Abstract
Despite affecting millions of individuals, the etiology of hot flushes remains unknown. Here we review the physiology of hot flushes, CNS pathways regulating heat-dissipation effectors, and effects of estrogen on thermoregulation in animal models. Based on the marked changes in hypothalamic kisspeptin, neurokinin B and dynorphin (KNDy) neurons in postmenopausal women, we hypothesize that KNDy neurons play a role in the mechanism of flushes. In the rat, KNDy neurons project to preoptic thermoregulatory areas that express the neurokinin 3 receptor (NK3R), the primary receptor for NKB. Furthermore, activation of NK3R in the median preoptic nucleus, part of the heat-defense pathway, reduces body temperature. Finally, ablation of KNDy neurons reduces cutaneous vasodilatation and partially blocks the effects of estrogen on thermoregulation. These data suggest that arcuate KNDy neurons relay estrogen signals to preoptic structures regulating heat-dissipation effectors, supporting the hypothesis that KNDy neurons participate in the generation of flushes.
Keywords: Menopause, Reproduction, Thermoregulation, Estrogen, LH, GnRH, Estrogen
1. Introduction
Menopause is characterized by degeneration of ovarian follicles and loss of ovarian estrogen secretion. In the majority of menopausal women, estrogen withdrawal causes hot flushes (also known as hot flashes), a disorder of hypothalamic thermoregulation. Despite the millions of individuals affected, there has been limited basic research on the physiology of hot flushes and their etiology has remained an enigma (Kronenberg, 2010). Because of their adverse impact on the quality of life, hot flushes are the major reason that menopausal women seek medical attention. The most effective treatment of hot flushes is estrogen replacement therapy, but the Women’s Health Initiative Study raised questions about the long-term safety of this treatment (Rossouw et al., 2002). Without knowing the mechanism of flushes, it is difficult to design targeted treatments that are alternatives to hormone replacement.
Morphologic studies of postmortem hypothalamic tissues from premenopausal and postmenopausal women have shown hypertrophy of a subpopulation of neurons in the infundibular (arcuate) nucleus of the postmenopausal group (Rance et al., 1990; Sheehan and Kovács, 1966). These neurons express estrogen receptor, kisspeptin and neurokinin B (NKB) and the cellular hypertrophy is accompanied by increased neurokinin B and kisspeptin gene expression (Fig. 1) (Rance et al., 1990; Rance and Young, 1991; Rometo et al., 2007). Dynorphin neurons are also enlarged in the infundibular nucleus of postmenopausal women, but the number of dynorphin mRNA-expressing neurons is reduced (Rometo and Rance, 2008). The hypertrophy and increased kisspeptin and NKB gene expression in postmenopausal women are secondary to estrogen withdrawal, because these changes are induced by ovariectomy (Fig. 2) and reversed by estrogen replacement of young cynomolgus monkeys (Abel et al., 1999; Rometo et al., 2007; Sandoval-Guzmán et al., 2004). Moreover, two of the peptides in the hypertrophied neurons, kisspeptin and neurokinin B, are essential for pubertal development and fertility in the human (Chan et al., 2011; Topaloglu et al., 2009, 2012).
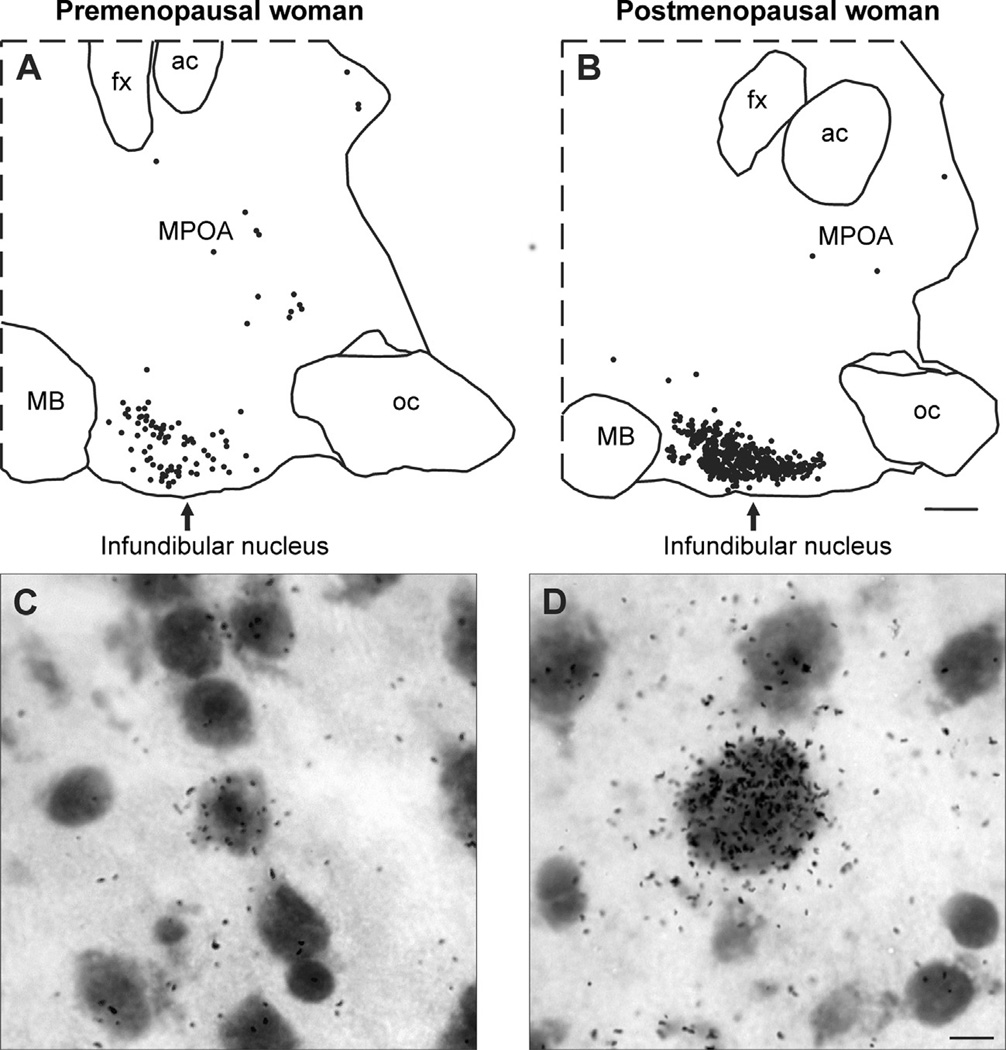
Fig. 1.
(A and B) Computer-assisted maps of kisspeptin mRNA-expressing neurons in sagittal hypothalamic sections from a premenopausal (A) and a postmenopausal (B) woman hybridized with cDNA probes complimentary to kisspeptin mRNA and visualized using autoradiography. Each symbol represents one labeled neuron. In the infundibular nucleus of postmenopausal women, there is a marked increase in the number of neurons expressing kisspeptin mRNA. Small numbers of kisspeptin neurons are also identified within the MPOA, but these did not change in postmenopausal women. (C and D) Photomicrographs of kisspeptin mRNA-expressing neurons in the infundibular nucleus of a premenopausal (C) or postmenopausal (D) woman. In postmenopausal women, kisspeptin neurons are hypertrophied and display increased numbers of audioradiographic grains, indicative of increased gene expression. Nearly identical changes can be seen in NKB neurons in the infundibular nucleus of postmenopausal women (Rance and Young, 1991). Scale Bar = 2 mm in B and applies to A and B. Scale bar = 10 µm in D and applies to C and D. ac, anterior commissure; fx, fornix; MB, mammillary body; MPOA, medial preoptic area; oc, optic chiasm Modified with permission from Rometo et al. (2007).
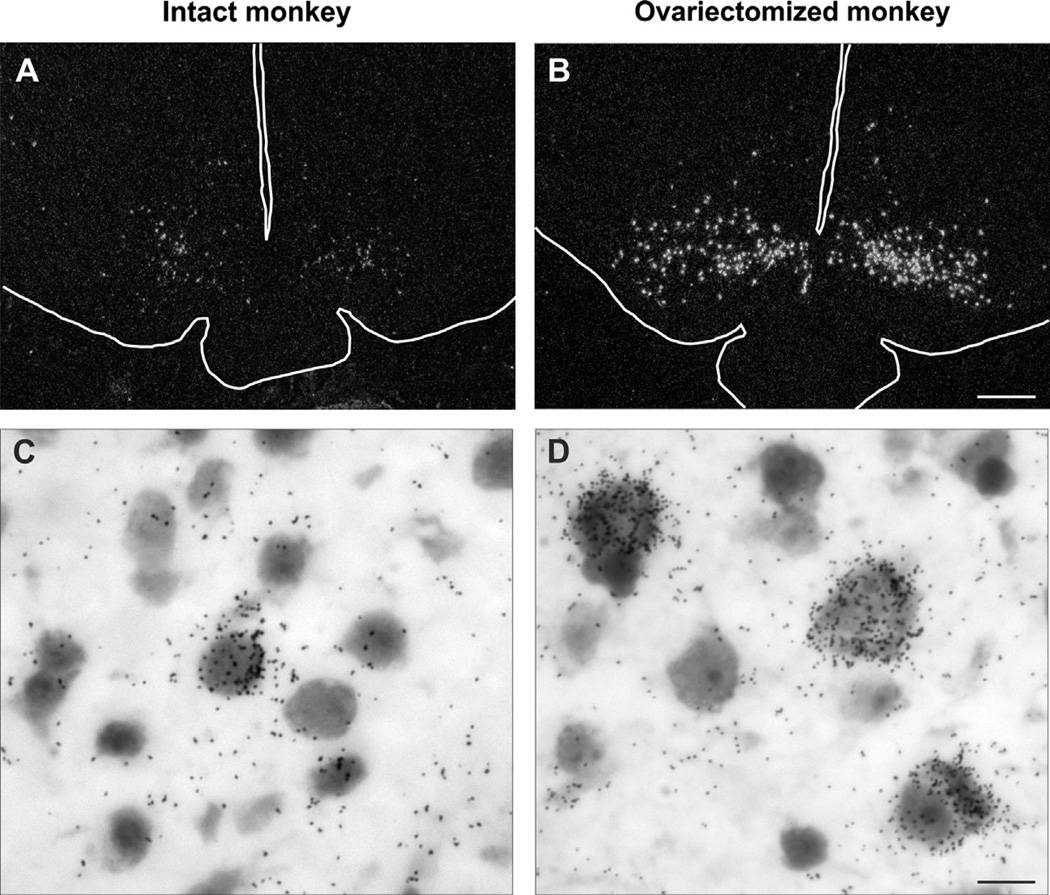
Fig. 2.
(A and B) Darkfield photomicrogaphs of coronal hypothalamic sections from an intact (A) or ovariectomized (B) cynomolgus monkey hybridized with cDNA probes complimentary to NKB mRNA (Tac3 in the monkey) and visualized using autoradiography. The outlines of the base of the brain, pituitary stalk and third ventricle have been drawn on these photomicrographs. Note the marked increase in the number of labeled neurons in arcuate nucleus of the ovariectomized monkey. (C and D) Brightfield photomicrogaphs of neurons expressing NKB mRNA visualized using in situ hybridization in the arcuate nucleus of an intact (C) or ovariectomized (D) cynomolgus monkey. The increase in cell size in the ovariectomized monkeys is accompanied by increased numbers of labeled cells and autoradiographic grains/neuron, indicative of a marked increase in gene expression in response to ovariectomy. These findings mimic the changes in NKB cell size and gene expression observed in the infundibular nucleus of postmenopausal women. Scale bar = 0.5 mm in B (applies to A and B), Scale bar = 25 µm in D (applies to C and D). Modified with permission from Sandoval-Guzmán et al. (2004).
Coexpression of kisspeptin, NKB and dynorphin (the KNDy peptides) have been identified in the arcuate nucleus of the mouse, rat, goat, monkey and sheep (Burke et al., 2006; Goodman et al., 2007; Navarro et al., 2009; Ramaswamy et al., 2010; Rance and Bruce, 1994; Rometo et al., 2007; Smith, 2009; Smith et al., 2005a; Wakabayashi et al., 2010). The arcuate nucleus is the homologue of the infundibular nucleus in the human. Complete coexpression of kisspeptin, NKB and dynorphin is not always found (Hrabovszky et al., 2012; Navarro et al., 2011), but this issue is complicated by limitations in the sensitivity of detection (Ruka et al., 2013) and differential expression depending on developmental stage (Gill et al., 2012) or physiological state of the animal (Foradori et al., 2005; Navarro et al., 2011; Rance and Bruce, 1994; Smith et al., 2005b). Nevertheless, coexpression of kisspeptin, NKB and ERα has only been identified in arcuate (infundibular) neurons (Lehman et al., 2010) and this term clearly refers to a distinct functional sub-population of estrogen-responsive neurons within the arcuate nucleus. Because KNDy neurons have been identified in multiple experimental animals, information on the role of these neurons in LH secretion is accumulating at a rapid pace (Kinsey-Jones et al., 2012; Lehman et al., 2010; Mittelman-Smith et al., 2012b; Rance et al., 2010; Wakabayashi et al., 2010).
We hypothesized that KNDy neurons could contribute to the generation of flushes (Rance and Young, 1991) because estrogen withdrawal leads to such remarkable changes in cell size and gene expression in the hypothalamus of postmenopausal women (Abel et al., 1999; Rance and Young, 1991; Rometo et al., 2007; Sandoval-Guzmán et al., 2004). There was, however virtually no information on the role of KNDy neurons in thermoregulation in any species. This state of affairs was reminiscent of our early studies, where there was no data on the relationship of NKB neurons to the hypothalamic circuitry regulating reproduction and pulsatile LH secretion (Rance and Bruce, 1994; Rance and Young, 1991). To address this issue, we established methods in our laboratory to examine how estrogen modulates thermoregulation and related neural pathways in the rat (Dacks et al., 2011b; Dacks and Rance, 2010; Williams et al., 2010). We were then able to conduct studies to determine if KNDy neurons and/or NKB signaling played a role in thermoregulation. This review summarizes the physiology of flushes, estrogen control of heat-defense mechanisms and our recent studies implicating KNDy neurons and neurokinin 3 receptor (NK3R, the primary NKB receptor) signaling in the estrogen modulation of body temperature. For alternative theories of flushes, please see other reviews (Archer et al., 2011; Freedman, 2001; Stearns et al., 2002).
2. Physiology of hot flushes
Successful reproduction requires the integration of many hypothalamic control systems, including thermoregulation, to optimize homeostasis. In women, core temperature varies by 0.3–0.5 °C across the menstrual cycle (Baker and Driver, 2007; Stephenson and Kolka, 1993). Core temperature is higher in the mid-luteal phase due to elevated progesterone secretion from the corpus luteum (Charkoudian and Johnson, 2000; Kolka and Stephenson, 1997). It has been hypothesized that progesterone influence on core temperature facilitates implantation of the blastocyst (Charkoudian and Johnson, 2000; Stephenson and Kolka, 1993). There is also modulation of body temperature during pregnancy, which may protect the developing fetus from hyperthermia (Charkoudian and Johnson, 2000; Eliason and Fewell, 1997a,b). Moreover, toward the end of pregnancy, exogenous pyrogens are less effective in producing fever (Martin et al., 1995, 1996). Whatever the evolutionary advantage, gonadal hormones have complex effects on thermoregulation in mammals. Hot flushes, in fact, are a dramatic example of the integration of the reproductive and thermoregulatory systems.
Hot flushes are characterized by a transient and intense sensation of heat accompanied by the activation of heat-loss effectors including skin vasodilatation, sweating, and cold-seeking behavior (Fig. 3) (Freedman, 2001; Kronenberg, 2010). In many women, the hallmark of a flush is the reddening of the face and upper torso due to cutaneous vasodilatation. The increased peripheral blood flow may be accompanied by an increased heart rate, lower blood pressure and reduced blood flow to the brain (Kronenberg, 2010; Lucas et al., 2013). Vasodilatation causes heat loss from the body by increasing the skin temperature, and thus can be objectively monitored by a temperature probe on the surface of the skin (Meldrum et al., 1979; Molnar, 1975). Hot flushes can also be assessed by increased skin conductance due to sweating (Fig. 3)(Freedman, 2001). However, there may be limited concordance between self-reports of hot flushes and skin conductance, and registering of events other than flushes, especially in an ambulatory setting (Carpenter et al., 2012; Mann and Hunter, 2011). Thus, further efforts to improve the objective measurements of flushes are needed.
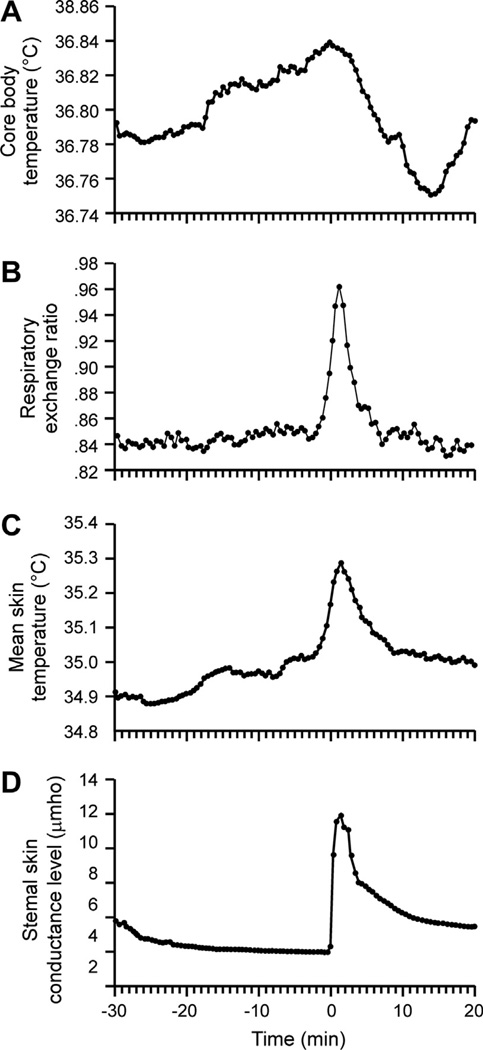
Fig. 3.
(A) Average core body temperature, (B) respiratory exchange ratio (C) skin temperature and (D) sternal skin conductance recorded from 29 hot flushes in 14 postmenopausal women. Time 0 is the onset of the rise in sternal skin conductance. Vasodilatation and sweating (reflected in C and D, respectively) are physiological heat-dissipation effectors that are activated during hot flushes. A small increase in core temperature is seen before the flush, followed by a larger drop due to the activation of heat-dissipation effectors (A). Adapted with permission from Freedman (1998).
Hot flushes occur in response to ovariectomy in young women and are effectively treated by estrogen replacement (Santoro, 2008). It is not the absolute level of estrogens, but the rate of estrogen withdrawal that determines the onset of flushes (Kronenberg, 2010; Santoro, 2008). As an example, the abrupt hormone withdrawal after oophorectomy (‘‘surgical menopause’’) is associated with increased severity and frequency of hot flushes (Casper and Yen, 1985; Gelfand, 1999; Özdemir et al., 2009). Hot flushes do not occur in situations where estrogen is chronically absent from birth, unless exogenous estrogens are administered and then subsequently withdrawn (Kronenberg, 1994). Withdrawal of gonadal steroids induces flushes in a variety of patients: women treated with tamoxifen for breast cancer, women with premature ovarian failure, aging men with diminished testosterone, and men with prostate carcinoma undergoing androgen ablation therapy (Santoro, 2008). Flushes often begin during the perimenopausal period when there are wide fluctuations in serum estrogen (Burger et al., 2002). Eventually, most (but not all) postmenopausal women will acclimate to the low estrogen level, and the flushes will disappear (Kronenberg, 1990). However, even in these women, hot flushes may reoccur if estrogens are administered and then withdrawn (Jensen and Christiansen, 1983).
Hot flushes can be triggered by a variety of stimuli including thermal (increased ambient temperature or hot beverages), chemical (caffeine or spicy food) or other (stress or anxiety) (Archer et al., 2011; Freedman, 2001). Placing women in a cold environment inhibits the occurrence of hot flushes (Kronenberg and Barnard, 1992). Conversely, raising the ambient temperature increases the frequency of flushes in symptomatic postmenopausal women, and this feature has been used to stimulate flushes in a laboratory setting (Freedman, 2001). When placed in a warm environment, symptomatic postmenopausal women exhibited lower core temperatures at the onset of sweating, compared to asymptomatic women (Freedman and Krell, 1999). These data suggest that hot flushes may be triggered by increased temperature sensitivity of the thermoregulatory pathways that control heat dissipation effectors.
Despite the sensation of intense heat, the core temperature preceding the flush is normal. Using an ingestible thermometer (radio-telemetry pill), a very small increase in core temperature was detected before the onset of 65% to 76% of flushes (Fig. 3) (Freedman, 1998, 2001). Although this increase in core temperature is still well within the range of normal body temperature fluctuations, this small change could facilitate a flush in postmenopausal women with a lower core temperature threshold for the induction of heat dissipation (Freedman and Krell, 1999). After a hot flush is triggered, the core temperature drops (Fig. 3) (Freedman, 2001; Kronenberg, 1990; Molnar, 1975), as expected with the activation of heat dissipation effectors.
Compelling evidence that flushes involve abnormal hypothalamic function comes from studies that measure serum LH concurrently with flushes (Fig. 4). Blood sampling at frequent intervals have revealed synchronization of LH pulses and hot flushes (Casper et al., 1979; Tataryn et al., 1979). There are also changes in serum levels of catecholamine metabolites and calcitonin gene related peptide associated with flushing (Chen et al., 1993; Chen and Shiraki, 2003; Freedman, 1998; Wyon et al., 2000), but these substances have not been shown to exhibit the precise timing associated with LH pulses and flushes.
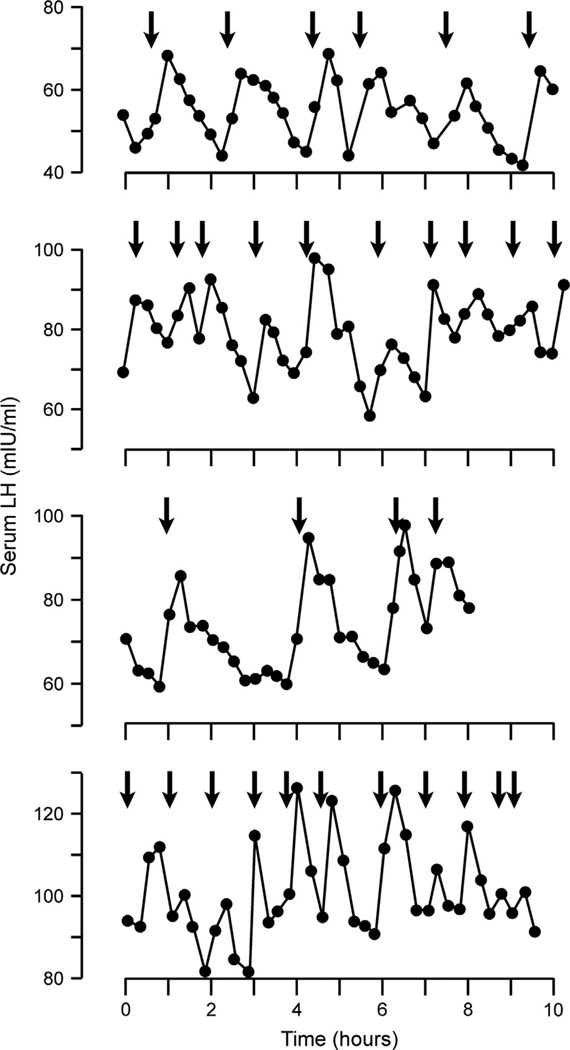
Fig. 4.
Pattern of pulsatile LH release and associated flush episodes in four postmenopausal women. The arrows indicate flush onset. Each graph illustrates a separate 8- to 10-h study in which blood samples were obtained at 15-min intervals. The close timing of LH pulses and hot flushes provides evidence that the mechanism of flushes is linked with the hypothalamic control of pulsatile GnRH secretion. Reproduced with permission from Casper et al. (1979).
Although pulses of LH accompany hot flushes, this relationship is not causative. Estrogen administration and withdrawal causes hot flushes in women who do not have substantial LH because their pituitary gland has been surgically removed (Casper and Yen, 1985) or because of LH suppression using gonadotropin-releasing hormone (GnRH) analogs (Casper and Yen, 1981; DeFazio et al., 1983). Similarly, GnRH does not cause the flushes, as estrogen withdrawal produces hot flushes in women who lack hypothalamic GnRH neurons due to Kallman’s syndrome (Gambone et al., 1984; Schwanzel-Fukuda et al., 1989). Because it is well established that LH pulses are due to pulses of GnRH (Clarke and Cummins, 1982), the close timing of LH pulses and flushes provides a clue that the mechanism of flushes is tied to the hypothalamic control of pulsatile GnRH secretion. Emerging evidence that KNDy neurons are an important part of the GnRH pulse generator (Lehman et al., 2010; Rance et al., 2010; Wakabayashi et al., 2010) strongly supports the hypothesis that they could also be involved in the generation of flushes.
3. Changes in KNDy neuron morphology and neuropeptide gene expression in the hypothalamus of postmenopausal women
The postmenopausal state is characterized by virtually absent ovarian follicles and increased LH secretion due to withdrawal of ovarian estrogen (Hansen et al., 2008). With increasing age after the menopause, LH secretion declines (Hall, 2007; Rossmanith et al., 1991), but many neuroendocrine functions remain intact. For example, if estrogen is administered in an appropriate regimen, positive feedback effects that stimulate LH secretion can still be demonstrated after the last menstrual cycle (Shaw et al., 2011). Furthermore, negative feedback effects of estrogen on LH secretion are not diminished with age (Gill et al., 2002a,b). GnRH mRNA is increased in the medial basal hypothalamus of postmenopausal women (Rance and Uswandi, 1996), and menopause in monkeys is accompanied by increased GnRH secretion into portal blood (Gore et al., 2004). Therefore, the rise in serum LH in postmenopausal women is likely mediated by increased GnRH secretion from the hypothalamus (Rance, 2009). Interestingly, in postmenopausal women, estrogen negative feedback on LH secretion is accompanied by decreased metabolic activity in the medial basal hypothalamus, the site of KNDy and GnRH neurons (Ottowitz et al., 2008).
More than 40 years ago, Sheehan and Kovacs described neuronal hypertrophy in the hypothalamus of postmenopausal women (Sheehan and Kovács, 1966). The neuronal hypertrophy was characterized by enlargement of the somata, increased size of nuclei and nucleoli and increased Nissl substance (rough endoplasmic reticulum). The hypertrophied neurons were located in the infundibular nucleus, a region known as the arcuate nucleus in other species. Classic studies in the rhesus monkey showed that the arcuate nucleus/medial basal hypothalamus is essential for the control of reproduction in the primate and the pulsatile release of GnRH (Knobil, 1980, 1990; Krey et al., 1975). Our initial studies used computer microscopy to confirm that neurons in the infundibular nucleus were significantly enlarged in postmenopausal women, and using in situ hybridization, we determined that the hypertrophied neurons expressed estrogen receptor alpha (ERa) mRNA, but not GnRH mRNA (Rance et al., 1990). Subsequent studies showed that the hypertrophied neurons expressed NKB mRNA and that menopause is associated with a dramatic increase in NKB gene expression (Rance and Young, 1991). Stereological analysis revealed no neuronal cell loss in the infundibular nucleus of post-menopausal women (Abel and Rance, 2000). Thus, the increase in NKB gene expression and the neuronal hypertrophy in the hypothalamus of postmenopausal women is not a compensatory response to degeneration of neurons.
The striking increase in NKB gene expression in postmenopausal women provided the impetus for more than 20 years of research into the role of NKB in the regulation of LH secretion (Rance et al., 2010). The significance of this line of inquiry became apparent with the discovery that individuals with mutations in the genes encoding NKB or its receptor (NK3R) are infertile and do not transition through puberty (Gianetti et al., 2010; Topaloglu et al., 2009; Young et al., 2010). The hypertrophied neurons in the infundibular nucleus of postmenopausal women also express kisspeptin (Hrabovszky et al., 2010, 2011; Rometo et al., 2007), another essential peptide for puberty and reproduction (de Roux et al., 2003; Seminara et al., 2003; Topaloglu et al., 2012) and similar to NKB, kisspeptin mRNA is increased in postmenopausal women (Fig. 1)(Rometo et al., 2007). In addition, the gene encoding substance P and neurokinin A (preprotachykinin A , TAC1) is expressed in the hypertrophied neurons and accompanied by increased preprotachykinin A mRNA (Rance and Young, 1991). Dynorphin neurons in the infundibular nucleus of postmenopausal women also exhibit hypertrophy, but the number of neurons expressing dynorphin mRNA is markedly reduced, compared to premenopausal women (Rometo and Rance, 2008). Although neurons expressing NKB mRNA are located throughout the hypothalamus (Chawla et al., 1997) kisspeptin neurons in the human are located predominantly in the infundibular nucleus (Hrabovszky et al., 2010; Rometo et al., 2007), and this is the only site where neurons express both NKB and kisspeptin. The marked changes in KNDy neurons in the infundibular nucleus of postmenopausal women, combined with essential role of kisspeptin and NKB for human reproduction, clearly indicates that these neurons are key components of the reproductive axis in the human.
Ovariectomy produces neuronal hypertrophy and increased NKB and kisspeptin gene expression in the arcuate nucleus of young monkeys, and these findings are identical to that seen in postmenopausal women (Fig. 2) (Alçin et al., 2013; Rometo et al., 2007; Sandoval-Guzmán et al., 2004). These data indicate that the neuronal hypertrophy and increased NKB and kisspeptin gene expression in the hypothalamus of postmenopausal women are due to estrogen withdrawal. Estrogen has been shown to suppress NKB and kisspeptin gene expression in the arcuate nucleus of ovariectomized monkeys, sheep, goats, rats, and mice (Abel et al., 1999; Alçin et al., 2013; Dellovade and Merchenthaler, 2004; Pillon et al., 2003; Rance and Bruce, 1994; Rometo et al., 2007; Smith et al., 2007). In parallel to the findings in postmenopausal women, ovariectomy decreased dynorphin gene expression in the arcuate nucleus of the ewe (Foradori et al., 2005), but ovariectomized mice exhibited increased dynorphin mRNA, compared to estrogen treated mice (Navarro et al., 2009). Finally, neurons expressing preprotachykinin A mRNA have been identified in the arcuate nucleus of the rat, but these neurons did not exhibit changes in gene expression in response to ovariectomy (Rance and Bruce, 1994). Although beyond the scope of this review, there is substantial evidence that KNDy neurons are part of the circuitry mediating estrogen negative feedback on pulsatile LH secretion, via modulation of the activity of GnRH neurons (Lehman et al., 2010; Mittelman-Smith et al., 2012b; Ohkura et al., 2009; Popa et al., 2008; Rance, 2009; Rance et al., 2010)] (Fig. 5).
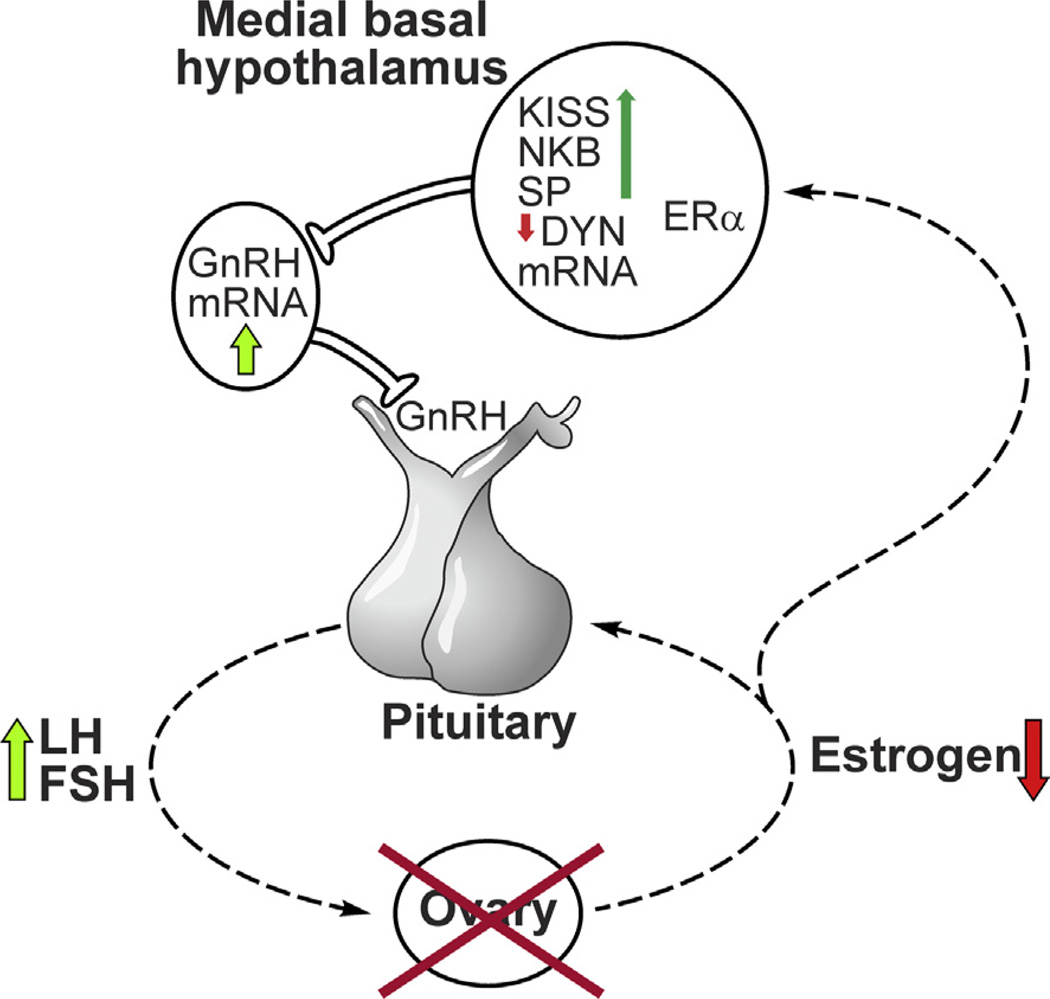
Fig. 5.
Schematic diagram showing the relationship between KNDy neurons and the neuroendocrine circuits controlling LH secretion in postmenopausal women. Degeneration of ovarian follicles results in loss of estrogen secretion (Hansen et al., 2008). Removal of estrogen negative feedback increases LH secretion from the anterior pituitary gland into the systemic circulation. In the infundibular nucleus, there is hypertrophy of a subpopulation of neurons expressing ERα, kisspeptin, NKB and substance P mRNA accompanied by increased kisspeptin, NKB and substance P gene expression (Rance and Young, 1991; Rometo et al., 2007; Rometo and Rance, 2008; Sandoval-Guzmán et al., 2004). Neurons expressing dynorphin mRNA also exhibit neuronal hypertrophy, but the number of neurons expressing dynorphin gene transcripts is reduced (Rometo and Rance, 2008). Dual-labeled NKB/kisspeptin fibers are closely apposed to GnRH cell bodies and processes in the human medial basal hypothalamus (Hrabovszky et al., 2011). GnRH mRNA is increased in a separate subpopulation of neurons in the medial basal hypothalamus of postmenopausal women (Rance and Uswandi, 1996). Reproduced with permission from Rance (2009).
4. CNS pathways controlling heat dissipation effectors
Because hot flushes are characterized by the activation of heat-dissipation effectors, understanding the basic biology of heat defense is a critical requirement for elucidating the etiology of hot flushes. Humans and other mammals tightly regulate their internal temperature (Romanovsky, 2007a,b). The regulated level of core temperature varies depending on the time of day, hormone balance, immune system activation, and numerous other factors (Refinetti and Menaker, 1992). Heat-defense mechanisms are critically important because an increase in brain temperatures of only a few degrees Celsius can disrupt cellular function (Nakamura and Morrison, 2010).
4.1. Thermosensors
Thermoregulatory systems must defend core temperature against a wide range of ambient temperatures (Kanosue et al., 2010; Yang and Gordon, 1996). The ambient temperature strongly affects skin temperature, which is monitored by primary sensory nerve endings. Thermosensitive elements in these cutaneous nerve endings include at least some transient receptor potential (TRP) channels, particularly those TRP channels that are highly sensitive to temperature (thermoTRP). Different thermoTRP channels respond to different ranges of skin temperature with different sensitivity (Romanovsky et al., 2009). Peripheral temperature information reaches the brain through two pathways. The spinothalamic pathway, critical for temperature perception, relays information from the dorsal horn of the spinal cord to the basal ventromedial thalamus and then to insular neocortex (Craig, 2002, 2003). In the second pathway, information critical for autonomic thermoregulation is relayed from the dorsal horn of the spinal cord to the lateral parabrachial nucleus of the brainstem and, from there, to the median preoptic nucleus (MnPO) of the hypothalamus (Nakamura and Morrison, 2008, 2010).
Core body temperature is monitored by thermoreceptors in deep tissues of the body, including the brain (Romanovsky, 2007a). Unlike cutaneous sensors, neuronal thermosensitivity is unlikely to be mediated by thermoTRP channels (Romanovsky et al., 2009). Thermosensitive neurons are located throughout the brain, including the cerebral cortex, but most of them are not involved in thermoeffector pathways and do not drive thermoeffector responses (Barker and Carpenter, 1970). Thermosensitive neurons that do participate in physiological thermoregulation are predominantly warm-sensitive cells located mostly in the preoptic hypothalamus (Boulant, 2000; Nakayama et al., 1961). Activation of warm-sensitive neurons by heating of the preoptic hypothalamus results in skin vasodilatation and evaporative cooling (Ishikawa et al., 1984; McAllen, 2004), indicating that temperature signals from these neurons contribute to driving heat-dissipation effectors.
Thermal and nonthermal inputs converge on thermosensitive neurons, providing a basis for coordination between thermoregulatory and other responses (Werner, 2010). In addition to sensing brain temperature, warm-sensitive neurons receive inputs from peripheral thermosensors (Boulant and Hardy, 1974). Thermosensitive neurons also receive information related to blood pressure, osmolarity, pyrogens, glucose concentration, and gonadal hormones, including estrogen (Boulant, 2000; Silva and Boulant, 1986). For example, evaporative cooling is a critical heat-loss mechanism, but it can be detrimental for the survival of animals that are already dehydrated, so it is inhibited by dehydration (Nagashima, 2006). Another example is that animals in shock (with low blood pressure) do not increase cutaneous blood flow to defend their body temperature against heat; instead, they use cold-seeking behavior (Romanovsky et al., 1996). Thus, convergence of information on warm-sensitive neurons provides a mechanism to modulate the activation of thermoregulatory effectors, based on the physiological state of the animal.
4.2. The thermoneutral zone and thermoregulatory effectors
The thermoneutral zone is an important factor to consider for the interpretation of each experiment. The thermoneutral zone is defined as the range of ambient temperatures in which the physiological control of body temperature is achieved only by control of sensible (dry) heat loss (Commission for Thermal Physiology, 2001). Within the thermoneutral zone, core temperature is regulated by skin blood vessels fluctuating between constriction and dilatation to modify heat exchange with the environment (Fig. 6) (Romanovsky et al., 2002). The ambient temperature at which an experiment is performed greatly influences which effectors are recruited (Romanovsky et al., 2002). For example, rats treated with antagonists of the TRP vanilloid-1 channel (Steiner et al., 2007) or with pyrogens (Székely and Szelényi, 1979; Szélenyi et al., 1992) use tail skin vasoconstriction alone to mount a rise in deep body temperature in a supraneutral (warm) environment, but use thermogenesis alone to achieve the same body temperature rise in a subneutral (cool) environment. As a result, knowing the relationship of the ambient temperature to the thermoneutral zone is important for interpretation of thermoregulatory studies. In addition, the effective ambient temperature depends on a number of factors such as humidity, airflow, bedding and cage type (Gordon, 2004; Romanovsky et al., 2002). Therefore, the thermoneutral zone for the same animal varies widely, depending on the experimental setup (Romanovsky et al., 2002).
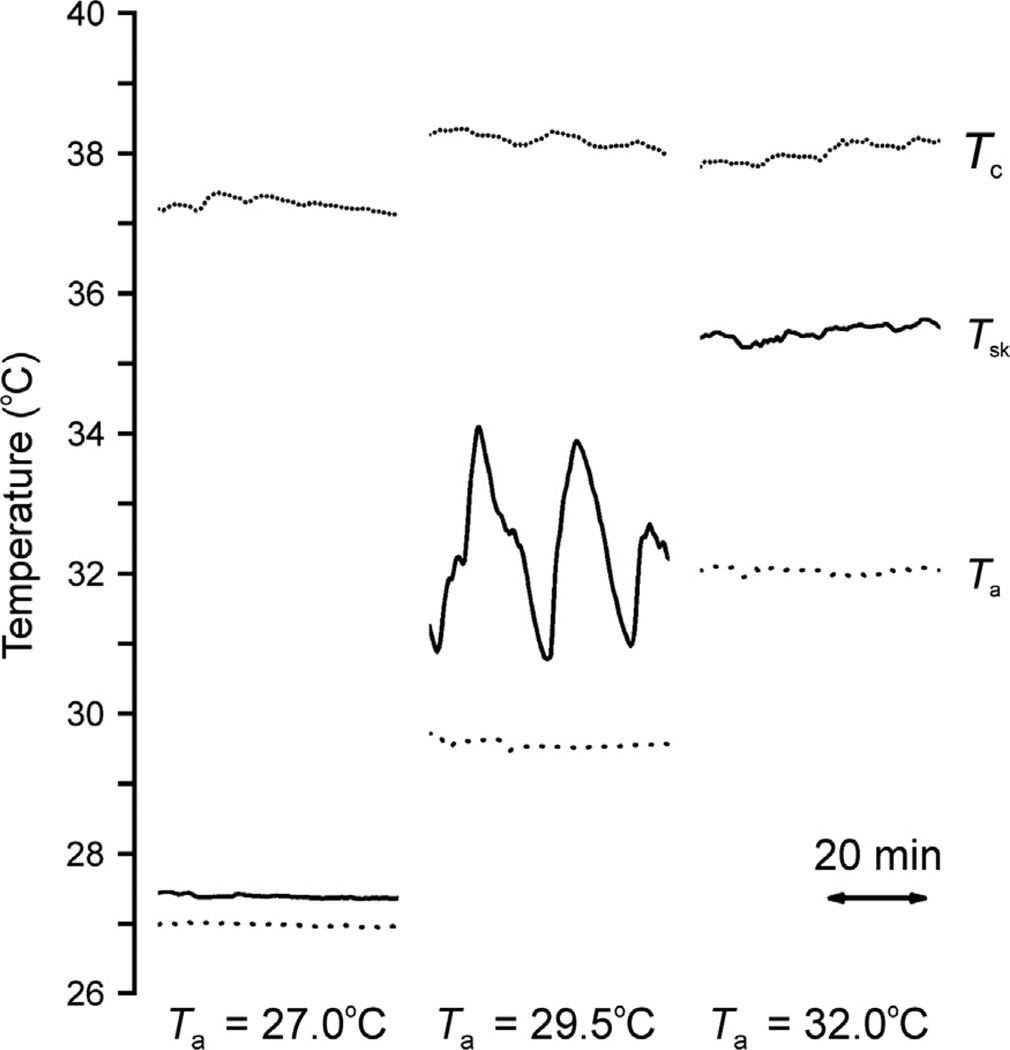
Fig. 6.
Typical records of core temperature (Tc, dotted line), tail skin temperature (Tsk, solid line) and ambient temperature (Ta dashed line) from three different Zucker lean rats at ambient temperatures of 27 °C (left), 29.5 °C (middle), or 32 °C (right). Note the large-amplitude fluctuations in tail skin temperature that occur at 29.5 °C, characteristic of tail skin vasomotion within the thermoneutral zone. Reproduced with permission from Romanovsky et al. (2002).
Diverse autonomic and behavioral effectors involved in body temperature regulation are controlled through independent neural pathways (McAllen et al., 2009; Romanovsky, 2007b; Tanaka et al., 2007). Below the thermoneutral zone, autonomic thermoeffectors are activated to minimize heat loss through skin vasoconstriction and to generate heat through non-shivering thermogenesis in brown adipose tissue (more important in rodents) or shivering thermogenesis (more important in humans) (Romanovsky, 2007a). Subneutral temperatures also increase food intake, blood pressure and heart rate (Overton, 2010). Above the thermoneutral zone, all mammals exhibit cutaneous vasodilatation, but they also use other autonomic and behavioral thermoeffectors to dissipate heat by evaporative cooling. The methods for evaporative cooling vary between species and include sweating (humans), panting (dogs) and saliva-spreading (rats) (Gordon, 1990; Romanovsky, 2007a).
4.3. Hypothalamic and downstream neural pathways for thermoregulation
Autonomic thermoeffectors are controlled by neural pathways passing through the preoptic anterior hypothalamus, specifically, through the MnPO and the medial preoptic area (Boulant, 2000; Romanovsky et al., 2009). Warm-sensitive neurons located in the medial preoptic area project to the rostral raphe pallidus in the medulla, either directly or through the dorsomedial hypothalamus (Morrison and Nakamura, 2011; Tanaka et al., 2009; Yoshida et al., 2009). The rostral raphe pallidus contains premotor nuclei that project to the intermediolateral cell column of the spinal cord and then to the sympathetic ganglia (Nagashima, 2006; Nagashima et al., 2000). The rostral raphe pallidus contains neurons that stimulate sympathetic vasoconstriction and brown adipose tissue thermogenesis (Nakamura and Morrison, 2008; Tanaka et al., 2009). The preoptic warm-sensitive neurons are GABAergic (Eberwine and Bartfai, 2011), and activation of these neurons inhibits the rostral raphe pallidus, resulting in cutaneous vasodilatation.
The MnPO has recently been shown to be an important part of the thermoregulatory pathways (Nakamura and Morrison, 2010; Saper et al., 2012). Signals from cutaneous warm-sensitive thermoreceptors (presumably, thermoTRP channels) reach the MnPO via glutamatergic neurons originating in the lateral parabrachial nucleus (Nakamura and Morrison, 2010). The MnPO may influence skin vasomotion by projections to other preoptic nuclei, the rostral periaqueductal gray (Yoshida et al., 2005), or the raphe pallidus in the medulla, the site of sympathetic premotor neurons (Tanaka et al., 2009; Yoshida et al., 2005, 2009). Excitation of the heat-defense pathway by skin warming inhibits the activity of sympathetic vasoconstrictor neurons, resulting in tail skin vasodilatation (Nakamura and Morrison, 2010). Conversely, blocking transmission through the MnPO blocks the stimulatory effects of skin warming on cutaneous vasodilatation (Nakamura and Morrison, 2008, 2010; Romanovsky et al., 2009). Finally, lesions that include the MnPO result in hyperthermia, supporting a role for this nucleus in the control of heat-defense effectors (Almeida et al., 2006b; Romanovsky et al., 2003; Yoshida et al., 2009).
Cold-seeking behavior is a prominent component of the menopausal flush in women, but the neural control of behavioral thermoregulation is poorly understood. Furthermore, studies of the pathways involved in behavioral thermoregulation are scarce, even in laboratory rats. Unlike autonomic thermoregulation, most thermoregulatory behaviors, including cold seeking, are not impaired by lesions of the medial preoptic area (Nagashima et al., 2000): Pathways for behavioral thermoregulation do not travel through this part of the hypothalamus (Nakamura and Morrison, 2008; Romanovsky, 2007b). A notable exception from this rule is the relaxed postural extension that occurs in rats in response to extended heat exposure; this behavior is triggered by the preoptic temperature and involves the medial preoptic area (Roberts and Martin, 1977). Some pathways for behavioral thermoregulation may involve other hypothalamic structures. For example, lesion studies suggest that neurons in the dorsomedial hypothalamus and neural fibers traveling though the paraventricular hypothalamic nucleus may play a role in cold-seeking behavior during endotoxin shock (Almeida et al., 2006b). In addition, the MnPO may be involved in operant cold-seeking behavior following systemic salt loading (Konishi et al., 2007).
5. Effects of estrogen on hypothalamic thermoregulation in the rat
5.1. Effects of estrogen on tail skin temperature
Because cutaneous vasodilatation is the hallmark of a hot flush, studies of this parameter have been used to develop animal models to study flushes. In rats, the tail is the primary heat exchange organ. This is due to its extensive vascularization, the presence of arterio-venous anastomoses, lack of fur, and high surface area to volume ratio (Gordon, 1990; Smith et al., 1998). Cutaneous vasodilatation of the tail can be monitored by measuring several different parameters, including tail skin temperature. A variety of instruments can be used to monitor tail skin temperature: infrared thermography (Almeida et al., 2006a; Garami et al., 2011; Ikeda et al., 2008), temperature-sensitive dyes (Romanovsky et al., 2002), thermocouples attached to the surface of the tail (Dacks and Rance, 2010; Hosono et al., 2001; Romanovsky et al., 2002) or implanted subcutaneously (Szélenyi et al., 1992), telemetry probes or Subque dataloggers mounted on the surface of the tail (Gordon et al., 2002; Williams et al., 2010), or surgically implanted telemetry probes with sensor leads tunneled to the base of the tail (Berendsen et al., 2001; Bowe et al., 2006; Cosmi et al., 2009).
For many years, tail skin vasodilatation secondary to acute opioid withdrawal was used as the primary model of hot flushes, based on the observation that heroin withdrawal leads to flushing, sweating and impaired sleep in humans (Casper and Yen, 1985; Simpkins et al., 1983). Administration of naloxone (an opioid receptor antagonist) causes cutaneous vasodilatation in guinea pigs (Romanovsky et al., 1994), and this effect is drastically exaggerated in morphine-dependent animals. In the hot flush model, naloxone treatment of morphine-dependent rats induces acute tail skin vasodilatation and elevated serum LH, and both of these responses are reduced by prior estrogen treatment (Deecher et al., 2007; Katovich et al., 1986; Merchenthaler et al., 1998; Simpkins et al., 1983). A limitation of the model is the occurrence of the severe adverse effects of morphine withdrawal, necessitating the use of ketamine sedation in many experimental protocols (Deecher et al., 2007; Merchenthaler et al., 1998).
An alternative animal model to study the mechanism of hot flushes relies on changes in tail skin temperature in response to estrogen withdrawal and replacement. Tail skin temperature is increased by ovariectomy (Berendsen et al., 2001; Kobayashi et al., 1995; Opas et al., 2004) and reduced by estrogen treatment of ovariectomized rats (Berendsen et al., 2001; Bowe et al., 2006; Dacks and Rance, 2010; Hosono et al., 2001; Kobayashi et al., 1995). The reduction in tail skin temperature by estrogens cannot be explained by changes in body weight or core temperature (Williams et al., 2010). Estrogens also reduce tail skin vasodilatation during the light phase (Fig. 7), but this effect is smaller in magnitude than during the dark phase, delayed until 7–8 days after treatment and dependent on the ambient temperature (Dacks and Rance, 2010; Hosono et al., 2001; Kobayashi et al., 1995; Williams et al., 2010). Perhaps for this reason, the reduction in tail skin temperature by estrogen during the light phase has not been reported in all studies, and even within a study, there may be variations in achieving statistical significance (Dacks and Rance, 2010; Williams et al., 2010). In contrast, the dark phase reduction in tail skin temperature by estrogen is large (>3 °C) and consistent (Fig. 7). The robust reduction of tail skin temperature by estrogen during the active (dark) phase increases the amplitude of the circadian rhythm (Berendsen et al., 2001; Girbig et al., 2012; Williams et al., 2010).
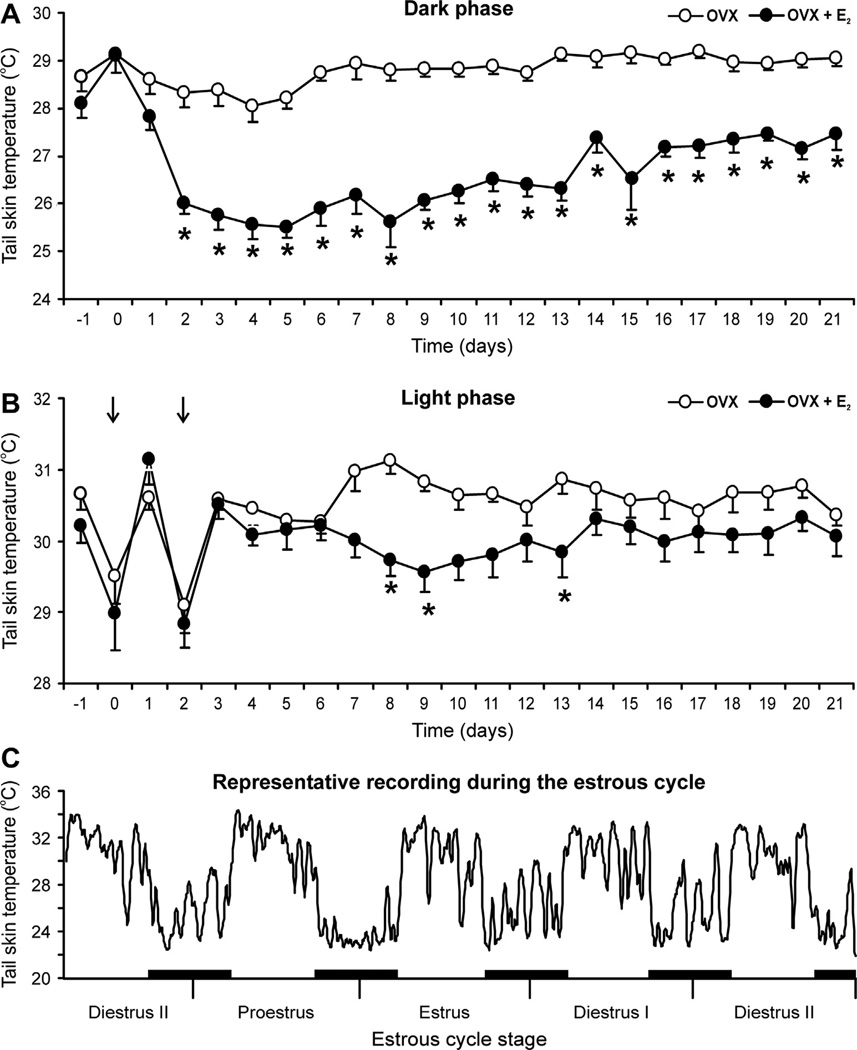
Fig. 7.
Effects of subcutaneous capsules of 17β-estradiol (E2) on average (±SEM) tail skin temperature of ovariectomized rats during the dark (A) or light (B) phase (n = 8–10 rats/group). In the dark phase (A), E2 treatment of ovariectomized (OVX) rats markedly reduces tail skin temperature from experimental day 2 through the end of the experiment. The reduction in tail skin temperature by E2 in the light phase (B) is delayed and smaller in magnitude than that seen in the dark phase. The acute drop in tail skin temperature during the light phase of days 0 and 2 (arrows) reflects sympathetic vasoconstriction at the time of capsule implantation. (C) Recording of tail skin temperature of an individual intact rat during the estrous cycle (moving average of 5 data points). This recording illustrates circadian rhythms, large fluctuations during most of the cycle and decreased tail skin temperature with lower fluctuations on proestrous night. The black bars represent the dark phase. * Significantly different in OVX + E2 rats compared to OVX. Reproduced with permission from Williams et al. (2010).
Tail skin temperature also exhibits an infradian rhythm over the rat estrous cycle. Using a Subcue Mini datalogger attached to the surface of tail, tail skin temperatures were recorded throughout the estrous cycle in freely-moving rats (Williams et al., 2010). These recordings showed circadian rhythms with spontaneous large-amplitude fluctuations over most of the cycle. Interestingly, on proestrous night (when endogenous estrogen levels are high), the average tail skin temperature was significantly decreased, with decreased variability (Fig. 7). These data provide evidence that the reduction in tail skin temperature by estrogen is part of the normal estrous cycle and physiologically relevant (Williams et al., 2010).
Whether or not a rat actually experiences hot flushes after estrogen withdrawal is unknown. A major limitation is that a rat cannot report the onset of flush, so experimental studies must rely on objective measurements such as changes in tail skin temperature. However, even in women, objective findings, such as detection of changes in sternal conductance (sweating) may be a sign of physiological event other than a flush (Mann and Hunter, 2011). Another limitation is that there are wide fluctuations of tail skin temperature in normal female rats (Fig. 7C) (Williams et al., 2010). Fluctuations in tail skin temperature are a defining feature of the thermoneutral zone (Fig. 6) (Romanovsky et al., 2002), which is shifted by estrogen (Fig. 8) (Dacks and Rance, 2010) but also by many other factors (Garami et al., 2011). Thus, caution should be used before interpreting fluctuations of tail skin temperature after ovariectomy to represent flushes (Puri et al., 2012). Although an increase in the average tail skin temperature after ovariectomy is not episodic like a flush, the ability of a compound to reduce cutaneous vasodilatation has been extensively used to screen drugs for reducing flushes in women (Alfinito et al., 2006; Berendsen and Kloosterboer, 2003; Berendsen et al., 2001; Bowe et al., 2006; Deecher et al., 2007; Girbig et al., 2012).
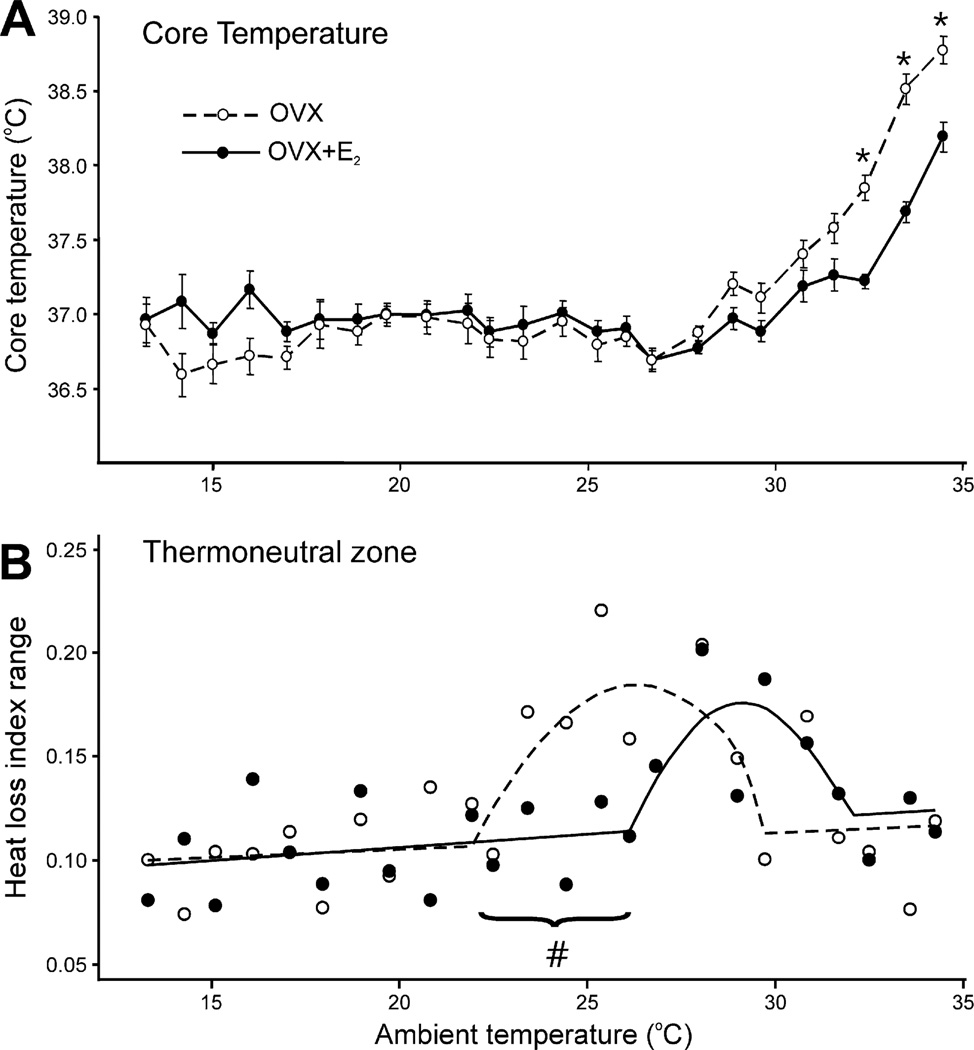
Fig. 8.
(A) Average (±SEM) core temperature of ovariectomized (OVX) and OVX + estradiol−17β (OVX + E2) rats at ambient temperatures from 13 °C to 34 °C (n = 11–12 rats/group). At most ambient temperatures, estradiol has no effect on core temperature. However, at ambient temperatures above 32.5 °C, the core temperature of OVX rats is significantly higher than OVX + E2 rats. These data show that estradiol-17β treatment of OVX rats improves core temperature regulation during heat exposure. (B) Heat loss index (HLI) range of OVX and OVX + E2 rats at ambient temperatures from 13 °C to 34 °C. HLI is a measure of tail skin vasomotion.An increase in HLI range indicates increased fluctuations in tail skin vasomotion, a characteristic finding within the thermoneutral zone (see Fig. 6 and Romanovsky et al., 2002). The straight line and parabola were fitted to these points using breakpoint analysis. The range of the parabola approximates the thermoneutral zone. These data show a shift in thermoneutral zone to lower ambient temperatures in OVX rats compared to OVX + E2 rats. The circles represent median values of 11–12 rats/group. * Significantly different OVX vs OVX + E2#Non-overlapping 95% confidence intervals comparing the first breakpoint for OVX vs. OVX + E2 rats. Adapted with permission from Dacks and Rance (2010).
5.2. Effects of estrogen on core temperature, the thermoneutral zone and hypothalamic Fos activation
Core temperature has been reported to be increased (Hosono et al., 1997a; Marrone et al., 1976), decreased (Wilkinson et al., 1980) or unaffected by estrogen treatment of ovariectomized rats (Hosono et al., 2001; Laudenslager et al., 1980; Williams et al., 2010). In guinea pigs, core temperature is increased by ovariectomy and reduced by treatment with estrogen or a ligand for a membrane-associated estrogen receptor (Roepke et al., 2010). The variable effects of estrogens on core temperature may be due to the animal model or methodological differences, such as the temperature probes, the presence of restraint stress, or the various hormone replacement regimens. Moreover, ambient temperature has a profound impact on the estrogen modulation of core temperature. At supraneutral ambient temperatures, ovariectomized rats exhibit higher core temperatures compared to those receiving estrogen (Fig. 8) (Baker et al., 1994; Dacks et al., 2011b; Dacks and Rance, 2010; Mittelman-Smith et al., 2012a). Thus, similar to postmenopausal women (Tankersley et al., 1992), estrogen replacement of ovariectomized rats improves the maintenance of core temperature during heat stress. The mechanism whereby estrogen improves body temperature defense against high ambient temperature is not understood (Dacks and Rance, 2010; Mittelman-Smith et al., 2012a).
In symptomatic postmenopausal women, exposure to a warm environment provokes hot flushes (Freedman, 2001; Freedman and Roehrs, 2006; Kronenberg and Barnard, 1992; Molnar, 1981). Moreover, postmenopausal women who have hot flushes have a lower core temperature threshold for sweating than women who do not experience hot flushes (Freedman and Krell, 1999). These data suggest that hot flushes may be due to increased sensitivity of heat-dissipation responses to temperature. Based on this hypothesis, we predicted that heat dissipation effectors would be activated at lower environmental temperatures in ovariectomized rats (compared to those receiving estrogen), resulting in a shift in the thermoneutral zone. To evaluate the thermoneutral zone, tail skin and core temperatures were measured in rats exposed to a wide range of ambient temperatures, using a method described by Romanovsky et al. (2002). Using this paradigm, ovariectomized rats without estrogen replacement showed a shift in the thermoneutral zone to lower ambient temperatures (Fig. 8) (Dacks and Rance, 2010). Remarkably, the ambient temperature threshold for skin vasodilatation was 4 °C lower in ovariectomized rats, compared to ovariectomized rats receiving estrogen (Dacks and Rance, 2010). Thus, in both humans and rats, an estrogen deficient state increases the sensitivity of the heat-defense pathway, leading to the activation of heat-loss effectors at lower environmental temperatures.
From the studies described above, it is clear that estrogen has pronounced effects on body temperature regulation, with suppression of vasodilatation in ovariectomized rats in parallel with the inhibition of flushes in the human. Based on this information, we reasoned that the rat would be a useful model for determining sites in the central nervous system for estrogen modulation of heat-dissipation effectors. To investigate potential sites, we used Fosimmunocytochemistry, a tool previously used to investigate the activation of brain areas in response to warm or cold stimuli (Bratincsák and Palkovits, 2004; Kiyohara et al., 1995; McKitrick, 2000; Yoshida et al., 2005). Based on our studies of the thermoneutral zone (Dacks and Rance, 2010), we exposed rats to mild changes in ambient temperature, to minimize the effects on core body temperature and reduce stress. Interestingly, the MnPO was the only area (out of 14) that exhibited increased numbers of Fos-immuno-reactive nuclei when the ambient temperature was raised to a supraneutral temperature of 31 °C (Dacks et al., 2011b). Moreover, at the ambient temperature of 24 °C, estrogen treatment of ovariectomized rats decreased the Fos expression in the MnPO, in parallel with the reduction in tail skin temperature. These data implicate the MnPO as a site where estrogen signaling may influence thermoregulatory heat-dissipation pathways.
6. Evidence that KNDy neurons modulate LH secretion and body temperature in the rat
6.1. KNDy neurons project to thermoregulatory structures (MnPO, medial preoptic area) and GnRH axons in the median eminence
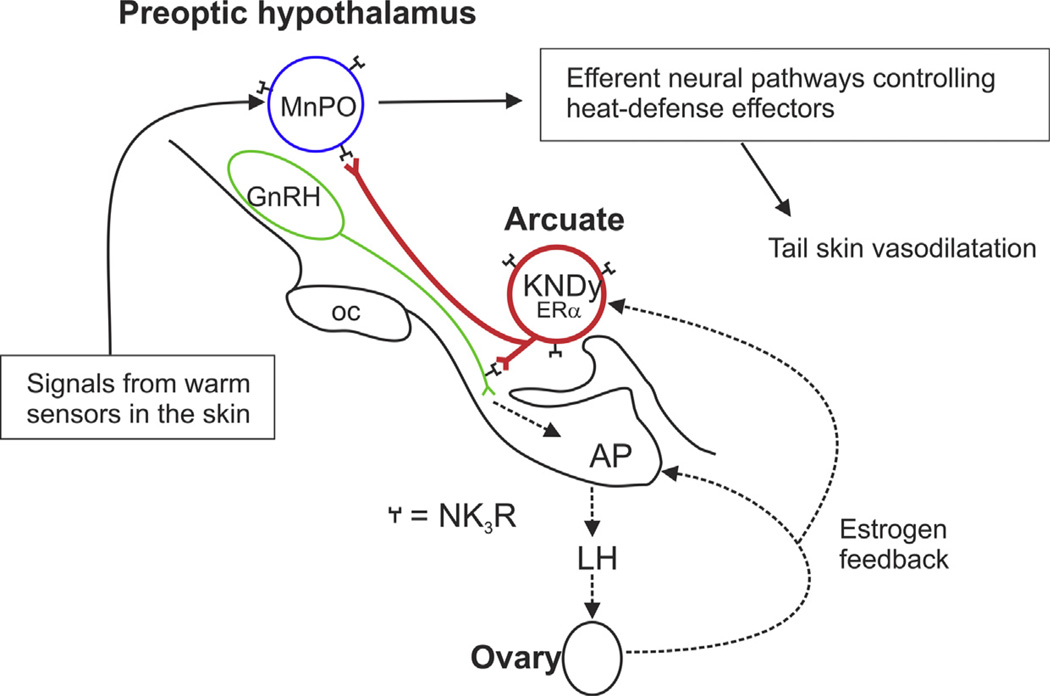
As discussed above, the close temporal relationship between LH pulses and hot flushes suggests a trigger for hot flushes that is related to pulsatile GnRH secretion from the hypothalamus. KNDy neurons may play a role in this pathway because estrogen strongly suppresses kisspeptin and neurokinin B gene expression, as well as LH pulses and flushes. For KNDy neurons to play a role in the generation of hot flushes, they should influence GnRH neurons and also connect, directly or indirectly, with the pathways that control heat-defense effectors. As explained above, such a connection should likely involve the MnPO. To explore the possibility of an anatomic connection between KNDy neurons and thermoregulatory pathways, we performed a series of studies using dual-label immunofluorescence, anterograde tract-tracing and monosodium glutamate ablation in the rat. Four projection pathways of KNDy neurons were demonstrated:
KNDy neurons branch extensively within the arcuate nucleus, projecting to other KNDy neurons and to the contralateral arcuate nucleus, forming a bilateral, interconnected network via the expression of NK3R (Burke et al., 2006; Krajewski et al., 2010).
KNDy neurons project to the median eminence (Burke et al., 2006; Krajewski et al., 2010) where they come in close proximity to GnRH terminals (Ciofi et al., 2006; Krajewski et al., 2005). Because KNDy neurons do not take up FlouroGold after systemic injections, they are unlikely to secrete peptides into the portal circulation to directly influence serum LH (Krajewski et al., 2005).
KNDy neurons project via a dense periventricular pathway to multiple hypothalamic areas, including preoptic structures. Projections from the periventricular pathway extend to the MnPO, medial preoptic area, dorsomedial nucleus, paraventricular nucleus, lateral hypothalamus, organum vasculosum of the lamina terminalis, anteroventral periventricular nucleus and septal nuclei (Burke et al., 2006; Krajewski et al., 2010).
KNDy neurons project via a ventral hypothalamic tract to the medial forebrain bundle and lateral hypothalamus (Krajewski et al., 2010).
Our prediction that KNDy neurons project to preoptic regions that control heat dissipation effectors was correct, as demonstrated by KNDy neuron projections to the MnPO and medial preoptic area (Krajewski et al., 2010; Yeo and Herbison, 2011). As discussed above, the MnPO is a site that is altered by both ambient temperature and estrogen (Dacks et al., 2011b) and MnPO neurons express NK3R mRNA and protein (Dacks et al., 2011a; Shughrue et al., 1996). Moreover, preliminary data using single-cell transcriptomics, suggests that the Tacr3 gene encoding NK3R is expressed by warm-sensitive GABAergic neurons in the medial preoptic area (Eberwine and Bartfai, 2011). Warm-sensitive neurons in the medial preoptic area have horizontally oriented dendrites that extend toward the periventricular region and the medial forebrain bundle (Griffin et al., 2001) both of which are projection pathways for arcuate KNDy neurons (Krajewski et al., 2010). Therefore, NKB signaling (from KNDy neurons) could influence heat-defense effectors through projections to NK3R-expressing MnPO neurons or NK3R-expressing warm-sensitive neurons in the medial preoptic area. A schematic diagram showing the anatomic relationship between KNDy neurons, GnRH neurons and the heat-defense pathway in the rat is shown in Fig. 9. For recent reviews of the neuroanatomy and function of KNDy neurons in other species, see Goodman and Lehman (2012) and Lehman et al. (2013).
Fig. 9.
Schematic diagram showing the relationship between KNDy neurons, GnRH neurons and the heat-defense pathway in the rat. KNDy neurons branch and project to GnRH terminals in the median eminence and preoptic structures that regulate body temperature (Krajewski et al., 2005, 2010; Nakamura and Morrison, 2010; Romanovsky et al., 2009; Tanaka et al., 2009; Yeo and Herbison, 2011; Yoshida et al., 2009). Secretion of GnRH into portal capillaries stimulates LH secretion from the anterior pituitary gland, which stimulates the secretion of estrogen (E2) from the ovaries. E2 negative feedback reduces serum LH and decreases NKB and kisspeptin mRNA in KNDy neurons (Rance and Bruce, 1994; Smith et al., 2005a). ERa, the isoform required for estrogen negative feedback (Dorling et al., 2003), is expressed in arcuate KNDy neurons (Burke et al., 2006) but not GnRH neurons (Hrabovszky et al., 2001). NK3R is expressed on arcuate KNDy neurons (Burke et al., 2006) and GnRH terminals in the median eminence (Krajewski et al., 2005). GnRH neurons express kisspeptin receptor mRNA (Han et al., 2005), but the location of the kisspeptin receptor protein on GnRH neurons has not been described. MnPO neurons express NK3R. The MnPO receives information from warm-sensitive, cutaneous thermoreceptors and project to CNS structures to modulate heat-dissipation effectors (Nakamura and Morrison, 2010; Yoshida et al., 2009). Not shown are KNDy neuron projections to the medial preoptic area, which contains warm-sensitive GABAergic neurons which may also express NK3R (Eberwine and Bartfai, 2011). Modified with permission from (Mittelman-Smith et al., 2012a).
6.2. Activation of NK3R in the MnPO lowers body temperature in the rat
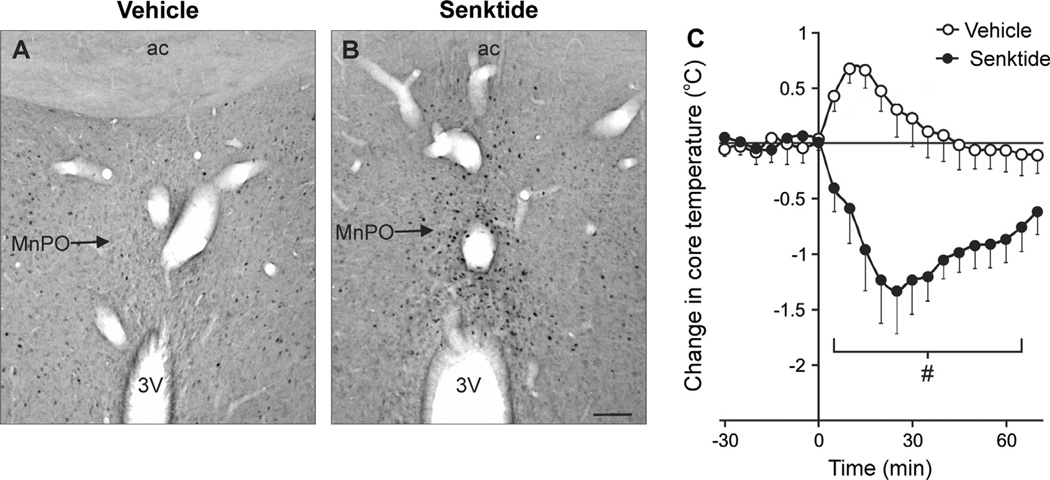
The above data provide an anatomical framework for the hypothesis that the effects of estrogen on thermoregulation occur via projections of KNDy neurons to NK3R-expressing neurons in the MnPO. However, no studies had linked central NK3R activity to temperature regulation. To determine if activation of NK3R in the MnPO would affect body temperature, we infused senktide, a selective NK3R agonist, directly into the MnPO. Small volumes (300 nl) were infused via chronically-implanted cannulas in freely-moving rats in an environmental chamber. Remarkably, microinfusion of senktide (90 pmol) into the MnPO reduced body temperature by about 1.5 °C (Fig. 10). In addition, senktide markedly increased the number of Fos-ir nuclei in the MnPO, with minimal effects on adjacent nuclei (Dacks et al., 2011a). The hypothermia induced by senktide is relevant to the study of hot flushes, because the activation of heat dissipation effectors in flushing women has been shown to reduce core temperature (Fig. 3).
Fig. 10.
(A and B) Photomicrographs of Fos expression in the MnPO, 90 min after focal microinfusion of either vehicle (A, aCSF) or senktide (B). Note the marked increase in Fos-immunoreactive nuclei in senktide-microinfused rats. (C) Core temperature before and after vehicle or senktide microinfusion into the MnPO of ovariectomized, estradiol-treated rats (ambient temperature of 21.5 °C, mean ± SEM, n = 6–8 microinfusions/group). Senktide microinfusion into the MnPO markedly decreased core temperature. Scale bar in B = 100 microns and applies to A and B. aCSF, artificial cerebrospinal fluid, 3V, Third ventricle; ac, anterior commissure; MnPO, median preoptic nucleus. # Significantly different, senktide compared with vehicle. Modified with permission from Dacks et al. (2011a).
Although senktide consistently reduced core temperature, the effects of senktide on tail skin temperature were complex. We have observed that even a relatively non-stressful interaction, such as changing a cage, produces vasoconstriction of the tail (Williams et al., 2010). Thus, it is not surprising that microinfusion of either vehicle or senktide in the MnPO resulted in a consistent drop in tail skin temperature at the subneutral ambient temperature of 21.5 °C (Dacks et al., 2011a). Similarly, in a recent study, administration of nine different drugs caused acute tail skin vasoconstriction, although a graph of the vehicle controls was not shown (Prelle et al., 2012). In our study, however, at the neutral ambient temperature of 29.0 °C, vasoconstriction occurred in vehicle-infused animals, but not in rats in which senktide was microinfused in the MnPO. Because vasodilatation in the rat is accomplished by inhibiting vasoconstriction (O’Leary et al., 1985), these data show a vasodilator effect of NK3R MnPO neuron activation in neutral ambient temperatures, that is suppressed in the subneutral environment.
Although we have strong evidence implicating NKB signaling in thermoregulation, these data do not exclude participation of other KNDy peptides, which are likely to be released from the same terminals. Microdialysis of dynorphin A1–17 (a κ-opioid agonist) into the preoptic area results in a dose-dependent hypothermia (Xin et al., 1997). Central injection of kisspeptin raises body temperature (Csabafi et al., 2012) and increases tail skin temperature (Kinsey-Jones et al., 2010) and local injections of kisspeptin into the skin produces vasoconstriction (Sawyer et al., 2011). Infusion of substance P into the vasculature causes flushing in humans (Schaffalitzkey de Muckadell et al., 1986) but this is likely due to the potent peripheral vasodilator effects of substance P (Charkoudian, 2010). GnRH neurons have also been implicated in tail skin vasomotion (Hosono et al., 1997b; Noguchi et al., 2008) but are not required for flushing in women (Gambone et al., 1984; Schwanzel-Fukuda et al., 1989). A recent study showed a GnRH antagonist to reduce flushes in menopausal women by 40% (van Gastel et al., 2012), but these studies lacked placebo controls. On the whole, much work remains to explore potential roles for kisspeptin, dynorphin, substance P or GnRH neurons in the estrogen modulation of body temperature.
6.3. Effects of KNDy neuron ablation on the estrogen modulation of LH secretion and thermoregulation
To investigate the role of KNDy neurons in the regulation of body temperature and LH secretion, we developed a method to selectively ablate these neurons. KNDy neurons were targeted based on their expression of NK3R (Amstalden et al., 2009; Burke et al., 2006; Navarro et al., 2009). The arcuate nucleus is densely packed with numerous subpopulations of neurons regulating a variety of physiological processes (Chronwall, 1985; Meister et al., 1989). The vast majority of arcuate neurons do not express NK3R protein (Burke et al., 2006) or NK3R mRNA (Shughrue et al., 1996). For example, arcuate neurons that project to the fenestrated capillaries of the pituitary portal system (neuroendocrine neurons) do not express NK3R (Krajewski et al., 2005). Proopiomelanocortin neurons or tyrosine hydroxylase-immunoreactive neurons in the arcuate nucleus also do not express NK3R (Krajewski and Rance, unpublished observations). Moreover, in the mouse arcuate nucleus, NK3R mRNA was reported to be exclusively expressed in kisspeptin mRNA-expressing neurons (Navarro et al., 2009). These data suggested that ablation of NK3R-expressing cells in the arcuate nucleus would selectively destroy KNDy neurons, with limited impact on other arcuate cell populations.
Arcuate KNDy neurons were ablated using stereotaxic injections of NK3-saporin (SAP), a selective NK3R agonist ([MePhe7]NKB) conjugated with SAP, a ribosome-inactivating toxin (Wiley and Lappi, 2005). [MePhe7]NKB, like senktide, has greater affinity for NK3R than NKB, and is 1000 times more selective for NK3R than other tachykinin receptors (Corboz et al., 2010; Drapeau et al., 1987). Stereotaxic injections of NK3-SAP destroyed nearly all the kisspeptin and NKB-immunoreactive neurons in the arcuate nucleus (Mittelman-Smith et al., 2012b). Arcuate dynorphin neurons were reduced by 76% (Mittelman-Smith et al., 2012b), consistent with the percentage of these neurons that express NK3R (Burke et al., 2006). Target selectivity was also demonstrated by the preservation of proopriomelanocortin, neuropeptide Y and GnRH-immunoreactive neurons in NK3-SAP injected rats. Nissl-stained sections showed no qualitative change in the cellular architecture between rats injected with NK3-SAP and Blank-SAP (a scrambled peptide conjugated to SAP), indicating that the vast majority of arcuate neurons were preserved (Mittelman-Smith et al., 2012b).
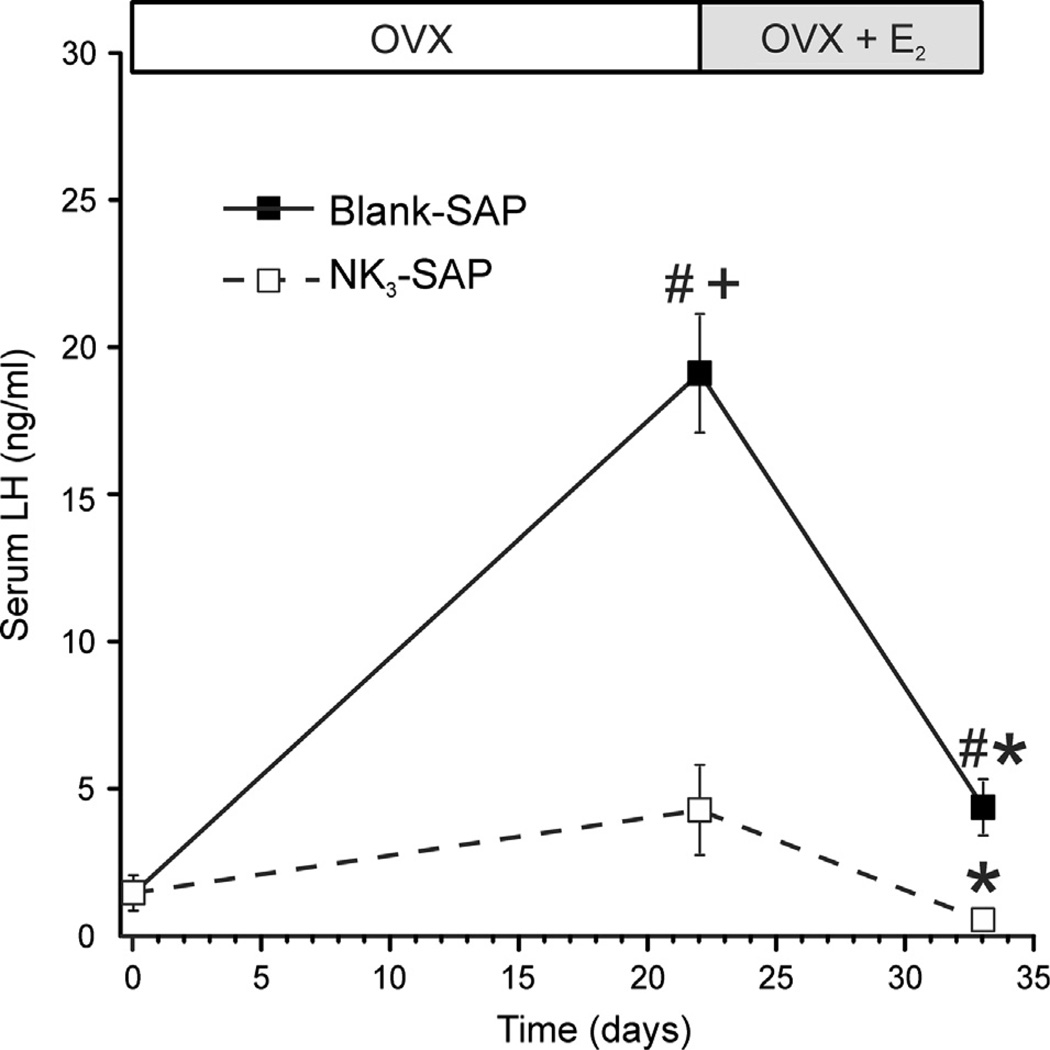
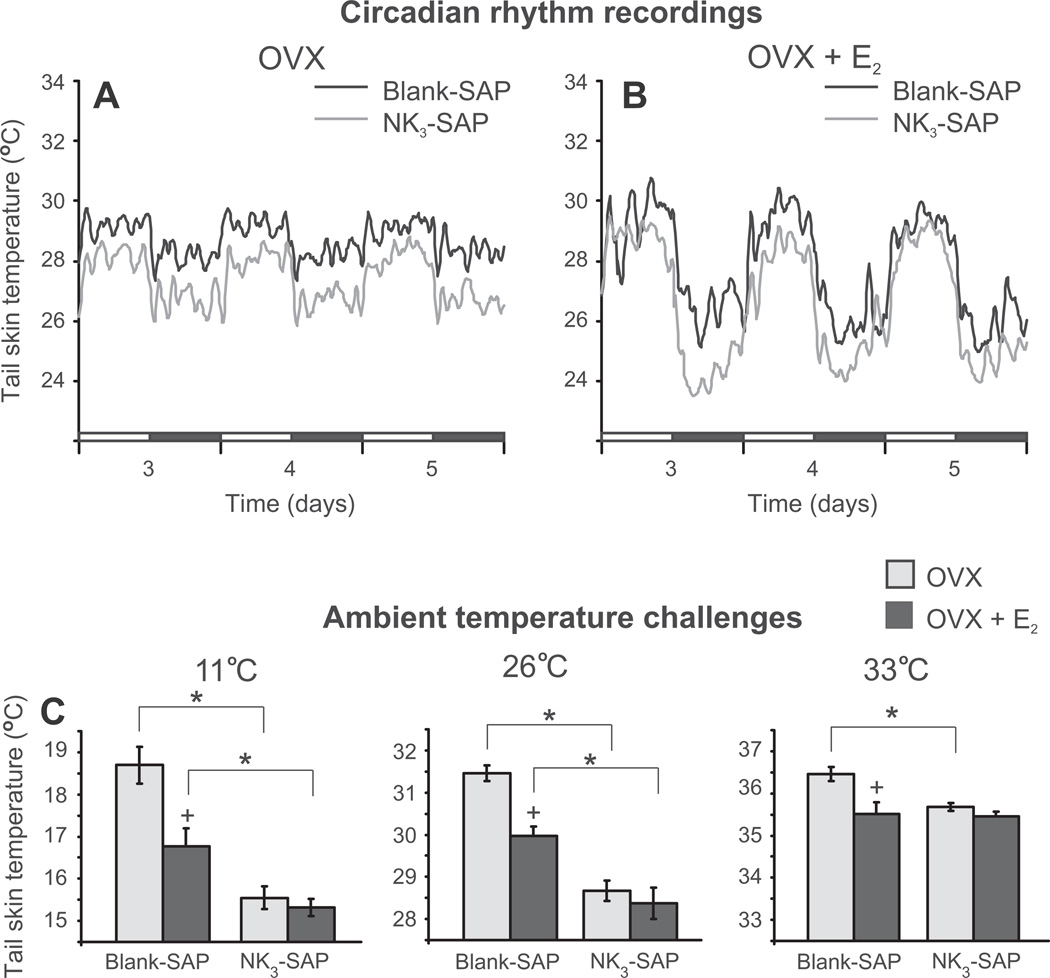
Ablation of KNDy neurons resulted in pronounced alterations in LH secretion (Fig. 11). Control rats exhibited a marked increase in serum LH after ovariectomy, which returned to intact levels 11 days after estrogen treatment. In contrast, LH did not significantly increase after ovariectomy in KNDy ablated rats, and their serum LH was lower regardless of estrogen replacement. Remarkably, KNDy neuron ablation consistently reduced tail skin temperatures, indicating that KNDy neurons facilitate cutaneous vasodilatation (Fig. 12) (Mittelman-Smith et al., 2012a). The decrease in tail skin temperature in KNDy-ablated rats occurred regardless of the time of day or estrogen treatment. Moreover, KNDy-ablated rats were able to better defend their core temperatures against heat than control rats, and unlike controls, did not respond to estrogen treatment with a reduction in core temperature. Finally, KNDy ablation blocked the effects of estradiol on tail skin temperature during the light phase in the environmental chamber, but not the dark phase. These data provide the first evidence that KNDy neurons promote cutaneous vasodilatation and participate in estrogen modulation of body temperature in the rat (Mittelman-Smith et al., 2012a). Because cutaneous vasodilatation is a cardinal sign of a hot flush, these data support the hypothesis that KNDy neurons could play a role in the generation of flushes.
Fig. 11.
Effects of KNDy neuron ablation on Serum LH (mean ± SEM, n = 6–8 rats/ group). On day 0, rats were ovariectomized (OVX) and injected with NK3-SAP (KNDy ablated) or Blank-SAP in the arcuate nucleus. From 20 to 23 days later and they were implanted s.c. with E2 capsules and sacrificed 11 days later. In Blank-SAP controls, serum LH was markedly increased after ovariectomy and reduced by E2 treatment. In KNDy-ablated rats, LH did not significantly rise three weeks after OVX. Serum LH was also lower in KNDy ablated rats, vs Blank-SAP controls after estrogen treatment. These data show that KNDy neurons are essential for the rise in LH secretion after ovariectomy and tonic LH secretion in estrogen-treated rats. # Significantly different, Blank-SAP vs. NK3-SAP. + Significantly different from intact values on day 0. * Significantly reduced after E2 treatment (compared to OVX). Modified with permission from Mittelman-Smith et al. (2012b).
Fig. 12.
(A and B) Circadian rhythms of tail skin temperature in KNDy-ablated (NK3-SAP) and control (Blank-SAP) rats that are ovariectomized (OVX, A) or OVX and treated with 17β-estradiol (OVX + E2, B). Circadian rhythms of tail skin temperature and activity were unchanged, but tail skin temperature was consistently lower in KNDy ablated rats than Blank-SAP controls. In both KNDy-ablated and control rats, estradiol decreased tail skin temperature during the dark phase (compare A and B). (C) Average tail skin temperature (±SEM) of KNDy-ablated (NK3-SAP) and control (Blank-SAP) rats subjected to different ambient temperatures in an environmental chamber (during the light phase). At the ambient temperature of 11 °C and 26 °C, the tail skin temperature of NK3-SAP animals was significantly lower than in Blank-SAP animals. Tail skin temperature of the Blank-SAP rats was reduced by E2 at all ambient temperatures, but not lowered by E2 treatment in NK3-SAP rats. The lower levels of tail skin temperature in KNDy-ablated rats provides evidence that KNDy neurons facilitate cutaneous vasodilatation, a cardinal sign of a hot flush. * Significantly different, NK3-SAP vs Blank-SAP. + Significantly different, OVX vs OVX+ 17β-estradiol. Adapted with permission from Mittelman-Smith et al. (2012a).
7. Conclusions
One of the most prominent symptoms of menopause is the hot flush, a manifestation of a central (hypothalamic) disorder of thermoregulation. Given the millions of individuals who experience these symptoms and the significant impact on the quality of life, it is surprising how little is known about the etiology of hot flushes. Because KNDy neurons change so dramatically in the hypothalamus of postmenopausal women, we hypothesized that they could contribute to the generation of flushes (Rance and Young, 1991). This hypothesis is supported by the close timing of LH pulses and flushes in women (Casper et al., 1979), the putative role of KNDy neurons in LH pulses in multiple species (Kinsey-Jones et al., 2012; Lehman et al., 2010; Rance et al., 2010; Wakabayashi et al., 2010) and the demonstration that estrogen suppresses flushes in humans (Santoro, 2008) and suppresses NKB and kisspeptin gene expression in ovariectomized monkeys (Abel et al., 1999; Rometo et al., 2007). In this review, we summarize the evidence gained in a rat model that estrogen-responsive KNDy neurons play a role in body temperature regulation and may activate thermoregulatory vasodilatation through projections to preoptic regions expressing NK3R.
Our approach combines information on KNDy neurons in post-menopausal women (Rance and Young, 1991; Rometo et al., 2007) with emerging knowledge on their role in GnRH secretion (Lehman et al., 2010; Rance et al., 2010), recent data on the neural pathways regulating heat-defense (Nakamura and Morrison, 2010) and studies on the effects of estrogen on thermoregulation in a rat model. By investigating how arcuate KNDy neurons may relay estrogen signals to the CNS pathways that regulate heat-dissipation effectors, these studies provide a tentative mechanism for triggering a hot flush in postmenopausal women. Moreover, projections of KNDy neurons to both GnRH neurons and preoptic structures involved in thermoregulation could explain the temporal link between hot flushes and LH pulses in postmenopausal women. Continued progress will require a deeper understanding of the control of body temperature by estrogen, further information on the modulation of heat-dissipation effectors by KNDy neurons, and the application of this knowledge to a clinical laboratory setting. The design of targeted therapies ultimately depends on understanding the mechanism of hot flushes.
Acknowledgments
The work of the authors on hot flushes was supported by the National Institutes of Health (NIH) National Institute on Aging Grant R01 AG032315 (NER). We have also reviewed studies on thermoregulation that were supported by the NIH National Institute of Neurological Disorders and Stroke Grant R01 NS41233 (AAR). The authors are grateful for the expert advice and continuing support of Dr. Nathaniel T. McMullen. We would also like to acknowledge the Graduate Interdisciplinary Programs in Neuroscience and Physiology at the University of Arizona.
References
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J. Comp. Neurol. 2000;424:679–688. doi: 10.1002/1096-9861(20000904)424:4<679::aid-cne9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J. Clin. Endocrinol. Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- Alçin E, Sahu A, Ramaswamy S, Hutz ED, Keen KL, Terasawa E, Bethea CL, Plant TM. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (Macaca mulatta) J. Neuroendocrinol. 2013;25:488–496. doi: 10.1111/jne.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfinito PD, Huselton C, Chen X, Deecher DC. Pharmacokinetic and pharmacodynamic profiles of the novel serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized Sprague-Dawley rats. Brain Res. 2006;1098:71–78. doi: 10.1016/j.brainres.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur. J. Neurosci. 2006a;23:3359–3367. doi: 10.1111/j.1460-9568.2006.04854.x. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One. 2006b;1:e1. doi: 10.1371/journal.pone.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2009;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, Gompel A, Hickey M, Hunter MS, Lobo RA, Lumsden MA, MacLennan AH, Maki P, Palacios S, Shah D, Villaseca P, Warren M. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14:515–528. doi: 10.3109/13697137.2011.608596. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Baker MA, Dawson DD, Peters CE, Walker AM. Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994;267:R673–R677. doi: 10.1152/ajpregu.1994.267.3.R673. [DOI] [PubMed] [Google Scholar]
- Barker JL, Carpenter DO. Thermosensitivity of neurons in the sensorimotor cortex of the cat. Science. 1970;169:597–598. doi: 10.1126/science.169.3945.597. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Kloosterboer HJ. Oestradiol and mirtazapine restore the disturbed tail-temperature of oestrogen-deficient rats. Eur. J. Pharmacol. 2003;482:329–333. doi: 10.1016/j.ejphar.2003.09.061. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Weekers AHJ, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur. J. Pharmacol. 2001;419:47–54. doi: 10.1016/s0014-2999(01)00966-9. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J. Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O’Byrne K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol. 2006;191:399–405. doi: 10.1677/joe.1.06919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratincsák A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–397. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J. Comp. Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Newton KM, Sternfeld B, Joffe H, Reed SD, Ensrud KE, Milata JL. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the menopause strategies finding lasting answers for symptoms and health network. Menopause. 2012;19:664–671. doi: 10.1097/gme.0b013e31823dbbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RF, Yen SSC. Menopausal flushes: effect of pituitary gonadotropin desensitization by a potent luteinizing hormone-releasing factor agonist. J. Clin. Endocrinol. Metab. 1981;53:1056–1058. doi: 10.1210/jcem-53-5-1056. [DOI] [PubMed] [Google Scholar]
- Casper RF, Yen SSC. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin. Endocrinol. (Oxf.) 1985;22:293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]
- Casper RF, Yen SSC, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science. 1979;205:823–825. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]
- Chan YM, Broder-Fingert S, Paraschos S, Lapatto R, Au M, Hughes V, Bianco SD, Min L, Plummer L, Cerrato F, De Guillebon A, Wu IH, Wahab F, Dwyer A, Kirsch S, Quinton R, Cheetham T, Ozata M, Ten S, Chanoine JP, Pitteloud N, Martin KA, Schiffmann R, Van der Kamp HJ, Nader S, Hall JE, Kaiser UB, Seminara SB. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1 . J. Clin. Endocrinol. Metab. 2011;96:E1771–E1781. doi: 10.1210/jc.2011-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010;109:1221–1228. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc. Sport Sci. Rev. 2000;28:108–112. [PubMed] [Google Scholar]
- Chawla MK, Gutierrez GM, Young WS, III., McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J. Comp. Neurol. 1997;384:429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Chen JT, Shiraki M. Menopausal hot flash and calciotonin gene-related peptide; effect of Keishi-bukuryo-gan, a kampo medicine, related to plasma calciotonin gene-related peptide level. Maturitas. 2003;45:199–204. doi: 10.1016/s0378-5122(03)00128-2. [DOI] [PubMed] [Google Scholar]
- Chen JT, Hirai Y, Seimiya Y, Hasumi K, Shiraki M. Menopausal flushes and calcitonin-gene-related peptide. Lancet. 1993;342:49. doi: 10.1016/0140-6736(93)91911-5. [DOI] [PubMed] [Google Scholar]
- Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl. 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteininzing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- Commission for Thermal Physiology, I.U.o.P.S. Glossary of terms for thermal physiology. Jpn. J. Physiol. (third ed.) 2001;51:245–280. [Google Scholar]
- Corboz MR, Rivelli MA, Eckel SP. Bronchoconstrictor effect of the tachykinin NK(3)-receptor agonists [MePhe(7)]-neurokinin B and senktide in the isolated guinea pig lung. Exp. Lung Res. 2010;36:509–521. doi: 10.3109/01902141003777582. [DOI] [PubMed] [Google Scholar]
- Cosmi S, Pawlyk AC, Alfinito PD, Roman J, Zhou T, Deecher DC. Simultaneous telemetric monitoring of tail-skin and core body temperature in a rat model of thermoregulatory dysfunction. J. Neurosci. Methods. 2009;178:270–275. doi: 10.1016/j.jneumeth.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Csabafi K, Jászberényi M, Bagosi Z, Lipták N, Telegdy G. Effects of kisspeptin-13 on the hypothalamic–pituitary–adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav. Brain Res. 2012;241C:56–61. doi: 10.1016/j.bbr.2012.11.039. [DOI] [PubMed] [Google Scholar]
- Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151:1187–1193. doi: 10.1210/en.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011a;152:4894–4905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Krajewski SJ, Rance NE. Ambient temperature and 17β-estradiol modify fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology. 2011b;152:2750–2759. doi: 10.1210/en.2010-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecher DC, Alfinito PD, Leventhal L, Cosmi S, Johnston GH, Merchenthaler I, Winneker R. Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endocrinology. 2007;148:1376–1383. doi: 10.1210/en.2006-1163. [DOI] [PubMed] [Google Scholar]
- DeFazio J, Meldrum DR, Laufer L, Vale W, Rivier J, Lu JKH, Judd HL. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J. Clin. Endocrinol. Metab. 1983;56:445–448. doi: 10.1210/jcem-56-3-445. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor a. Endocrinology. 2004;145:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Drapeau G, d’Orléans-Juste P, Dion S, Rhaleb N-E, Regoli D. Specific agonists for neurokinin B receptors. Eur. J. Pharmacol. 1987;136:401–403. doi: 10.1016/0014-2999(87)90313-x. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Bartfai T. Single cell transcriptomics of hypothalamic warm sensitive neurons that control core body temperature and fever response: Signaling asymmetry and an extension of chemical neuroanatomy. Pharmacol. Ther. 2011;129:241–259. doi: 10.1016/j.pharmthera.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason HL, Fewell JE. Influence of pregnancy on the febrile response to ICV administration of PGE1 in rats studied in a thermocline. J. Appl. Physiol. 1997a;82:1453–1458. doi: 10.1152/jappl.1997.82.5.1453. [DOI] [PubMed] [Google Scholar]
- Eliason HL, Fewell JE. Thermoregulatory control during pregnancy and lactation in rats. J. Appl. Physiol. 1997b;83:837–844. doi: 10.1152/jappl.1997.83.3.837. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil. Steril. 1998;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- Freedman RR. Physiology of hot flashes. Am. J. Human Biol. 2001;13:453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am. J. Obstet. Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576–583. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- Gambone J, Meldrum DR, Laufer L, Chang RJ, Lu JKH, Judd HL. Further delineation of hypothalamic dysfunction responsible for menopausal hot flashes. J. Clin. Endocrinol. Metab. 1984;59:1097–1102. doi: 10.1210/jcem-59-6-1097. [DOI] [PubMed] [Google Scholar]
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J. Neurosci. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MM. Role of androgens in surgical menopause. Am. J. Obstet. Gynecol. 1999;180:S325–S327. doi: 10.1016/s0002-9378(99)70728-3. [DOI] [PubMed] [Google Scholar]
- Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LFG, Costa EMF, Bilharinho de Mendonca B, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 2010;95:2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J. Clin. Endocrinol. Metab. 2002a;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J. Clin. Endocrinol. Metab. 2002b;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, Clifton DK, Carroll RS, Steiner RA, Kaiser UB. Increased Neurokinin B (Tac2) Expression in the Mouse Arcuate Nucleus Is an Early Marker of Pubertal Onset with Differential Sensitivity to Sex Steroid-Negative Feedback than Kiss1. Endocrinology. 2012 doi: 10.1210/en.2012-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbig D, Keller K, Prelle K, Patchev V, Vonk R, Igl BW. A dynamic model of circadian rhythms in rodent tail skin temperature for comparison of drug effects. J. Circadian Rhythms. 2012;10:1–11. doi: 10.1186/1740-3391-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermal biology of the laboratory rat. Physiol. Behav. 1990;47:963–991. doi: 10.1016/0031-9384(90)90025-y. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp. Med. 2004;54:63–68. [PubMed] [Google Scholar]
- Gordon CJ, Puckett E, Padnos B. Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J. Pharmacol. Toxicol. Methods. 2002;47:107–114. doi: 10.1016/s1056-8719(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J. Physiol. 2001;537:521–535. doi: 10.1111/j.1469-7793.2001.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine changes with reproductive aging in women. Semin. Reprod. Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen X-M, Zhang Y-H, Kanosue K. Effects of estrogen on thermoregulatory responses in freely moving female rats. Ann. N. Y. Acad. Sci. 1997a;813:207–210. doi: 10.1111/j.1749-6632.1997.tb51695.x. [DOI] [PubMed] [Google Scholar]
- Hosono T, Yanase-Fujiwara M, Zhang YH, Xiao-ming C, Fukuda Y, Asaki Y, Yamaji K, Kanosue K. Effect of gonadotropin releasing hormone on thermoregulatory vasomotor activity in ovariectomized female rats. Brain Res. 1997b;754:88–94. doi: 10.1016/s0006-8993(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Hosono T, Chen X-M, Miyatsuji A, Yoda T, Yoshida K, Yanase-Fujiwara M, Kanosue K. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1341–R1347. doi: 10.1152/ajpregu.2001.280.5.R1341. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur. J. Neurosci. 2010;31 doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Molnar CS, Sipos MT, Vida B, Ciofi P, Borsay BA, Sarkadi L, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Kallo I, Liposits Z. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front. Endocrinol. 2011;2:80. doi: 10.3389/fendo.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153:4978–4989. doi: 10.1210/en.2012-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Egawa T, Yamauchi A, Sohda Y, Koga A, Tominaga K, Shuto H, Kataoka Y. Selective serotonin reuptake inhibitors fluvoxamine and paroxetine restore forced exercise-induced temperature dysregulation in ovariectomized mice. Eur. J. Pharmacol. 2008;579:439–444. doi: 10.1016/j.ejphar.2007.11.043. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Nakayama T, Kanosue K, Matsumura K. Activation of central warm-sensitive neurons and the tail vasomotor response in rats during brain and scrotal thermal stimulation. Pflugers Arch. 1984;400:222–227. doi: 10.1007/BF00581551. [DOI] [PubMed] [Google Scholar]
- Jensen J, Christiansen C. Dose-response and withdrawal effects on climacteric symptoms after hormonal replacement therapy. A placebo-controlled therapeutic trial. Maturitas. 1983;5:125–133. doi: 10.1016/0378-5122(83)90008-7. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Crawshaw LI, Nagashima K, Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur. J. Appl. Physiol. 2010;109:5–11. doi: 10.1007/s00421-009-1256-6. [DOI] [PubMed] [Google Scholar]
- Katovich MJ, Simpkins JW, Berglund LA, Omeara J. Regional skin temperature-changes in a rat model for the menopausal hot flush. Maturitas. 1986;8:67–76. doi: 10.1016/0378-5122(86)90009-5. [DOI] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Lin Y, Knox AM, Milligan SR, Lightman SL, Millar RP, O’Byrne KT. Kisspeptin signalling and tail vasomotor function in the female rat. Society for Neuroscience Abstracts. 2010 Program No. 695.6. [Google Scholar]
- Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315. doi: 10.1210/en.2011-1641. [DOI] [PubMed] [Google Scholar]
- Kiyohara T, Miyata S, Nakamura T, Shido O, Nakashima T, Shibata M. Differences in Fos expression in the rat brains between cold and warm ambient exposures. Brain Res. Bull. 1995;38:193–201. doi: 10.1016/0361-9230(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm. Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Knobil E. The GnRH pulse generator. Am. J. Obstet. Gynecol. 1990;163:1721–1727. doi: 10.1016/0002-9378(90)91435-f. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ushijima O, Chen J-T, Shiraki M, Ohta T, Kiyoki M. Basal tail skin temperature elevation and augmented response to calcitonin gene-related peptide in ovariectomized rats. J. Endocrinol. 1995;146:431–437. doi: 10.1677/joe.0.1460431. [DOI] [PubMed] [Google Scholar]
- Kolka MA, Stephenson LA. Resetting the thermoregulatory set-point by endogenous estradiol or progesterone in women. Ann. N. Y. Acad. Sci. 1997;813:204–206. doi: 10.1111/j.1749-6632.1997.tb51694.x. [DOI] [PubMed] [Google Scholar]
- Konishi M, Kanosue K, Kano M, Kobayashi A, Nagashima K. The median preoptic nucleus is involved in the facilitation of heat-escape/cold-seeking behavior during systemic salt loading in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R150–R159. doi: 10.1152/ajpregu.00769.2005. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J. Comp. Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. NeuroScience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- Kronenberg F. Hot flashes: epidemiology and physiology. Ann. N. Y. Acad. Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp. Gerontol. 1994;29:319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Kronenberg F. Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J. Nutr. 2010;140:1380S–1385S. doi: 10.3945/jn.109.120840. [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Barnard RM. Modulation of menopausal hot flashes by ambient temperature. J. Therm. Biol. 1992;17:43–49. [Google Scholar]
- Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estrogen replacement. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1980;238:R400–R405. doi: 10.1152/ajpregu.1980.238.5.R400. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/ neurokinin B/dynorphin (KNDy) cells of the arucate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv. Exp. Med. Biol. 2013;784:27–62. doi: 10.1007/978-1-4614-6199-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RA, Ganio MS, Pearson J, Crandall CG. Brain blood flow and cardiovascular responses to hot flashes in postmenopausal women. Menopause. 2013;20:299–304. doi: 10.1097/GME.0b013e31826e45f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E, Hunter MS. Concordance between self-reported and sternal skin conductance measures of hot flushes in symptomatic perimenopausal and postmenopausal women: a systematic review. Menopause. 2011;18:709–722. doi: 10.1097/gme.0b013e318204a1fb. [DOI] [PubMed] [Google Scholar]
- Marrone BL, Gentry RT, Wade GN. Gonadal hormones and body temperature in rats: effects of estrous cycles, castration and steroid replacement. Physiol. Behav. 1976;17:419–425. doi: 10.1016/0031-9384(76)90101-3. [DOI] [PubMed] [Google Scholar]
- Martin SM, Malkinson TJ, Veale WL, Pittman QJ. Fever in pregnant, parturient, and lactating rats. Am. J. Physiol. 1995;268:R919–R923. doi: 10.1152/ajpregu.1995.268.4.R919. [DOI] [PubMed] [Google Scholar]
- Martin SM, Malkinson TJ, Veale WL, Pittman QJ. Prostaglandin fever in rats throughout the estrous cycle late pregnancy and post parturition. J. Neuroendocrinol. 1996;8:145–151. doi: 10.1111/j.1365-2826.1996.tb00835.x. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Preoptic thermoregulatory mechanisms in detail. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R272–R273. doi: 10.1152/ajpregu.00141.2004. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur. J. Appl. Physiol. 2009 doi: 10.1007/s00421-009-1295-z. [DOI] [PubMed] [Google Scholar]
- McKitrick DJ. Expression of Fos in the hypothalamus of rats exposed to warm and cold temperatures. Brain Res. Bull. 2000;53:307–315. doi: 10.1016/s0361-9230(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Meister B, Ceccatelli S, Hökfelt T, Andén NE, Andén M, Theodorsson E. Neurotransmitters, neuropeptides and binding sites in the rat mediobasal hypothalamus: effects of monosodium glutamate (MSG) lesions. Exp. Brain Res. 1989;76:343–368. doi: 10.1007/BF00247894. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Shamonki IM, Frumar AM, Tataryn IV, Chang RJ, Judd HL. Elevations in skin temperature of the finger as an objective index of postmenopausal hot flashes: standardization of the technique. Am. J. Obstet. Gynecol. 1979;135:713–717. doi: 10.1016/0002-9378(79)90380-6. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Funkhouser JM, Carver JM, Lundeen SG, Ghosh K, Winneker RC. The effect of estrogens and antiestrogens in a rat model for hot flush. Maturitas. 1998;30:307–316. doi: 10.1016/s0378-5122(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewsk-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc. Natl. Acad. Sci. USA. 2012a;109:19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012b;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar GW. Body temperatures during menopausal hot flashes. J. Appl. Physiol. 1975;38:499–503. doi: 10.1152/jappl.1975.38.3.499. [DOI] [PubMed] [Google Scholar]
- Molnar GW. Menopausal hot flashes: their cycles and relation to air temperature. Obstet. Gynecol. 1981;57:52S–55S. [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front. Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K. Central mechanisms for thermoregulation in a hot environment. Ind. Health. 2006;44:359–367. doi: 10.2486/indhealth.44.359. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton. Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/ dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, Yuzurihara M, Kase Y, Yasui T, Irahara M. Involvement of cytokine-induced neutrophil chemoattractant in hypothalamic thermoregulation of luteinizing hormone-releasing hormone. Endocrinology. 2008;149:2899–2906. doi: 10.1210/en.2007-1521. [DOI] [PubMed] [Google Scholar]
- Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, Maeda KI, Tsukamura H. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides. 2009;30:49–56. doi: 10.1016/j.peptides.2008.08.004. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Johnson JM, Taylor WF. Mode of neural control mediating rat tail vasodilation during heating. J. Appl. Physiol. 1985;59:1533–1538. doi: 10.1152/jappl.1985.59.5.1533. [DOI] [PubMed] [Google Scholar]
- Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A. Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas. 2004;48:463–471. doi: 10.1016/j.maturitas.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J. Clin. Endocrinol. Metab. 2008;93:3208–3214. doi: 10.1210/jc.2008-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int. J. Obes. 2010;34(Suppl. 2):S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- Özdemir S, Çelik Ç, Görkemli H, Kiyici A, Kaya B. Compared effects of surgical and natural menopause on climacteric symptoms, osteoporosis, and metabolic syndrome. Int. J. Gynaecol. Obstet. 2009;106:57–61. doi: 10.1016/j.ijgo.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J. Neuroendocrinol. 2003;15:749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu. Rev. Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Prelle K, Igl BW, Obendorf M, Girbig D, Lehmann T, Patchev VK. Endpoints of drug discovery for menopausal vasomotor symptoms: interpretation of data from a proxy of disease. Menopause. 2012;19:909–915. doi: 10.1097/gme.0b013e318245533f. [DOI] [PubMed] [Google Scholar]
- Puri P, Wuttke W, Seidlova-Wuttke D. 20-OH-ecdysone prevents hot flushes in ovariectomized rats. Planta Med. 2012;78:109–114. doi: 10.1055/s-0031-1280317. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. NeuroEndocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J. Clin. Endocrinol. Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- Rance NE, Young WS., III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., III. Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J. Clin. Endocrinol. Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol. Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Martin JR. Effects of lesions in central thermosensitive areas on thermoregulatory responses in rat. Physiol. Behav. 1977;19:503–511. doi: 10.1016/0031-9384(77)90226-8. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA. Temperature regulation. In: Petersen O, editor. Lecture Notes on Human Physiology. Oxford, UK: Blackwell; 2007a. pp. 603–615. [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007b;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Shido O, Ungar AL, Blatteis CM. Peripheral naloxone attenuates lipopolysaccharide fever in guinea pigs by an action outside the blood-brain barrier. Am. J. Physiol. 1994;266:R1824–R1831. doi: 10.1152/ajpregu.1994.266.6.R1824. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am. J. Physiol. 1996;270:R693–R703. doi: 10.1152/ajpregu.1996.270.4.R693. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R420–R428. doi: 10.1152/ajpregu.00757.2002. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol. Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J. Neuroendocrinol. 2008;20:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Scherbaum WA, Lauritzen C. Gonadotropin secretion during aging in postmenopausal women. NeuroEndocrinology. 1991;54:211–218. doi: 10.1159/000125878. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013 doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J. Neuroendocrinol. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- Santoro N. Symptoms of menopause: hot flushes. Clin. Obstet. Gynecol. 2008;51:539–548. doi: 10.1097/GRF.0b013e31818093f6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer I, Smillie S-J, Bodkin JV, Fernandes E, O’Byrne KT, Brain SD. The vasoactive potential of kisspeptin-10 in the peripheral vasculature. PLoS One. 2011;6:e14671. doi: 10.1371/journal.pone.0014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffalitzkey de Muckadell Ob, Aggestrup S, Stentoft P. Flushing and plasma substance P concentrations during infusion of synthetic substance P in normal man. Scand. J. Gastroenterol. 1986;21:498–502. doi: 10.3109/00365528609015169. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res. Mol. Brain Res. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr., Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Srouji SS, Histed SN, Hall JE. Differential effects of aging on estrogen negative and positive feedback. Am. J. Physiol. Endocrinol. Metab. 2011;301:E351–E355. doi: 10.1152/ajpendo.00150.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan HL, Kovács K. The subventricular nucleus of the human hypothalamus. Brain. 1966;89:589–614. doi: 10.1093/brain/89.3.589. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J. Comp. Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986;250:R625–R632. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Katovich MJ, Song IC. Similarities between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32:1957–1966. doi: 10.1016/0024-3205(83)90047-4. [DOI] [PubMed] [Google Scholar]
- Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen ASP, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF. Hot flushes. Lancet. 2002;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJS, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J. Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LA, Kolka MA. Thermoregulation in women. Exerc. Sport Sci. Rev. 1993;21:231–262. [PubMed] [Google Scholar]
- Székely M, Szelényi Z. Endotoxin fever in the rat. Acta Physiol. Acad. Sci. Hung. 1979;53:265–277. [PubMed] [Google Scholar]
- Szélenyi Z, Székely M, Romanovskii AA. The central thermoregulatory action of cholecystokinin-8 and prostaglandin E1. Fizziol. Zh. SSSR im I. M. Sechenova. 1992;78:94–101. (in Russian) [PubMed] [Google Scholar]
- Tanaka M, Ootsuka Y, McKinley MJ, McAllen RM. Independent vasomotor control of rat tail and proximal hairy skin. J. Physiol. 2007;582:421–433. doi: 10.1113/jphysiol.2007.131292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1248–R1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J. Appl. Physiol. 1992;73:1238–1245. doi: 10.1152/jappl.1992.73.4.1238. [DOI] [PubMed] [Google Scholar]
- Tataryn IV, Meldrum DR, Lu KH, Fruraar AM, Judd HL. LH, FSH and skin temperature during the menopausal hot flash. J. Clin. Endocrinol. Metab. 1979;49:152–154. doi: 10.1210/jcem-49-1-152. [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 2012;366:629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- van Gastel P, van der Zanden M, Telting D, Filius M, Bancsi L, de Boer H. Luteinizing hormone-releasing hormone receptor antagonist may reduce postmenopausal flushing. Menopause. 2012;19:178–185. doi: 10.1097/gme.0b013e31822542ca. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J. System properties, feedback control and effector coordination of human temperature regulation. Eur. J. Appl. Physiol. 2010;109:13–15. doi: 10.1007/s00421-009-1216-1. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Lappi DA. Molecular Neurosurgery with Targeted Toxins. Totowa, New Jersey: Humana Press; 2005. [Google Scholar]
- Wilkinson CW, Carlisle HJ, Reynolds RW. Estrogenic effects on behavioral thermoregulation and body temperature of rats. Physiol. Behav. 1980;24:337–340. doi: 10.1016/0031-9384(80)90096-7. [DOI] [PubMed] [Google Scholar]
- Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151:5389–5394. doi: 10.1210/en.2010-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyon YA-M, Spetz A-C, Theodorsson GE, Hammar ML. Concentrations of calcitonin gene-related peptide and neuropeptide Y in plasma increase during flushes in postmenopausal women. Menopause. 2000;7:25–30. doi: 10.1097/00042192-200007010-00005. [DOI] [PubMed] [Google Scholar]
- Xin L, Geller EB, Adler MW. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J. Pharmacol. Exp. Ther. 1997;281:499–507. [PubMed] [Google Scholar]
- Yang Y, Gordon CJ. Ambient temperature limits and stability of temperature regulation in telemetered male and female rats. J. Therm. Biol. 1996;21:353–363. [Google Scholar]
- Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J. Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Bouligand J, Francou B, Raffin-Sanson M-L, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J. Clin. Endocrinol. Metab. 2010;95:2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]