PDTC improved long-term neurological outcome after neonatal brain HI
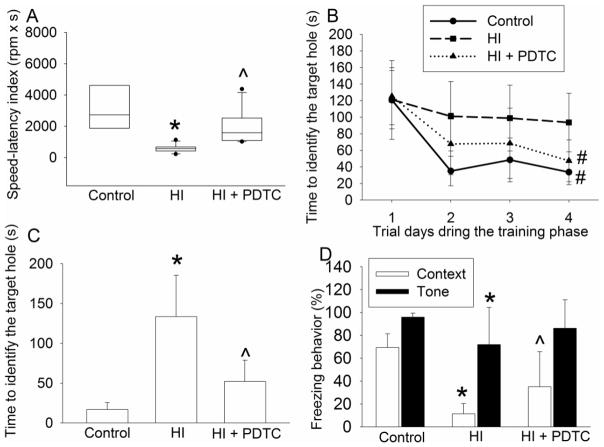
To determine whether PDTC improves the long-term neurological outcome, the brain structures and neurological functions were evaluated at one month after the brain HI. No rats from the HI and HI plus PDTC groups died during the one-month period. PDTC at 50 mg/kg applied at 15 min after brain HI reduced brain tissue loss (Figs. 2A and 2B) and hippocampal neuronal loss (Figs. 2C and 2D). Consistent with these brain structure results, PDTC improved the neurological functions of rats as reflected by their performance in rotarod test (Fig. 3A). In Barnes maze, the latency to identify the target box during training phase was gradually decreased with the increased training sessions in control rats or rats treated with PDTC after brain HI but not in the rats with HI only (Fig. 3B). There was a significant effect of HI on this learning process when control and HI only rats were compared [F(1,15) = 12.534, P = 0.003]. The effect of PDTC on this learning process was also significant in rats with HI [F(1,20) = 5.872, P = 0.025). Similarly, HI rats took much longer time than control rats to identify the target box during the memory phase test on day 5 of Barnes maze test. This HI effect was attenuated by PDTC (Fig. 3C). These results suggest that HI impairs the spatial learning and memory of rats and PDTC attenuates this impairment. Also, HI reduced the freezing behavior in the context- and tone-related fear conditioning test. This effect was attenuated by PDTC (Fig. 3D). Since context- and tone-related fear conditioning tests hippocampus-dependent and hippocampus–independent learning and memory, respectively (Kim and Fanselow, 1992), our results suggest that PDTC reduces brain HI-impaired hippocampus-dependent and hippocampus–independent learning and memory.
Fig. 3.
Improvement of long-term neurological functions by PDTC. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Their functions were assessed at one month after the brain HI. A: Motor functions assessed by rotarod test. Results are presented in a box plot format (n = 6–11) because they fail normality test. B and C: performance in Barnes maze. D: performance in context- and tone-related fear conditioning. Results are means ± S.D. (n = 6–11). * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only. # P < 0.05 compared with the values on the first trial day.
Intranasal PDTC reduced oxidative stress and inflammatory mediator expression after neonatal brain HI
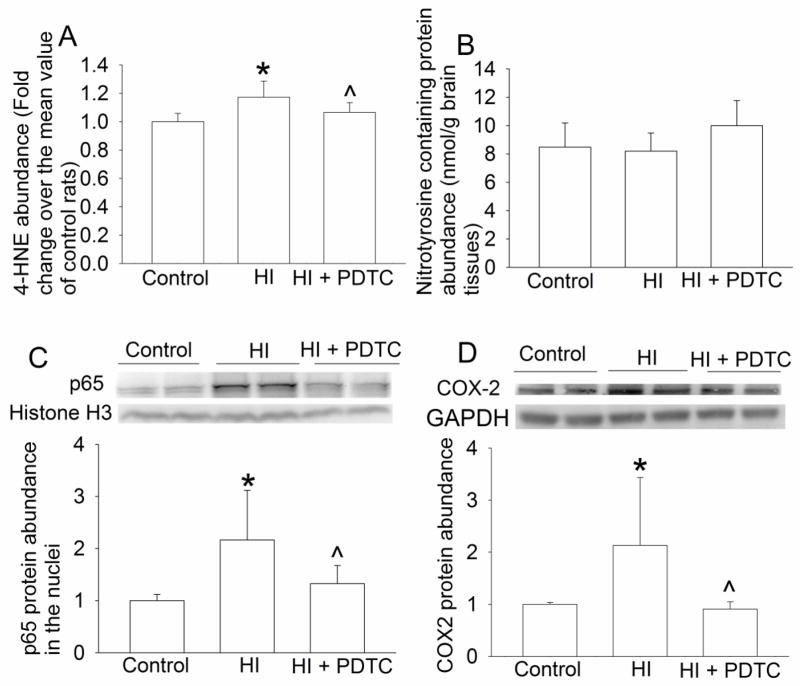
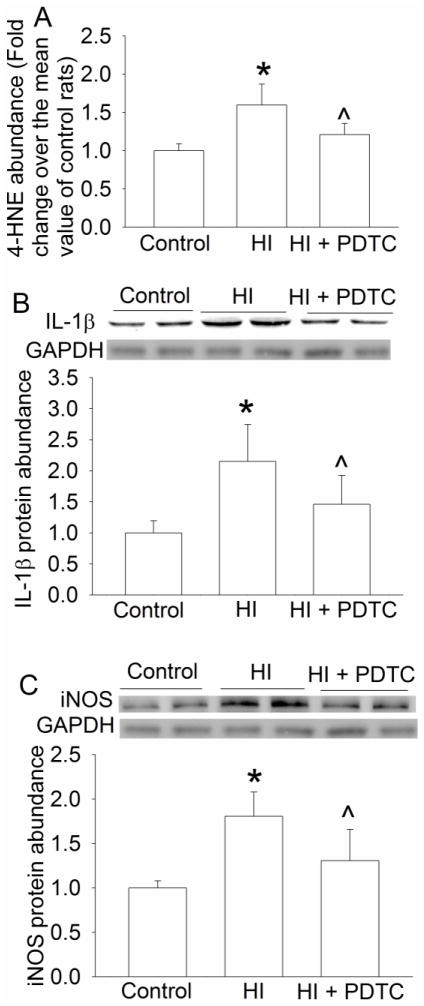
The levels of 4-HNE, a lipid oxidative stress indicator (Li and Zuo, 2011), in the brain were increased at 24 h after the brain HI. This effect was attenuated by PDTC (Fig. 4A). However, HI and PDTC did not affect the levels of nitrotyrosine containing proteins (Figs. 4B), an indicator for protein oxidative stress (Li and Zuo, 2011).
Fig. 4.
Reduction of lipid oxidative stress, nuclear translocation of p65 and COX-2 expression by PDTC. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Right hemisphere was harvested at 6 h (nuclear translocation of p65, panel C) or 24 h (expression of oxidative stress indicators and COX-2, panels A, B and D) after the brain HI. Results are means ± S.D. (n = 8). * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only.
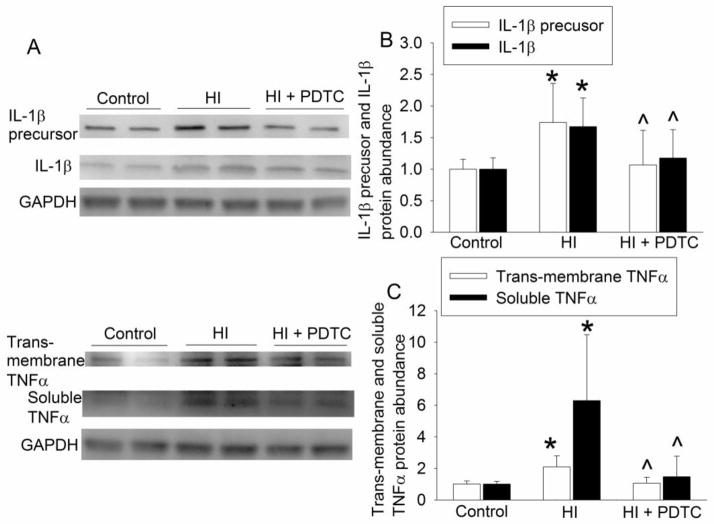
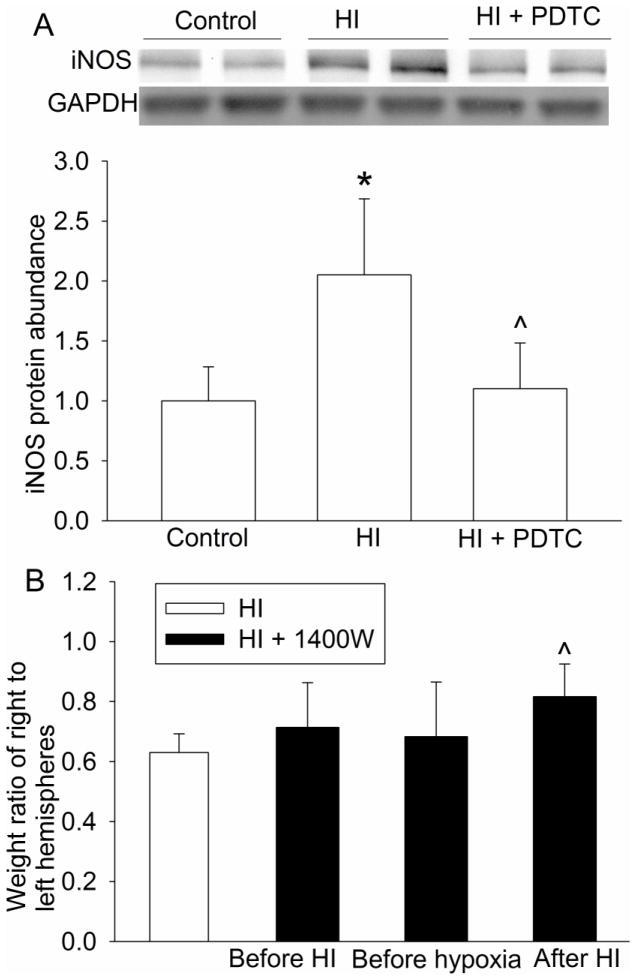
HI significantly increased the nuclear translocation of p65, a component of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). This increase was inhibited by PDTC (Fig. 4C). Consistent with this change of p65 nuclear translocation, the expression of COX-2, IL-1β, TNFα and iNOS in the brain was increased by brain HI and this increase was inhibited by PDTC (Figs. 4D, 5A, 5B 5C and 6A). Of note, 2 protein bands at 17 and 27 KDa were detected with the anti-IL-1β antibody. These brands correspond to the molecular weights of IL-1β and IL-1β precursor. Similarly, two protein bands at 17 and 32 KDa, corresponding to the molecular weights of TNFα and trans-membrane TNFα, were detected by the anti-TNFα antibody.
Fig. 5.
Inhibition of the expression of IL-1β and TNFα by PDTC. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Right frontal hemisphere was harvested at 24 h after the brain HI to measure the expression of IL-1β precursor, IL-1β, trans-membrane TNFα and TNFα. Representative Western blot images are presented in panel A and graphic presentation of the IL-1β precursor, IL-1β, trans-membrane TNFα and TNFα protein abundance quantified by integrating the volume of autoradiograms from 8 rats for each experimental condition is shown as fold change over the control rats in panels B to C. * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only.
Since our results showed that PDTC reduced HI-induced impairment of hippocampus-dependent learning and memory, we analyzed the oxidative stress status and inflammatory mediators in the hippocampal dentate gyrus, a region that is involved in neurogenesis and neuronal plasticity (Mongiat and Schinder, 2011). Similar to the results from using the right hemisphere, right brain HI increased 4-HNE, IL-1β and iNOS and PDTC reduced this increase in the right dentate gyrus (Fig. 7).
Fig. 7.
Reduction of lipid oxidative stress, IL-1β and iNOS expression by PDTC in the hippocampal dentate gyrus. Seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Right dentate gyrus was harvested at 24 h after the brain HI to measure 4-HNE (panel A) and the protein expression of IL-1β (panel B) and iNOS (panel C). Results are means ± S.D. (n = 8). * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only.
To determine whether inhibition of brain HI-induced iNOS expression by PDTC contributes to its neuroprotection, 1400W, a specific iNOS inhibitor (Zhao et al., 2007), was applied to the rats. Application of 1400W at 15 min after the HI reduced brain tissue loss. However, injection of 1400W at 30 min before the initiation of brain HI or 30 min before the hypoxia did not reduce the brain tissue loss (Fig. 6B).
Fig. 6.
Inhibition of the expression of iNOS by PDTC and the neuroprotection by an iNOS inhibitor. A: seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without intranasal 50 mg/Kg PDTC at 15 min after the HI. Right frontal hemisphere was harvested at 24 h after the brain HI to measure the iNOS expression of COX-2. Representative Western blot images are presented at top and graphic presentation of the iNOS protein abundance quantified by integrating the volume of autoradiograms from 8 rats for each experimental condition is shown at bottom as fold change over the control rats. * P < 0.05 compared with control rats. ^ P < 0.05 compared with rats that had brain HI only. B: seven-day old rats were subjected to or were not subjected to the brain HI (right common carotid ligation plus 2-h hypoxia at 8% oxygen) treated with or without 1 mg/Kg 1400W at 30 min before the brain HI, 30 min before the hypoxia and 15 min after the HI. Brain was harvested at 7 days after the brain HI. Results are means ± S.D. (n = 9–11). ^ P < 0.05 compared with rats that had brain HI only.
Intranasal PDTC did not cause sciatic nerve demyelination
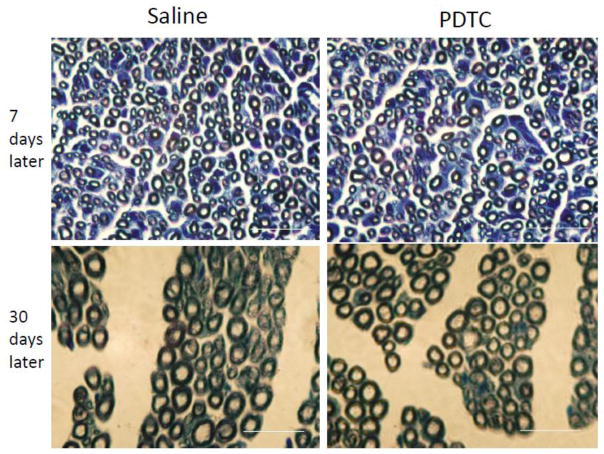
Chronic exposure to PDTC can cause peripheral nerve damage includes demyelination and axonal degeneration (Valentine et al., 2006). However, there was no evidence of these pathological changes in the sciatic nerves of the neonatal rats exposed to intranasal application of 50 mg/Kg PDTC once no matter whether the observation was performed at 7 or 30 days after the application (Fig. 8).
Fig. 8.
Micrographs of rat sciatic nerve sections stained with toluidine blue. Saline or 50 mg/Kg PDTC was applied intranasally for only once and then the right sciatic nerve was harvested at 7 or 30 days after the application. Scale bars = 20 μm in each pane.
Discussion
Birth asphyxia presents a unique situation. Many of these newborns are healthy before the birth asphyxia. However, significant brain injury from birth asphyxia can result in death and long-term disabilities for the rest of their life. Oral feeding for medication is not possible during the resuscitation of these newborns immediately after birth asphyxia. Intravenous access takes time to be established even in the best hands. Thus, a route of drug application that can be used immediately for neuroprotective medication is ideal in this case. Intranasal application meets this requirement. Our results showed that intranasal PDTC application can provide neuroprotection against brain HI in neonatal rats, suggesting a potential intervention to reduce brain injury in newborns with birth asphyxia.
Our results showed that intranasal PDTC needed to be applied within 45 min after the brain HI. This effective time-window makes it possible to practically use this intervention in clinical situation if this effect is confirmed in humans. Intranasal application of PDTC can be applied easily by any care providers within the effective time-window during the resuscitation of an asphyxiated newborn. Consistent with our finding that a delayed application of PDTC provides neuroprotection, a previous study showed that PDTC intraperitoneally injected at 6 h after a transient focal brain ischemia in young adult rats reduced brain infarct size (Nurmi et al., 2004b). Also, intraperitoneal application of PDTC at 2 h after brain HI in neonatal rats reduced brain lesion size detected by T2-weighed magnetic resonance imaging (Nurmi et al., 2006). Detailed time-course experiment was not performed in these previous studies. Nevertheless, these studies suggest a wider effective time-window than did our study. The reason for this discrepancy is not known and may be related to different animal models, drug administration routes and methods used to detect the brain lesions between our study and the previous studies.
It has been recommended by expert panel to determine the effectiveness of an intervention in improving long-term neurological outcome in preclinical testing phase during the establishment of the intervention for possible human use (Fisher et al., 2009). Structurally, the right brain HI caused significant injury to the cerebral cortex and hippocampus in the right hemisphere. Correspondingly, the rats with brain HI had worse performance in rotarod than control rats. Also, the hippocampus-dependent and hippocampus-independent learning and memory were impaired in the rats with brain HI. Our results showed that intranasal PDTC reduced brain tissue and neuronal loss and improved neurological and cognitive functions assessed at one month after the brain HI. These results suggest that PDTC improved the long-term neurological outcome after brain HI.
In addition to the primary insult that is brain ischemia and hypoxia, multiple secondary insults, such as oxidative stress and inflammation, can induce cell injury after brain HI. Free radicals can damage the functions and structures of cellular proteins, lipids and nucleic acids. Inflammation causes the complement system activation, which leads to proteolytic reaction and cell lysis. Inflammation also induces cell apoptosis (Iadecola and Anrather, 2011). Consistent with the idea that oxidative stress and inflammation play a role in the brain injury after brain HI, our results showed that brain HI increased the 4-HNE levels and the expression of multiple inflammatory mediators including iNOS and that PDTC provided neuroprotection and also attenuated the brain HI-induced increase of 4-HNE and inflammatory mediators in the ischemic brain hemisphere or dentate gyrus. In addition, 1400W, a specific iNOS inhibitor, applied after brain HI reduced brain injury. These results suggest that reducing oxidative stress and inflammatory mediators contributes to the neuroprotective effects of PDTC.
PDTC is a known NF-κB inhibitor (Liu et al., 1999). Activation of NF-κB is a critical step to induce inflammatory mediator production (Giacomini et al., 2011; Liu et al., 1999). Our results showed that brain HI increased the expression of multiple inflammatory mediators including IL-1β, TNFα, iNOS and COX-2 and also the nuclear translocation of p65, a NF-κB component. Nuclear translocation is a necessary step for NF-κB to regulate protein expression. The brain HI-induced nuclear translocation of NF-κB was reduced by PDTC, consistent with its known function as an NF-κB inhibitor. Together, these results suggest that PDTC inhibits NF-κB to reduce inflammatory mediator expression.
Our results did not show an increase of nitrotyrosine containing proteins in the right cerebral hemisphere after the right brain HI. The amount of nitrotyrosine containing proteins has been used as an indicator for the level of oxidative stress in proteins (Li and Zuo, 2011). It is not clear why brain HI does not affect the nitrotyrosine containing protein levels in the ischemic brain. We harvested the whole right cerebral hemisphere to provide a large amount of sample for measuring the nitrotyrosine containing proteins and 4-HNE by ELISA. This method of tissue harvest may have diluted the tissues with increased nitrotyrosine containing protein levels by the surrounding tissues. It is also possible that most tissues/proteins are damaged in the ischemic brain tissues.
Interestingly, 1400W applied before the brain HI or hypoxia did not provide neuroprotection but 1400W applied after the brain HI provided neuroprotection. This situation is similar to that in a previous study showing that 1400W applied before lipopolysaccharide did not affect the increased nitric oxide production but the application of 1400W at 3 h after lipopolysaccharide attenuated the nitric oxide production in rats (Rota et al., 2002). Both 1400W and lipopolysaccharide are injected intraperitoneally. These results suggest that 1400W has a relatively short half-life.
We included both male and female rats in this study because both genders can suffer from birth asphyxia. However, there are gender differences in the responses to brain ischemia and rehabilitative training after brain ischemia (Tsuji et al., 2010; Zhu et al., 2006). Future studies will focus on determining the gender difference in the effectiveness of PDTC to provide neuroprotection in the neonatal animals.
Exposure of rats to PDTC for months is known to cause peripheral nerve degeneration and demyelination (Valentine et al., 2006). Our results did not show these pathological changes after a single dose of PDTC. These results suggest the safety of the identified potential regimen for neonatal brain HI.
In summary, our study showed that intranasal PDTC induced a dose- and time-dependent neuroprotective effect against brain HI in neonatal rats. This effect was maximized by one dose of PDTC and was translated into improved long-term neurological outcome. This protection may be mediated by inhibiting the expression of inflammatory mediators and reducing oxidative stress. Since PDTC has a low toxicity profile and intranasal application is easily achieved, our results suggest the need of clinical trials to determine the effectiveness of intranasal PDTC in reducing brain injury after birth asphyxia in humans.
Research highlights.
Intranasal pyrrolidine dithiocarbamate dose-dependently reduces ischemic brain injury in neonatal rats
Pyrrolidine dithiocarbamate-induced neuroprotection against neonatal brain hypoxia/ischemia is long-lasting
Pyrrolidine dithiocarbamate-induced neuroprotection may be mediated by reducing oxidative stress and neuroinflammation
Acknowledgments
Grant support: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Footnotes
Conflict of interest: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Bagger MA, Bechgaard E. The potential of nasal application for delivery to the central brain-a microdialysis study of fluorescein in rats. Eur J Pharm Sci. 2004;21:235–242. doi: 10.1016/j.ejps.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, Reddy HM, Deshmukh MD. Management of birth asphyxia in home deliveries in rural Gadchiroli: the effect of two types of birth attendants and of resuscitating with mouth-to-mouth, tube-mask or bag-mask. J Perinatol. 2005;25(Suppl 1):S82–91. doi: 10.1038/sj.jp.7211275. [DOI] [PubMed] [Google Scholar]
- Chabicovsky M, Prieschl-Grassauer E, Seipelt J, Muster T, Szolar OH, Hebar A, Doblhoff-Dier O. Pre-clinical safety evaluation of pyrrolidine dithiocarbamate. Basic Clin Pharmacol Toxicol. 2010;107:758–767. doi: 10.1111/j.1742-7843.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini E, Remoli ME, Scandurra M, Gafa V, Pardini M, Fattorini L, Coccia EM. Expression of proinflammatory and regulatory cytokines via NF-kappaB and MAPK-dependent and IFN regulatory factor-3-independent mechanisms in human primary monocytes infected by Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:841346. doi: 10.1155/2011/841346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Li J, Sheng W, Feng C, Zuo Z. Pyrrolidine dithiocarbamate attenuates brain Abeta increase and improves long-term neurological outcome in rats after transient focal brain ischemia. Neurobiol Dis. 2012;45:564–572. doi: 10.1016/j.nbd.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neurosci. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13:499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40:1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004a;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004b;91:755–765. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- Rota C, Bergamini S, Daneri F, Tomasi A, Virgili F, Iannone A. N-Acetylcysteine negatively modulates nitric oxide production in endotoxin-treated rats through inhibition of NF-kappaB activation. Antioxid Redox Signal. 2002;4:221–226. doi: 10.1089/152308602753625988. [DOI] [PubMed] [Google Scholar]
- Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol. 2005;79:8014–8023. doi: 10.1128/JVI.79.13.8014-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sran SK, Baumann RJ. Outcome of neonatal strokes. Am J Dis Child. 1988;142:1086–1088. doi: 10.1001/archpedi.1988.02150100080031. [DOI] [PubMed] [Google Scholar]
- Sreenan C, Bhargave R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term outcome. J Pediatrics. 2000;137:351–355. doi: 10.1067/mpd.2000.107845. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Aoo N, Harada K, Sakamoto Y, Akitake Y, Irie K, Mishima K, Ikeda T, Fujiwara M. Sex differences in the benefits of rehabilitative training during adolescence following neonatal hypoxia-ischemia in rats. Exp Neurol. 2010;226:285–292. doi: 10.1016/j.expneurol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Valentine HL, Amarnath K, Amarnath V, Valentine WM. Dietary copper enhances the peripheral myelinopathy produced by oral pyrrolidine dithiocarbamate. Toxicol Sci. 2006;89:485–494. doi: 10.1093/toxsci/kfj047. [DOI] [PubMed] [Google Scholar]
- World-Health. The World Health Report Shaping the future. World Health Organization; 2003. [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in the neonatal rats. Anesthesiology. 2004;101:695–702. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]