Abstract Abstract
Immunocytochemical and electron microscopic analysis of synaptonemal complexes (SCs) was carried out for the first time in homozygotes and complex Robertsonian heterozygotes (hybrids) of the common shrew, Sorex araneus Linnaeus, 1758, from a newly discovered hybrid zone between the Moscow and the Neroosa chromosomal races. These races differ in four monobrachial homologous metacentrics, and closed SC tetravalent is expected to be formed in meiosis of a hybrid. Indeed, such a multivalent was found at meiotic prophase I in hybrids. Interactions between multivalent and both autosomes and/or the sex chromosomes were observed. For the first time we have used immunocytochemical techniques to analyse asynapsis in Sorex araneus and show that the multivalent pairs in an orderly fashion with complete synapsis. Despite some signs of spermatocytes arrested in the meiotic prophase I, hybrids had large number of active sperm. Thus, Moscow – Neroosa hybrid males that form a ring-of-four meiotic configuration are most likely not sterile. Our results support previous demonstrations that monobrachial homology of metacentrics of the common shrew does not lead to complete reproductive isolation between parapatric chromosomal races of the species.
Keywords: Synaptonemal complex, MSCI, γH2AX, centromeres, Sorex araneus
Introduction
The concept of chromosomal speciation implies occurrence of reproductive isolation as a result of chromosomal rearrangements (Vorontsov 1960, White 1978, 1982, King 1993). The most common type of chromosome rearrangements in mammalian evolution is represented by the Robertsonian translocations – fusion of two acrocentric chromosomes into a single submeta – or metacentric chromosome. It was first described in orthopterous insects (Robertson 1916). Species with a so-called “Robertsonian fan” represent unique models for studying chromosomal speciation. The term was introduced by R. Matthey for description of a wide-range chromosomal variation caused by multiple Robertsonian translocations (Matthey 1970). Among mammals, there are several species that demonstrate the Robertsonian fan: the Subsaharan pygmy mouse, Mus (Nannomys) musculoides Temminck, 1853 (Matthey 1970, Jotterand 1972), the house mouse, Mus musculus domesticus Schwarz et Schwarz, 1943 (Gropp et al. 1972, Gropp and Winking 1981), the Eastern mole vole, Ellobius tancrei Blasius, 1884 (Lyapunova et al. 1980), the Nigerian gerbil, Gerbillus nigeriae Thomas et Hinton, 1920 (Volobouev et al. 1988), the Daghestan pine vole, Pitymys daghestanicus Shidlovsky, 1919(Tembotov et al. 1976), and the common shrew, Sorex araneus Linnaeus, 1758 (Searle and Wójcik 1998).
Due to its high level of karyotype variability, the common shrew Sorex araneus is subdivided into at least 72 parapatric chromosomal races, each characterised by a unique set of metacentric chromosomes formed by Robertsonian fusions and/or whole-arm reciprocal translocations (WARTs) (Hausser et al. 1994, White et al. 2010). Three metacentrics (af, bc, tu) and sex chromosomes (XX in females and XY1Y2 trivalent in males) are invariant in all chromosomal races, while another ten autosomal arms (g-r) may occur as acrocentrics and/or combined together as metacentrics (Searle et al. 1991). XY1Y2 sex trivalent formed by the X-autosome translocation (Sharman 1956) is specific for the species of the ‘Sorex araneus’ group (Zima et al. 1998). The sex trivalent has original parts (e “true” arm of X chromosome and the Y1) and autosomal parts (d translocated arm of X chromosome homologous to the Y2) (Fredga 1970, Pack et al. 1993).
Hybrids between parapatric chromosomal races of the common shrew are often expected to be complex Robertsonian heterozygotes with monobrachial homology, which form chain (C) or ring (R) configurations of three or more elements at prophase I of meiosis. Such complex meiotic configurationsare considered to be more susceptible to irregularity. As a consequence, complex heterozygotes are expected to be less fertile than homozygotes of pure chromosomal races. At present, interracial hybrids with different types of meiotic configurations from CIII and RIV up to CXI and RVI have been revealed from seventeen well-studied hybrid zones (Searle and Wójcik 1998, Bulatova et al. 2011, Polyakov et al. 2011, Orlov et al. 2012). Studies so far have shown that hybrids with long chain or ring configurations have more abnormalities during meiosis than hybrids with shorter configurations; however even in these cases the complex meiotic configurations do not appear to be associated with complete sterility (Mercer et al. 1992, Narain and Fredga 1997, Jadwiszczak and Banaszek 2006, Pavlova et al. 2008). There is a need to document more fully the match between complexity of karyotype and degree of regularity of the meiotic configurations expected.
A new chromosomal hybrid zone between the Moscow race (gm, hi, jl, kr, no, pq, 2na=18) and the Neroosa race (go, hi, jl, kr, mn, pq, 2na=18) has been found recently in the centre of European Russia (Pavlova et al. 2012, in press). Karyotypes of the races differ in four metacentrics with monobrachial homology so that the complex heterozygotes should form a ring-of-four (RIV) configuration at meiosis I. On the basis of karyotype differences of the races one can suggest that fixation of just one WART, between metacentrics gm and no or between go and mn, could have separated these races in the past.
This paper presents a comparative synaptonemal complex (SC)analysis of prophase I of meiosis using electron microscopy and immunofluorescence in homozygotes and complex Robertsonian heterozygotes from this hybrid zone. A combination of both methods together for SC analysis is used for the first time in Sorex araneus.
Material and methods
Animals and karyotypes. A total of eight adult male common shrews were collected from the Moscow-Neroosa hybrid zone, located in the south-eastern part of the Moscow Region near Ozyory town (the left bank of the River Oka), in April 2012, at the beginning of the breeding season. Each specimen was processed according to the field procedure described in Bulatova et al. (2009). Mitotic chromosomes were prepared from bone marrow and spleen following Ford and Hamerton (1956) with modifications. A trypsin - Giemsa staining technique of Kràl & Radjabli (1974) was used for identification of chromosome arms according to the standard nomenclature for the Sorex araneus karyotype (Searle et al. 1991). Only three of eight karyotyped males were used for the meiotic analysis.
Synaptonemal Complex Analysis. Synaptonemal complex (SC) preparations were prepared and fixed using the technique of Navarro et al. (1981) with modifications (Kolomiets et al. 2010). Measurements of autosomal bivalents and their ranking in each cell were made in order to determining relative lengths (MicroMeasure 3.3, Colorado, USA).
Electron microscopy. Slides were stained with a 50% AgNO3 solution in a humid chamber for 3 h at 56°C, washed 4 times in distilled water and air dried. Stained slides were observed under a light microscope to select suitably spread cells. Once selected, plastic (Falcon film) circles were cut out with a diamond tap and transferred onto grids and examined in a JEM 100B electron microscope.
Immunofluorescence. Poly-L-lysine-coated glass was used for immunostaining. The slides were placed in phosphate-buffered saline (PBS) and incubated overnight at 4°C with the following primary antibodies diluted in antibody dilution buffer (ADB: 3% bovine serum albumin - BSA, 0.05% Triton X-100 in PBS): rabbit anti-SCP3 1:200 (Abcam,Ab15093), human anti-centromere antibodies, ACA 1:200 (Antibody Incorporated, 15-235) and mouse anti-phospho-histone γH2AX 1:500 (Abcam,Ab26350). After rinsing in PBS (3 times for 10 min), the slides were incubated with appropriate secondary antibodies diluted 1:800 in PBS: goat anti-rabbit Alexa Fluore 488 conjugated antibodies, goat anti-human Alexa Fluore 546 conjugated antibodies and FITC-conjugated horse anti-mouse IgG (all Abcam) at 37°C for 90 min. After a final rinse in PBS, the slides were mounted in Vectashield with DAPI (Vector Laboratories). Slides were analyzed in an Axioimager D1 microscope CHROMA filter sets (Carl Zeiss, Jena, Germany) equipped with an Axiocam HRm CCD camera (Carl Zeiss), and image-processing AxioVision Release 4.6.3. software (Carl Zeiss, Germany). Images were processed using Adobe Photoshop CS3 Extended.
Results
Karyotypes. Three of the eight karyotyped shrews were complex heterozygotes, i.e. F1 hybrids. They showed the expected arm combinations of Rb metacentrics - go/gm/mn/no, hi, jl, kr, pq. Five other shrews were homozygotes with Moscow race karyotype (gm, hi, jl, kr, no, pq). Hybrid individuals and homozygotes of the pure race had 2n=21, NF=40, XY1Y2. Only two hybrids and one homozygote were subject to comparative SC analysis.
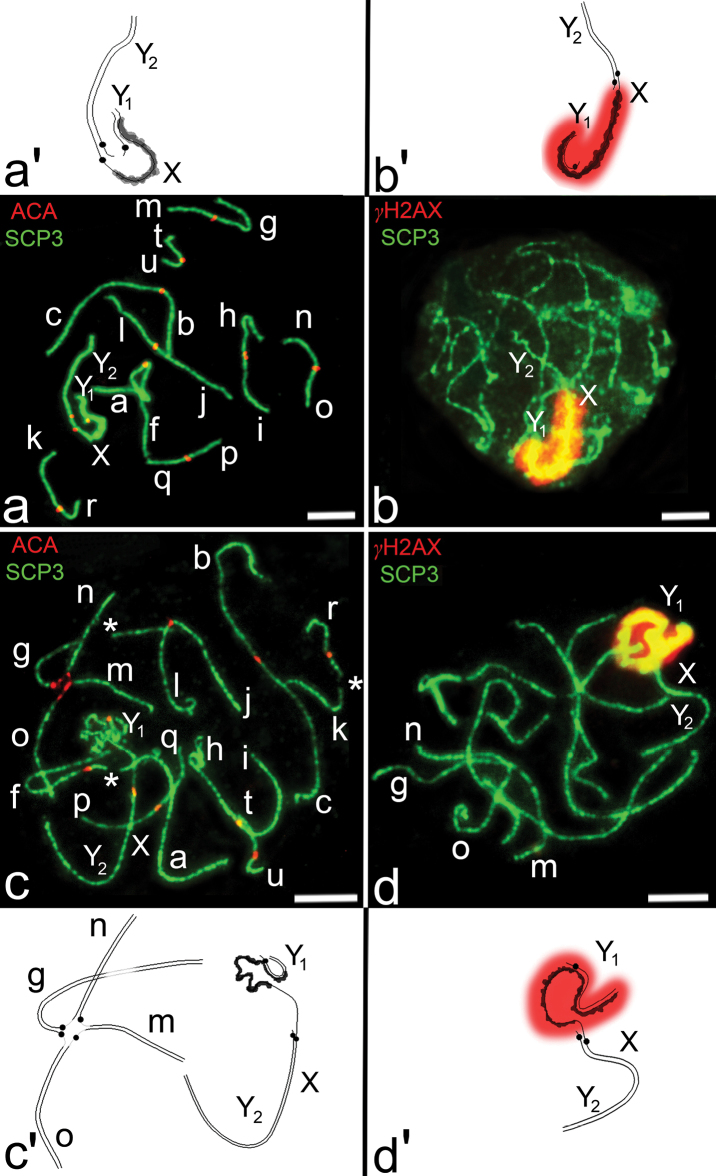
SC analysis of a homozygote of the common shrew. Immunocytochemical analysis of SCs in pachytene spermatocytes of the homozygote revealed nine SC bivalents (af, bc, jl, hi, gm, no, kr, pq, tu) and the sex trivalent (XY1Y2), as expected from the G-banded karyotype of the Moscow race. Centromeres of hi SC bivalent and centromeres in the sex trivalent were not aligned. The sex trivalent exhibited irregular thickenings of the “true” arm of the X chromosome. The autosomal arm of the X chromosome formed a typical SC (Fig. 1a, a’). γH2AX covered only the synaptic region of the X and the Y1 chromosomes and the thickened part of the X chromosome. The autosomal arm of the X chromosome is not involved in inactivation (Fig. 1b,b’).
Figure 1.
a–d Synaptonemal complexes of homozygotes and complex heterozygotes of the common shrew. Immunostaining with antibodies against axial elements of SC - SCP3 (green), polyclonal antibodies to centromeric protein ACA (red) and antibodies to γH2AX (red) marking chromosome asynaptic regions. Bar = 5 μm a, b SCs from spermatocyte pachytene nuclei (the Moscow race) a Nine SC bivalents (af, bc, jl, hi, gm, no, kr, pq, tu) and sex trivalent XY1Y2. Sex trivalent contains irregular thickening of the “true” arm of X-chromosome (scheme a’). The autosomal arm of the X-chromosome forms a typical SC. Centromeres within hi bivalent and XY1Y2 trivalent are displaced relative to each other b Anti-γH2AX antibodies recognize chromatin in the synaptic zone of X and Y1 chromosomes and unsynapsed thickened region of the “true” arm of X-chromosome (scheme b’) c, d SCs from spermatocyte pachytene nuclei obtained from Moscow-Neroosa hybrids c Seven SC bivalents (af, bc, jl, hi, kr, pq, tu), sex trivalent XY1Y2 and SC tetravalent (g/o/n/m) were revealed in spermatocyte nuclei of complex heterozygotes. Gaps were detected in SC bivalents af, kr and in g arm of SC-tetravalent (indicated with asterisks). Gaps were also detected in pericentromeric regions of all metacentrics of the SC tetravalent (scheme c’). af SC bivalent is associated with sex trivalent; d Anti-γH2AX antibodies identify chromatin in the synaptic region of X and Y1 chromosomes and asynaptic thickening of the “true” arm of the X-chromosome (scheme d’), as for common shrew spermatocytes from Moscow race (Fig. 2b). One of the SC bivalents is associated with the true part of sex trivalent. The SC tetravalent is usually associated with one or two autosomes (c, d).
SC analysis of complex heterozygotes of the common shrew. As expected, seven SC bivalents (af, bc, jl, hi, kr, pq, tu), an SC tetravalent (g/o/n/m) and the sex trivalent XY1Y2 were detected in spermatocyte nuclei at pachytene stage (Fig. 1c, c’, 2). According to the previously elaborated classification, the SC tetravalent represents a closed SC multivalent which was formed due to monobrachial homology (Matveevsky and Kolomiets 2011). Arms af, kr of SC bivalents, the g arm and pericentromeric regions of the SC tetravalent contain gaps (Fig. 1c). Sex trivalents were recurrently located at the periphery of the pachytene nuclei of spermatocytes. The “true” X arm of the sex trivalent was irregularly thickened and covered with γH2AX (Fig. 1d, d’).
Figure 2.
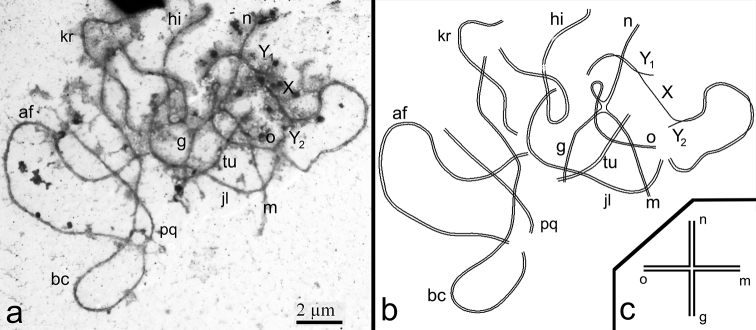
a–d A pachytene spermatocyte of the Moscow-Neroosa hybrid. a An electron micrograph. Seven SC bivalents (af, bc, jl, hi, kr, pq, tu), the sex trivalent XY1Y2 and the SC tetravalent (g/o/n/m) are detected. Closed SC tetravalent is composed of four monobrachial homologous metacentrics go, on, nm, mg. SC tetravalent is associated with two autosomes and sex trivalent. Bar = 2 μm b A scheme of chromosome synapsis on the basis of Fig. 2a c A scheme of SC tetravalent.
Suspension of testis cells. There are spermatocytes and active spermatozoa in testis cell suspension from common shrews of both Moscow race and hybrids (Fig. 3). Chromosome spreads also contained a significant amount of spermatozoa (Fig. 3a’, b’).
Figure 3.
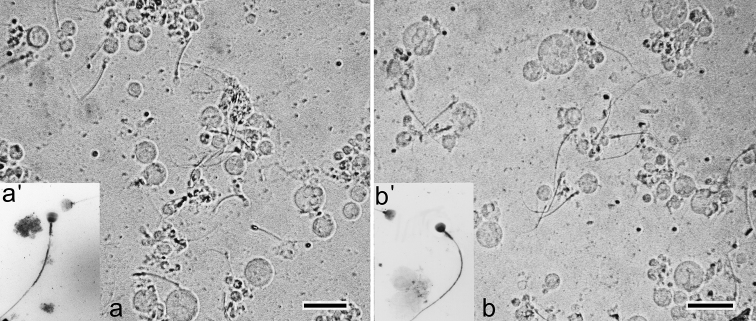
a–b Cell suspension of common shrew testis a – homozygote (the Moscow race) b – complex heterozygote from interracial hybrid zone a’, b’ Inverted image of spermatozoa (non-specific binding of anti-SCP3 antibodies after immunocytochemistry). Bar = 20 μm.
Discussion
Hybrid zones of Sorex araneus represent unique natural laboratories for studying Robertsonian chromosomal polymorphism. Complex cytogenetic studies have been carried out in 17 known chromosomal hybrid zones; however only seven of them have been subjected to analysis of early stages of meiosis including synaptonemal complexes analysis (see Table 1). Such studies provide information about the peculiarities of chromosomal synapsis and separation of multivalents in meiosis, which determine a hybrid’s sterility/fertility. The latter is important for estimation of reproductive isolation level between different chromosomal races.
Table 1.
SC analysis in chromosomal hybrid zones of the common shrew.
| Hybrid zone | Examined karyotypic categories | Detected SC-configuration | Reference |
|---|---|---|---|
| Oxford Hermitage | SH (chain-of-three) (k/q), (n/o), (p/r) | SC trivalents | Wallace and Searle 1990 |
| Oxford Wirral | SH (chain-of-three) (k/q), (n/o), (k/o), (j/l) | SC trivalents | Borodin et al. 2008 |
| Abisko Sidensjö | CH (chain-of-four) i/ih/hn/n | SC tetravalent | Narain and Fredga 1998 |
| Aberdeen/ Oxford | CH (chain-of-seven) r/rp/pn/no/ok/kq/q | SC chain with 7 elements | Mercer et al. 1992 |
| Novosibirsk/Tomsk | CH (chain-of-eight) + (chain-of-three) o/og/gk/ki/ih/hn/nm/m, q/r | SC chain with 8 elements and SC trivalent | Karamysheva et al. 2007 |
| Moscow Seliger | CH (chain-of-eleven) g/gm/mh/hi/ik/kr/rp/pq/qn/no/o | SC chain with 11 elements | Pavlova et al. 2008 |
| Uppsala Hällefors | CH (ring-of-four) qp/pk/ko/oq | SC tetravalent | Narain and Fredga 1997 |
| Moscow Neroosa | CH (ring-of-four) og/gm/mn/no | SC tetravalent | this study |
SH – simple heterozygotes, CH – complex heterozygotes
The model of chromosomal speciation by monobrachial centric fusion has been proposed by Baker and Bickham (1986). Fixation of metacentric chromosomes with monobrachial (single-arm) homology formed as a result of independent fusion of acrocentric chromosomes can entail reproductive isolation of population and further speciation due to the accumulation of genetic differences (Capanna 1982, Baker and Bickham 1986). It is considered that such a mechanism of speciation occurs among some mammal species: lemur genus Eulemur Simons et Rumpler, 1989 (Djlelati et al. 1997, Rumpler 2004), bat genus Rhogeessa H. Allen, 1866 (Baker et al. 1985), mole vole of the Ellobius tancrei (Bakloushinskya et al. 2010), beaver genus Castor Linnaeus, 1758 (Ward et al. 1991), mouse of the Mus musculus domesticus (Capanna et al. 1976), rat genus Rattus Fischer de Waldheim, 1803 (Baverstock et al. 1983). Searle (1988) suggested that monobrachial fusions may contribute to speciation in Sorex araneus.
It was assumed that interracial hybrids of the common shrew (complex heterozygotes with multiple Rb rearrangements) had either significantly reduced fertility or were completely sterile (Searle 1988, 1993, Aniskin and Lukianova 1989). Mice and mole voles that differed in several Rb translocations, exhibited reduced fertility too (Capanna 1975, Lyapunova et al. 1990, Hauffe and Searle 1998, Bakloushinskaya et al. 2010). Furthermore, reduced fertility and presence of aneuploid cells were revealed in heterozygotes from different hybrid zones of house mouse chromosomal races that varied in monobrachial homologous metacentrics (Said et al. 1993, Nunes et al. 2011). The fertility of hybrids most likely depends on the amount of monobrachial homologous metacentrics. Indeed, complex heterozygotes of Sorex araneus from the contact zone of Oxford and Hermitage races had an increased content of defective testicular tubes and testis with reduced weight, whereas simple heterozygotes were similar to homozygotes (Garagna et al. 1989). However, in the common shrew, even extremely long meiotic chain configurations may not necessarily lead to complete sterility (Mercer et al. 1992). For example, hybrids from a contact zone of Moscow and Seliger races that exhibited the most diverse pattern of monobrachial homologous metacentrics with an additional WART translocation had mature spermatozoa (Pavlova et al. 2008).
Previous studies have also demonstrated that association of autosomes and complex SC configurations with sex chromosomes in meiotic prophase I could cause reduction of fertility or even complete sterility (Forejt et al. 1981, Burgoyne and Baker 1984). It should be noted that, unlike SC trivalents, complex SC configurations are often associated with autosomes and sex trivalents (Narain and Fredga 1997, Pavlova et al. 2008). We also revealed that sometimes the SC tetravalent interacted with the sex trivalent in the complex heterozygotes that we examined. In previous works similar contact sites (or physically interactions) of autosomes, SC multivalents and sex bivalents were interpreted as associations (Mercer et al. 1992, Narain and Fredga 1997).
To reveal the signs of defects in spermatogenesis in our specimens, we studied the dynamics of meiotic prophase I focusing on the sex trivalent. Normally, sex chromosomes of male mammals contain a short SC in pseudoautosomal region and long unpaired axes in meiotic prophase I. Also, sex chromosomes often move to the periphery of pachytene nuclei and undergo MSCI (meiotic sex chromosome inactivation), which is required for successful progression of meiosis (Forejt 1984, Burgoyne et al. 2009). We used SCP3 antibodies to identify the SC axial elements and γH2AX antibodies to mark chromosome asynaptic regions. This marker was revealed in chromosome asynaptic regions starting from leptotene and up to late diplotene in cases of incomplete synapsis (Turner et al. 2006).
We found that the behavior of the sex trivalent in homozygotes (Moscow race) was similar to that of sex chromosomes in meiotic prophase I in other mammals. However, in complex heterozygotes, the sex trivalent interacted with autosomes in some prophase nuclei, which was typical of hybrids and heterozygotes with chromosomal rearrangements and reduced fertility (Forejt et al. 1981). Thus, we do not exclude a possibility of partial loss of spermatocytes due to this condition.
Formation of complex SC configurations is known to be associated with a high degree of asynapsis. In such cases, chromosome asynaptic regions undergo transcriptional inactivation MSUC (meiotic silencing of unsynapsed chromatin), which in its turn results in meiotic arrest and reduction of fertility (Homolka et al. 2007, Mahadevaiah et al. 2008). Nonetheless, no MSUC signs were detected in the hybrids of Sorex araneus. In the gap regions (SC tetravalent, af, kr SC bivalents), γH2AX was not detected. Most probably, gaps in pericentromeric regions of the SC tetravalent do not reflect asynaptic regions, but may result from the extension of chromosome axial elements due to the alteration of nucleus organization and retention of telomere links with nuclear envelope. Similar trends in tetravalent dynamics were revealed in the progeny of radiation-exposed male mice (Kolomiets et al. 1992). Closed SC multivalents associate with the sex bivalent to a lesser extent and therefore do not cause meiotic failures.
Probably, the four metacentrics that form SC tetravalent in interracial Moscow-Neroosa hybrids undergo successful separation, spermatocytes are not arrested (or are arrested partially) and balanced gametes are formed in the end. This is also supported by the presence of numerous spermatozoa in hybrid testis cell suspensions. Further studies are needed to measure the level of aneuploidy.
Our data conform to the results of other authors. For example, no defects of sex body formation were detected in most spermatocyte nuclei of mice that were heterozygous for eight Rb translocations, which indicated moderate activity of pachytene arrest (Manterola et al. 2009). Association of the SC trivalent carrying asynaptic regions with the XY bivalent in early-middle pachytene, which also did not result in the reduction of fertility, was revealed with immunocytochemical methods in laboratory mice with a single translocation (Saferali et al. 2010). Apparently, in case of Rb translocations the reduced efficiency of checkpoint in pachytene determines the possibility of Rb metacentric circulation in natural populations and their role in karyotype evolution (Matveevsky and Kolomiets 2011).
In our study, centromeres of homologues in the SC bivalent formed between two Rb metacentrics (hi) were not aligned. We assume that this pattern might result from different mechanisms of Rb metacentric formation in the past. One of the ancestors might have retained centromere of h chromosome after formation of Rb metacentric, while another ancestor might have retained centromere of i chromosome. Previous works reported a presence of two centromeric foci in other Rb metacentrics (Borodin et al. 2008). We suggest that in case of extensive chromosomal variability of Sorex araneus, centromere polymorphism of Rb metacentrics might be often observed in different populations.
Our synaptonemal complex results suggest regularity in formation of the ring-of-four configuration produced by hybrids between the Moscow and the Neroosa chromosome races of Sorex araneus. This relates well to the previous findings that chromosome races differing by monobrachial homology in Sorex araneus does not lead to complete sterility in hybrids. In particular, our immunocytochemical demonstration of an absence of asynapsis in the ring-of-four configuration relates well to the production of sperm in such hybrids.
Acknowledgments
We would like to thank Dr Nina Bulatova for discussion on the structure of the manuscript and valuable comments in karyology. We are especially grateful to Prof Jeremy B. Searle for valuable comments on the manuscript and the English. This work was supported by President Grant for Russian Distinguished Young Scientists MK-2500.2011.4 (for SP) and research grants of the Russian Foundation for Basic Research № 12-04-31425, 12-04-31200.
References
- Aniskin VM, Lukianova IV. (1989) New chromosome race and analysis of the hybrid zone between two karyomorphs of Sorex araneus (Insectivora, Soricidae). Proceedings of the USSRAcademyofSciences 309: 1260-1262 [In Russian] [Google Scholar]
- Baker RJ, Bickham JW. (1986) Speciation by Monobrachial Centric Fusions. Proceedings of the National Academy of Sciences of USA 83: 8245-8248.10.1073/pnas.83.21.8245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RJ, Bickham JW, Arnold ML. (1985) Chromosomal evolution in Rhogeessa (Chiroptera: Vespertilionidae): Possible speciation by centric fusions. Evolution 39: 233-243.10.2307/2408359 [DOI] [PubMed] [Google Scholar]
- Bakloushinskya IYu, Romanenko SA, Graphodatsky AS, Matveevsky SN, Lyapunova EA, Kolomiets OL. (2010) The role of chromosome rearrangements in the evolution of mole voles of the genus Ellobius (Rodentia, Mammalia). Russian Journal of Genetics 46: 1143-1145.10.1134/S1022795410090346 [Google Scholar]
- Baverstock PR, Gelder M, Jahnke A. (1983) Chromosome evolution in Australian Rattus - G-banding and hybrid meiosis. Genetica 60: 93-103.10.1007/BF00127495 [Google Scholar]
- Borodin PM, Karamysheva TV, Belonogova NM, Torgasheva AA, Rubtsov NB, Searle JB. (2008) Recombination map of the common shrew, Sorex araneus (Eulipotyphla, Mammalia). Genetics 178 (2): 621-32.10.1534/genetics.107.079665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulatova N, Searle JB, Nadjafova RS, Pavlova SV, Bystrakova NV. (2009) Field protocols for the genomic era. Comparative Cytogenetics 3: 57-62.10.3897/compcytogen.v3i1.9 [Google Scholar]
- Bulatova N, Jones RM, White TA, Shchipanov NA, Pavlova SV, Searle JB. (2011) Natural hybridization between extremely divergent chromosomal races of the common shrew (Sorex araneus, Soricidae, Soricomorpha): hybrid zone in European Russia. Journal of Evolutionary Biology 24 (3): 573-586.10.1111/j.1420-9101.2010.02191.x [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Baker TG. (1984) Meiotic pairing and gametogenic failure. Symposia of the Society for Experimental Biology 38: 349-362 [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM. (2009) The consequences of asynapsis for mammalian meiosis. Nature Reviews Genetics 10: 207-216.10.1038/nrg2505 [DOI] [PubMed] [Google Scholar]
- Capanna E. (1975) Gametic aneuploidy in mouse hybrids. Chromosomes Today 5: 83-89 [Google Scholar]
- Capanna E. (1982) Robertsonian Numerical variation in Animal Speciation: Mus musculus an emblematic model. In: Barigozzi C. (ed). Mechanisms of Speciation, Alan R.Liss, New York: 155-177 [PubMed]
- Capanna E, Gropp A, Winking H, Noack G, Civitelli MV. (1976) Robertsonian metacentrics in the mouse. Chromosoma 58: 341-353.10.1007/BF00292842 [DOI] [PubMed] [Google Scholar]
- Djlelati R, Brun B, Rumpler Y. (1997) Meiotic study of hybrids in the genus Eulemur and taxonomic considerations. American Journal of Primatology 42 (3): 235-245.10.1002/(SICI)1098-2345(1997)42:3<235::AID-AJP6>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Ford CE, Hamerton JL. (1956) A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technology 31: 247-251 [DOI] [PubMed] [Google Scholar]
- Forejt J. (1984) X-inactivation and its role in male sterility. Chromosomes Today 8: 17-22 [Google Scholar]
- Forejt J, Gregorov S, Goetz P. (1981) XY pair associates with the synaptonemal complex of autosomal male-sterile translocations in pachytene spermatocytes of the mouse (Mus musculus). Chromosoma 82: 41-53.10.1007/BF00285748 [DOI] [PubMed] [Google Scholar]
- Fredga K. (1970) Unusual sex chromosome inheritance in mammals. Philosophical Transactions of the Royal Society of London. Biological Sciences 259: 15-36.10.1098/rstb.1970.0042 [DOI] [PubMed] [Google Scholar]
- Garagna S, Zuccotti M, Searle JB, Redi CA, Wilkinson PJ. (1989) Spermatogenesis in heterozygotes for Robertsonian rearrangements from natural population of the common shrew Sorex araneus. Journal of Reproduction and Fertility 87: 431-438.10.1530/jrf.0.0870431 [DOI] [PubMed] [Google Scholar]
- Gropp A, Winking H, Zech L, Müller HJ. (1972) Robertsonian chromosomal variation of metacentric chromosomes in feral mice. Chromosoma 39: 265-288.10.1007/BF00290787 [DOI] [PubMed] [Google Scholar]
- Gropp A, Winking H. (1981) Robertsonian translocations: Cytology, meiosis, segregation patterns and biological consequences of heterozygosity. Symposia of the Zoological Society of London 47: 141-181 [Google Scholar]
- Hausser J, Fedyk S, Fredga K, Searle JB, Volobouev V, Wójcik J, Zima J. (1994) Definition and nomenclature of the chromosome races of S. araneus. Folia Zoologica 43 (1): 1-9 [Google Scholar]
- Hauffe HC, Searle JB. (1998) Chromosomal heterozygosity and fertility in house mice (Mus musculus domesticus) from northern Italy. Genetics 150: 1143-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolka D, Ivanek R, Capkova J, Jansa P, Forejt J. (2007)Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Research 17 (10): 1431-1437.10.1101/gr.6520107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadwiszczak KA, Banaszek A. (2006) Fertility in the male common shrews, Sorex araneus, from the extremely narrow hybrid zone between chromosome races. Mammalian Biology 71: 257-267.10.1016/j.mambio.2006.02.004 [Google Scholar]
- Jotterand M. (1972) Le polymorphisme chromosomique des Mus (Leggadas) africains: cytogénétique, zoogéographie, évolution. Revue Suisse de Zoologie 79: 287-359 [PubMed] [Google Scholar]
- Karamysheva TV, Belonogova NM, Rodionova MI, Rubtsov NB, Polyakov AV, Searle JB, Borodin PM. (2007)Temporal and spatial distribution of Rad51 protein in spermatocytes of the common shrew Sorex araneus L. (Soricidae, Eulipotyphla). Russian Journalof Theriology 6 (1): 15-19 [Google Scholar]
- King M. (1993) Species Evolution: The Role of Chromosomal Change. Cambridge, 360 pp. [Google Scholar]
- Kolomiets OL, Matveevsky SN, Bakloushinskaya IYu. (2010) Sexual dimorphism in prophase I of meiosis in mole vole (Ellobius talpinus Pallas) with isomorphic (XX) chromosomes in males and females. Comparative Cytogenetics 4 (1): 55-66.10.3897/compcytogen.v4i1.25 [Google Scholar]
- Kolomiets OL, Mazurova TF, Pomerantseva MD, Chekhovich AV, Bogdanov YuF. (1992) Electron microscopic analysis of the synaptonemal complexes in male laboratory mice exposed in the Chernobyl Region during embryogenesis. Russian Journal of Genetics 28 (9): 120-127 [In Russian] [PubMed] [Google Scholar]
- Král B, Radjabli SI. (1974) Banding patterns and Robertsonian fusions in the western Siberian population of Sorex araneus (Insectivora, Soricidae). Folia Zoologica 23: 217–227 [Google Scholar]
- Lyapunova EA, Vorontsov NN, Korobitsina KV, Ivanitskaya EYu, Borisov YuM, Yakimenko L V, Dovgal VY. (1980) A Robertsonian fan in Ellobius talpinus. Genetica 52/53: 239–247.10.1007/BF00121833 [Google Scholar]
- Lyapunova EA, Bakloushinskaya IYu, Kolomiets OL, Mazurova TF. (1990) Analysis of fertility of hybrids of multi chromosomal forms in mole voles of the superspecies Ellobius tancrei differing in a single pair of Robertsonian metacentrics. Proceedings of the USSRAcademyofSciences 310 (3): 721-723 [In Russian] [Google Scholar]
- Mahadevaiah SK, Bourc’His D, de Rooij DG, Bestor TH, Turner JM, Burgoyne PS. (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. Journal of Cell Biology 182: 263-276.10.1083/jcb.200710195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manterola M, Page J, Vasco C, Berríos S, Parra M, Viera A, Rufas J, Zuccotti M, Garagna S, Fernández-Donoso R. (2009) A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple Robertsonian translocations. PLoS Genetics 8: 1-14.10.1371/journal.pgen.1000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthey R. (1970) The “Robertsonian fan” in African Mus (Leggada) of the group minutoides-musculoides). Revue Suisse de Zoologie 77: 625-629 [PubMed] [Google Scholar]
- Matveevsky SN, Kolomiets OL. (2011) Types of synaptonemal complex chains in heterozygotes for multiple Robertsonian translocations. Factors of Experimental Evolution of Organisms 10: 42-47 [In Russian] [Google Scholar]
- Mercer SJ, Wallace BMN, Searle JB. (1992) Male common shrews (Sorex araneus) with long meiotic chain configurations can be fertile: implications for chromosomal models of speciation. Cytogenetic and Cell Genetics 60: 68-73.10.1159/000133298 [DOI] [PubMed] [Google Scholar]
- Narain Y, Fredga K. (1997) Meiosis and fertility in common shrew, Sorex araneus, from a chromosomal hybrid zone in central Sweden. Cytogenetic and Cell Genetics 78: 253-259.10.1159/000134668 [DOI] [PubMed] [Google Scholar]
- Narain Y, Fredga K. (1998) Spermatogenesis in common shrews, Sorex araneus, from a hybrid one with extensive Robertsonian polymorphism. Cytogenetic and Cell Genetics 80: 158-164.10.1159/000014973 [DOI] [PubMed] [Google Scholar]
- Navarro J, Vidal F, Quitart M. (1981) A method for the sequential study of SC by light and electron microscopy. Human Genetics 59: 419-423.10.1007/BF00295483 [DOI] [PubMed] [Google Scholar]
- Nunes AC, Catalan J, Lopez J, M da Graça Ramalhinho, M da Luz Mathias, Britton-Davidian J. (2011) Fertility assessment in hybrids between monobrachially homologous Rb races of the house mouse from the island of Madeira: implications for modes of chromosomal evolution. Heredity 106 (2): 348-356.10.1038/hdy.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov VN, Borisov YuM, Cherepanova EV, Grigor’eva OO, Shestak AG, Sycheva VB. (2012) Narrow hybrid zone between Moscow and Western Dvina chromosomal races and specific features of population isolation in common shrew Sorex araneus (Mammalia). Russian Journal of Genetics 48 (1): 70-78.10.1134/S1022795412010152 [PubMed] [Google Scholar]
- Pack SD, Borodin PM, Serov OL, Searle JB. (1993) The X-autosome translocation in the common shrew (Sorex araneus L.): late replication in female somatic cells and pairing in male meiosis. Chromosoma 102: 355-360.10.1007/BF00661279 [DOI] [PubMed] [Google Scholar]
- Pavlova SV, Kolomiets OL, Bulatova N, Searle JB. (2008) Demonstration of a WART in a hybrid zone of the common shrew (Sorex araneus Linnaeus, 1758). Comparative Cytogenetics 2 (2): 115-120 [Google Scholar]
- Pavlova SV, Matveevsky SN, Acaeva MM, Kolomiets OL, Bulatova N. (2012) Can chromosomal hybrid zones promote speciation in the common shrew Sorex araneus: what does the study of meiosis? International Conference "Chromosome 2012". Novosibirsk, September 2–7, 2012. [in press]
- Polyakov A, White Th, Jones RM, Borodin PM, Searle JB. (2011) Natural hybridization between extremely divergent chromosomal races of the common shrew (Sorex araneus, Soricidae, Soricomorpha): hybrid zone in Siberia. Journal of Evolutionary Biology 24: 1393-1402.10.1111/j.1420-9101.2011.02266.x [DOI] [PubMed] [Google Scholar]
- Robertson WRB. (1916) Chromosome studies. I. Taxonomic relationships shown in the chromosomes of Tettigidae and Acrididae: V-shaped chromosomes and their significance in Acrididae, Locustidae, and Gryllidae: chromosomes and variation. Journal of Morphology 27: 179-181.10.1002/jmor.1050270202 [Google Scholar]
- Rumpler Y. (2004) Complementary approaches of cytogenetics and molecular biology to the taxonomy and study of speciation processes in Lemurs. Evolutionary Anthropolgy 13: 67–78.10.1002/evan.20010 [Google Scholar]
- Saferali A, Berlivet S, Schimenti J, Bartolomei MS, Taketo T, Naumova AK. (2010) Defective imprint resetting in carriers of Robertsonian translocation Rb (8.12). Mammalian Genome 21: 377-387.10.1007/s00335-010-9271-9 [DOI] [PubMed] [Google Scholar]
- Said K, Saad A, Auffray J-C, Britton-Davidian J. (1993) Fertility estimates in the Tunisian all-acrocentric and Robertsonian populations of the house mouse and their chromosomal hybrids. Heredity 71: 532-538.10.1038/hdy.1993.172 [DOI] [PubMed] [Google Scholar]
- Searle JB. (1988) Selection and Robertsonian variation in nature: the case of the common shrew. In: Daniel A. (Ed.) The Cytogenetics of Mammalian Autosomal Rearrangements. New York, 507–531 [Google Scholar]
- Searle JB. (1993) Chromosomal hybrid zones in eutherian mammals. In: Harrison RG. (Ed.). Hybrid Zones and the Evolutionary Process.Oxford University Press, New York: 309-353
- Searle JB, Fedyk S, Fredga K, Hausser J, Volobouev VT. (1991) Nomenclature for the chromosomes of the common shrew (Sorex araneus). Mémoires de la Société vaudoise des sciences naturelles 19: 13-22 [Google Scholar]
- Searle JB, Wójcik JM. (1998) Chromosomal evolution: the case of Sorex araneus. In: Wójcik JM, Wolsan M. (Eds) Evolution of Shrews. Białowieża, 219–268 [Google Scholar]
- Sharman GB. (1956) Chromosomes of the common shrew. Nature 177: 941-942.10.1038/177941a0 [DOI] [PubMed] [Google Scholar]
- Tembotov AK, Khatukhov AM, Ivanov VG, Grigorieva GI. (1976) An ecological and geographical aspect of the evolution of the pine vole in the Caucasus. In: Fauna, ecology and protection of North Caucasus animals, Nalchik, 3: 1-35 [In Russian] [Google Scholar]
- Turner JMA, Mahadevaiah SK, Ellis PJI, Mitchell MJ, Burgoyne PS. (2006) Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Developmental Cell 10 (4): 521-529.10.1016/j.devcel.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Volobouev V, Viegas-Péquignot E, Petter F, Gautun JC, Sicard B, Dutrillaux B. (1988) Complex chromosomal polymorphism in Gerbillus nigeriae (Rodentia, Gerbillidae). Journal of Mammalia 69: 131-134.10.2307/1381757 [Google Scholar]
- Vorontsov NN. (1960) Species of Palaearctic hamsters (Cricetinae, Rodentia) in statu nascendi. Proceedings of the USSRAcademyofSciences 132: 1065-1069 [In Russian] [Google Scholar]
- Wallace BMN, Searle JB. (1990) Synaptonemal complex studies of the common shrew (Sorex araneus). Comparison of Robertsonian heterozygotes and homozygotes by light microscopy. Heredity 65: 359-367.10.1038/hdy.1990.105 [Google Scholar]
- Ward OG, Graphodatsky AS, Wurster-Hill DH, Ermina VR, Park JP, Yu Q. (1991) Cytogenetics of beavers: a case of speciation by monobrachial centric fusions. Genome 34: 324-328.10.1139/g91-053 [Google Scholar]
- White MJD. (1978)Modesofspeciation. San Francisco, California, 455 pp. [Google Scholar]
- White MJD. (1982)Rectangularity, speciation and chromosome architecture. In: Barigozzi C. (ed). Mechanisms of Speciation, Alan R.Liss, New York: 75-103 [PubMed]
- White TA, Bordewich M, Searle JB. (2010) A network approach to study karyotypic evolution: the chromosomal races of the common shrew (Sorex araneus) and house mouse (Mus musculus) as model systems. Systematic Biology 59: 262-276.10.1093/sysbio/syq004 [DOI] [PubMed] [Google Scholar]
- Zima J, Lukacova L, Macholan M. (1998) Chromosomal evolution in shrews. In: Wójcik JM, Wolsan M. (Eds). Evolution of shrews.Białowieża: 175-218