Abstract
The treatment of osteosarcoma (OS) pulmonary metastases remains a challenge. T cells genetically modified to express a chimeric antigen receptor (CAR), which recognizes a tumor-associated antigen, have shown activity against hematopoetic malignancies in clinical trials, but this requires the identification of a specific receptor on the tumor cell. In the current study, we found that interleukin (IL)-11Rα was selectively expressed on 14 of 16 OS patients’ lung metastases and 4 different human OS cell lines, indicating that IL-11Rα may be a novel target for CAR-specific T-cell therapy. IL-11Rα expression was absent or low in normal organ tissues, with the exception of the GI track. IL-11Rα-CAR-specific T cells were obtained by non-viral gene transfer of Sleeping Beauty DNA plasmids and selectively expanded ex vivo using artificial antigen presenting cells derived from IL-11Rα + K562 cells genetically modified to co-express T-cell co-stimulatory molecules. IL-11Rα-CAR+ T cells killed all 4 OS cell lines in vitro; cytotoxicity correlated with the level of IL-11Rα expression on the tumor cells. Intravenous injection of IL-11Rα-CAR+ T cells into mice resulted in the regression of OS pulmonary metastases with no organ toxicity. Together, the data suggest that IL-11Rα-CAR T cells may represent a new therapy for OS patients with pulmonary metastases.
Keywords: Osteosarcoma, lung metastasis, IL-11Rα, chimeric antigen receptor, T cells
Introduction
Despite multiple changes in the adjuvant chemotherapy regimens used to treat patients with osteosarcoma (OS), both the 2-year metastasis-free survival and the overall survival rates have remained stagnant at 65-70% for the past 20 years [1-6]. Disease relapsed usually occurs in the lung and is resistant to salvage chemotherapy, which indicates that OS patients may benefit from new approaches and/or the addition of new agents to currently available adjuvant chemotherapy regimens. New therapies are particularly needed for OS patients with lung metastases. In preclinical studies, we found that liposome-encapsulated MTP-PE (L-MTP-PE) activated the tumoricidal properties of human monocytes (7) and could be used to treat OS lung metastases (8). L-MTP-PE is an immune therapy that targets monocytes and pulmonary macrophages and activates them to recognize and kill tumor cells. We have further demonstrated that microscopic OS lung metastases are sensitive to this immune-based therapy and that the combination of L-MTP-PE and chemotherapy in the adjuvant setting results in a clinically significant improvement in the overall survival rate 6-12 years after initial diagnosis (8, 9). These studies and the clinical success of L-MTP-PE support the investigations of other immune-based therapies such as engineered T cells to improve the treatment of OS patients.
The use of genetically modified T cells for the treatment of solid tumors is an emerging field. The genetic modification of primary T cells with tumor-specific immunoreceptors such as chimeric antigen receptors (CARs) can redirect T cells against tumor cells (10). The adoptive transfer of these tumor-specific T cells into patients provides a novel way to deliver specific antigen–targeted cancer therapy and has been used in preclinical and clinical trials (11). Such an approach requires the identification of tumor–specific antigen targets that are expressed in OS. One such potential molecular target is interleukin 11 receptor α-chain (IL-11Rα).
IL-11 is a member of a family of pleiotropic cytokines that include IL-6, leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor (12,13). The binding of IL-11 specifically to IL-11Rα mediates the assembly of a multi-subunit receptor complex that initiates intracellular signaling by association with the transmembrane signal transducer glycoprotein gp-130 (13, 14). The IL-11/IL-11Rα signaling pathway is involved in several biological activities such as adipogenesis, osteoclastogenesis, neurogenesis, and megakaryocyte maturation and platelet production (15, 16). It has recently been demonstrated that human IL-11Rα is overexpressed in colon cancer, gastric cancer, breast cancer, prostate cancer, and OS (12,14,17-20). In addition, IL-11/IL-11Rα signaling may mediate the activation of the JAK-STAT pathway, resulting in an anti-apoptosis effect in human colonic epithelial cells (14) and prostate cancer cells (12). IL-11Rα can also function as a therapeutic target using a cyclic IL-11 nanopeptide, c(CGRRAGGSC), conjugated to a suicide peptide (KLAKLA) to eliminate prostate cancer in vivo (17).
In the current study, we found that IL-11Rα was overexpressed on OS lung metastases from patients and expressed in 4 different OS cell lines at levels ranging from 20% to 60%. Engineered T cells expressing specific IL-11Rα-CAR that we propagated ex-vivo killed OS cells in vitro; cytotoxicity correlated with the cells’ level of IL-11Rα expression. We further demonstrated that the i.v. injection of IL-11Rα-CAR T cells resulted in the regression of established OS lung metastases.
Materials and Methods
Cell lines and cell culture
The human OS cell lines CCH-OS-D, KRIB, SAOS-2 and LM7 were evaluated for cell surface expression of IL-11Rα. SAOS-2, LM7 and KRIB cells were cultured in Eagle’s modified essential medium supplemented with 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 1×nonessential amino acids, 2×minimal essential medium vitamin solution, and 10% heat-inactivated (56°C for 30 minutes) fetal bovine serum (FBS). Luciferase–expressing KRIB cells were kindly provided by Dr. Dennis PM Hughes (The University of Texas M.D. Anderson Cancer Center, Houston, TX). SAOS-2 cell line were derived from a primary osteosarcoma from an 11 year-girl, was purchased from the American Type Culture Collection (Rockville, Maryland). LM7 cells were OS lung metastases type derived from SAOS-2 in our Lab (21). CCH-OS-D cells were also provided by Dr. Dennis PM Hughes’s Lab. The CCH-OS-D is an osteosarcoma cell line derived from patients under an IRB-approved protocol at the Children’s Cancer Hospital at The University of Texas M.D. Anderson Cancer Center. The cells were cultured in DMEM supplemented with 10% FBS, penicillin, streptomycin, and 1% insulin/transferring selenium (Gibco). CCH-OS-D cells have a unique signature by DNA microsatellite fingerprinting, with no overlap with any described or previously tested cell lines. A monolayer culture of cells was maintained at 37°C in a humidified 5% CO2 incubator. All these four cell line fingerprints were performed on December 17th, 2010 in M.D. Anderson Cancer Center.
Human T cells were harvested from healthy adult volunteer donors (Gulf Coast Regional Blood Center, Houston, TX) after informed consent was obtained and prepared as described previously (22). K562 clone 4 cells were kindly provided by C. June (University of Pennsylvania, Philadelphia, PA). T cells and K562 clone 4 cells were cultured in RPMI medium supplemented with 10% FBS and 2 mmol/L glutamine. K562 clone 4 cells were used as artificial antigen presenting cells (aAPCs) after 100 Gy γ-irradiation for in vitro expansion of genetically modified T cells in culture medium.
Immunohistochemistry
Paraffin-embedded blocks of tissues from 16 patients with pulmonary OS metastases were obtained from MD Anderson tissue banks. Paraffin embedded microarray slides of human normal tissues were purchased from US Biomax Inc. (Rockville). Resected tissues from mice were washed in saline, fixed in 10% formalin buffer, and embedded in paraffin. Imunohistochemistry staining for IL-11Rα expression was performed as described previously (17) with modification. Briefly, tissue sections (5-μm-thick) were deparaffinized in xylene and rehydrated. Trypsin incubation at 37°C for 30 min was performed for antigen retrieval. Following biotin and protein blocking (DAKO Corp., Carpinteria), sections were incubated with a rabbit anti–IL-11Rα antibody at a 1:50 dilution (N20; Santa Cruz Biotechnology) for 1 h. The sections were then developed using a Mach 4 Universal Polymer Detection Kit (Biocare Medical).
Plasmid constructs
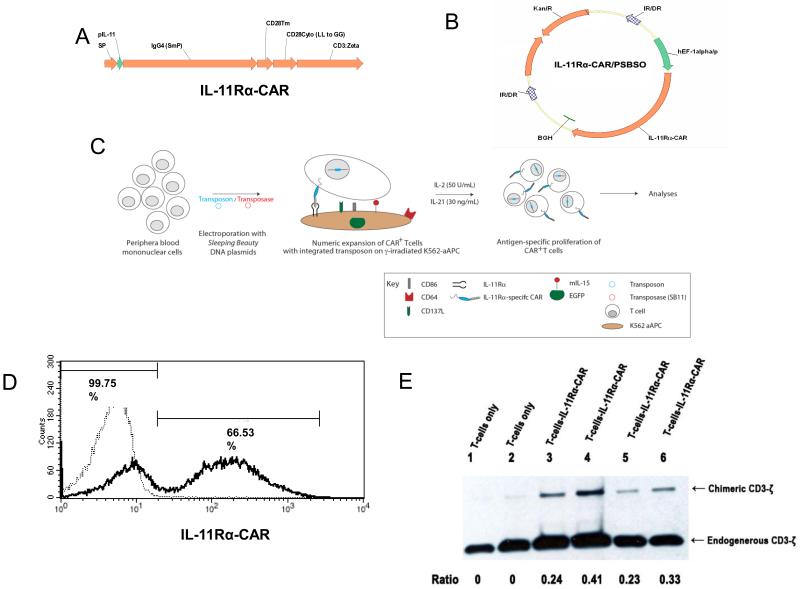
IL-11Rα-CAR was generated by connecting an IL-11 peptide (CGRRAGGSC) (17, 23) as an extracellular domain to T-cell activation endodomains. The cDNA encoding for IL-11Rα-CAR (Fig. 1A) was assembled by PCR using splicing by overlap extension (24) and cloned into a DNA Sleeping Beauty (SB) expression plasmid pSBSO [22] to create the transposon plasmid pSBSO-IL-11-CAR (Fig. 1B). The DNA plasmid pCMV-SB11 was used to expresses the SB11 transposase (22).
Figure 1. Schematic for IL-11Rα-CAR and genetic modification of primary human T cells.
(A) IL-11Rα–CAR including a signal peptide (SP), IL-11 peptide as an extracellular domain (pIL-11), an Fc domain (IgG4 [SmP]), a transmembrane domain (CD28Tm and CD28Cyto), and an intracellular signal transduction domain CD3-ζ. (B) A cDNA of IL-11Rα-CAR was cloned into the Sleeping Beauty expression DNA plasmid pSBSO (22) to create the transposon plasmid pSBSO-IL-11Rα-CAR (IL-11Rα/pSBSO). IL-11Rα-CAR, IL-11Rα-specific chimeric antigen receptor; EF-1α/p, human elongation factor-1α promoter; IR/DR, SB inverted repeats/direct repeats; BGH, polyadenylation signal from bovine growth hormone; Kan R, kanamycin resistance gene. (C) Human T cells (derived from PBMCs) were transfected with the pSBSO-IL-11 Rd-CAR and transposase plasmid pCMV-SB 11 (22) using electroporation. The transposon was cleaved by transposase and integrated into the host genome as previously described (22). Following overnight incubation, the cells were stimulated with γ-irradiated antigen presenting cells (aAPCs) and IL-2 and IL-21 (22). K562 clone 4 cells (genetically modified to coexpress CD64, CD86, CD137L, and surface membrane–bound IL-15 with EGFP) are IL-11Rα+ and served as aAPCs. The numeric expansion of the IL-11Rα-CAR T cells was then analyzed by flow cytometry. (D) A representative histogram of the expression of IL-11Rα- CAR on the T cells 24 h after electroporation as analyzed by flow cytometry (black line, 66.5% IL-11Rα-CAR+ T cells). T cells without genetic modification and propagated by cross-linking CD3 were used as controls (dotted line, 99.7% negative for IL-11Rα-CAR). (E) The expression of the IL-11Rα-CAR in control T cells (lanes 1 and 2) and IL-11Rα-CAR T cells (lanes 3–6) was analyzed by Western blot after expansion for 2 weeks (lanes 1, 3, and 5) and 4 weeks (lanes 2, 4, and 6). Lanes 2 and 4, stimulated with IL-2; lanes 3 and 5, stimulated with IL-2 and IL-21.
Electroporation and ex-vivo expansion of IL-11Rα-CAR T cells
Human T cells isolated from peripheral blood were transfected with the pSBSO-IL-11Rα-CAR transposon and the pCMV-SB11 transposase plasmids by electroporation to express IL-11Rα-CAR. T cells were then propagated ex-vivo using γ-irradiated aAPCs, IL-2 and IL-21. The schematic for the genetic modification and expansion of T cells is shown in Fig. 1 C. T cells (1×107) were suspended in 100 μL of nucleofector solution (Human CD34+ Cell Nucleofector Kit, Amaxa), mixed with 15 μg of pSBSO-IL-11 CAR plasmid and 5 μg of pCMV-SB11 plasmid, transferred to a cuvette, and electroporated (Program U-14, Amaxa). The cells were transferred to a 6-well plate containing 4 mL of phenol-free RPMI containing 2 mM Glutamax and 20% FBS and incubated overnight. On day 1 (1 day after electroporation), the T cells were stimulated with γ-irradiated aAPCs at a 1:2 T cell–aAPC ratio. aAPCs were added to T cell every 7 days during expansion. Fifty units/ml recombinant human IL-2 (rhIL-2; Chiron, Emeryville, CA) alone or combined with 30 ng/mL IL-21 (IL-21; eBioscience, Inc. San Diego, CA) were added to the cultures every 48 h beginning on day 7. T cells were stimulated for 5 cycles, and viable cells were counted based on trypan blue exclusion. T cells that were not genetically modified were used as control T cells; these cells were propagated on γ-irradiated aAPCs preloaded with anti-CD3 antibody (OKT3), IL-2 and IL-21 (22).
The expression of IL-11Rα-CAR in T cells was detected 24h after electroporation (day 1). The mean percentage of T cells that expressed IL-11Rα-CAR in 4 experiments was 58.2%. A representative experiment is shown in Fig. 1D. The expression of IL-11Rα-CAR was further confirmed by Western blotting using an anti-CD3-ζ antibody (Fig 1E). The expression of IL-11Rα-CAR was higher in the T cells that were simulated by both IL-2 and IL-21 (Fig. 1E, lane 4 and 6) then in the cells that were simulated by IL-2 alone (Fig. 1E, lane 3 and 5). This indicated that adding IL-21 was beneficial in the expansion of the IL-11Rα-CAR T cells, which was consistent with previous results suggesting that IL-21 improves CD19-CAR T cell expansion (25). Therefore, IL-21 and IL-2 were used for IL-11Rα-CAR T-cell expansion.
Flow cytometry
The phenotypes of T cell populations were analyzed weekly using flow cytometry. Fluorochrome-conjugated anti-CD3, anti-CD4, and anti-CD8 antibodies were obtained from BD Biosciences. PE-conjugated anti-IL-11Rα was obtained from Santa Cruz Biotechnology. PE or FITC-conjugated goat anti-human Fcγ (Jackson Immunoresearch) was used to detect the cell surface expression of IL-11Rα-CAR. The blocking of nonspecific antibody binding was achieved using FACS wash buffer (2% FCS in PBS). Data acquisition was performed on a FACScalibur (BD Biosciences), and the percentage of cells in a region ofanalysis was calculated using CellQuest version 3.3 software (BD Biosciences).
Western Blot
IL-11Rα signaling was determined by Western blot analysis of phospho-Stat3. Osteosarcoma LM-7 and KRIB cells were treated with 10 ng/ml or 25ng/ml rhIL-11 (RD Minneapolis) for five minutes. Following cytokine treatment, LM7 and KRIB cells were harvested and lysed with RIPA lysis buffer supplemented with protease and phosphatase inhibitors (Santa Cruz Biotechnology, Inc.). Lysates were cleared by centrifugation at 12,000 × g at 4°C. Total protein concentration was measured using a bicinchoninic acid assay kit (Bio-Rad Laboratories) with bovine serum albumin as a standard. The protein was denatured by boiling at 100°C for 5 min in the presence of sample buffer (0.5 m Tris [pH 6.8], 10% glycerol, 10% SDS, 5% 2-mercaptoethanol, and 1% bromophenol). For each immunoblot, 30 μg of protein were resolved on a 10% or 12% polyacrylamide gel for 90 min at 100 V. The protein was then transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Following transfer, the membrane was blocked with 5% nonfat milk in TBST (138 mmol/L NaCl, 2.7 mmol/L KCl [pH 7.4], and 0.1% Tween 20) for 1 h. Phospho-Stat3 was detected with a rabbit anti-human phospho-Stat3 antibody (Cell Signaling). Membranes were then washed and incubated with secondary antibody conjugated to HRP. The secondary antibody was visualized using an enhanced chemiluminescence detection Western blotting analysis system (Amersham Pharmacia Biotech). T cell lysates were obtained for Western blot analysis in the same manner as KRIB and LM7 cells. CD3 protein in T cells was detected with a mouse anti-human CD monoclonal antibody (BD Biosciences)
Chromium release assay
The cytolytic activity of the T cells was determined using 4-hr chromium release assay (22). IL-11Rα-specific T cells were incubated with 5 × 103 51Cr-labeled target cells in a V-bottomed, 96-well plate. The percentage of specific cytolysis was calculated from the release of 51Cr using a TopCount NXT (Perkin-Elmer Life and Analytical Sciences, Inc.). Data are reported as means ± SDs.
Animal model
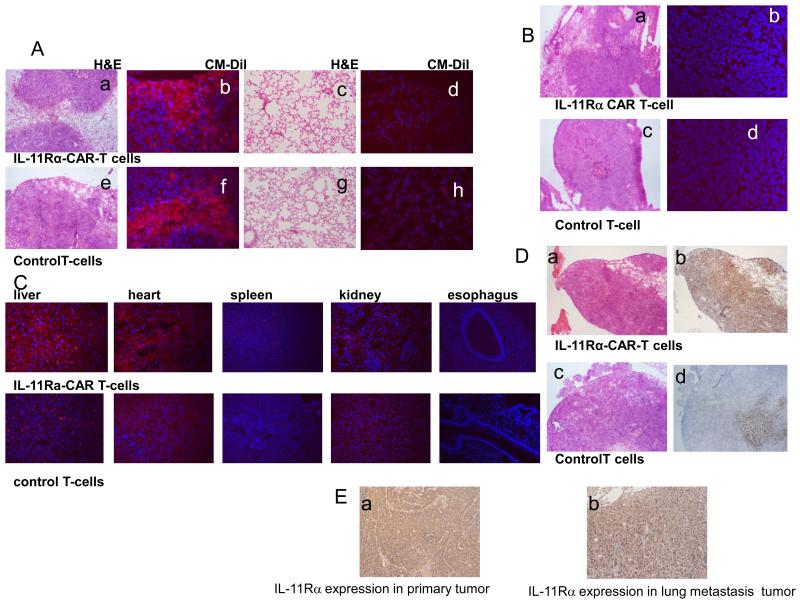
All animal experiments were approved by the Institutional Animal Care and Use Committee at MD Anderson Cancer Center. Six-week-old athymic nu/nu nude mice were purchased from the National Cancer Institute. Luciferas-expressing human KRIB cells (2×105 cells/10uL/mouse) were injected into the proximal tibia of the nu/nu mice. Tumor growth was monitored by bioluminescence imaging using a cryogenically cooled IVIS 100 imaging system coupled to a data acquisition computer running Living Image Software (Xenogen Corp) (26). For the investigation of T cell infiltration into the primary and metastatic lung tumors, lung metastases were allowed to develop for 8 weeks after KRIB cell injection. IL-11Rα-CAR T cells (1×107 cells/mouse) or control T cells (1×107 cells/mouse) were labeled with CM-Dil (Molecular Probes, Inc.) and i.v. injected into the mice at 8 weeks. The mice were killed 72 h later. The primary bone and metastatic lung tumor as well as liver, heart, spleen, kidney and esophagus tissues were removed and examined using hematoxylin and eosin (H&E) staining for morphology and florescence microscopy for the presence of the CM-Dil–labeled T cells. We also determined whether T-cell therapy induced tumor cell apoptosis. Mouse lung tissues were embedded in Tissue-Tek optimum cutting temperature compound and frozen. Apoptosis was measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay.
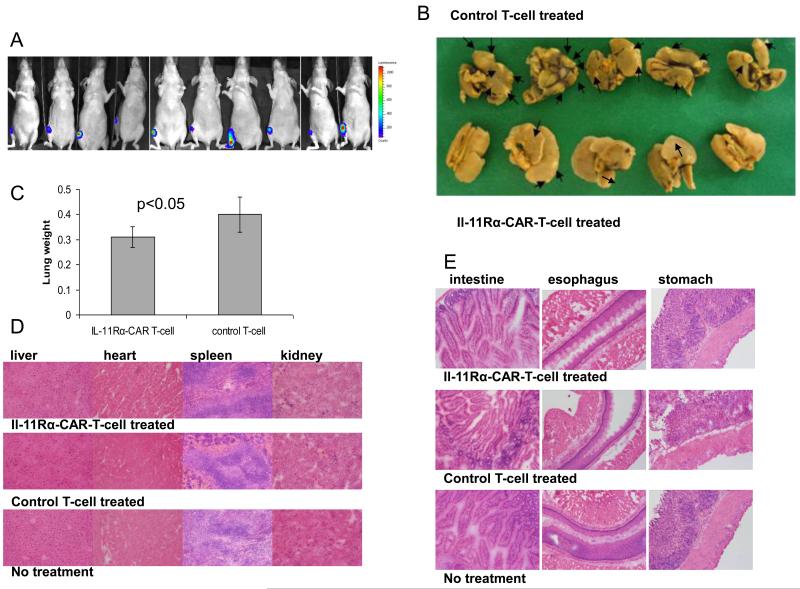
To evaluate the therapeutic effect of the IL-11Rα-CAR T cells on lung metastasis ten days after KRIB cell injection, the presence of tumor in the tibia was confirmed by luciferase signals. Mice with comparable tumor loads were selected for the therapy study (10 mice/group). Four days later (14 days after tumor injection), IL-11Rα-CAR T cells (1×107 cells/mouse) or control T cells (1×107 cells/mouse) were i.v. injected into the mice two times per week for 7 weeks. Limb amputation was performed 8 weeks after tumor cell injection due to the size of the tumor. Mice were killed 7 weeks after therapy completion. The lungs were then extracted and evaluated for metastases.
Statistical Analysis
Cell culture experiments were done at least in triplicate and repeated at least three times. Western blot and inmmunofluorescence experiments were repeated at least three times. Flow cytometry and chromium release assay were done at least in triplicate and repeated at least three times. Differences in IL-11Rα-CAR T cell-treated and control T cell-treated mouse lung weights were analyzed using the student’s paired t test. All the results were considered to be statistically significant at values of P<0.05.
Results
IL-11Rα overexpression in OS lung metastases and OS cell lines
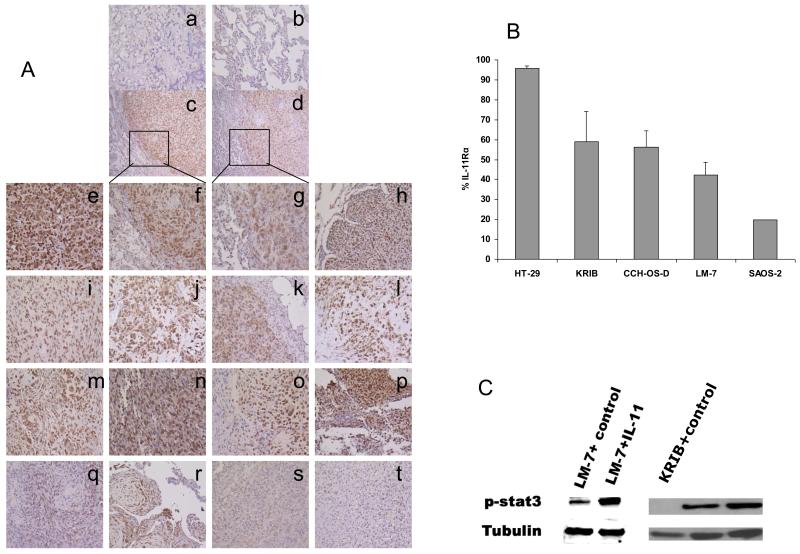
The expression of IL-11Rα in lung metastasis specimens from 16 different OS patients was analyzed by immunohistochemistry. IL-11Rα was expressed in 14 of 16 patient specimens (Fig. 2A, e-r were positive; s and t were negative). IL-11Rα was seen in the cytoplasm and on the cell surface. The OS cells in the metastatic tumor nodule were positive for IL-11Rα, whereas the surrounding normal lung was negative for IL-11Rα (Fig. 2A-c, A-d). We examined 4 different OS cell lines for cell surface expression of IL-11Rα using flow cytometry (Fig. 2B). IL-11Rα was expressed in all 4 OS cell lines, with expression levels ranging from 20% to 60%. To assess whether the IL-11Rα signaling pathway was functional, LM-7 and KRIB cells were incubated with IL-11. As shown in Fig. 2C, this led to the phosphorylation of Stat3 (p-Stat3).
Figure 2. Expression of IL-11Rα in lung metastases from OS patients.
(A) Immunohistochemical staining for IL-11Rα was performed in formalin-fixed paraffin-embedded OS lung metastasis specimens from 16 different patients. (Subpanel a) Tumor tissue stained without the first antibody served as the negative control. (Subpanel b) Surrounding normal lung tissue. (Subpanels e-t) OS lung metastases from 16 different patients. (Subpanels f and g) 100× magnification from the square areas in Subpanels c and d, respectively. Subpanels and specimens a, b, and e-t, ×100; Subpanels and specimens c and d ×40. (B) The expression of cell-surface IL-11Rα on tumor cells was analyzed by flow cytometry. HT-29 human colon cancer cells served as the positive control. (C) The expression of the phospho-Stat3 (p-Stat3) in LM-7 cells and KRIB cells treated with IL-11 or control medium was analyzed by Western blotting. Tubulin served as internal control.
IL-11Rα was not present in the normal lung tissue surrounding the tumor (Fig. 2A-c, 2A-d). To confirm this finding and determine whether IL-11Rα was expressed in other organs, we analyzed normal human tissue microarrays and mouse tissues. Immunohistochemical staining of the human micro-arrays revealed no IL-11Rα in the parenchymal cells of the brain, heart, or kidney or in the epithelial cells of the lung (data not shown). IL-11Rα was also absent in the hemopoietic cells in the lymph nodes and spleen. IL-11Rα expression was limited to the GI tract in the stroma tissues, endothelial cells, and surface and gland epithelial cells. There was weak positive staining in the liver. Similar results were found with immunohistochemical staining of mouse tissues. Western blot analysis revealed low expression levels of IL-11Rα in mouse esophagus only.
Ex-vivo expansion of IL-11Rα-CAR T cells using aAPCs
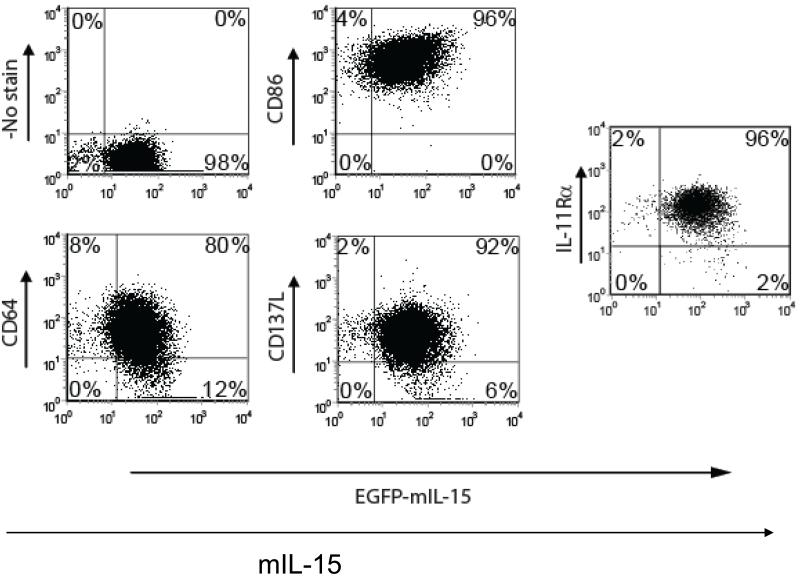
K562 clone 4 cells [22] transduced to coexpress membrane-bound IL-15 (mIL-15), CD86, CD64, and CD137 ligand (CD137L) were >98% IL-11Rα+ as measured by flow cytometry (Fig. 3). Therefore, the K562 clone 4 cells were used as the aAPCs for the expansion of IL-11Rα-CAR T cells in which CD64, CD86, CD137L, and mIL-15 served as co-stimulatory cytokines [22] and IL-11Rα served as a specific antigen for T-cell growth. Following incubation with γ-irradiated aAPCs and expansion, the number of IL-11Rα-CAR T cells increased by 2.8 ×102-, 1.3 ×104-, 4 ×105-, and 1.0 ×106-fold on days 14, 21, 28, and 35, respectively. The total number of CD3+ T cells similarly increased (data not shown). IL-11Rα-CAR T cells constituted 53%, 36%, 78%, 88%, and 87% of the total number of CD3+ T cells on days 1, 14, 21, 28, and 35, respectively. A higher percentage of the IL-11Rα-CAR T cells were CD8+ then CD4+. The percentage of CD8+ IL-11Rα-CAR T cells increased from 19% on day 14 to 67%, 77%, and 73% on days 21, 28, and 35, respectively. In contrast, the percentage of the CD4+ cells remained constant at ≤10%.
Figure 3. Characterization of aAPCs.
The K562 aAPCs (clone 4) were analyzed for the expression of membrane-bound IL-15 (mIL15), CD86, CD64, CD137L, and endogenous IL-11Rα by flow cytometry. A representative dot plot is shown. The numbers represent the percentages of the aAPC cells in each quadrant. The total percentage of the aAPC cells positive for each molecule is also indicated.
Sensitivity of OS cells in vitro to IL-11Rα-CAR T cells
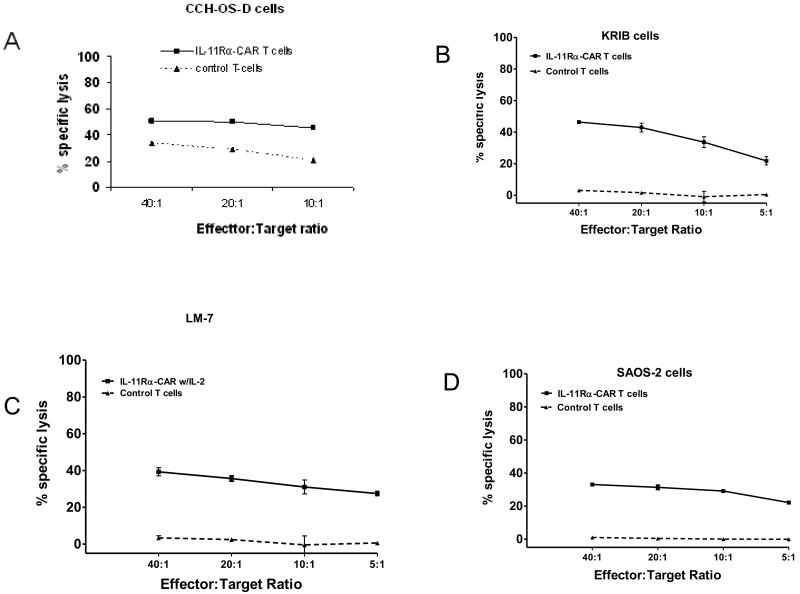
Having confirmed the expression of IL-11Rα-CAR by the genetically modified T cells, we next evaluated the ability of IL-11Rα-CAR T cells to kill SAOS-2, LM7, CCH-OS-D, and KRIB cells. IL-11Rα-CAR T cells were cytotoxic to all 4 cell lines (Fig. 4).
Figure 4. Cytotoxicity of IL-11Rα CAR T cells against OS cells in vitro.
CCH-OS-D, KRIB, LM-7, and SAOS-2 OS cells were incubated with IL-11Rα-CAR T cells or control T cells. Lysis of the tumor cells was quantified using chromium release assay.
Inhibition of OS lung metastases by IL-11Rα-CAR T cells
To evaluate the efficacy of IL-11Rα-CAR T cells against OS lung metastasis in vivo, we injected KRIB cells into the tibias of nude mice, which produced palpable tumors within 2 weeks and visible lung metastases by 6 weeks. The primary tumor must be removed at 8 weeks due to tumor size. This model mimics the clinical pathogenesis of osteosarcoma. We first investigated the ability of T cells to infiltrate the primary tumor and lung metastases. IL-11Rα-CAR T-cells or control T cells labeled with CM-Dil were i.v. injected 8 weeks after tumor cell inoculation. Three days after injection of the IL-11Rα-CAR T cells, CM-Dil+ cells were present in the tumor (Fig. 5A-b) but not the surrounding normal lung tissues (Fig 5. A-d). Control T cells were also present in the tumor (Fig. 5A-f). Neither CM-Dil+ IL-11R-CAR T-cells nor control T-cells were found in the primary bone tumor or any other organs including the esophagus (5B, C). TUNEL assay was used to assess apoptosis. TUNEL-positive (brown cells) were seen in lung metastasis from mice treated with IL-11Rα-CAR T cells (Fig. 5D-b). There were significantly fewer TUNEL-positive cells in the tumors from mice treated with control T cells (Fig. 5D-d). There was equivalent expression of IL-11Rα in the primary tumor (Fig 5. E-a) and lung metastasis (Fig 5. E-b). After confirming that T cells could reach targeted tumor cells in the lung, we next investigated the effect of IL-11Rα-CAR T-cell therapy on OS lung metastasis. Ten days following the intra-tibia injection of luciferase-labeled KRIB cells, tumor growth was confirmed by bioluminescence signals (Fig. 6A). T-cell therapy with either IL-11Rα-CAR T cells or control T cells was started 14 days after tumor cell injection and given 2 times per week for 7 weeks. Mice were killed and their lungs evaluated after the completion of the therapy. IL-11Rα-CAR T-cell treatment reduced the number of visible lung metastases (Fig. 6B). Three of the five mice treated with IL-11Rα-CAR T-cells had no visible metastases, whereas all of the five mice treated with control T cells had metastases. The number and size of the lung nodules (Fig. 6B) and the lung weight (Fig. 6C) were also lower in the mice treated with IL-11Rα-CAR T cells than in the mice treated with control T cells. There were no morphological changes or inflammatory cells in the liver, heart, kidney, spleen, esophagus, stomach, or intestine tissues obtained from the mice treated with IL-11Rα-CAR T cells as compared with the mice treated with control T cells (Fig. 6D, E).
Figure 5. IL-11Rα CAR T cells infiltrate lung metastases and induce apoptosis.
IL-11Rα-CAR T cells or control T cells labeled with CM-Dil were i.v. injected into nude mice with human KRIB primary bone and OS lung metastases. (A) After 72 h, lung metastases (Subpanels a, b, e, and f) and lung tissues (Subpanels c, d, g, and h) were analyzed using H&E staining (Subpanels a, c, e, and g) and florescent microscopy (Subpanels b, d, f, and h) for the presence of CM-Dil+ cells (red). The cellular nuclei were identified with Hoechst 33258 (blue; Subpanels b, d, f, and h). (B) Primary bone tumor was also analyzed using H&E staining (Subpanels a and c), CM-Dil (Subpanels b and d), and Hoechst 33245 (Subpanels b and d) as described in A. (C) other organs examined for presence of CM-Dil+ cells. (D) Tumor lung metastases were analyzed with H&E staining (Subpanels a and c), and the same tumor areas were analyzed using TUNEL assay (Subpanels b and d). (E) Immunohistochemical staining for IL-11Rα was performed in formalin-fixed paraffin-embedded KRIB OS primary tumor (Subpanels a) and lung metastasis (Subpanel b) from nude mice. Tumor tissue stained without the first antibody served as the negative control.
Figure 6. Effect of IL-11Rα CAR T cells on OS lung metastases.
(A) Ten days after intra-tibia injection with luciferase-expressing KRIB cells, luminescence signals were detected. (B) Two weeks following KRIB cell injection the mice were treated with IL-11Rα-CAR T cells or control T cells twice a week for 7 weeks. After therapy completion, mice were killed and their lungs extracted and analyzed for the presence of visible lung metastases. Representative lung tissue and lung metastases from 5 of 10 mice/group. Arrows identify visible metastatic nodules. (C) The mean wet-lung weight was calculated. One of three representative experiments (10mice/group) is shown. (D, E) H&E staining.
Discussion
The death rate from OS lung metastases has not changed for more than 20 years. Salvage chemotherapy has made little impact on the survival of relapsed disease in the lung (27). Patients that are cured often suffer from the late effects of chemotherapy particularly cardiac failure and osteoporosis. This underscores the need for the development of new tumor-specific therapy. Our findings indicate that genetically modified T cells engineered to recognize IL-11Rα may have therapeutic potential against OS lung metastases. We demonstrated that IL-11Rα was expressed by OS cell lines and on lung metastases from OS patients. The IL-11Rα signaling pathway was functional, as evidenced by the stimulation of p-Stat3 following incubation of both LM7 and KRIB cells with IL-11. In contrast, IL-11Rα was not expressed in the adjacent normal lung tissue or in the brain, heart, kidney, lymph nodes, or spleen. IL-11Rα has previously been identified in primary OS patient samples (20). In addition IL-11Rα-targeting phage injected i.v. resulted in the accumulation of these phage particles in the OS bone tumor tissue but not in normal bone (20). Finally, the therapeutic potential of targeting IL-11Rα was shown using a cyclic IL-11 nanopeptide conjugated to a suicide peptide (17). These data taken together with the findings in our study support the selection of IL-11Rα as a target for T-cell directed therapy.
CARs, which can redirect T-cell specificity to a tumor cell surface antigen (10), usually consist of an extracellular antigen-recognition domain, a trans-membrane, and an intracellular activation domain. Engagement of the target molecule by the extracellular antigen-recognition domain initiates a downstream signal that results in T-cell activation. CAR-initiated T-cell activation is not MHC restricted. Therefore, these T cells are able to kill tumor cells with downregulated HLA class I. The extracellular antigen-recognition domain is commonly from an antibody-derived signal-chain fragment (scFv) that can bind to a targeted antigen motif (10). In the current study we used an IL-11 peptide to construct IL-11Rα-CAR. The peptide we used has been shown to bind IL-11Rα as an extracellular receptor recognition signal (17, 28). The intracellular signaling is mediated through a chimeric CD28 co-stimulatory domain and a CD3-ζ activation domain that activates T cells. A second-generation CAR using dual endodomains CD28 and CD3-ζ improves T-cell activation (29).
We engineered IL-11Rα-CAR T-cells by transfecting human peripheral blood T cells with an IL-11R-CAR transposon and then propagated these transfected T cells ex vivo with aAPCs. The expression of IL-11Rα-CAR was confirmed by both Western blot analysis and flow cytometry, which indicated that the IL-11Rα-CAR was on the T-cell surface. These IL-11Rα-CAR T-cells killed OS cells in vitro and accumulated in the OS lung metastases but not in the normal surrounding lung tissue following i.v. injection. This demonstrates selective localization to the tumor tissue. Treating mice with established OS lung metastases with IL-11Rα-CAR T cells resulted in significant tumor cell apoptosis and tumor regression. While accumulation of control T-cells was also seen in the lung tumors with some apoptosis there was no significant therapeutic effect. The mice treated with control T cells had large pulmonary metastases compared to those treated with IL-11Rα-CAR T-cells.
It is important to evaluate the efficacy of potential therapies aimed at treating OS lung metastases using an in vivo model in which pulmonary metastases arise from a primary tumor. Evaluating the therapeutic efficacy against a primary bone tumor alone may not be adequate because the biological characteristics of the metastatic nodules may differ from those of the primary tumor. Such differing characteristics may be important not only in the metastatic process but also in the growth of metastatic tumor cells in a distant organ microenvironment. For example, we demonstrated in a previous study that while primary OS cells growing in the bone are Fas+, the cells comprising the lung metastases are Fas− which permits these cells to survive in the FasL+ lung microenvironment (30). We have similarly shown that this FasL+ organ microenvironment is critical to the efficacy of certain chemotherapeutic agents, including gemcitabine and liposomal 9-nitrocamptothecin (31, 32). Other investigators have shown that OS pulmonary metastases are less sensitive to chemotherapy than the primary OS tumors are (33). The human KRIB OS model used in the current study to test the efficacy of IL-11Rα-CAR T cells fits the criteria stated above (34). A primary bone tumor developed within 2 weeks of intra-tibia tumor cell injection, and spontaneous lung metastases developed within 6 weeks. KRIB lung metastases were IL-11Rα+ and Fas−. The histologies of both the primary tumor and lung metastases in the mouse model as judged by H&E were similar to those of patient samples.
Other potential targets have been proposed for therapy against OS. A monoclonal antibody against IGF-1R has been shown to induce the regression of subcutaneous OS (35, 36), but its effectiveness against OS lung metastases is unknown. Although Her-2 has also been proposed as a therapeutic target for OS lung metastases (37), its expression is weak and mainly seen in the cytoplasm (as opposed to the cell surface), indicating that Her-2 may be an inferior target compared with IL-11Rα. Constructing bi-specific T cells with IL-11Rα and Her-2 CARs on the same cell may prove beneficial since the expression of IL-11Rα is not 100% on every OS cell. However, this will require future studies investigating whether Her-2 is expressed in IL-11Rα− OS cells and whether Her-2–directed T cells can recognize and kill IL-11Rα− OS cells.
T-cell therapy is particularly relevant to OS because peripheral lymphocyte numbers correlate with OS patient outcomes (38). Patients who have high lymphocyte counts were shown to have a better outcome. T-cell infusions can increase the number of T lymphocytes raising the total number of T cells which again, was shown to be beneficial to the patients (38). T-cell infusions have also been shown to recruit other cytotoxic effector cells which again may have therapeutic benefit (39, 40). This is significant because OS is an orphan disease that is in dire need of new therapies. Salvage chemotherapy has failed to improve survival (27). We are using the same chemotherapy agents that were used over 20 years ago. The only documented successful approach for relapsed disease is multiple thoracotomies (41, 42). Identifying a new therapeutic approach that is not chemotherapy can have a significant impact on the lives of these patients. The data presented here suggest that CAR-specific T-cell therapy targeting IL-11Rα on OS cells may have therapeutic potential for the treatment of patients with OS pulmonary metastases.
Acknowledgements
We thank Margaret J. Dawson, Yanwen Yang, Harjeet Singh, Simon Olivares, and Ling Zhang, Ge Yang, for technical help.
Financial Support: This work was supported by National Cancer Institute grant R01 CA 42992 (to E.S.K.) and Cancer Center Support Grant CA 16672.
Footnotes
No conflicts of interest exist
References
- 1.Jaffe N. Chemotherapy in osteosarcoma: advances and controversies. In: Muggia FM, editor. Experimental and clinical progress in chemotherapy. Martinus Nijhoff Publishers; Boston: 1986. pp. 223–33. [Google Scholar]
- 2.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Balasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 3.Eiber F, Giuliano A, Echardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–6. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Goorin AM, Perez Atayde A, Gebhardt M, Andersen JW, Wilkinson RH, et al. Weekly high-dose methotrexate and doxorubicin for osteosarcoma: the Dana-Farber Cancer Institute/The Children’s Hospital – Study III. J Clin Oncol. 1987;5:1178–84. doi: 10.1200/JCO.1987.5.8.1178. [DOI] [PubMed] [Google Scholar]
- 5.Hudson M, Jaffe MR, Jaffe N, Ayala A, Raymond AK, Carrasco H, et al. Pediatric osteosarcoma: Therapeutic strategies, results and prognostic factors derived from a 10-year experience. J Clin Oncol. 1990;8:1988–97. doi: 10.1200/JCO.1990.8.12.1988. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcome R, et al. Chemotherapy for non-metastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Kleinerman ES, Erickson KL, Schroit AJ, Fogler WE, Fidler IJ. Activation of tumoricidal properties in human blood monocytes by liposomes containing lipophilic muramyl tripeptide. Cancer Res. 1983;43:2010–4. [PubMed] [Google Scholar]
- 8.Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol (CCT) 1995;18:93–9. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Meyers PA, Schwartz C, Krailo M, Healey JH, Bernstein M, Betcher D, et al. A report from the Children’s Oncology Group Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival. J Clin Oncol. 2008;26:119–26. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 10.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper LJ, Al-Kadhimi Z, DiGiusto D, Kalos M, Colcher D, Raubitschek A, et al. Development and application of CD19-specific T cells for adoptive immunotherapy of B cell malignancies. Blood Cells Mol Dis. 2004;33:83–9. doi: 10.1016/j.bcmd.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Campbell CL, Jiang Z, Savarese DM, Savarese TM. Increased expression of interleukin-11 receptor and evidence of STAT3 activation in prostate carcinoma. Am J Pathol. 2001;158:25–32. doi: 10.1016/S0002-9440(10)63940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and pg 130. Blood. 1995;86:1243–54. [PubMed] [Google Scholar]
- 14.Kiessling S, Muller-Newen G, Leeb SN, Hausmann M, Rath HC, Strater J, et al. Functional expression of the interleukin-11 receptor alpha-chain and evidence of antiapoptotic effects in human colonic epithelial cells. J Biol Chem. 2004;279:10304–15. doi: 10.1074/jbc.M312757200. [DOI] [PubMed] [Google Scholar]
- 15.Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effect of interleukin-11. Leukemia. 1999;13:1307–15. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- 16.Teramura M, Kobayashi S, Yoshinaga K, Iwabe K, Mizoguchi H. Effect of interleukin 11 on normal and pathological thrombopoiesis. Cancer Chemother Pharmacol. 1996;38:99–102. doi: 10.1007/s002800051048. [DOI] [PubMed] [Google Scholar]
- 17.Zurita AJ, Troncoso P, Cardo-Vila M, Logothetis CJ, Pasqualini R, Arap W. Combinatorial screenings in patients: the interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Res. 2004;64:435–9. doi: 10.1158/0008-5472.can-03-2675. [DOI] [PubMed] [Google Scholar]
- 18.Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. Expression of interleukin 11 and its receptor and their prognostic value in human breast cancer. Ann Surg Oncol. 2006;13:802–8. doi: 10.1245/ASO.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T, Yoshizaki A, Izumida S, Suehiro T, Miura S, Uemura T, et al. Expression of interleukin-11 (IL-11) and IL-11 receptor alpha in human gastric carcinoma and IL-11 upregulates the invasive activity of human gastric carcinoma cells. Int J Oncol. 2007;30:825–33. [PubMed] [Google Scholar]
- 20.Lewis VO, Ozawa MG, Deavers MT, Wang G, Shinta T, Arap W. The interleukin-11 receptor alpha as a candidate ligand-directed target in osteosarcoma: consistent data from cell lines, orthotopic models and human tumor samples. Cancer Res. 2009;69:1995–9. doi: 10.1158/0008-5472.CAN-08-4845. [DOI] [PubMed] [Google Scholar]
- 21.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;6:501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 22.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68(8):2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arap W, Kolonin MG, Trepel M, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–27. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 24.Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 25.Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage alignancies. Cancer Res. 2011;71:3516–27. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Ulrich Exner G. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Ke S, Kwon S, Yallampalli S, Cameron AG, Adams KE, et al. A new optical and nuclear dual-labeled imaging agent targeting interleukin 11 receptor alpha-chain. Bioconjug Chem. 2007;18:397–402. doi: 10.1021/bc0602679. [DOI] [PubMed] [Google Scholar]
- 29.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;15(66):10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 30.Gordon N, Kleinerman ES. The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat Res. 2009;152:497–508. doi: 10.1007/978-1-4419-0284-9_29. [DOI] [PubMed] [Google Scholar]
- 31.Gordon N, Koshkina NV, Jia S-F, Khanna C, Mendoza A, Worth LL. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential and reduces the effect of aerosol gemcitabine. Clin Cancer Res. 2007;13:4503–10. doi: 10.1158/1078-0432.CCR-07-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshkina NV, Khanna C, Mendoza A, Guan H, DeLauter L, Kleinerman ES. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role the Fas pathway in metastatic process of osteosarcoma. Molecular Cancer Res. 2007;10:991–9. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]
- 33.Posthumadeboer J, Witlox MA, Kaspers GJ, van Royen BJ. Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature. Clin Exp Metastasis. 2011;28:493–503. doi: 10.1007/s10585-011-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berlin O, Samid D, Donthineni-Rao R, Akeson W, Amiel D, Woods VL., Jr. Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer Res. 1993;53:4890–95. [PubMed] [Google Scholar]
- 35.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1190–7. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 36.Kolb EA, Kamara D, Zhang W, Lin J, Hingorani P, Baker L, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer. 2010;55:67–75. doi: 10.1002/pbc.22479. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–87. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore C, Eslin D, Levy A, Roberson J, Guisti V, Sutphin R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatric Blood and Cancer. 2010;55:1041–2. doi: 10.1002/pbc.22673. [DOI] [PubMed] [Google Scholar]
- 39.Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 40.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Putnam JB, Jr, Roth JA, Wesley MN, Johnston MR, Rosenberg SA. Survival following aggressive resection of pulmonary metastases from osteogenic sarcoma: analysis of prognostic factors. Ann Thorac Surg. 1983;36:516–23. doi: 10.1016/s0003-4975(10)60679-0. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins DS, Arndt CS. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]