Abstract
WNV has become the leading vector-borne cause of meningoencephalitis in the United States. Although the majority of WNV infections result in asymptomatic illness, approximately 20% of infections result in West Nile fever and 1% in West Nile neuroinvasive disease (WNND), which causes encephalitis, meningitis, or flaccid paralysis. The elderly are at particular risk for WNND, with more than half the cases occurring in persons older than sixty years of age. There is no licensed treatment for WNND nor is there any licensed vaccine for humans for the prevention of WNV infection. The Laboratory of Infectious Diseases at the National Institutes of Health has developed a recombinant live attenuated WNV vaccine based on chimerization of the wild-type WNV NY99 genome with that of the live attenuated DENV-4 candidate vaccine rDEN430. The genes encoding the prM and envelope proteins of DENV-4 were replaced with those of WNV NY99 and the resultant virus was designated rWN/DEN4Δ30. The vaccine was evaluated in healthy flavivirus-naïve adult volunteers age 18 – 50 years in two separate studies, both of which are reported here. The first study evaluated 103 or 104 PFU of the vaccine given as a single dose; the second study evaluated 105 PFU of the vaccine given as two doses 6 months apart. The vaccine was well-tolerated and immunogenic at all three doses, inducing seroconversion to WNV NY99 in 74% (103 PFU), 75% (104 PFU), and 55% (105 PFU) of subjects after a single dose. A second 105 PFU dose of rWN/DEN4Δ30 given 6 months after the first dose increased the seroconversion rate 89%. Based on the encouraging results from these studies, further evaluation of the candidate vaccine in adults older than 50 years of age is planned.
Keywords: West Nile virus (WNV), live attenuated WNV vaccine, clinical trial
INTRODUCTION
West Nile virus (WNV) is a member of the Japanese encephalitis virus serogroup of the genus Flavivirus belonging to the Flaviviridae family [1]. Humans are thought to be only incidental hosts in the transmission cycle of WNV in which birds serve as the amplifying hosts of the virus and Culex mosquitoes serve as the primary vector [1]. Although the majority of cases of WNV are asymptomatic, approximately 20% of infections result in symptomatic West Nile fever or neuroinvasive disease (WNND) manifesting as encephalitis, meningitis, or flaccid paralysis resembling poliomyelitis [2– 4]. The first outbreak of WNV in the Western Hemisphere occurred in New York in 1999, and since that time, WNV has become the leading vector-borne cause of viral encephalitis in the United States [1, 5]. By 2010, the number of adults infected with WNV in the U.S. was estimated to be nearly 3 million, with approximately 13,000 cases of WNND, almost half of which occurred in persons more than 60 years of age [6]. Importantly, the second largest recorded outbreak of WND in the United States occurred in 2012 with the CDC reporting 5,674 cases, including 2,873 (50.6%) of which were severe neurologic WNND and 286 deaths [7].
Economic analyses of WNV epidemics have demonstrated them to be costly [8, 9] and there is currently no licensed treatment or human vaccine for WNV. A low cost, efficacious vaccine may provide a cost-effective alternative for the prevention of WNV disease. Based on the success of the yellow fever and Japanese encephalitis vaccines, scientists at the Laboratory of Infectious Diseases have developed numerous live attenuated candidate flavivirus vaccines [10–15]. Many of these were evaluated in clinical trial and were demonstrated to be attenuated and immunogenic in adult flavivirus-naïve subjects [16–20]. A similar strategy was employed to develop a recombinant live attenuated chimeric WNV vaccine designated rWN/DEN4Δ30. The vaccine was highly attenuated for neurovirulence and neuroinvasiveness in mice compared with its wild-type parent virus WNV NY99 [21, 22]. Importantly, non-human primates immunized with a single dose of rWN/DEN4Δ30 were completely protected against challenge with wild-type WNV NY99 [21]. rWN/DEN4Δ30 also demonstrated reduced ability to infect, replicate, and disseminate in both Culex and Aedes mosquitoes, diminishing its risk of transmission from vaccinees to other hosts [23]. These data encouraged further evaluation of the rWN/DEN4Δ30 vaccine in healthy flavivirus-naïve adult subjects. Here we describe two Phase I clinical trials of rWN/DEN4Δ30 designed to examine the safety, immunogenicity, and dosing regimen of this promising candidate vaccine.
MATERIALS AND METHODS
Two studies of the live attenuated chimeric vaccine rWN/DEN4Δ30 were conducted under an investigational new drug application (BB-IND #11940) reviewed by the US Food and Drug Administration. The studies were conducted at the Center for Immunization Research (CIR) at the Johns Hopkins Bloomberg School of Public Health and the Vanderbilt University School of Medicine and were approved by the Institutional Review Boards and Biosafety Committees of both institutions. The National Institute of Allergy and Infectious Diseases (NIAID) Intramural Data Safety Monitoring Board was convened for periodic review of all study data. The trials were registered with Clinicaltrials.gov as NCT00094718 and NCT00537147.
Study population
Healthy adult male and non-pregnant female subjects were recruited from the Baltimore, MD and Nashville, TN metropolitan areas. Informed consent was obtained from each subject in accordance with the Code of Federal Regulations (21 CFR 50). Healthy subjects between the ages of 18 and 50 years were enrolled if they met the following eligibility criteria: normal findings during physical examination; negative for antibodies to DENV-1, DENV-2, DENV-3, DENV-4, yellow fever, WNV, and St. Louis encephalitis viruses; negative for hepatitis B and C viruses; negative for HIV; normal values for complete blood count (CBC) with differential, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, creatinine phosphokinase, coagulation studies, and urinalysis. Female subjects were required to have a negative result on a urine pregnancy test at screening and on vaccination day and were required to use a reliable method of contraception.
Study design and clinical monitoring
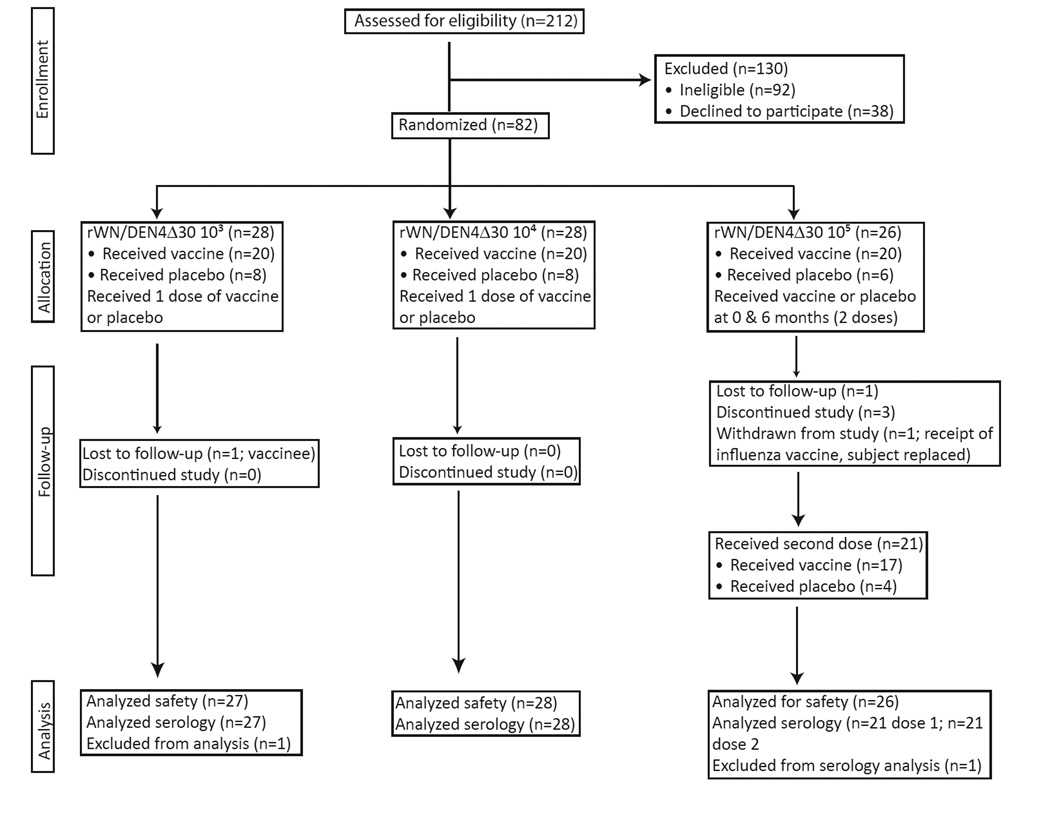
Both studies were randomized, double-blind, placebo-controlled trials designed to assess the safety and immunogenicity of rWN/DEN4Δ30. Three doses of the rWN/DEN4Δ30 vaccine (103 PFU, 104 PFU, or 105 PFU), given as a single subcutaneous dose, were to be studied in a single trial. However, due to the limited availability of fully potent vaccine virus (see below), only the 103 PFU and 104 PFU cohorts were enrolled (Figure 1). After manufacture of a new vaccine lot, a second study was initiated to evaluate 105 PFU of rWN/DEN4Δ30, given at 0 and 6 months, (Figure 1).
Figure 1.
Consort diagram illustrating the number of subjects screened, enrolled, and followed in the study as well as the number of subjects included in the safety and serologic analyses. A subject could be replaced if the immunogenicity data out to day 42 was not collected or could not be evaluated as defined by the protocol. Because one subject was replaced (a placebo recipient) a total of twenty-six subjects were enrolled in the 105 PFU cohort.
On day of vaccination, subjects were randomly assigned to receive either vaccine or placebo (vaccine diluent) given as a single 0.5 ml subcutaneous injection. Volunteers were given a digital thermometer and a diary card to record their oral temperature three times per day for 16 days. After each vaccination, clinical assessments for local and solicited reactogenicity (Table 2) were performed every other day through study day 16 (and on day 19, single dose study) and again on study days 21, 28 and 42. Blood was drawn at each assessment for detection of viremia through study day 16 or 19 and for antibody assay on study days 0, 28, 42 and 180. After each vaccination, a CBC with differential, serum ALT, and coagulation studies were done at periodic intervals through study day 28. The clinical and laboratory assessments following the second dose of vaccine were identical to the first dose follow-up schedule. All adverse events were graded for intensity and relationship to vaccine as previously described [24].
Table 2.
Percentage of rWN/DEN4Δ30 vaccine (all dose levels) or placebo recipients who experienced indicated injection site or systemic adverse eventa
| Adverse event | rWN/DEN4Δ30 (n=60) |
Placebo (n=22) |
p-value (2-tailed) |
|---|---|---|---|
| Injection site: | |||
| Erythema | 1.7% | 4.6% | 0.47 |
| Tenderness | 3.3% | 4.6% | 1.00 |
| Pain | 3.3% | 9.1% | 0.29 |
| Systemicb: | |||
| Fever | 5.0%c | 4.6% c | 1.00 |
| Headache | 25.0% | 27.3% | 1.00 |
| Rash | 15.0% | 13.6% | 1.00 |
| Neutropeniad | 6.7% | 4.6% | 1.00 |
| Elevated ALTe | 5.0% | 4.6% | 1.00 |
| Elevated CPK | 5.0% | 9.1% | 0.61 |
| Fatigue | 5.0% | 13.6% | 0.34 |
| Myalgia | 8.3% | 4.6% | 1.00 |
| Arthralgia | 1.7% | 9.1% | 0.17 |
| Photophobia | 1.7% | 0.0% | 1.00 |
Only local and solicited adverse events that occurred are presented in the table. Other solicited and local adverse events that did not occur are not presented in the table for brevity.
No subject experienced nuchal rigidity, neuropsychological, neurocerebellar, neuromotor, or neurosensory adverse events.
Temperature elevation was determined to be unrelated to vaccine in all subjects.
Absolute neutrophil count < 1,500/mm3.
Defined as a value > 1.25 above the clinical laboratory upper limit of normal.
Vaccine virus
rWN/DEN4Δ30 is a live attenuated chimeric virus vaccine produced using recombinant DNA technology and described previously [21, 22, 25]. The prM and E protein genes of the live attenuated DENV-4 vaccine candidate rDEN4Δ30 [26] have been replaced by those of the wild-type WNV strain NY99-35262 [25]. The remainder of the genome, including the capsid protein and all non-structural protein genes, is derived from the rDEN430 vaccine virus. Two rWN/DEN4Δ30 vaccine lots were generated and used in the two clinical studies. The first lot (WN/DEN4#1, titer of 3.1×105 PFU/ml) was produced and safety tested at Novavax, Inc. (Rockville, MD) with Good Manufacturing Practices (cGMP). The second lot (WN/DEN4#108A, titer of 2×107 PFU/ml) was produced and safety tested at Charles River Laboratories (Malvern, PA) with cGMP. The amino acid sequence of the polyprotein for these two vaccine lots did not differ [27]. Prior to administration, all vaccines were diluted to the appropriate titer with safety-tested L-15 medium (placebo). At each vaccination, aliquots of diluted and undiluted vaccine were titrated as described below to ensure potency of the vaccine administered.
Virus quantitation
The amount of virus in blood (viremia) was determined using a standard plaque- forming assay as previously described [26]. On the last day of detectable viremia for each subject, viral genomic RNA was isolated and reverse transcribed and a PCR cDNA fragment corresponding to the end of NS5 gene and the 3′UTR of the DEN4-part of chimeric genome (nts 9735 – 10644; GenBank accession no. AY376438) was generated. A region (nts 9830 – 10434) around the Δ30 deletion in the virus genome was sequenced to evaluate the stability of the Δ30 mutation.
Serologic assessment
Serum neutralizing antibody titers against wt WNV strain NY99 were measured by the 60% plaque-reduction neutralization assay as previously described [21, 22]. Neutralizing antibody titer was defined as the highest dilution of serum that reduced the number of WNV plaques by 60% (PRNT60). Seroconversion to WNV was defined as a ≥ 4-fold rise in serum neutralizing antibody titer to the wild-type WNV parent virus at either study day 28 or 42 post-vaccination, compared with the pre-vaccination PRNT60. The durability of the antibody response was determined by measurement of the PRNT60 at study day 180 following the first dose.
Data analysis
The purpose of this study was to describe the safety and immunogenicity of this novel vaccine candidate rather than to test formal statistical hypotheses. Secondary purposes were to determine the safety and immunogenicity of a booster dose of vaccine and to assess the safety profile of the different lots of the vaccine. Baseline characteristics and frequency of vaccine-related adverse events were compared between vaccine and placebo groups, between the subjects receiving one injection of vaccine and two injections of vaccine, and between subjects receiving the three different doses of the vaccine (103 PFU, 104 PFU and 105 PFU). Data from the two studies are presented together to improve the statistical validity of the results and because an important outcome of both studies was to determine which dose of vaccine (103 PFU, 104 PFU, or 105 PFU) should be further evaluated in older subjects. Statistical significance was determined using the Fishers exact test (adverse event data) or according to Tukey-Kramer multiple comparison test (viremia data) using JMP 7 software version 5.0.1.2 (SAS Institute, Cary, NC).
RESULTS
Demographics
A total of 212 subjects were recruited and 82 subjects were enrolled in the two studies (Figure 1). In total, 60 subjects received vaccine while 22 received placebo. Vaccinees ranged in age from 19 to 50 years while subjects receiving placebo ranged in age from 20 to 49 years. There was no statistically significant difference in mean age between subjects who received vaccine (31.3 years) and those who received placebo (31.6 years) (Table 1). There was also no statistically significant difference in mean age of subjects between the dose groups. There was no significant difference in the race or gender of vaccine recipients between dose groups and compared with placebo recipients. A total of 76 subjects were followed for the duration of the studies; six subjects were removed or withdrew from the study (Figure 1).
Table 1.
Demographics of vaccine and placebo recipients
| 103 PFU rWN/DEN4Δ30 (n=20) |
104 PFU rWN/DEN4Δ30 (n=20) |
105 PFU rWN/DEN4Δ30 (n=20) |
Placebo (n=22) |
|
|---|---|---|---|---|
| Age ± SE (years) | 29.4 ± 2.1 | 31.7 ± 2.1 | 33.1 ± 2.2 | 31.6 ± 1.7 |
| Female (%) | 55 | 40 | 75 | 68 |
| Race (%) | ||||
| Black | 45 | 20 | 70 | 50 |
| Caucasian | 50 | 75 | 30 | 45 |
| Asian | 5 | 5 | 0 | 0 |
| Other | 0 | 0 | 0 | 5 |
Vaccine virus
Undiluted and diluted vaccine was titrated on each vaccine day within 4 hours of vaccine virus removal from the −80°C freezer. For every vaccination except one, the titer of diluted vaccine virus was within 0.5 log10 PFU/ml of the target titer. However, when WN/DEN4#1 vaccine was prepared for 3 subjects in the 104 PFU cohort, the resulting titer of the undiluted vaccine was approximately 100-fold below the expected titer and the diluted vaccine was approximately 25-fold below the expected titer. Although these three subjects received a dose of vaccine approximately 102.6 PFU instead of the intended dose of 104 PFU, the safety and immunogenicity results have been included and will be commented on below.
Local reactogenicity and solicited adverse events
The vaccine was well tolerated by all vaccine recipients and no subjects experienced a systemic WNV-like or dengue-like illness. There was one serious adverse event that was unrelated to vaccine. A subject was hospitalized and diagnosed with diabetes mellitus 94 days after vaccination. There was no significant difference in the incidence of any solicited or local adverse event between vaccinees and placebo- recipients (Table 2) or by dose cohort (data not shown). The most commonly reported adverse events experienced by subjects were headache and rash. No subject experienced signs or symptoms of encephalopathy or meningitis. Three vaccine recipients and one placebo recipient developed fever following first vaccination. The fevers were due to inter-current illness, including acute influenza, and were determined to be unrelated or unlikely related to the vaccine. One vaccinee developed a fever following second vaccination. This fever was due to mild cholecystitis and was determined to be not related to vaccine. During physical examination it was noted that nine vaccine recipients and three placebo recipients developed a macular rash following first vaccination. The rashes were observed between 2 and 19 days following receipt of vaccine with a mean duration of 8 days. There were no rashes following revaccination. The rashes observed in the placebo recipients occurred on days 5 to 10 post-vaccination with a mean duration of 2.6 days.
There were no significant differences in the occurrence of abnormal laboratory values in vaccinees compared with placebo recipients. Four vaccinees and one placebo recipient developed transient mild neutropenia [defined as an absolute neutrophil count (ANC) of 1,000 – 1500/mm3] lasting an average of four days. One vaccine recipient developed severe neutropenia (ANC nadir of 600/mm3study day 6) and elevated CPK secondary to confirmed acute influenza B infection.
Viremia
Viremia was detected in eight vaccine recipients following first vaccination (Table 3). No subject developed detectable viremia after second vaccination. The peak viral titer (0.5 log10 PFU/ml) was at the lower level of detection and did not differ significantly by study or by vaccine dose. There was no significant difference in the onset or duration of viremia between cohorts. Virus isolates were prepared from serum collected from each vaccinee on the last day of detectable viremia (study day 8 to 19). Sequence analysis of these isolates revealed that the engineered Δ30 mutation remained unchanged in all virus isolates. Only one point mutation at nt 10308 (U → C) in the area adjacent to the Δ30 mutation was identified in one isolate.
Table 3.
Summary of viremia in subjects vaccinated with rWN/DEN4Δ30 (first dose)
| Vaccine candidate |
Dose (log10 PFU) |
No. subjects |
No. infecteda (%) |
No. viremic (%) |
Mean peak titer, log10 PFU/ml ± SEb |
Mean day of onset of viremia ± SEb |
Mean no. days of viremia ± SEb |
|---|---|---|---|---|---|---|---|
| rWN/DEN4Δ30 | 3.0 | 19c | 14 (74) | 3 (16) | 0.5 ± 0.0 | 15.3 ± 1.9 | 2.3 ± 0.7 |
| rWN/DEN4Δ30 | 4.0 | 20 | 15 (75) | 4d (20) | 0.5 ± 0.0 | 9.2 ± 1.8 | 3.2 ± 0.9 |
| rWN/DEN4Δ30 | 5.0 | 20 | 11 (55) | 1 (5) | 0.5 ± 0.0 | 8.0 ± 0.0 | 1.0 ± 0.0 |
Infection is defined as recovery of vaccine virus from the blood or a ≥ 4-fold rise in serum neutralizing antibody titer to vaccine virus at day 28 or 42 compared with day 0.
Mean peak titer is calculated only for those subjects who were viremic.
One subject withdrew from the study prior to the collection of samples for testing of viremia.
One of these viremic subjects received only ~ 102.6 PFU due to diminished potency of the vaccine
Serological Response
Following one dose, 40 of 59 vaccinees (68%) seroconverted to wt WNV/NY99. The frequency of seroconversion by dose cohort ranged from 55% (105 PFU) to 75% (104 PFU) (Table 4). Two of the three subjects who received ~ 102.6 PFU did not develop detectable neutralizing antibody against WNV NY99 however the third subject had detectable viremia following vaccination and developed a peak PRNT60 of 1:155. Following a second dose of 105 PFU of vaccine, 5 of 7 (71%) subjects who had not seroconverted to wild-type WNV/NY99 following the first dose, seroconverted following the second dose. In addition, two vaccinees who had not met the definition of seroconversion by study day 42 did so by study day 180. Following two doses of vaccine, 89% of vaccinees met the definition of seroconversion (Table 5).
Table 4.
PRNT60 against WNV(NY99) induced by a single dose of rWN/DEN4Δ30 given at 103, 104, or 105 PFU
| Geometric mean PRNT60 to WNV(NY99) (range)a |
|||||||
|---|---|---|---|---|---|---|---|
| Vaccine candidate |
Dose (log10 PFU) |
No. subjects |
Day 0 | Day 28 | Day 42 | Day 180 | # sero- converting (%)b |
| rWN/DEN4Δ30 | 3.0 | 19 | <5 | 64 (<5–1270) | 161 (8–1530) | 76 (<5–290) | 14 (74) |
| rWN/DEN4Δ30 | 4.0 | 20 | <5 | 117 (5–3218) | 107 (<5–854) | 35 (<5–232) | 15 (75) |
| rWN/DEN4Δ30 | 5.0 | 20 | <5 | 44 (18–183) | 39 (9–330) | 15c (<5–120) | 11 (55)d |
Reciprocal titer. Geometric mean PRNT60 calculated only for those subjects who seroconverted at study day 28 or 42.
Defined as a ≥ 4-fold rise in serum neutralizing antibody against WNV(NY99) at day 28 or 42.
17/20 vaccinees returned for study day 180.
Two vaccinees who did not have a ≥ 4-fold rise in serum neutralizing antibody titer by study day 42 did so by study day 180. These subjects were not included in % seroconversion per the protocol definition of seroconversion.
Table 5.
A second dose of rWN/DEN4Δ30 at day 180 boosts the antibody response against WNV
| Geometric mean PRNT60 to WNV(NY99) virus (range)a |
||||||
|---|---|---|---|---|---|---|
| Vaccine candidate |
Dose (log10 PFU) |
No. subjects |
180 | 208 | 222 | # sero- converting (%)b |
| rWN/DENΔ30 | 5.0 | 17 | 15 (<5–120) | 39 (6–154) | 57 (17–134) | 15 (89) |
Reciprocal titer. Calculated for all subjects who seroconverted after first or second dose of rWN/DEN4Δ30.
Defined as a ≥ 4-fold rise in serum neutralizing antibody against WNV(NY99) by day 222 compared with day 0.
DISCUSSION
This vaccine candidate is a live chimeric flavivirus comprised of the prM and E protein genes of WNV NY99 and the capsid and non-structural protein genes of the attenuated DENV rDEN4Δ30. The vaccine is attenuated by two mechanisms: chimerization of WNV with a non-neuroinvasive flavivirus, DENV-4, and a 30-nucleotide deletion in the 3′UTR. Chimerization is a potent attenuation strategy and was the major factor that led to the satisfactory balance between attenuation and immunogenicity of rWN/DEN4 for mice and monkeys [21, 22, 25]. The advantage of a dual attenuation strategy is that the mutations are independently attenuating and make reversion to a wild-type WNV or DENV phenotype within a vaccinated host nearly impossible.
Overall, the vaccine was well tolerated with no significant differences noted in the occurrence of any systemic or local adverse event in vaccinees compared with placebo recipients. Importantly, there were no clinical signs or symptoms indicative of neurotropism of the vaccine in healthy adult volunteers. Following one dose of vaccine, the frequency of seroconversion against wild-type WNV NY99 was highest in the 103 and 104 PFU dose groups (74% and 75%, respectively) and lowest, surprisingly, in the 105 PFU dose group (55%), although these differences did not reach statistical significance. The rates of seroconversion to wild-type WNV induced by the WN/DEN4Δ30 vaccine were somewhat lower than that induced by another live attenuated chimeric WNV vaccine, ChimeriVax-WN02 [28]. However, the target virus used in the PRNT50 assay for that vaccine trial was the immunizing vaccine virus itself and not wild-type WNV, a less stringent assessment. We found higher seroconversion rates and higher neutralizing antibody titers when WN/DEN4Δ30 was used as the target virus in the PRNT60 assay (data not shown) but have presented the results of the assay with wild-type WNV as the target virus because we believe that it may be a more relevant assessment of immunogenicity.
Interestingly, two volunteers who received the 105 PFU dose of rWN/DEN4Δ30 and who had not seroconverted to WNV/NY99 by study 42, did seroconvert by study day 180. The first had a peak PRNT60 of only 1:7 to WNV/NY99 on day 28 following first vaccination which increased to 1:22 on day 180 (prior to second dose). The second vaccinee did not have detectable antibody to WNV/NY99 by study day 42 following the first dose but developed a titer of 1:120 by study day 180. This volunteer received the first dose of vaccine on 9/10/08 and the second dose 3/11/09 and it is unlikely that the volunteer was exposed to WNV in the interim as it would have occurred during the very late fall period. Overall, 13/20 (65%) subjects who received the first 105 PFU dose of rWN/DEN4Δ30 seroconverted to WNV/NY99 by study day 180, which is comparable to that observed for recipients of 103 and 104 PFU of vaccine. We have observed a delayed development of peak antibody titer in some recipients of other chimeric flavivirus vaccines. In particular, 36% of recipients of a live attenuated tetravalent dengue vaccine containing a chimeric DENV-2 component developed peak titers to DENV-2 after study day 56. Unfortunately in our current study, serum was not collected between study day 42 and study day 180 and we therefore cannot establish the kinetics of the antibody responses in these two volunteers.
Only recipients of the 105 PFU dose received a second dose of vaccine and the overall frequency of seroconversion in this cohort improved to 89% following the second dose. Antibody titers were boosted following the second dose, increasing from a GMT of 1:15 at day 180 to 1:57 six weeks following second vaccination. It is unclear whether the observed antibody boost was secondary to undetected replication of the vaccine virus or caused by the antigen load delivered by the 105 PFU dose. Based on the evidence of boosting with a second dose of rWN/DEN4Δ30 given at 6 months, a second dose given at an earlier time-point such as 3 or 4 months after the first dose may provide a boost in antibody titer and would be a more useful vaccination schedule.
A satisfactory balance between sufficient vaccine attenuation and immunogenicity can be difficult to achieve. Although rWN/DEN4Δ30 is highly attenuated, it was able to induce seroconversion to wild-type WNV/NY99 in 55% to 75% of vaccinees after a single dose. Higher seroconversion frequencies were observed with lower doses of the vaccine, something that has been demonstrated for other flavivirus vaccines [29–31]. The reduced viremia and immune response of volunteers who received the high dose of WNV vaccine might have been due to differences in the two manufacturing productions and subsequently, in accumulation of quasi-species and defective interfering particles, in particular, in the WN/DEN4#108 virus prepared at the higher titer. It should be noted that evidence for interference in high-dose infection of flaviviruses such as tick-borne Langat virus [29] and DENV-2 [32] was reported in monkeys and mice, respectively.
In summary, the rWN/DEN4Δ30 vaccine candidate was well tolerated and immunogenic. A single dose of 103 or 104 PFU of vaccine was able to induce seroconversion to WN/NY99 in ~75% of vaccinees. However, the titer of antibody required for protection against WND is not known and efficacy studies may be difficult because the incidence rate is low in the U.S. and outbreaks are sporadic. Because the majority of WNND occurs in persons older than 60, the target population for a vaccine would most likely be adults ≥ 50 years of age. Future studies are being designed to evaluate the safety and immunogenicity of rWN/DEN4Δ30 in adults older than 50. It will also be important to determine if a second dose of vaccine will improve either the seroconversion frequency or the durability of the antibody response and if so, establish the optimal interval between vaccinations.
HIGHLIGHTS.
We evaluated two lots of a novel chimeric live attenuated WNV candidate vaccine in two Phase I clinical trials.
The first trial evaluated 103 or 104 PFU of vaccine given as a single subcutaneous dose.
The second trial evaluated a new lot of the vaccine given at a dose of 105 PFU with a booster dose given 6 months later.
A single sub-cutaneous dose induced seroconversion to wild-type WNV NY99 in 55% – 75% of vaccinees.
Acknowledgments
These studies were supported by the National Institute of Allergy and Infectious Diseases Intramural Research Program, National Institutes of Health, through a contract with the Johns Hopkins Bloomberg School of Public Health and a subcontract with Vanderbilt University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 1192–1197. [Google Scholar]
- 2.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 3.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, et al. Neurologic manifestations and outcome of West Nile virus infection. Jama. 2003 Jul 23;290(4):511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Sejvar JJ, Leis AA, Stokic DS, Van Gerpen JA, Marfin AA, Webb R, et al. Acute flaccid paralysis and West Nile virus infection. Emerging infectious diseases. 2003 Jul;9(7):788–793. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey NP, Kuhn S, Campbell GL, Hayes EB. West nile virus neuroinvasive disease incidence in the United States: 2002–2006. Vector Borne Zoonotic Dis. 2008 Feb;8(1):35–40. doi: 10.1089/vbz.2007.0137. [DOI] [PubMed] [Google Scholar]
- 6.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiology and infection. 2012 May;28:1–5. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. [cited 2013 May 20];West Nile Virus (WNV) human infections reported to ArboNET, by State, United States. 2012 2012 Available from: http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm.
- 8.Barber LM, Schleier JJ, 3rd, Peterson RK. Economic cost analysis of West Nile virus outbreak, Sacramento County, California, USA, 2005. Emerging infectious diseases. 2010 Mar;16(3):480–486. doi: 10.3201/eid1603.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohrabian A, Meltzer MI, Ratard R, Billah K, Molinari NA, Roy K, et al. West Nile virus economic impact, Louisiana, 2002. Emerging infectious diseases. 2004 Oct;10(10):1736–1744. doi: 10.3201/eid1010.030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaney JE, Jr, Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006 Spring;19(1):10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 11.Blaney JE, Jr, Sathe NS, Goddard L, Hanson CT, Romero TA, Hanley KA, et al. Dengue virus type 3 vaccine candidates generated by introduction of deletions in the 3' untranslated region (3'-UTR) or by exchange of the DENV-3 3'-UTR with that of DENV-4. Vaccine. 2008 Feb 6;26(6):817–828. doi: 10.1016/j.vaccine.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaney JE, Jr, Speicher J, Hanson CT, Sathe NS, Whitehead SS, Murphy BR, et al. Evaluation of St. Louis encephalitis virus/dengue virus type 4 antigenic chimeric viruses in mice and rhesus monkeys. Vaccine. 2008 Aug 5;26(33):4150–4159. doi: 10.1016/j.vaccine.2008.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehead SS, Falgout B, Hanley KA, Blaney Jr JE, Jr, Markoff L, Murphy BR. A Live, Attenuated Dengue Virus Type 1 Vaccine Candidate with a 30-Nucleotide Deletion in the 3' Untranslated Region Is Highly Attenuated and Immunogenic in Monkeys. J Virol. 2003 Jan 15;77(2):1653–1657. doi: 10.1128/JVI.77.2.1653-1657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai CJ, Monath TP. Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis. Adv Virus Res. 2003;61:469–509. doi: 10.1016/s0065-3527(03)61013-4. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead SS, Hanley KA, Blaney JE, Jr, Gilmore LE, Elkins WR, Murphy BR. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine. 2003 Oct 1;21(27–30):4307–4316. doi: 10.1016/s0264-410x(03)00488-2. [DOI] [PubMed] [Google Scholar]
- 16.Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE, Jr, Thumar B, et al. rDEN4 Delta 30, a Live Attenuated Dengue Virus Type 4 Vaccine Candidate, Is Safe, Immunogenic, and Highly Infectious in Healthy Adult Volunteers. J Infect Dis. 2005 Mar 1;191(5):710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 17.Durbin AP, McArthur J, Marron JA, Blaney JE, Jr, Thumar B, Wanionek K, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006 Jul-Aug;2(4):167–173. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 18.Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, et al. rDEN2/4Delta30(ME), A Live Attenuated Chimeric Dengue Serotype 2 Vaccine Is Safe and Highly Immunogenic in Healthy Dengue-Naive Adults. Hum Vaccin. 2006 Nov 5;2(6):255–260. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 19.Wright PF, Ankrah S, Henderson SE, Durbin AP, Speicher J, Whitehead SS, et al. Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers. Vaccine. 2008 Feb 13;26(7):882–890. doi: 10.1016/j.vaccine.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, et al. A Single Dose of Any of Four Different Live Attenuated Tetravalent Dengue Vaccines Is Safe and Immunogenic in Flavivirus-naive Adults: A Randomized, Double-blind Clinical Trial. J Infect Dis. 2013 Mar;207(6):957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pletnev AG, Claire MS, Elkins R, Speicher J, Murphy BR, Chanock RM. Molecularly engineered live-attenuated chimeric West Nile/dengue virus vaccines protect rhesus monkeys from West Nile virus. Virology. 2003 Sep 15;314(1):190–195. doi: 10.1016/s0042-6822(03)00450-1. [DOI] [PubMed] [Google Scholar]
- 22.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006 Sep 29;24(40–41):6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Hanley KA, Goddard LB, Gilmore LE, Scott TW, Speicher J, Murphy BR, et al. Infectivity of west nile/dengue chimeric viruses for west nile and dengue mosquito vectors. Vector Borne Zoonotic Dis. 2005 Spring;5(1):1–10. doi: 10.1089/vbz.2005.5.1. [DOI] [PubMed] [Google Scholar]
- 24.Durbin AP, Whitehead SS, Shaffer D, Elwood D, Wanionek K, Blaney JE, Jr, et al. A single dose of the DENV-1 candidate vaccine rDEN1Ä30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS neglected tropical diseases. 2011 Aug;5(8):e1267. doi: 10.1371/journal.pntd.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pletnev AG, Putnak R, Speicher J, Wagar EJ, Vaughn DW. From the Cover: West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):3036–3041. doi: 10.1073/pnas.022652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. The American journal of tropical medicine and hygiene. 2001 Nov;65(5):405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 27.Laassri M, Bidzhieva B, Speicher J, Pletnev AG, Chumakov K. Microarray hybridization for assessment of the genetic stability of chimeric West Nile/dengue 4 virus. Journal of medical virology. 2011 May;83(5):910–920. doi: 10.1002/jmv.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults >/= 50 years of age. Vaccine. 2012 Oct 19;30(47):6656–6664. doi: 10.1016/j.vaccine.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 29.Pletnev AG, Bray M, Hanley KA, Speicher J, Elkins R. Tick-borne Langat/mosquito-borne dengue flavivirus chimera, a candidate live attenuated vaccine for protection against disease caused by members of the tick-borne encephalitis virus complex: evaluation in rhesus monkeys and in mosquitoes. J Virol. 2001;75(17):8259–8267. doi: 10.1128/JVI.75.17.8259-8267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guirakhoo F, Zhang ZX, Chambers TJ, Delagrave S, Arroyo J, Barrett AD, et al. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 1999 May 10;257(2):363–372. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- 31.Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. Elsevier; 2008. pp. 959–1055. [Google Scholar]
- 32.Schlesinger RW. Dengue viruses. Virol Monogr. 1977;(16):1–132. doi: 10.1007/978-3-7091-8466-0. [DOI] [PubMed] [Google Scholar]