Abstract
Targeted drug delivery to tumor sites is one of the ultimate goals in drug delivery. Recent progress in nanoparticle engineering has certainly improved drug targeting, but the results are not as good as expected. This is largely due to the fact that nanoparticles, regardless of how advanced they are, find the target as a result of blood circulation, like the conventional drug delivery systems do. Currently, the nanoparticle-based drug delivery to the target tumor tissues is based on wrong assumptions that most of the nanoparticles, either PEGylated or not, reach the target by the enhanced permeation and retention (EPR) effect. Studies have shown that so-called targeting moieties, i.e., antibodies or ligands, on the nanoparticle surface do not really improve delivery to target tumors. Targeted drug delivery to tumor sites is associated with highly complex biological, mechanical, chemical and transport phenomena, of which characteristics vary spatiotemporally. Yet, most of the efforts have been focused on design and surface manipulation of the drug carrying nanoparticles with relatively little attention to other aspects. This article examines the current misunderstandings and the main difficulties in targeted drug delivery.
Keywords: Targeted drug delivery, Nanoparticles, EPR effect, Tumor, Cancer
1. Introduction
Controlled drug delivery systems have advanced over the last 60 years [1]. Numerous delivery systems have been developed, and literally hundreds of controlled release formulations have been in clinical use. Such successful applications, however, have been limited to only a few types of formulations, such as oral and transdermal delivery systems. There are still many delivery systems to be optimized, including poorly soluble drug formulations, protein delivery systems, self-regulated insulin delivery devices and targeted drug delivery systems. Of these, targeted delivery to tumors has received great attention recently, partly due to the increased anticipation of achieving it using nanotechnology-based delivery systems. The emergence of nanotechnology has resulted in development of numerous nanostructures; various terms have been used to describe them such as nanocarrier, nanovehicle, nanosystem, nanodisc, nanoworm, nanorod, and nanotube. For simplicity, all nanostructures, regardless of their actual shape and nature, will be described in this paper as nanoparticles. The term “nanoparticles” also include drug–polymer conjugates, drug– protein conjugates, liposomes, polymer micelles, dendrimers, and drug nanocrystals, all of which have existed since the 1960s [2].
The ultimate goal of targeted drug delivery is to deliver most of the administered drug to the target, while eliminating or minimizing the accumulation of the drug at any non-target sites. This, however, is a challenge that may not be achieved in the near future. Targeted delivery may occur if the drug formulation is injected directly to the target site, and such localized administration is not feasible or practical in most cases. Unless specifically mentioned otherwise, targeted delivery here means delivery of the intravenously (iv) administered dose to the target site, e.g., solid tumors. Targeted drug delivery systems are designed to facilitate drug delivery to the tumor sites with minimum side effects associated with the use of free drugs [3]. This has been one of the main reasons of using nanoparticles. As pointed out by Florence in 2007, however, nanoparticle-based targeted drug delivery has not fulfilled its expectations [4], and this remains true today.
Almost the whole decade of 2000s has been consumed by developing various nanoparticles for targeted drug delivery to tumors, and the results are not, on the whole, encouraging. This is not surprising since advances in any field come in small increments and over time the cumulative advances make big differences. At the same time, however, it needs to be clearly understood why nanoparticle delivery systems have not been able to meet our expectation even remotely. Only with such an understanding we will be able to design the right nanoparticles for true targeted delivery. For example, the so-called nanotechnology-based formulations, such as paclitaxel-albumin complex and paclitaxel in polymer micelles, are as good as the conventional formulation of Cremophor/ethanol-based formulation, but not any better in treating cancers. If targeted drug delivery has been achieved, the treatment should have been better with much less side effects. The lower side effect should allow increase in the administered dose for superior results, but this has not happened. It seems, for example, that the nanoparticle formulation of paclitaxel is simply for solubilizing poorly soluble drug for iv administration, rather than for achieving true targeted delivery. It will be beneficial to review current understanding, as well as misunderstanding, of issues in targeted drug delivery.
2. A simplified version of current targeted drug delivery by nanoparticles
As the field of nanotechnology-based drug delivery has advanced, it is necessary to remove unjustified reliance on PEGylated nanoparticles accumulating in tumors by the enhanced permeation and retention (EPR) effect. Developing truly targeted drug delivery systems has to be based on clear understanding on the problems that must be solved. Treating tumors requires delivering therapeutic agents in optimal quantities to tumors. The iv administered agent will have to circulate in the blood stream, extravasate (i.e., cross the vascular walls) into the interstitium, and penetrate tumors [5]. As described in Fig. 1-A, one has to understand that the majority of the iv administered drug is not going to the target tumor. The actual accumulation of the administered dose varies depending on the nanoparticle formulation, drug, drug assay method, and other experimental parameters, but it usually is less than 5% [6]. Sometimes higher values are observed depending on the assay method [7] or the type of nanoparticles [8]. Fig. 1-B shows three main factors affecting the overall drug delivery to tumors.
Fig. 1.
Delivery of intravenously (iv) administered nanoparticles to the target tumor. (A)The majority of the administered nanoparticles end up in the non-target organs and only a small fraction reaches the target tumor. (B) The nanoparticles circulate in blood to reach target tumors by extravasation. The drug leaks out of the nanoparticles during circulation, and the majority of the nanoparticles end up in non-target organs. The nanoparticles reaching the target tumor face a tumor microenvironment different from that of normal tissues.
For iv administered nanoparticles to reach the target, they have to circulate in the blood. Since nanoparticles have no means of self-propulsion, reaching the target is purely based on the movement of blood passing through the target, wherever it may be [4]. The presence of ligands on the nanoparticle surface, so-called “active targeting”, does not change the chances of nanoparticles reaching the target. During blood circulation, a significant portion of the nanoparticles are taken up by the reticuloendothelial system (or mononuclear phagocyte system) of the spleen, liver, and lungs [9]. A conventional idea is that the chances of reaching the target would increase by prolonging the blood circulation time, such as through PEGylation [9,10]. PEGylation is also thought to prevent or decrease the uptake by the reticuloendothelial system. The nanoparticles used for drug delivery are usually larger than 100 nm. Because the threshold size for efficient renal clearance is known to the kinetics of uptake [11] or 70 kDa [12], the renal clearance of nanoparticles may not be a dominant mechanism for elimination of nanoparticles from blood. The presumed threshold sizes, however, need confirmation by more data. One may anticipate that the drug remains inside the nanoparticles and is released only at the target site, but in reality the drug is released during circulation. Depending on their nature, the nanoparticles may not remain intact in the blood, which results in premature dumping of the encapsulated drugs.
The extravasation of nanoparticles from blood vessel to the interstitium of tumors is thought to be achieved by the enhanced permeation and retention (EPR) effect. The term “EPR effect,” however, has been abused by applying it ubiquitously as if all of the iv administered nanoparticles go only to the tumors. According to the original paper by Maeda, proteins accumulate better in tumors than do nanoparticles [13]. Even if nanoparticles reach the tumor site, they have to penetrate the tumor microenvironment, known to be significantly different from that of normal tissues. The dense extracellular matrix and elevated interstitial fluid pressure of tumors makes drug penetration more difficult than in normal tissues.
3. Current uncomfortable facts in targeted drug delivery
3.1. There is no targeted delivery, but simply random distribution
A minor portion of iv administered nanoparticles end up in tumors, primarily as a result of blood circulation (Fig. 1-A). Nanoparticles do not have any propulsive force leading the system to the target [14], and thus, the so-called “homing” mechanism by the antibody or ligand grafting is at the mercy of blood circulation. There has been some misunderstanding in targeting, expressed in the concept of passive and active targeting. When these terms were first used, they were intended to distinguish delivery systems having ligands from those without. As time passed by, however, the original reasoning was lost, and the terms were used as if the delivery systems are really designed to target any particular site in the body. Perhaps this has misled the subsequent development on targeted drug delivery.
One example illustrating the irrelevance of the presence of ligand on the liposomes in targeting is described by Kirportin et al. [8]. The presence of the monoclonal antibody (anti-HER2) of immunoliposomes did not increase their accumulation at the tumor tissue significantly over the control, as shown in Fig. 2. In fact, the pharmacokinetic profiles of the control (non-targeted) and immunoliposomes are almost identical. Furthermore, it is important to note that the liposome levels reached 7–8% of the injected dose/g tumor tissue whether the tumor overexpressed HER2 or not. The only thing that the antibody or ligand on the nanoparticle surface presents is the higher chance of binding and/or internalizing into tumor cells, if the cells happen to overexpress a receptor at the time when nanoparticles are nearby. As shown in Fig. 2-insert, uptake of the immunoliposomes by the HER2-overexpressing tumor cells was an order of magnitude higher than the control liposome. HER2-coated poly(DL-lactic acid) nanoparticles also showed no difference in biodistribution as compared with the control nanoparticle [15]. Another recent study also showed that many targeting ligands for brain targeting did not really target to the brain in vivo when attached to liposome [16].
Fig. 2.
Tumor pharmacokinetics of anti-HER2 immunoliposomes (Anti-HERs ILs) versus control PEGylated liposomes (Ls) in s.c. BT-474 breast cancer xenografts in nude mice. (Inset) uptake of anti-HER2 immunoliposomes (cross-hatched column on the right) versus control liposomes (empty column on the left) in HER2-overexpressing breast cancer cells (SK-Br-3) in vitro (redrawn from reference [8]).
3.2. Overexpression of receptors has little to do with targeted delivery
The inability to change the biodistribution of liposomes by the presence of surface antibody suggests that the biodistribution is not likely to be altered by the presence of other ligands either. In fact, the tumor accumulation of an albumin–folate conjugate was not any different from that of albumin in rodent tumor models [17]. No significant differences were observed between folate-conjugated PLGA nanoparticles in pharmacokinetics and biodistribution as compared with the control micelle [18]. Such lack of differences may be due to another fact that the majority of the folate conjugate is captured by the liver which is a major storage organ of excess folate [17]. A significant portion of pH-sensitive micelles with surface-grafted folate is known to accumulate in the liver and spleen [19]. Since the accumulation in tumor is based on blood circulation, the presence of antibody or ligand on nanoparticle surface may be irrelevant in determining biodistribution. This leads to another point that overexpression of the receptors on cancer cells is actually not associated with increasing the amount of nanoparticles reaching tumor sites.
The presence of antibody or ligand on the nanoparticle surface may increase the cellular uptake if the cells in contact with nanoparticles happen to have overexpressed receptors. Overexpression on cancer cells means that the normal cells also express the receptors. Since the total number of normal cells is much larger than the number of cancer cells, it is reasonable to consider that most of the ligand is captured by the normal cells. Considering the fact that not all tumor cells overexpress receptors all the time, it is plausible that inconsistent results are achieved by antibody/ligand-conjugated nanoparticles. In addition, the kinetics of uptake and receptor recycling need to be considered. The goal of nanoparticle delivery is to maximize the drug concentration within the tumor tissue. Thus, the faster cellular uptake is preferred. Comparative studies between receptor-mediated endocytosis (i.e., receptor-specific) and fluid phase endocytosis (i.e., non-specific) have shown that the former occurs at a significantly faster rate and more efficiently than the latter [20,21]. This information, however, should be interpreted cautiously, since its primary relevance to drug delivery is whether the receptor-mediated endocytosis can occur fast but repeatedly in the presence of high concentrations of antibody/ligand-conjugated nanoparticle. The receptor-mediated endocytosis is saturable, so that the efficiency of endocytosis decreases once the receptors are saturated [22] In addition, the rate of receptor recycling time also affects the drug uptake efficiency. For example, the folate receptor recycling in tumors varies from 6 h to 20 h depending on the tumor cell type [23]. The efficiency of receptor-mediated endocytosis depends on the concentration of the nanoparticle near target cells and dosing frequency.
Cancer is not one but a highly heterogeneous set of diseases, and thus no one cancer form can represent all cancers [24]. Even for a specific cancer, inter-individual variation is extremely large. Even in the same patient, cancer cells may behave differently depending on different stages of the progression [24]. The receptors on cancer cells are highly variable in density or structure [25]. This is one of the reasons why identifying a molecular target on cancer cells does not always lead to successful treatment of the disease. It is noted, however, the presence of ligand on nanoparticles can enhance receptor-mediated transcytosis [26], and it can be effective in transporting nanoparticles beyond 40–50 µm (3–5 cell layers) away from the tumor vasculature [27]. This suggests that transcytosis into the core of tumors can be possible using antibody/ligand-grafted nanoparticles.
3.3. The EPR effect results in improved delivery, but not targeted delivery
Nanoparticles have been assumed to target tumors, and this misunderstanding is most likely due to the unrestricted use of the EPR effect concept. The EPR effect, as clearly indicated in a recent review by Professor Maeda and his group [28], improves the therapeutic effects of nanoparticles as compared with the conventional chemotherapy with low molecular weight drugs. The key point here is the relative improvement in accumulation at tumors as compared with conventional small drug molecules. The EPR effect does not explain the absolute accumulation of nanoparticles.
Small molecules do not show the EPR effect, because they can freely pass through the blood vessels into the tumor as well as the normal tissue, and diffuse back into blood capillaries [13]. In contrast, macromolecular drugs, e.g., drug–albumin conjugates, pass through the blood vessels around the tumor and do not diffuse back into blood capillaries or end up in lymphatic system. One of the drug conjugates first used by Professor Maeda was SMANCS (a conjugates of styrene and maleic acid copolymer (SMA) and neocarzinostatin (NCS)). The molecular weight of NCS is 12,000 Da and two SMA chains of 2000 Da each were conjugated to make the 16,000 Da molecule. In comparison, the molecular weight of albumin and IgG is 68,000 Da and 150,000 Da, respectively. Matsumura and Maeda concluded that macromolecules of a certain molecular range (15,000 to 70,000) and with certain properties can effectively accumulate in a solid tumor [13].
The study by Matsumura and Maeda presents the data on the percent of the recovered protein [13], as shown in Table 1. SMANCS accumulated in the tumor only at the level of 5% during 72 h of observation. During this time, SMANCS was mostly recovered in the liver and spleen (60–90% of the injected dose, as indicated by bold font in Table 1). On the other hand, for bovine albumin about 7.0% of the injected dose was found in the tumor, while only about 15% and 6% was found in the liver and spleen, respectively. A similar trend was observed with mouse IgG. Collectively, the data clearly indicate that albumin and IgG accumulate more and are retained in the tumor tissue longer, with less accumulation in the liver and spleen. The comparison here is for only one new synthetic system, SMANCS, and a general conclusion may require more head-to-head comparisons between novel nanoparticles and other formulations [9]. Nevertheless, the information in Table 1 suggests that synthetic materials may not be as good as endogenous protein in escaping the reticuloendothelial system.
Table 1.
Tissue distribution of two 51Cr-labeled proteins in tumor-bearing mice after iv injection (from reference [13]).
| Tissue/organ | Proteins recovered as % of injected doses/g of specimen at 3 different times (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SMANCS | Bovine albumin | Mouse IgG | |||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Tumor | 4.92 | 4.0 | 4.71 | 6.85 | 6.95 | 7.22 | 7.98 | 5.65 | 6.56 |
| Blood | 0.86 | 0.49 | 0.24 | 4.06 | 1.90 | 0.95 | 5.27 | 2.28 | 2.24 |
| Liver | 42.42 | 50.71 | 42.92 | 16.05 | 13.28 | 15.09 | 6.88 | 2.56 | 4.55 |
| Kidney | 2.43 | 2.8 | 2.75 | 4.17 | 3.46 | 3.47 | 5.15 | 2.25 | 2.00 |
| Spleen | 24.21 | 40.40 | 36.4 | 6.09 | 5.63 | 4.7 | 4.09 | 1.91 | 2.88 |
| Lung | 2.36 | 2.98 | 2.13 | 2.47 | 1.76 | 1.6 | 4.36 | 1.91 | 2.12 |
| Heart | 1.25 | 1.58 | 1.70 | 1.80 | 1.53 | 1.17 | 2.4 | 1.25 | 1.58 |
Nanoparticles are much larger than albumin or IgG. Thus, nanoparticles will be much less efficient in passing through the blood vessel as compared with the proteins and water-soluble polymers. This will drastically decrease the amount of nanoparticles leaking to the tumor tissue. Furthermore, nanoparticles, in particular made of artificial materials such as PLGA, block copolymer, dendrimer, and polymer conjugates, may be more prone to elimination by the reticuloendothelial system. This may be one of the reasons why the total amount of nanoparticles accumulating in tumor tissues is still only in the order of 5% of the injected dose as described in the vast majority of data in the literature. Thus, it will not do any good for advancing the targeted drug delivery if we continue to assume, without any scientific basis, that nanoparticles, regardless of their properties, will automatically go to the tumor tissue by the EPR effect. Although nanoparticles have been reported to deliver more drugs to the tumor tissue than the same drugs in a solution formulation, this does not mean that nanoparticles have targeted drug delivery properties. Since more than 90% of the injected dose does not end up in the target, nanoparticle-based therapy relying on the EPR effect may be better called “improved delivery.”
3.4. Drug release can occur before reaching the target site
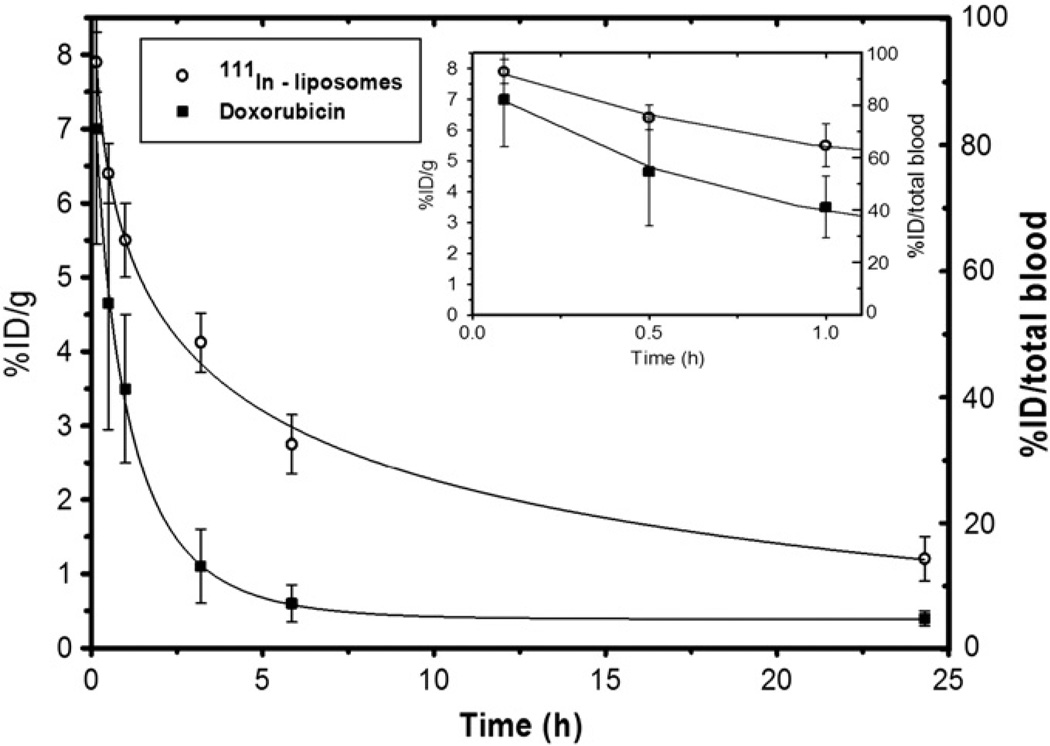
It has been assumed, without any clear reason, that drug molecules loaded in nanoparticles are released only after the nanoparticles reach the target, i.e., around the tumor cells or after endocytosed into the tumor cells. Only a few studies have examined the pharmacokinetics of a drug independent of its delivery system. Studies have reported that the drug loaded in nanoparticles, such as PEGylated liposomes and polymer micelles, release the loaded drug right after iv administration. When doxorubicin-loaded liposomes were iv administered, doxorubicin was cleared faster than the liposomal carrier. As shown in Fig. 3, doxorubicin starts to leak from liposome soon after administration. In 3 h, only about 10% of the total administered doxorubicin remains, while about 50% of the liposome circulates in the blood [29].
Fig. 3.
Blood kinetics of 111In labeled temperature-sensitive liposomes containing doxorubicin. The percentage of the injected dose (%ID) is plotted per gram blood (left axis) and for the total blood (right axis) (redrawn from reference [29]).
The faster clearance of the drug compared with the nanoparticles leads to a question on the usefulness of PEGylation. If the loaded drug is released before nanoparticles reach the tumor site, the long-term circulation by the PEGylated nanoparticles, even if it occurs, may not be as useful as presumed. This is aside from the fact that the percentage of the PEGylated nanovehicles circulating after 24 h is much less than 10% of the injected dose. Furthermore, another point to be considered for PEGylated nanoparticles is that PEGylation may result in accelerated blood clearance (ABC) phenomenon. The ABC phenomenon refers to the decline of the prolonged blood circulation of PEGylated nanoparticles following the second dose a few days later, resulting from the formation of anti-PEG IgM [30]. Further understanding on the effect of PEGylation on the ABC phenomenon is still warranted [31]. The problems associated with premature drug release, however, can be alleviated if nanoparticles are properly modified. For example, recent studies on polymer micelles have shown that drug release can be delayed until they are endocytosed by cancer cells, if either the shell or core of polymer micelles is crosslinked [32,33].
3.5. Reaching the tumor tissue is not the same as improved delivery
The ultimate goal of targeted drug delivery is to achieve both systemic and intracellular targeting. Intracellular targeting, unless the nanoparticles are introduced directly to the target cells, occurs only after the systemic targeting is achieved. For this reason, systemic targeting has been the target of extensive studies. Reaching the tumor tissue following blood circulation and extravasation is not the endpoint of the targeted drug delivery.
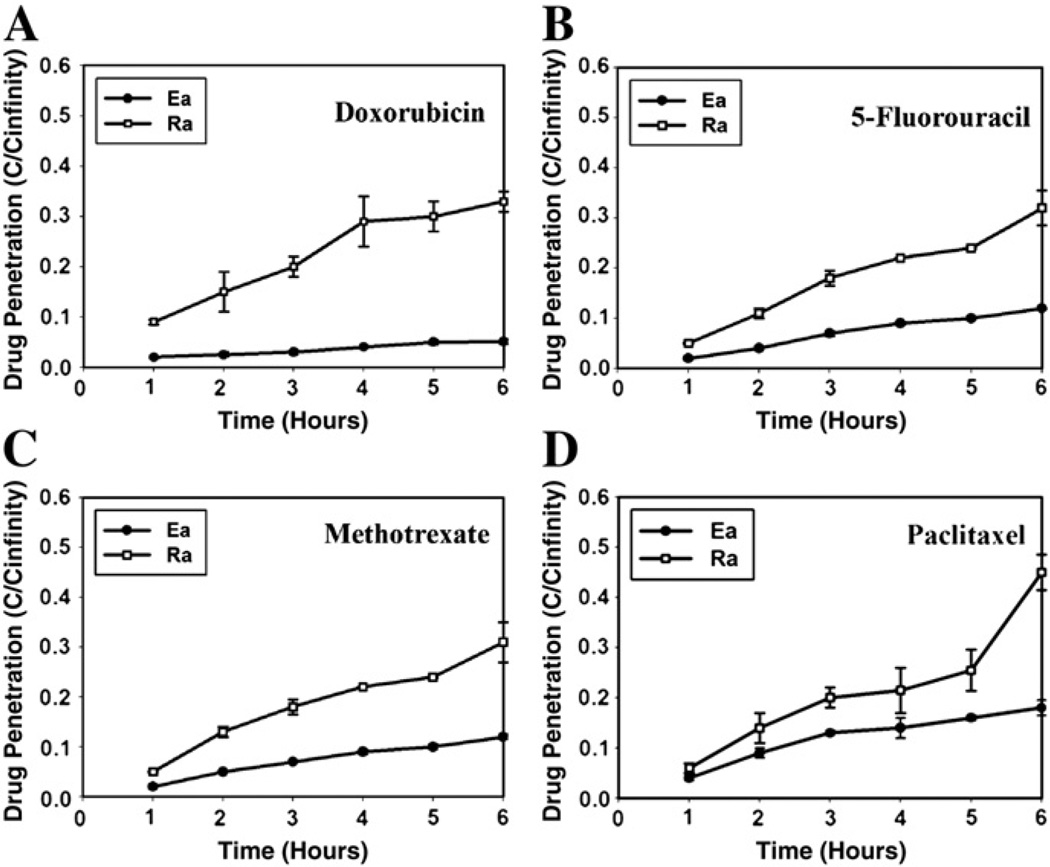
Nanoparticles face a tumor microenvironment much different from the extracellular matrix (ECM) of normal tissues. It is well known that cancer cells develop drug resistance, and this is attributed to unique features of tumor-associated stromal ECM and ECM remodeling [3]. The tumor microenvironment has denser ECM, making drug penetration more difficult, than the normal ECM. The cellular packing density is another factor to consider in efficient drug delivery. This point is clearly demonstrated by a simple experiment using multicellular layers of cancer cells of different cellular packing density [5]. As shown in Fig. 4, the penetration of four anticancer drugs (paclitaxel, doxorubicin, methotrexate, and 5-fluorouracil) through the multicellular layers of cancer cells has been shown to be a function of cellular packing density. Even for those small molecular drugs penetration through tightly packed epithelioid sublines was significantly lower than through the loosely packed round sublines. This leads to a question as to how nanoparticles, which are much larger than the drugs, will be able to diffuse through the tumor microenvironment and tightly packed tumor cells.
Fig. 4.
Penetration of anticancer drugs as a function of time through multicellular layers derived from human colon carcinoma cell lines. The penetration of doxorubicin (A), 5-FU (B), methotrexate (C), and paclitaxel (D) through highly packed (Ea, ●) and loosely packed (Ra, ○) sublines (redrawn from reference [5]).
More difficulties are caused by transport hindrances due to elevated tumor interstitial fluid pressure (IFP) and abnormal ECM structure [34–36]. As shown in Fig. 5, the IFP of a solid tumor stays at elevated level and sharply decreases at the periphery of the tumor. Even though it is not fully understood, this elevated IFP is thought to result from anomalous characteristics of tumor vascular structure including high vascular permeability and lack of well-developed lymphatic vessels. This elevated IFP adversely affects the transport of therapeutic agents in several different levels: (i) less extravasation of the agents [37]; and (ii) radially outward interstitial fluid movement at the periphery of tumor. Consequently, the elevated IFP contributes to insufficient delivery of drugs to the interior of tumors. Moreover, it not only diminishes the significance of convection in the interstitial transport, but also induces adverse convection — oozing drugs out of tumors. The magnitude of this outward interstitial fluid movement is estimated to be 0.1–0.2 µm/s for a 1 cm isolated tumor [34], and an order of magnitude less for a subcutaneous tumor [38].
Fig. 5.
Interstitial fluid pressure (IFP) and velocity distribution of a tumor grown in subcutaneous tissue. The center and the boundary of the tumor are indicated by r/R=0, and r/R=1, respectively. IFP ( ) stays at elevated level at the interior of the tumor and sharply decreases at the periphery. Due to this pressure gradient, radially outward interstitial fluid motion is induced at approximately 0.02 µm/s (
) stays at elevated level at the interior of the tumor and sharply decreases at the periphery. Due to this pressure gradient, radially outward interstitial fluid motion is induced at approximately 0.02 µm/s ( ). This outward convection in conjunction with less extravasation due to elevated IFP is believed to lead insufficient delivery of therapeutic agents (redrawn from reference [38]).
). This outward convection in conjunction with less extravasation due to elevated IFP is believed to lead insufficient delivery of therapeutic agents (redrawn from reference [38]).
Thus, the elevated tumor IFP makes diffusion a primary transport mechanism in tumor interstitium. Higher collagen content and the consequent dense organization of collagen fibrils, however, result in low diffusivity for therapeutic agents. Moreover, since diffusion decreases drastically as the size of drug increases, transport of macromolecules and nanoparticles is significantly limited in tumor interstitial space [35,39–41].
For successful interstitial drug transport, therefore, these physiological barriers must be overcome. A wide variety of methods have been proposed and investigated to enhance the interstitial transport, but the main underlying strategies are either lowering tumor IFP [42–44], or modulating tumor ECM structure [35,45]. However, due to complex interaction involving various physiological parameters, the control or manipulation of tumor IFP and ECM structure still warrants further research. The xenograft models have mainly been used to study these, but new models to allow systemic study of the effects of these physiological parameters are highly desired.
Thus, nanoparticles may accumulate in the tumor tissue, confirmed with improved fluorescence signals or other imaging signals, but it is not clear whether this is associated with improved drug delivery to the tumor cells. As mentioned above, the majority of the drug is released from liposome, and most likely from other nanoparticles, within several hours [29]. The drug release from nanoparticles before reaching tumor tissues works against targeted drug delivery to tumor sites. In fact, the accumulation of empty nanoparticles at tumors may in fact negatively impact the drug delivery. The liposomes accumulating at the tumor site by way of the EPR effect are known to linger for days, and the extravasated liposomes can remain near blood vessels for a week [46], providing physical barriers for the subsequent liposomes and/or free drugs.
4. Future
To develop truly targeted drug delivery systems we have to realize that there may be a limit in drug delivery to the targeting of tumors by nanoparticles [14]. The current overestimation of targeted drug delivery stems from a few oversimplifications of the complex biotransport phenomena. It should be recognized that targeted drug delivery cannot be tested using an in vitro cell culture model which lacks these complex transport processes. When nanoparticles are directly introduced to the cultured cells, the interaction between the two is virtually guaranteed due to the physical proximity. The presence of ligands on the nanoparticles facilitates cellular interaction, leading to internalization into cancer cells. This, however, is hardly targeted drug delivery. Assessing targeted drug delivery absolutely requires using in vivo models in which the iv administered nanoparticles undergo distribution throughout the body, in both normal and neoplastic tissues, and elimination by the reticuloendothelial system. Xenografts are the most commonly used model for the in vivo biodistribution study for drug development as well as testing of drug delivery systems. The wide use of xenografts is, in large part, due to their high degree of reproducibility while maintaining some biological properties of human tumors [24].
It is not uncommon to see that the drug delivery to tumors has increased substantially by the nanoparticles in the xenograft models. Whether we call this targeted drug delivery or not, it must be recognized that more than 90% of the injected does end up in or near normal tissues and organs. Another challenge of targeted drug delivery, as any other drug delivery strategies, is that therapeutic agents have to be delivered in sufficient quantities at a rate designed to produce beneficial results [4]. Although the tumor size usually shrinks by administration of the nanoparticle formulations, the still remaining question is whether nanoparticle formulations can eradicate tumors.
Albumin is the main energy and nutrition source for the tumor growth [47], and it can be used as a valuable biomarker of cancer [48]. Considering the excellent properties of albumin allowing accumulation and retention in the tumor tissue, the use of albumin for targeted drug delivery is not surprising [49–52]. What is surprising, however, is that albumin has not been exploited more extensively in targeted drug delivery. It may be due to the fact that the drug delivery field has been occupied by the notion of nanoparticles and did not pay too much attention to albumin [53]. While albumin may not be a perfect solution either [54], it provides an alternative approach [55].
Development of truly targeted drug delivery systems which are not simply better than the control but deliver the majority of the injected dose to the target tumor tissue requires drastic changes in our current approaches. Changes in strategies, experimental methods, evaluation criteria on successful delivery are required. The drug delivery community needs to escape from the cozy assumption that PEGylated nanoparticles with the aid of the EPR effect will somehow magically eliminate the target tumor. It is too good to be true, and in fact, it has not been shown that the nanoparticles are any better than traditional non-nano systems and formulations. The difficulty in targeted drug delivery to tumor tissue is that the problem is beyond the preparation of new types of nanoparticles. Nanoparticles will have to transport through highly complex tumor-associated ECM which varies its biological, mechanical, and chemical properties in a spatiotemporal manner. There has been only a few study on the quantitative cellular uptake of nanoparticles in vivo [56], and thus, it is not clear whether the results of in vitro studies have any bearing in the real targeted drug delivery in animals, not to mention in humans [57]. Only a deeper understanding of the human physiology and tumor biology will allow us to develop truly targeted delivery systems. Accepting the fact that it is extremely difficult to achieve targeted drug delivery would allow us to find new ways to treat cancer. It is time to think beyond PEGylated nanoparticles and the support of the EPR effect. It is time to think beyond the approach we have been taking.
Acknowledgments
This study was supported in part by NIH through grants CA129287 and GM095879, and the Showalter Research Trust Fund.
References
- 1.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J. Control. Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Park K. Nanotechnology: what it can do for drug delivery. J. Control. Release. 2007;120:1–3. doi: 10.1016/j.jconrel.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cukierman E, Khan DR. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem. Pharmacol. 2010;80(80):762–770. doi: 10.1016/j.bcp.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florence AT. Pharmaceutical nanotechnology: more than size. Ten topics for research. Int. J. Pharm. 2007;339:1–2. doi: 10.1016/j.ijpharm.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Grantab R, Sivananthan S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 2006;66:1033–1039. doi: 10.1158/0008-5472.CAN-05-3077. [DOI] [PubMed] [Google Scholar]
- 6.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality, and possibility. J. Control. Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand N, Leroux J-C. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J. Control. Release. 2012:152–163. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 10.Bansal R, Post E, Proost JH, de Jager-Krikken A, Poelstra K, Prakash J. PEGylation improves pharmacokinetic profile, liver uptake and efficacy of interferon gamma in liver fibrosis. J. Control. Release. 2011;154:233–240. doi: 10.1016/j.jconrel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Tam JM, Tam JO, Murthy A, Ingram DR, Ma LL, Travis K, Johnston KP, Sokolov KV. Controlled assembly of biodegradable plasmonic nanoclusters for near-infrared imaging and therapeutic applications. ACS Nano. 2010;4:2178–2184. doi: 10.1021/nn9015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chames P, Regenmortel MV, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 14.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J. Control. Release. 2010;141:265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Cirstoiu-Hapca A, Buchegger F, Lange N, Bossy L, Gurny R, Delie F. Benefit of anti-HER2-coated paclitaxel-loaded immuno-nanoparticles in the treatment of disseminated ovarian cancer: therapeutic efficacy and biodistribution in mice. J. Control. Release. 2010;144:324–331. doi: 10.1016/j.jconrel.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 16.van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. J. Control. Release. 2011;150:30–36. doi: 10.1016/j.jconrel.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Shinoda T, Takagi A, Maeda A, Kagatani S, Konno Y, Hashida M. In vivo fate of folate-BSA in non-tumor and tumor bearing mice. J. Pharm. Sci. 1998;87:1521–1526. doi: 10.1021/js980215v. [DOI] [PubMed] [Google Scholar]
- 18.Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Control. Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Stromhaug PE, Berg TO, Gjoen T, Seglen PO. Differences between fluid-phase endocytosis (pinocytosis) and receptor-mediated endocytosis in isolated rat hepatocytes. Eur. J. Cell Biol. 1997;73:28–39. [PubMed] [Google Scholar]
- 21.Bareford LM, Swann PW. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kranenborg MH, Boerman OC, Oosterwijk-Wakka JC, Weijert MCD, Corstens FH, Oosterwijk E. Two-step radio-immunotargeting of renal-cell carcinoma xenografts in nude mice with anti-renal cell-carcinoma X anti-DTPA bispecific monoclonal antibodies. Int. J. Cancer. 1998;75:74–80. doi: 10.1002/(sici)1097-0215(19980105)75:1<74::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 24.Damia G, D'Incalci M. Contemporary pre-clinical development of anticancer agents — what are the optimal preclinical models? Eur. J. Cancer. 2009;45:2768–2781. doi: 10.1016/j.ejca.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Capone PM, Papsidero LD, Chu TM. Relationship between antigen density and immunotherapeutic response elicited bymonoclonal antibodies against solid tumors. J. Natl. Cancer Inst. 1984;72:673–677. [PubMed] [Google Scholar]
- 26.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Xiong C, Zhang R, Shi L, Huang M, Zhang G, Song S, Huang Q, Liu G, Li C. Receptor-mediated transcytosis: a mechanism for active extravascular transport of nanoparticles in solid tumors. J. Control. Release. 2012;161 doi: 10.1016/j.jconrel.2012.05.014. http://dx.doi.org/10.1016/j.jconrel.2012.1005.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 29.de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J. Control. Release. 2011;150:102–110. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J. Control. Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Park K. To PEGylate or not to PEGylate, that is not the question. J. Control. Release. 2010;142:147–148. doi: 10.1016/j.jconrel.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Kim SE, Kwon IK, Park C, Kim C, Yang J, Lee SC. Spatially mineralized self-assembled polymeric nanocarriers with enhanced robustness and controlled drug-releasing property. Chem. Commun. 2010;46:377–379. doi: 10.1039/b913732g. [DOI] [PubMed] [Google Scholar]
- 33.Koo AN, Min KH, Lee HJ, Lee S-U, Kim K, Chan Kwon I, Cho SH, Jeong SY, Lee SC. Tumor accumulation and antitumor efficacy of docetaxel-loaded core-shell-corona micelles with shell-specific redox-responsive cross-links. Biomaterials. 2012;33:1489–1499. doi: 10.1016/j.biomaterials.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 35.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 36.Brown E, Mckee TD, Tomaso ED, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 37.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors II. Role of heterogenous perfusion and lymphatics. Microvasc. Res. 1990;40:246–263. doi: 10.1016/0026-2862(90)90023-k. [DOI] [PubMed] [Google Scholar]
- 38.Baxter LT, Jain RK. Transport of fluid and macromoleculres in tumors I. Role of interstitial pressure and convection. Microvasc. Res. 1989;37:77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 39.Pluen A, Boucher Y, Ramanujan S, Mckee TD, Gohongi T, Tomaso ED, Brown EB, Izumi Y, Campbell RB, Berk DA, Jain RK. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramanujan S, Pluen A, Mckee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandrakis G, Brown E, Tong RT, Mckee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat. Med. 2004;10:203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- 42.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 43.Rubin K, Sjoquist M, Gustafsson A-M, Isaksson B, Salvessen G, Reed RK. Lowering of tumoral interstitial fluid pressure by prostaglandin E1 is paralleled by an increased uptake of Cr-EDTA. Int. J. Cancer. 2000;86:636–643. doi: 10.1002/(sici)1097-0215(20000601)86:5<636::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB, Sjöquist M, Ahlström H, Reed RK, Rubin K. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. FASEB J. 2003;17:1756–1758. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- 45.Davies CdL, Berk DA, Pluen A, Jain RK. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer. 2002;86:1639–1644. doi: 10.1038/sj.bjc.6600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan F, Leunig M, Huang SK, Berk DA, Papahad-jopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 47.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, Maier-Borst W, Heene DL. Plasma protein (albumin) catabolism by the tumor itself — implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 48.Fanali G, diMasi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serumalbumin: from bench to bedside. Mol. Aspects Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Di Stefano G, Fiume L, Baglioni M, Busi C, Bolondi L, Farina C, Mori F, Chieco P, Pariali M, Kratz F, Molin L, Salmaso S, Caliceti P. Doxorubicin coupled to lactosaminated albumin: effect of heterogeneity in drug load on conjugate disposition and hepatocellular carcinoma uptake in rats. Eur. J. Pharm. Sci. 2008;33:191–198. doi: 10.1016/j.ejps.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control. Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Elsadek B, Kratz F. Impact of albumin on drug delivery — new applications on the horizon. J. Control. Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 52.Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. J. Control. Release. 2012;161:429–445. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 53.Park K. Albumin: a versatile carrier for drug deliverys. J. Control. Release. 2011;157:3. doi: 10.1016/j.jconrel.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Soni N, Margarson M. Albumin. Where are we now? Curr. Anesth. Crit. Care. 2004;15:61–68. [Google Scholar]
- 55.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 56.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Mikhail AS, Allen C. Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J. Control. Release. 2009;138:214–223. doi: 10.1016/j.jconrel.2009.04.010. [DOI] [PubMed] [Google Scholar]