Abstract Abstract
Three new karyotypes (2n=40, 44, 52) are described revealing what are probably new cryptic species of Ethiopian spiny mice. Two other diploid numbers have already been reported for the country (2n=36 and 68) and, overall, the five known karyotypic forms constitute a common lineage differentiated by a Robertsonian process. Such arrays of karyotypic forms are known as a ‘Robertsonian fan’. This view of the situation in Ethiopian Acomys I. Geoffroy, 1838 is based on standard chromosomal morphology that reveals a constant FN (68) and needs further investigation of chromosome homology by differential staining and/or molecular cytogenetic techniques as well as further molecular phylogenetic analysis.
Keywords: mammalian karyotype, Robertsonian variation, Acomys
Introduction
Karyotypic studies on mammals of Ethiopia play an important role in species identification and in the interpretation of phylogenies of taxonomic groups (Lavrenchenko 2009). This in particular concerns the rodent taxa where karyotypic data constitute a powerful tool discriminating for an inventory of species diversity. Although the spiny mice of the widespread Afro-Asian genus Acomys I. Geoffroy, 1838 were in the focus of cytogenetic interests since the very beginning of current chromosome preparation era, i.e. the use of colchicine/hypotonic method (Wahrman and Zahavi 1953), the karyotype description of existing taxa in this group is far from complete. New data on karyotypic evolution are coming from the studies based on the chromosome banding techniques (Giagia-Athanasopoulou et al. 2011), and even the initial descriptions of chromosome numbers and morphology may be informative due to revealed wide chromosome variability and corresponding expectations of multiple chromosomal races or/and sibling species over wide generic distributional range (Volobouev et al. 1991).
It became clear from early descriptions of the Middle East populations that a special type of chromosomal variation, known as Robertsonian translocations, is to be regarded as the principal way of karyotypic evolution both between and within Acomys taxa. The differences in diploid numbers that was defined initially in representatives of Acomys cahirinus (É. Geoffroy, 1803) and Acomys russatus (Wagner, 1840) from Israel (2n=38 and 66, respectively), have shown almost exactly the 2n limits in the whole genus (Wahrman and Zahavi 1953). Further discoveries revealed even lower chromosome number (2n=36) in populations of Acomys cahirinus in the southern Sinai and had led to the discovery of a hybridization zone between the two intraspecific karyotypic forms (2n=36, 38) differed by a single whole-arm Robertsonian translocation (Wahrman and Goitein 1972). Data collected from species and populations of African Acomys since Matthey’s pioneer studies (Matthey 1956) up to now and in other parts of the generic range (for cytotaxonomic survey see Macholán et al. 1995, Volobouev et al. 1996, Zima et al. 1999, Dobigny et al. 2001, 2002, Granjon and Dobigny 2003, Corti et al. 2005, Sözen et al. 2008) show that diploid numbers for this genus vary from 2n=36 to 2n=68 via a series of intermediate 2n and the karyotypic constitution may be transformed from a totally acrocentric (2n=68) to almost fully metacentric (2n=36) complement.
The variation in chromosome arm numbers (the Fundamental Number, otherwise FN) is relatively low, from 66 to 78 (Fadda et al. 2001). The presence of FN numbers higher than 68 indicate that structural chromosome variations other than Robertsonian rearrangements, such as pericentric inversions and heterochromatin additions or deletions, occur in Acomys karyotypes.
In Ethiopia, only karyotypes with the extremal 2n values were previously found which have been reported for the two species inhabited the Lower Omo River Valley in the very south (2n=36 in Acomys percivali Dollman, 1911 and 2n=60 in Acomys wilsoni Thomas, 1892, according to Matthey 1968) and two species from the Rift Valley in the centre of the country (2n=36 in Acomys cahirinus and 2n=68 in Acomys sp., following Sokolov et al. 1993). Most close findings from Tanzania (Corti et al. 2005) show three different karyotypes with 2 nranged from 36 for Acomys ignitus Dollman, 1910 to 60 and 62 for Acomys spinosissimus Peters, 1852 and Acomys wilsoni, respectively. The different karyotype characteristics which were reported for the last taxon (2n=62 versus 60) were not considered in that publication.
In this communication, three new karyotypes of Acomys are presented which adds substantially to the 2n range known for taxa inhabiting this country (40, 44 and 52 versus 36 and 60, 68) and thus extends the series of Robertsonian transformations, known as a ‘Robertsonian fan’, performed in the related karyotypes. This study covers also three new geographic parts of Ethiopia where the spiny mice were never examined.
Materials and methods
Animals were collected during the 2008 and 2010 field seasons in the course of the Joint EthioRussian Biological Expedition (JERBE). Chromosome preparations were obtained from five Acomys specimens collected in two lowland regions on opposite sides of the Rift Valley. Among them, two specimens of both sexes were from a geographic site in the east (Babille Elephant Sanctuary, 9.0601°N; 42.2699°E, 1200 m a.s.l.) and three specimens from two sites of the Alatish National Park, north-western Ethiopia (Amjale, 12.3875°N; 35.7309°E, 533 m a.s.l., 2 males, and Bermil, 12.4958°N; 35.6382°E, 575 m a.s.l., 1 female).
For the karyotype analysis, bone marrow cell suspensions were prepared according to the standard colchicine, hypotonic solution and air-dried technique (Ford and Hamerton 1956) and stained on microscopic slides with Giemsa; each individual was preserved for further molecular analyses, following to a current research trends (Bulatova et al. 2009, Lavrenchenko 2009). Collected specimens were not specifically identified in the field, but deposited for further morphological or DNA examination.
Results
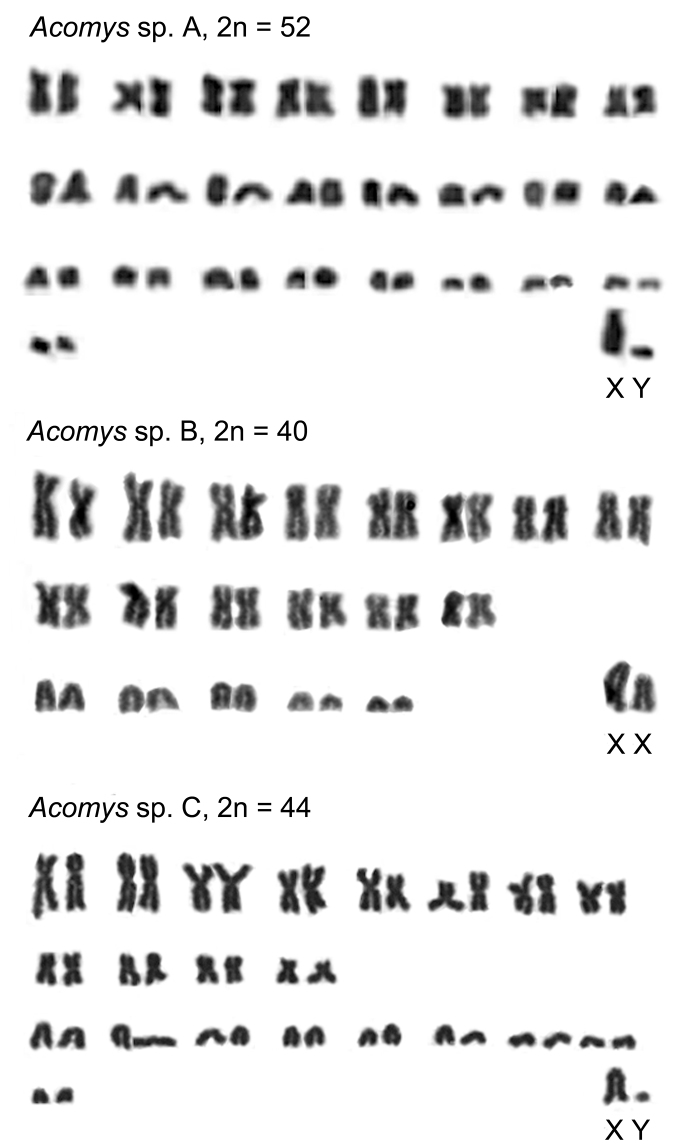
Three different karyotypes are found which characterize the Ethiopian Acomys of lowland savanna from three geographic samples in two different parts of the country. A higher chromosome number (2n=52) is detected in a male from Amjale. Its chromosome set contains lesser number of metacentric pairs than acrocentric ones (8 and 18, respectively; FN=68). Sex chromosomes cannot be reliably identified without differential staining, however, a larger acrocentric element with a distinguished short arm was recognized as the X chromosome, when one of the smaller acrocentrics may represent the Y chromosome. The autosomal arm number (FNa) characteristic for this karyotype is 66.
The lesser chromosome numbers are found in two other karyotypes (Figure 1). 2n=44 is characteristic for animals from the Babille Elephant Sanctuary. In this karyotype 12 metacentric and 10 acrocentric pairs are present (FN=68). The acrocentric chromosomes apparently include the XX pair in females and XY in males. NFa is 66 in this case. 2n=40 is reported from Bermil, and only 6 pairs of acrocentrics are identified in this karyotype (FN=68), one of which may be determined as the XX pair in a female studied, by analogy with the sex chromosomes of other Acomys species (Table 1). NFa is again 66.
Figure 1.
Karyograms of Ethiopian Acomys spp. A – Amjale, B – Bermil, C - Babille.
Table 1.
Karyotypic data on Acomys collected from Ethiopia.
| Species | 2n | FN | FNa | X | Location | Reference |
|---|---|---|---|---|---|---|
| Acomys wilsoni | 60 | 76 | 74 | A | S Ethiopia: Omo Valley | Matthey 1968 |
| *Acomys percivali | 36 | 68 | 66 | A | S Ethiopia: Omo Valley | Matthey 1968 |
| Acomys cahirinus | 36 | 68 | 66 | A | S Ethiopia: Rift Valley (Konso; Arba-Minch) | Sokolov et al. 1993 |
| Acomys cahirinus | 36 | 68 | 66 | A | Central Ethiopia: Rift Valley (2 sites along the middle Awash valley) | Sokolov et al. 1993 |
| Acomys sp. | 68 | 68 | 66 | A | Central Ethiopia: Rift Valley (Koka, upper Awash valley) | Sokolov et al. 1993 |
| Acomys sp. A | 52 | 68 | 66 | A | NW Ethiopia: Alatish National Park (Amjale) | This study |
| Acomys sp. B | 40 | 68 | 66 | A | NW Ethiopia: Alatish National Park (Bermil) | This study |
| Acomys sp. C | 44 | 68 | 66 | A | E Ethiopia: Babille | This study |
| Total: 6 or more species | 36–68 | 68 (76) | 66 (74) | A | Rift Valley and S, NW and E lowland Ethiopia | 3 publications |
* Referred to Acomys cahirinus in Sokolov et al. 1993.
Discussion
Description of three new karyotypes of Acomys presented in this study indicates that there is more karyotype diversity in Ethiopian Acomys than it was thought previously. Three additional 2n numbers that were found (40, 44, and 52) fill in the series of 2nchanges where only minimal (36) and maximal (60, 68) limits were known until now (Table 1).
The common feature for five out of six karyotypes is the same number of chromosome arms (FN). The FN value is invariably 68 for full chromosome complements and 66 for the autosomes (FNa) in the karyotypes with various 2n which were attributed to at least five species, including Acomys cahirinus (2n=36) and four unidentified taxa, probably cryptic species - Acomys sp. (2n=68), Acomys sp. A (2n=52), Acomys sp. B (2n=40), and Acomys sp. C (2n=44) (Matthey 1968, Sokolov et al. 1993, our data).
Regarding this Ethiopian group, interrelations based on Robertsonian rearrangements could be suggested. It is well known that it is usually difficult to decide whether Robertsonian fusions or fissions occurred in each given group, and the interpretation of karyotype evolution depends upon the context of the comparative analysis. It is often a working hypothesis that a chromosome set with the most numbers of acrocentrics is the primitive one (2n=68 in the case of Acomys) and that karyotypic evolution should pass via successive fusions of any twin acrocentrics into one metacentric. In fact, due to the bi-directional Robertsonian process and probable multiple fusions of original acrocentrics, the chromosomal sibling species may exist that should confuse a hypothetic common chain of karyotypic changes from maximal to minimal 2n, or, in our case, from 2n=68 to 2n=36 via 2ns such as 52, 44 and 40. Indeed, a clear indication on Robertsonian, or centric fission has been obtained through the karyotype comparison between the continental (Turkey) Acomys cilicicus Spitzenberger, 1978 and Mediterranean sea island (Cyprus) spiny mouse, Acomys nesiotes (Bate, 1903), which differ in 2n as 36 and 38, respectively (Zima et al. 1999).
As for the 60-chromosome karyotype of Acomys wilsoni, one of the first reported from the Omo Valley by Matthey (1968), it is to be regarded as distinguished from the group mentioned above even on the level of routine chromosome data because it has a different FN=76 (FNa=74, see Table 1 and Introduction). In reference to modern chromosome data, it should be, however, questioned to what species does each of the two initial karyotype descriptions of south Ethiopian Acomys - Acomys percivali and Acomys wilsoni (sensu Matthey cit.) - belong. The first of the two was attributed to Acomys cahirinus after the work of Sokolov et al. (1993) as it has the same chromosome number, 2n=36 (see Table 1). Following recent karyotype descriptions from Tanzania, another chromosome numbers, 2n=62 and FNa=76, are attributed to Acomys wilsoni which is in this karyotypic form included into the current mammal species checklists (Fadda et al. 2001, Corti et al. 2005).
The spiny mice with 2n=60 were found in central and southern parts of Africa, but the karyotype peculiarities do not allow to consider them conspecific. Comparing to Ethiopian karyotype with FNa=74, two other species with the same 2n=60 show not only differences in FNa – 68 in Acomys selousi De Winton, 1896 and 70 in Acomys spinosissimus – but reveal serious difference in the morphological structure of X chromosome (metacentric with a heterochromatic arm in the first case and submetacentric in the second one). Moreover, they are characterized by the unique XO system in both sexes, accompanied with the atypical inter- and intraindividual autosome number variation (2n=58-62) (Barome et al. 2001, Fadda et al. 2001, Corti et al. 2005). In Ethiopian Acomys no other sex chromosome constitution than standard XX/XY is observed and there was no other X chromosome in their karyotypes than a moderate size acrocentric which is considered as the original type for the genus (Table 1).
There are still more examples of 2n similarity in the karyotypes of different Acomys. Three of four 2n values that were found in Ethiopia have been reported for Acomys from other territories, i.e. the first finding of 2n=68 from Burkina-Faso (see Sokolov et al. 1993) or 2n=44 from Mali (Dobigny et al. 2001), 2n=40 from Crete (Giagia-Athanasopoulou et al. 2011), and only 2n=52 is shown for this genus for the first time.
The 36-chromosome karyotype is most widely distributed in Mediterranean area and presented among African taxa. The same FN characteristics of the karyotypes with the same 2n may indicate the karyotypic clusters of common origin in the related groups. It is, however, interesting that preliminary data from mt-DNA analysis (Lavrenchenko, in prep.) focused on Ethiopian – i.e. East African – karyotypic forms that we described in this paper do not support their genetic affiliation with taxa from western parts of Africa with the same 2n=40 or 44 and FNa=66 (Nicolas et al. 2009). For analysis of geographically distant taxa, high-resolution chromosome banding should be used in addition to other methods. It was already shown that externally the ‘same’ 36-chromosome karyotypes could be formed via the complex Robertsonian arm fusions leading to partial, i.e.monobrachial homology between the variable metacentrics (Volobouev et al. 2007). If so, any finding of the 2n=36 karyotype does not seem to be a reason to attribute a corresponding taxon immutably to Acomys cahirinus.
Besides the Robertsonian changes occured generally in autosomes, heterochromatin variability exists regarding the X chromosome. To avoid probable variation in FN due to the presence/absence of a short heterochromatic arm in the X-chromosome, Macholán et al. (1995) suggested that the autosomal number, FNa, is better to be used for the karyotype comparison. Whether such kind of variation presents in the X with or without clear short arm as we observed in 3 karyotypes (Fig. 1) is to be evidenced by application of differential staining of heterochromatin.
As a result of this preliminary chromosome study, we may conclude that 3 new karyotypes (2n=40, 44, 52) are to be added to at least three karyotypic forms of Ethiopian Acomys (2n=36, 60, 68), described previously (Matthey 1968, Sokolov et al. 1993, our data). It follows from the comparison of data that five out of six karyotypes, except the one with 2n=60, may form a Robertsonian fan, the reliability of which is to be checked by further chromosome analysis of Ethiopian populations. And, finally, wide taxonomic expertise of the genus is needed based on the complex morphological, fine karyological and, in particular, molecular investigation of spiny mice inhabiting this country.
Acknowledgements
The authors are indebted to the anonymous referee for valuable criticism of the early version of the submitted manuscript. The supporting grant from the RFBR (Russian Foundation of Basic Researches) is appreciated.
References
- Barome P-O, Volobouev V, Monnerot M, Mfune JK, Chitaukali W, Gautun J-C, Denys C. (2001) Phylogeny of Acomys spinosissimus (Rodentia, Muridae) from north Malawi and Tanzania: evidence from morphological and molecular analysis. Biological Journal of the Linnean Society 73: 321–340. http://www.idealibrary.com doi: 10.1006/bijl.2000.0546
- Bulatova N, Searle JB, Nadjafova RS, Pavlova SV, Bystrakova NV. (2009) Field protocols for the genomic era. Comparative Cytogenetics 3(1): 57–62. http://www.zin.ru/journals/compcyt/abstract.asp?k=54
- Corti M, Castiglia R, Colangelo P, Capanna E, Beolchini F, Bekele A, Oguge NO, Makundi RH, Sichilima AM, Leirs H, Verheyen W, Verhagen R. (2005) Cytotaxonomy of rodent species from Ethiopia, Kenya, Tanzania and Zambia. Belgian Journal of Zoology 135 (supplement): 197–216.
- Dobigny G, Moulin S, Cornette R, Gautun J-C. (2001) Rodents from Adrar des Iforas, Mali. Chromosomal data. Mammalia 65: 215-220 [Google Scholar]
- Dobigny G, Nomao A, Gautun J-C. (2002) A cytotaxonomic survey of Rodents from Niger: implications for systematics, biodiversity and biogeography. Mammalia 66: 495-523 [Google Scholar]
- Fadda C, Castiglia R, Colangelo P, Corti M, Machang’u R, Makundi R, Scanzani A, Tesha P, Verheyen W, Capanna E. (2001) The Rodent fauna of Tanzania: a cytotaxonomic report from the Maasai Steppe (1999). Rendiconti Lincei 9 (12): 29-49 [Google Scholar]
- Ford CE, Hamerton JL. (1956) A colchicine, hypotonic citrate squash sequence for mammalian chromosomes. Stain Technology 31: 247-251 [DOI] [PubMed] [Google Scholar]
- Giagia-Athanasopoulou EB, Rovatsos MTH, Mitsainas GP, Martimianakis S, Lymberakis P, Angelou L-XD, Marchal JA, Sánchez A. (2011) New data on the evolution of the Cretan spiny mouse, Acomys minous (Rodentia: Murinae), shed light on the phylogenetic relationships in the cahirinus group. Biological Journal of the Linnean Society 102: 498-509 doi: 10.1111/j.1095-8312.2010.01592.x Key:citeulike:8692629 [Google Scholar]
- Granjon L, Dobigny G. (2003) The importance of cytotaxonomy in understanding the biogeography of African rodents: Lake Chad murids as an example. Mammal Review 33: 77-91 [Google Scholar]
- Lavrenchenko LA. (2009) The mammals of the Ethiopian Plateau: general trends and peculiarities in forming of a tropical montane fauna. Dr. Sci. Dissertation, Moscow. Russian Federation: Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences. 302 pp. [In Russian]
- Macholán M, Zima J, Cervená A, Cervený J. (1995) Karyotype of Acomys cilicicus Spitzenberger, 1978 (Rodentia, Muridae). Mammalia 59 (3): 397-402 [Google Scholar]
- Matthey R. (1956) La formule chromosomique de quelques Murinae (Muridae - Rodentia - Mammalia). Archiv der Julius Klaus-Sitftung für Vererbungsforschung 31: 294-306 [Google Scholar]
- Matthey R. (1968) Cytogénétique et taxonomie du genre Acomys: A. percivali Dollmann et A. wilsoni Thomas, espèces d’Abyssinie. Mammalia 32: 621-627 [Google Scholar]
- Nicolas V, Granjon L, Duplantier J-M, Cruaud C, Dobigny G. (2009) Phylogeography of spiny mice (genus Acomys, Rodentia: Muridae) from the south-western margin of the Sahara with taxonomic implications. Biological Journal of the Linnean Society 98: 29-46 doi: 10.1111/j.1095-8312.2009.01273.x [Google Scholar]
- Sokolov VE, Orlov VN, Baskevich MI, Bekele A, Mebrate A. (1993) A karyological study of the spiny mouse Acomys Geoffroy 1838 (Rodentia, Muridae) along the Ethiopian Rift Valley. Tropical Zoology 6: 227-235 [Google Scholar]
- Sözen M, Karataş A, Alsheyab F, Shehab A, Amr Z. (2008) Karyotypes of seven rodents from Jordan (Mammalia: Rodentia). Zoology in the Middle East 44: 3-10 [Google Scholar]
- Volobouev V, Auffray JC, Debat V, Denys C, Gautun JC, Tranier M. (2007) Species delimitation in the Acomys cahirinus–dimidiatus complex (Rodentia, Muridae) inferred from chromosomal and morphological analyses. Biological Journal of the Linnean Society 91: 203-214 [Google Scholar]
- Volobouev V, Gautun J-C, Tranier M. (1996) Chromosome evolution in the genus Acomys (Rodentia, Muridae): chromosome banding analysis in Acomys cahirinus. Mammalia 60: 217-222 [Google Scholar]
- Volobouev V, Tranier M, Dutrillaux B. (1991) Chromosome evolution in the genus Acomys: chromosome banding analysis of Acomys cf. dimidiatus (Rodentia, Muridae). Bonner zoologischer Beiträge 42(3–4): 253-260 [Google Scholar]
- Wahrman J, Goitein R. (1972) Hybridization in nature between two chromosome forms of spiny mice. Chromosomes Today 3: 228-237 [Google Scholar]
- Wahrman J, Zahavi A. (1953) Intra-generic difference in chromosome numbers of spiny mice (Rodentia: Murinae). Bulletin of Research Council of Israel 3: 265.
- Zima J, Macholán M, Pialek J, Slivkova L, Suchomelova E. (1999) Chromosomal banding pattern in the Cyprus spiny mouse, Acomys nesiotes. Folia Zoologica 48: 149-152 [Google Scholar]