Abstract
Bulbar motor deterioration due to amyotrophic lateral sclerosis (ALS) leads to the eventual impairment of speech and swallowing functions. Despite these devastating consequences, no standardized diagnostic procedure for assessing bulbar dysfunction in ALS exists and adequate objective markers of bulbar deterioration have not been identified. In this paper, we consider objective measures of speech motor function, which show promise for forming the basis of a comprehensive, quantitative bulbar motor assessment in ALS. These measures are based on the assessment of four speech subsystems: respiratory, phonatory, articulatory, and resonatory. The goal of this research is to design a non-invasive, comprehensive bulbar motor assessment instrument intended for early detection, monitoring of disease progression, and clinical trial application. Preliminary data from an ongoing study of bulbar motor decline are presented, which demonstrate the potential clinical efficacy of the speech subsystem approach.
Keywords: Bulbar motor, amyotrophic lateral sclerosis, oral motor, speech assessment, speech intelligibility, speaking rate, speech movement analysis
Bulbar motor assessment in ALS: Challenges and future directions
Progressive bulbar motor deterioration as a result of amyotrophic lateral sclerosis (ALS) leads to the eventual impairment of speech and swallowing function. At time of diagnosis, up to 30% of patients with ALS present with bulbar symptoms (1). Regardless of the site of onset, most patients with ALS will experience bulbar symptoms, which significantly impact the quality of life and shorten survival (2,3). Despite these devastating consequences, no standardized diagnostic procedure for assessing bulbar dysfunction in ALS exists, and adequate objective markers of bulbar deterioration have yet to be identified. Consequently, little is known about the natural history of bulbar deterioration, and clinicians invariably find it challenging to predict the rate of speech and swallowing decline. Objective bulbar assessment strategies are needed for improving early disease detection, monitoring disease progression, and optimizing the efficacy of therapeutic ALS drug trials. Improved prediction accuracy of bulbar decline is also critical for clinical care because decisions regarding communication intervention and palliative care are most effective when made prior to the loss of speech intelligibility (4).
A significant obstacle, however, has been the logistical difficulties posed by the inaccessibility and complexity of the bulbar apparatus. Although advanced instrumentation-based methods of bulbar assessment exist (5), most are designed for the assessment of speech production and remain unfamiliar to neurologists or other members of the ALS clinical team. Moreover, their reliability, validity, and responsiveness to bulbar motor deterioration are not well established.
Neurologic examination and basic speech testing have been components of the standard bulbar assessment battery for decades, yet these assessments appear to be insensitive to the early phases of bulbar deterioration. Numerous studies have demonstrated, for example, that bulbar motor dysfunction occurs prior to perceived changes in speech intelligibility (4,6–11). Speaking rate, as measured in words per minute (wpm), has been observed to decline prior to speech intelligibility. As this rate drops below 100–120 wpm, speech intelligibility tends to decline rapidly (4,10). The diagnostic value of this measure for detecting disease onset, however, is questionable because even healthy individuals vary considerably in their habitual speaking rates (12) and are capable of using various strategies (11) for maintaining this rate as oromotor strength declines (8).
Over the past several decades, a relatively small number of speech researchers have worked to develop selective measures of speech subsystem motor involvement (see (5)). These speech subsystem measures are more sensitive than current best practices for detecting subclinical changes in bulbar motor performance. The extant work on these measures has, however, largely focused on the testing of a single speech subsystem (e.g. phonatory, articulatory, or resonatory) in populations with motor disorders other than ALS. The intent of this review is to integrate this disparate literature by 1) identifying promising instrumental measures of speech subsystem performance; and 2) presenting preliminary findings demonstrating the diagnostic potential of these measures relative to those currently used in translational research and clinical practice. The ultimate purpose of this research is to design a minimally invasive yet comprehensive bulbar motor assessment instrument that is readily adaptable to clinical settings, and intended for the monitoring of disease progression and clinical trial application.
Framework for the assessment of bulbar impairment: a speech subsystem approach
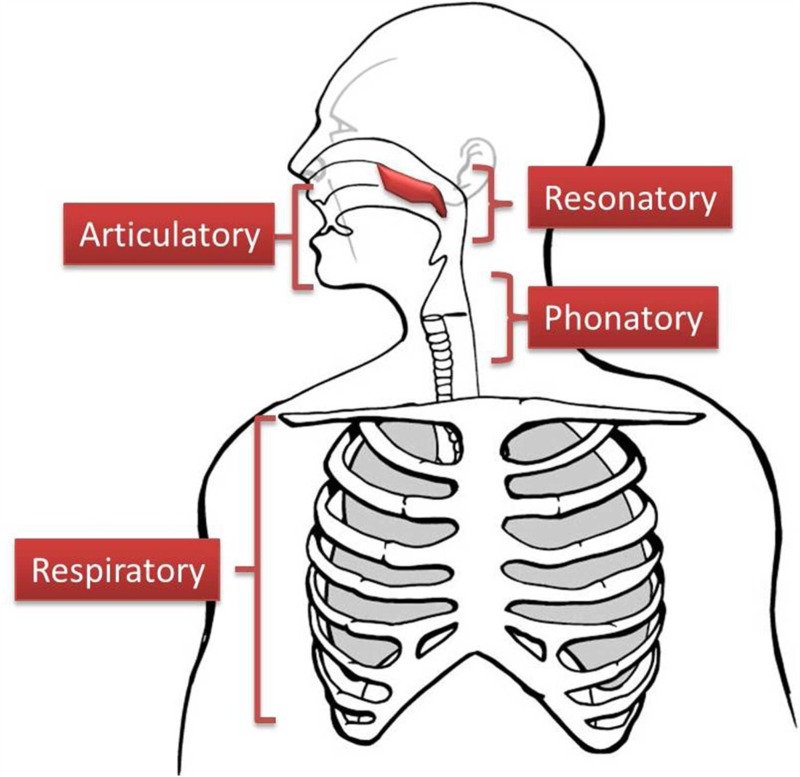
Speech researchers traditionally include the bulbar system as part of the larger speech production network, comprised of four distinct subsystems (13–15): respiratory, phonatory, articulatory, and resonatory (see Figure 1). In this review, we highlight several instrumental based measures that when combined, show promise for forming the basis of a comprehensive, quantitative bulbar motor assessment in ALS. In addition, preliminary data are presented that demonstrate the feasibility of this approach. The applied goal of this instrument is to improve early detection of bulbar involvement and to provide a reproducible, quantitative index of change in the rate of bulbar progression.
Figure 1.
The four speech subsystems.
The respiratory subsystem. ALS inevitably results in weakening of the respiratory musculature characterized by symptoms of dyspnea on exertion, orthopnea, and early morning headaches with fatigue. Respiratory failure remains the leading cause of death (16). The average rate of decline in vital capacity is reported to be 1.8% per month in patients with slowly progressive ALS and 3.5% per month in rapidly progressive disease (17,18). Although respiratory function is accurately documented through spirometry (19), the measured effects on speech communication remain poorly understood. Speech breathing patterns are quantified using technologies that record chest wall movements, oral pressures and flows, and speech acoustics. The most extensively studied aspect of speech breathing decline in persons with ALS has been the patterns of pauses in acoustic recordings of connected speech (9,20). Because respiratory muscle weakness necessitates more frequent inspirations during speech, persons with ALS exhibit longer and more frequent pauses than do healthy controls (20).
The phonatory subsystem. Perceptible changes in voice quality or loudness may be the first symptom of bulbar involvement, even in spinal-onset disease (21,22). The consistent pattern of vocal fold vibration is a function of muscular tension, the viscoelastic properties of vocal fold tissue, and the air pressure and flow generated by the lungs (23). Because voice quality is affected by multiple factors, the assessment of the laryngeal subsystem requires evaluation of multiple voice variables.
Auditory perceptual (i.e. listening-based) judgments of voice characteristics, including roughness, strain, breathiness, and loudness, are the most common measures of laryngeal involvement assessed by speech-language pathologists (24). Previous studies have suggested more than 60% of all patients with ALS exhibit changes to vocal quality such as tremor, breathiness, and harshness (25). Recordings of voice acoustics and aerodynamics provide a more objective assessment of phonatory function than subjective judgments of voice quality, which requires special training and can be unreliable (26). In persons with ALS, phonatory function decline has been detected prior to speech decline using acoustic measures of voice (27). Although abnormal phonatory characteristics associated with ALS vary considerably, phonatory dysfunction has been characterized as abnormal vocal pitch (too high or low), limited pitch range, instability of vocal fold vibration, and high noise-to-harmonic ratios (21,22,25,28–31). The latter measure quantifies the amount of noise in the voice signal.
The resonatory subsystem. Velopharyngeal muscle weakness leads to continual opening of the velopharyngeal port during speech. Acoustic energy then enters the nasal cavity producing abnormal nasal resonances. Consequently, speech becomes excessively nasal (or hypernasal) with speech sounds becoming less distinct and weak in intensity. In severe cases of velopharyngeal incompetence, speech can be rendered unintelligible. Although velopharygeal function has rarely been investigated in ALS (e.g. (32)), the existing data suggest that hypernasality may be a defining characteristic of bulbar disease. Carrow et al. (25) reported that 75% of the 79 patients they studied with motor neuron disease exhibited hypernasality.
Due to its inaccessibility, velopharygeal functioning is rarely studied directly. Aerodynamic or acoustic methods are traditionally used to detect spurious nasal airflow emissions or the presence of nasal resonances (see (33)). Nasalance, the relative proportion of nasal to oral acoustic energy in speech, is widely used by speech-language pathologists to assess velopharyngeal function. Nasalance scores correlate well with listeners’ perceptions of nasality due to ALS (32). The measure has been shown to be reliable (test-retest error = 2%), and has a high specificity (87%–93%) and a high sensitivity (83%–97%) in persons with velopharyngeal incompetence due to cleft lip and palate (34,35). Delorey et al. (32) reported significant changes across a six-month interval in nasalance for groups of participants with bulbar and spinal deterioration due to ALS. The measure was able to differentiate the bulbar group from the spinal group and healthy controls at both recordings, with the bulbar group exhibiting greater nasalance scores than the other groups.
The articulatory subsystem. Testing of articulatory involvement is achieved through measures of oral movement and strength. Speech is the product of highly coordinated movements of the tongue, lips, and jaw. The small movements of the lips and jaw can be non-invasively recorded using computer-based optical tracking systems (36). Magnetic (37,38) or imaging technologies such as dynamic MRI (39) and ultrasound (40) are currently required to capture the movements of the tongue inside the mouth. Multiple features of motor performance can be evaluated from speech movement recordings including movement extent, speed, and acceleration (see (41)). Other variables often measured include the regularity of the movement patterns (42,43) and the temporal coordination between the movements of different articulators (36).
As ALS progresses, speech movements become smaller in extent, slower in speed and longer in duration (8,44–47). In some individuals, the longitudinal course of decline is interrupted by a transient increase in movement extent and speed, particularly in the jaw (47,48). These studies also reported that the speed of speech movements was more consistently affected in ALS than several other measurable speech variables. Movement speed may be a particularly sensitive index of early motor deterioration in ALS as the disease appears to selectively affect fast-twitch muscles, as noted in early phases of the motor neuron disease mouse model when the animal is presymptomatic (49,50).
The force generating capacities of the jaw, lips and tongue can be measured using either a strain gauge (51) or pneumatic pressure bulb (52). Static measures are intended to test force generating capacity (strength and endurance) and dynamic measures are intended to assess force regulation (motor control). The maximum force and peak rate of change of force in the tongue and lips are markedly reduced in ALS (6,53). The test-retest reliability of oral strength measures varies across studies from moderate (54) to high (55). Common to all maximum performance assessments, oral strength scores are affected by age, gender, practice and motivation (56). These factors may drive the large population variance reported with these measures, limiting their usefulness in clinical trials (57).
Towards an objective bulbar assessment: preliminary data
Below we present preliminary data from an ongoing study of bulbar motor decline, demonstrating the use of subsystem measures for the assessment of bulbar disease onset and progression. The analyses were based on cross-sectional data from 30 participants: 10 healthy controls and 20 participants with ALS. The participants with ALS were stratified into two groups (10 participants per group) based on their speech intelligibility scores: ALS Normal Speech (100–97%) and ALS Impaired Speech (96–86%). Information about the characteristics of each participant group is provided in Table I.
Table I.
Participant characteristics for the Control, ALS Normal Speech, and ALS Impaired Speech groups. The values for Gender and Onset are counts, and the values for all other variables are means (standard deviations). FVC refers to predicted forced vital capacity.
| Group | Gender |
Age (years) | Months since onset | Onset |
ALSFRS-R Total | ALSFRS-R Bulbar | FVC | Speech Intell | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | bulbar | spinal | |||||||
| Healthy controls | 4 | 6 | 60.10 (10.54) | – | – | – | – | – | 3.00 (1.36) | 98.71 (1.23) |
| ALS Normal Speech | 6 | 4 | 55.69 (11.20) | 57.30 (50.74) | 2* | 9* | 33.00 (5.40) | 10.00 (1.70) | 2.67 (1.14) | 99.73 (0.61) |
| ALS Impaired Speech | 5 | 5 | 59.41 (11.79) | 33.30 (21.35) | 4 | 6 | 33.00 (9.29) | 8.60 (1.71) | 2.53 (1.13) | 91.45 (3.64) |
Onset for one subject was both bulbar and spinal.
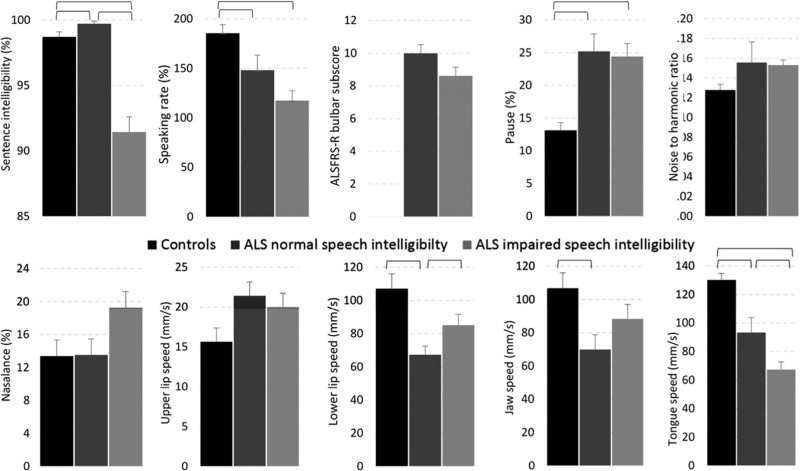
The respiratory, phonatory and resonatory subsystems were evaluated using the methods described above and previously in Yunusova et al. (5). The assessors were blinded to group assignment and ALS diagnosis. Table II shows the speech subsystem variables that were collected in addition to speech intelligibility (%), speaking rate (words per minute), and a bulbar severity score, which was derived by summing the scores from the three questions about speech, swallowing, and salivation on the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (58−60). The mean values for each group and variable are displayed in Figure 2. All group differences were tested using t-tests for independent samples using an alpha level of .05.
Table II.
Instrumentation-based speech subsystem variables recorded in this study.
| Speech subsystem | Measure | Experimental tasks |
|---|---|---|
| Respiratory | Percent pause (%) | Reading of Bamboo passage |
| Phonatory | Noise to harmonic ratio | Sustained “ah” |
| Resonatory | Nasalance (%) | Sentence repetition: “Buy Bobby a puppy” |
| Articulatory | ||
| Jaw | Peak movement speed (mm/s) | Word repetition: “uhpah” |
| Lip | Peak movement speed (mm/s) | Word repetition: “uhpah” |
| Tongue | Peak movement speed (mm/s) | Word repetition: “I owe you a yoyo today” |
Figure 2.
The mean values and standard error for each group and variable. Statistically significant comparisons are marked with horizontal brackets.
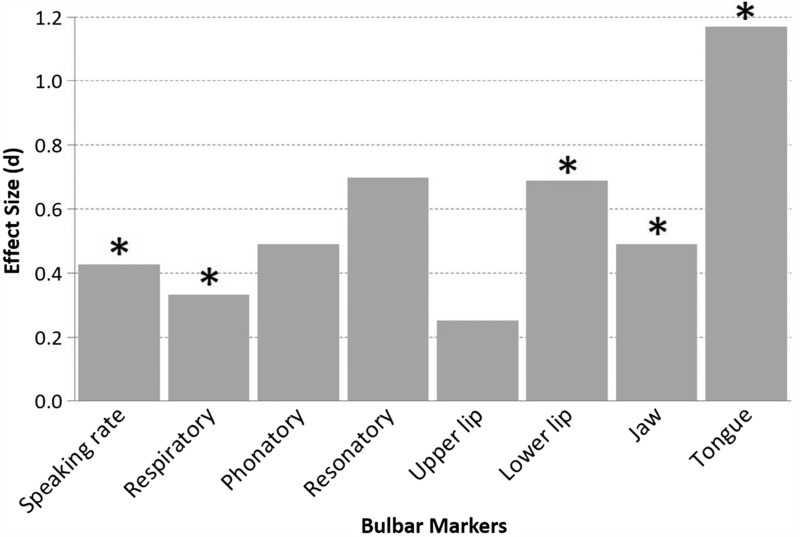
Early detection of bulbar involvement. As displayed in Figure 2, statistically significant differences were observed for speaking rate, lower lip, jaw, and tongue. The between-group effect sizes for these data are presented in Figure 3. For each speech system and subsystem measure, we computed the Cohen's d effect sizes (expressed in absolute value) between healthy controls and persons in the ALS Normal Speech group. Based on Cohen's conventions (61), the observed effect sizes were large for the tongue, medium for lower lip and jaw, and small for speaking rate. The observation of early tongue signs is consistent with prior reports from studies of muscle strength and articulatory movements (6,40,42,62).
Figure 3.
Effect sizes for each speech variable representing the differences between the healthy controls and the ALS participants with normal speech intelligibly. Statistically significant comparisons are marked with asterisks.
Changes during the mild to moderate phase of speech impairment. As displayed in Figure 2, statistically significant differences were observed for speaking rate, ALSFRS-R bulbar subscore, lower lip, and tongue. It is notable that the lower lip movement speed was faster for the ALS Impaired Speech group than for the ALS Normal Speech Group and that jaw movement speed showed a similar trend. This unexpected increase in lip and jaw movement speed with disease progression is thought to be a compensatory response to the slowing of tongue movements (47,48).
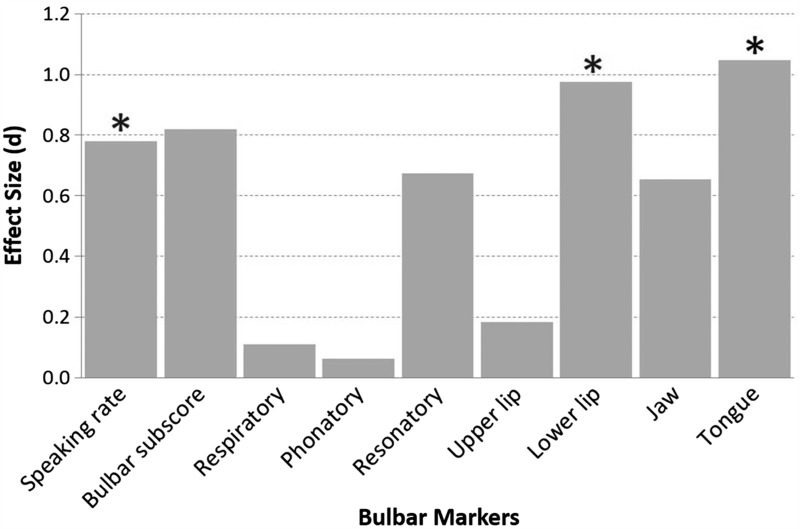
For each speech system and subsystem measure, the Cohen's d effect sizes (expressed in absolute value) between the ALS Normal Speech group and the ALS Impaired Speech group were also computed (Figure 4). Effect sizes were large for the lower lip and tongue speeds, and marginally large for speaking rate.
Figure 4.
Effect sizes for each speech variable representing the differences between participants with ALS who had normal speech and who had impaired speech. Statistically significant comparisons are marked with asterisks.
Conclusions
The reliable assessment of bulbar involvement in ALS continues to be a significant clinical and research challenge.
Overall, our preliminary findings suggest that measures of speech motor performance, in particular tongue movement speed, may be well suited for both early detection and progress monitoring. Speaking rate was also an effective measure for both early detection and progress monitoring, but was only associated with mild to moderate effect sizes. The ALSFRS-R bulbar subscore and several of the speech subsystem measures did not detect differences among the groups in this sample.
Longitudinal studies with a larger pool of participants are required to further interrogate the clinical efficacy of different bulbar measures, which should include determining each measure's psychometric properties (e.g. reliability, validity, specificity, sensitivity). Additional work also is needed to address barriers to clinical implementation including high equipment cost, equipment complexity, and the burden of data reduction. These issues are being actively investigated by multidisciplinary research teams focused on developing improved speech movement tracking hardware and intelligent algorithms to identify abnormal speech and voice patterns in large datasets (64).
Acknowledgements
The authors would like to thank Antje Mefferd, Cynthia Didion, Kimber Green, Michelle Falikowski, Vincci Tau, Daniele Thomas, Casey Willett, Toni Hoffer, Aaron Pattee, and Emily Foutch for their assistance with this project. They also thank Michael Jackson for illustrating Figure 1.
Declaration of interest: This research was funded by an NIH-NIDCD grant, Bernice Ramsey Discovery Grant from ALS Canada, and the Barkley Trust at the University of Nebraska- Lincoln. The authors do not have financial and/or business in any company that may be affected by the research reported in this manuscript. The authors alone are responsible for the content and writing of the paper.
References
- 1.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- 2.Armon C, Moses D. Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. J Neurol Sci. 1998;160:37–41. doi: 10.1016/s0022-510x(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 3.del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population based study. Neurology. 2003;60:813–9. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 4.Ball L, Beukelman DR, Pattee G. Timing of speech deterioration in people with amyotrophic lateral sclerosis. J Med Speech-Lang Pa. 2002;10:231–5. [Google Scholar]
- 5.Yunusova Y, Green JR, Wang J, Pattee G, Zinman L. A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS) J Vis Exp. 2011;21((48)) doi: 10.3791/2422. doi:pii: 2422. 10.3791/2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DePaul R, Brooks BR. Multiple orofacial indices in amyotrophic lateral sclerosis. J Speech Hear Res. 1993;36:1158–67. doi: 10.1044/jshr.3606.1158. [DOI] [PubMed] [Google Scholar]
- 7.Kent RD, Kent JF, Weismer G, Sufit RL, Rosenbek JC, Martin RE, et al. Impairment of speech intelligibility in males with amyotrophic lateral sclerosis. J Speech Hear Disord. 1990;55:721–8. doi: 10.1044/jshd.5504.721. [DOI] [PubMed] [Google Scholar]
- 8.Mefferd AM, Green JR, Pattee G. A novel fixed-target task to determine articulatory speed constraints in persons with amyotrophic lateral sclerosis. J Commun Disord. 2012;45:35–45. doi: 10.1016/j.jcomdis.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishio M, Niimi S. Changes over time in dysarthric patients with amyotrophic lateral sclerosis (ALS): a study of changes in speaking rate and maximum repetition rate. Clin Linguist Phonet. 2000;14:485–97. [Google Scholar]
- 10.Yorkston K, Strand E, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: implications for the timing of intervention. J Med Speech-Lang Pa. 1993;1:35–46. [Google Scholar]
- 11.Mefferd AS, Green JR, Ball L, Pattee G. A novel fixed-target task to determine articulatory speed constraints in persons with amyotrophic lateral sclerosis. J of Comm Dis. 2007;45:35–45. doi: 10.1016/j.jcomdis.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao Y-C, Weismer G, Iqbal K. Interspeaker variation in habitual speaking rate: additional evidence. J Speech Lang Hear R. 2006;49:1156–64. doi: 10.1044/1092-4388(2006/083). [DOI] [PubMed] [Google Scholar]
- 13.Barlow SM, Abbs JH. Force transducers for the evaluation of labial, lingual, and mandibular motor impairments. J Speech Lang Hear R. 1983;26:616–21. doi: 10.1044/jshr.2604.616. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch BE. Dysarthria: a physiological approach to assessment and treatment. Cheltenham, UK: Nelson Thornes;; 1998. [Google Scholar]
- 15.Netsell R, Lotz WK, Barlow SM. Recent advances in dysarthria. In: Yorkston KM, Beukelman DR, editors. A speech physiology examination for individuals with dysarthria. Boston, MA: Little, Brown and Co.; 1989. pp. 3–37. [Google Scholar]
- 16.Vender RL, Mauger D, Walsh S, Alam S, Simmons Z. Respiratory systems abnormalities and clinical milestones for patients with amyotrophic lateral sclerosis with emphasis upon survival. Amyotroph Lateral Scler. 2007;8:36–41. doi: 10.1080/17482960600863951. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman PL, Belsh JM. Pulmonary function at diagnosis of amyotrophic lateral sclerosis. Rate of deterioration. Chest. 1993;103:508–13. doi: 10.1378/chest.103.2.508. [DOI] [PubMed] [Google Scholar]
- 18.Sufit RL, Brooks BR. Longitudinal changes in pulmonary functions (PVF) in amyotrophic lateral sclerosis (ALS): a pilot study for natural history and clinical trials. Neurology. 1990;40((Suppl l)):315. [Google Scholar]
- 19.Singh D, Verma R, Garg RK, Singh MK, Shukla R, Verma SK. Assessment of respiratory functions by spirometry and phrenic nerve studies in patients of amyotrophic lateral sclerosis. J Neurol Sci. 2011;306:76–81. doi: 10.1016/j.jns.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Green JR, Beukelman DR, Ball LJ. Algorithmic estimation of pauses in extended speech samples of dysarthric and typical speech. J Med Speech-Lang Pa. 2004;12:149–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Robert D, Pouget J, Giovanni A, Azulay JP, Triglia JM. Quantitative voice analysis in the assessment of bulbar involvement in amyotrophic lateral sclerosis. Acta Oto-Laryngol. 1999;119:724–31. doi: 10.1080/00016489950180702. [DOI] [PubMed] [Google Scholar]
- 22.Strand EA, Buder EH, Yorkston K, Ramig L. Differential phonatory characteristics of four females with amyotrophic lateral sclerosis. J Voice. 1994;8:327–39. doi: 10.1016/s0892-1997(05)80281-4. [DOI] [PubMed] [Google Scholar]
- 23.Titze IR. Principles of voice production. Englewood Cliffs, NJ: Prentice Hall; 1994. [Google Scholar]
- 24.Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kramer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech-Lang Pat. 2009;18:124–32. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]
- 25.Carrow E, Rivera V, Mauldin M, Shamblin L. Deviant speech characteristics in motor neuron disease. Archiv Otolaryngol. 1974;100:212–8. doi: 10.1001/archotol.1974.00780040220014. [DOI] [PubMed] [Google Scholar]
- 26.Kreiman J, Gerratt B, Kempster G, Erman A, Berke G. Perceptual evaluation of voice quality: review, tutorial, and a framework for future research. J Speech Hear Res. 1993; 36:21–40. doi: 10.1044/jshr.3601.21. [DOI] [PubMed] [Google Scholar]
- 27.Silbergleit AK, Johnson AF, Jacobson BH. Acoustic analysis of voice in individuals with amyotrophic lateral sclerosis and perceptually normal vocal quality. J Voice. 1997;11:222–31. doi: 10.1016/s0892-1997(97)80081-1. [DOI] [PubMed] [Google Scholar]
- 28.Kent JF, Kent RD, Rosenbek JC, Weismer G, Martin R, Sufit R, et al. Quantitative description of the dysarthria in females with amyotrophic lateral sclerosis. J Speech Lang Hear R. 1992;35:723–33. doi: 10.1044/jshr.3504.723. [DOI] [PubMed] [Google Scholar]
- 29.Kent RD, Sufit RL, Rosenbek JC, Kent JF, Weismer G, Martin RE, et al. Speech deterioration in amyotrophic lateral sclerosis: a case study. J Speech Lang Hear R. 1991;34:1269–75. doi: 10.1044/jshr.3406.1269. [DOI] [PubMed] [Google Scholar]
- 30.Ramig LO, Scherer RC, Klasner ER, Titze IR, Horii Y. Acoustic analysis of voice in amyotrophic lateral sclerosis: a longitudinal case study. J Speech Hear Disord. 1990;55:2–14. doi: 10.1044/jshd.5501.02. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfield D, Viswanath N, Herbich K,, Nudelman H. Evaluation of the speech motor control system in amyotrophic lateral sclerosis. Journal of Voice. 1991;5:224–30. [Google Scholar]
- 32.Delorey R, Leeper HA, Hudson AJ. Measures of velopharyngeal functioning in subgroups of individuals with amyotrophic lateral sclerosis. J Med Speech-Lang Pa. 1999;7:19–31. [Google Scholar]
- 33.Zajac DJ, Warren DW, Hinton V. Aerodynamic assessment of motor speech disorders. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. 2nd edn. New York, NY: Thieme; 2009. pp. 64–79. [Google Scholar]
- 34.Dalston R, Neiman G, Gonzalez Landa G. Nasometric sensitivity and specificity: a cross-dialect and cross-culture study. Cleft Palate Craniofac J. 1993;30:285–91. doi: 10.1597/1545-1569_1993_030_0285_nsasac_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 35.Wermker K, Jung S, Joos U, Kleinheinz J. Nasopharyngeal development in patients with cleft lip and palate: a retrospective case-control study. Vol. 2012. International Journal of Otolaryngology; 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green JR, Moore CA, Higashikawa M, Steeve RW. The physiological development of speech motor control: lip and jaw coordination. J Speech Lang Hear R. 2000;43:239–55. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkell JS, Cohen MH, Svirsky MA, Matthies ML, Garabieta I, Jackson MT. Electromagnetic midsagittal articulometer systems for transducing speech articulatory movements. J Acoust Soc Am. 1992;92:3078–96. doi: 10.1121/1.404204. [DOI] [PubMed] [Google Scholar]
- 38.Yunusova Y, Green JR, Mefferd A. Accuracy assessment for AG500 electromagnetic articulograph. J Speech Lang Hear R. 2009;52:547–55. doi: 10.1044/1092-4388(2008/07-0218). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura SM, Freitas DR, Tavares JM. Toward dynamic magnetic resonance imaging of the vocal tract during speech production. J Voice. 2011;25:511–8. doi: 10.1016/j.jvoice.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Stone M, Lundberg A. Three-dimensional tongue surface shapes of English consonants and vowels. J Acoust Soc Am. 1996;99:3728–37. doi: 10.1121/1.414969. [DOI] [PubMed] [Google Scholar]
- 41.Tjaden K. Segmental articulation in motor speech disorders. In: Weismer G, editor. Motor speech disorders: essays for Ray Kent. San Diego, CA: Plural Publishing; 2007. pp. 151–86. [Google Scholar]
- 42.Green JR, Moore CA, Reilly KJ. Sequential development of jaw and lip control for speech. J Speech Lang Hear R. 2002;45:66–79. doi: 10.1044/1092-4388(2002/005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith A, Goffman L, Zelaznik H, Ying G, McGillem C. Spatiotemporal stability and patterning of speech movement sequences. Exp Brain Res. 1995;104:493–501. doi: 10.1007/BF00231983. [DOI] [PubMed] [Google Scholar]
- 44.Hirose H, Kiritani S, Sawashima M. Patterns of dysarthric movement in patients with amyotrophic lateral sclerosis and pseudobulbar palsy. Folia Phoniatr Logo. 1982;34:106–12. doi: 10.1159/000265636. [DOI] [PubMed] [Google Scholar]
- 45.Kuruvilla MS, Green JR, Yunusova JR, Hanford K. Coordinative flexibility of the tongue in ALS. J Speech Lang Hear R. 2012;55:1897–909. doi: 10.1044/1092-4388(2012/11-0259). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yunusova Y, Weismer G, Westbury JR, Lindstrom M. Articulatory movements during vowels in speakers with dysarthria and normal controls. J Speech Lang Hear R. 2008;51:596–611. doi: 10.1044/1092-4388(2008/043). [DOI] [PubMed] [Google Scholar]
- 47.Yunusova Y, Green JR, Lindstrom MJ, Ball LJ, Pattee GL, Zinman L. Kinematics of disease progression in bulbar ALS. J Commun Disord. 2010;43:6–20. doi: 10.1016/j.jcomdis.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yunusova Y, Green JR, Lindstrom MJ, Wang J, Pattee GL, Zinman L. J Med Speech-Lang Pa. Speech in ALS: longitudinal changes in lips and jaw movements and vowel acoustics. [PMC free article] [PubMed] [Google Scholar]
- 49.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motor neuron diseases. J Neurosci. 2000;20:2534–42. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon T, Putman CT, Hegedus J. Amyotrophic lateral sclerosis: evidence of early denervation of fast-twitch muscles. Basic Appl Myol. 2007;17:141–5. [Google Scholar]
- 51.Barlow SM, Rath EM. Maximum voluntary closing forces in the upper and lower lips of humans. J Speech Hear Res. 1985;28:373–6. doi: 10.1044/jshr.2803.373. [DOI] [PubMed] [Google Scholar]
- 52.Robin DA, Somodi LB, Luschei ES. Dysarthria and apraxia of speech: perspectives on management. In: Moore CA, Yorkston KM, Beukelman DR, editors. Measurement of tongue strength and endurance in normal and articulation disordered subjects. Baltimore, MD: Paul H. Brookes; 1991. pp. 173–84. [Google Scholar]
- 53.Dworkin JP, Aronson AE, Mulder DW. Tongue force in normal and in dysarthric patients with amyotrophic lateral sclerosis. J Speech Hear Res. 1980;23:828–37. doi: 10.1044/jshr.2304.828. [DOI] [PubMed] [Google Scholar]
- 54.Solomon NP, Clark HM, Makashay MJ, Newman LA. Assessment of orofacial strength in adults with dysarthria. J Med Speech-Lang Pa. 2008;16:251–8. [PMC free article] [PubMed] [Google Scholar]
- 55.Clark HM, Henson PA, Barber WD, Stierwalt JAS, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech-Lang Pat. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- 56.Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. J Speech Hear Disord. 1987;52:367. doi: 10.1044/jshd.5204.367. [DOI] [PubMed] [Google Scholar]
- 57.Brooks BR, Sufit RL, DePaul R, Tan YD, Sanjak M, Robbins J. Design of clinical therapeutic trials in amyotrophic lateral sclerosis. Adv Neurol. 1991;56:521–46. [PubMed] [Google Scholar]
- 58.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS: a revised ALS functional rating scale that incorporates assessment of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann P, Levy G, Thompson JLP, Delbene ML, Battista V, Gordon PH, et al. The ALSFRS-R predicts survival time in an ALS clinic population. Neurology. 2005;64:38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 60.Miano B, Stoddard GJ, Davis S, Bromberg MB. Inter-evaluator reliability of the ALS functional rating scale. Amyotroph Lateral Scler. 2004;5:235–9. doi: 10.1080/14660820410021302. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 62.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. J Speech Hear Res. 1994;37:28–37. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- 63.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1:293–300. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 64.Tsanas A, Little MA, McSharry PE, Spielman J, Ramig LO. Novel speech signal processing algorithms for high-accuracy classification of Parkinson’s disease. IEEE Trans Biomed Eng. 2012;59:1264–71. doi: 10.1109/TBME.2012.2183367. [DOI] [PubMed] [Google Scholar]