Abstract
Cancer-associated fibroblasts enhance cancer progression when activated by tumor cells through mechanisms not yet fully understood. Blocking mammary tumor cell-derived lysyl oxidase-like 2 (LOXL2) significantly inhibited mammary tumor cell invasion and metastasis in transgenic and orthotopic mouse models. Here we discovered that tumor-derived LOXL2 directly activated stromal fibroblasts in the tumor microenvironment. Genetic manipulation or antibody inhibition of LOXL2 in orthotopically grown mammary tumors reduced the expression of α-smooth muscle actin (α-SMA). Using a marker for reticular fibroblasts, it was determined that expression of α-SMA was localized to fibroblasts recruited from the host tissue. This marker also revealed that the matrix present in tumors with reduced levels of LOXL2 was more scattered compared to control tumors which exhibited matrices with dense, parallel alignments. Importantly, in vitro assays revealed that tumor-derived LOXL2 and a recombinant LOXL2 protein induced fibroblast branching on collagen matrices, as well as increased fibroblast-mediated collagen contraction and invasion of fibroblasts through extracellular matrix (ECM). Moreover, LOXL2 induced the expression of α-SMA in fibroblasts grown on collagen matrices. Mechanistically, it was determined that LOXL2 activated fibroblasts through integrin-mediated FAK activation. These results indicate that inhibition of LOXL2 in tumors not only reduces tumor cell invasion but also attenuates the activation of host cells in the tumor microenvironment.
Implications: These findings reveal new insight into the mechanisms of fibroblast activation, a novel function of LOXL2, and further highlight the importance of generating LOXL2-targeted therapies for the prevention of tumor progression and metastasis.
Keywords: lysyl oxidase-like 2 (LOXL2), fibroblast activation, alpha-smooth muscle actin (α-SMA), focal adhesion kinase (FAK), integrins, extracellular matrix (ECM), tumor microenvironment, breast cancer progression
INTRODUCTION
Fibroblasts are the principal cell type of normal connective tissues. They are embedded within the fibrillar extracellular matrix (ECM) and are primarily responsible for its deposition and remodeling, as well as synthesizing components of the basement membrane (1, 2). Not only do fibroblasts play a crucial role in the maintenance of normal ECM homeostasis, they are also integral to wound healing, organ fibrosis (2, 3) and tumor progression (reviewed in (4)). Cancer associated fibroblasts (CAFs), together with endothelial cells, pericytes, smooth muscle cells, immune cells, growth factors, cytokines, and a specialized ECM, make up the tumor microenvironment (5). CAFs are themselves a heterogeneous population of cells with a varying number being referred to as activated fibroblasts or myofibroblasts due to expression of α-smooth muscle actin (α-SMA) (6-8).
Importantly, CAFs appear to harbor unique cancer-promoting properties that “normal” fibroblasts lack. It has been shown that the growth of breast, prostate, pancreas and skin cancer cells in mice is significantly enhanced when they are co-implanted with α-SMA-positive CAFs isolated from solid tumors (9-14). Even non-tumorigenic prostate epithelial cells could be induced to form tumors in immunocompromised mice when mixed with CAFs (15).
The tumor-stroma crosstalk has been shown to play a fundamental role in tumor progression. Factors secreted from the tumor cells activate local fibroblasts, which in turn secrete increased levels of growth factors, chemokines, ECM proteins and matrix remodeling enzymes to modify the tumor microenvironment and promote proliferation, survival, invasion and metastasis of the tumor cells (5, 16-18). Fibroblasts sense changes in their microenvironment through integrins (19), and form focal adhesion complexes with intracellular molecules that engage the cytoplasmic tyrosine kinases, focal adhesion kinase (FAK) and Src, to activate multiple signaling pathways (20-22). FAK is also responsible for relaying signals from growth factors and cytokines, such as TGFβ, leading to the expression of α-SMA and concurrent activation of fibroblasts (23).
Despite their clear role in promoting metastasis, the tumor-fibroblast interactions needed for fibroblast activation are largely unknown. We have previously shown that blocking mammary tumor cell-derived lysyl oxidase-like 2 (LOXL2) significantly inhibits mammary tumor cell invasion and metastasis in transgenic and orthotopic mouse models (24). In this study we investigate the role of LOXL2 in the tumor stroma and demonstrate that LOXL2 mediates fibroblast activation through integrin engagement and FAK signaling.
MATERIALS AND METHODS
Generation of cell lines
C57Bl/6 fibroblasts were a kind gift from Joni Mäki and Johanna Myllyharju. These were isolated from the dermis of C57Black6 mice. 3T3 fibroblasts were obtained from the ATCC. Cells were grown in GIBCO®DMEM media (Invitrogen) supplemented with 10% fetal calf serum (FCS) at 37°C and 5% CO2.
4T1 mouse breast cancer cells were a kind gift from Fred Miller (25) and had been authenticated using short tandem repeat (STR) analysis within the last 6 months. Cells were grown in DMEM supplemented with 10% FCS at 37°C and 5% CO2. Cells were infected with lentiviruses expressing short hairpin RNA (shRNA) libraries specific to mouse Loxl2 (TRC-Mm1.0; Open Biosystems).
Preparation of conditioned media
Growth media was removed from cells at about 75% confluency in T75 flasks, cells were washed thoroughly and incubated in 10 mL of serum-free DMEM for 24 hours at 37°C and 5% CO2. Conditioned media (CM) was collected from cells and filtered through 45 μm filters (Millipore).
Fibroblast growth assays
6-well plates were prepared by adding 1 mL of collagen mix (50% 1 × DMEM, 1.5mg rat tail collagen I (BD Biosciences) with pH adjusted by addition of 5M NaOH and made up to 1mL with PBS) to each well and allowing it to set at 37°C. Fibroblasts were plated on the collagen at a density of 2 × 105 cells per well in normal growth media and allowed to settle overnight. Growth media was removed from the fibroblasts and replaced with serum-free media (SFM) or CM from 4T1 breast cancer cells. Cells were also incubated in SFM containing 10ng/ml TGFβ (Sigma) or 30μM recombinant LOXL2 protein. Specific antibodies were added at various concentrations: LOXL2 (N-15; Santa Cruz Biotechnology Inc.; 12μg/ml), β1 integrin (Millipore; 10μg/ml), α5β1 integrin (Abcam), or β3 integrin (Santa Cruz Biotechnology Inc.; 20μg/ml). Specific inhibitors to Src (Src inhibitor (26); 1:100) or FAK (FAK inhibitor 14 (27, 28); 1:50) were also used. Control Armenian hamster IgG (Santa Cruz Biotechnology Inc.) and sodium citrate buffer were added to SFM as controls for the antibodies and inhibitors respectively.
Invasion assays
Transwell invasion assays were carried out as previously described (29). Briefly, 2.5 × 104 fibroblasts were seeded in each Matrigel-coated transwell and 4T1 conditioned media (CM) placed in the bottom wells to act as a chemoattractant. Goat isotype control (Sigma) or LOXL2-specific (N-15; Santa Cruz Biotechnology Inc) antibodies were added to either the fibroblasts or the CM. In other experiments SFM containing 30μM recombinant human LOXL2 was placed in the bottom wells to act as chemoattractant. SFM alone was used as a control. Parallel assays were carried out in uncoated control transwell inserts to assess cell migration in the absence of ECM.
Collagen contraction assays
2×105 fibroblasts were suspended in a collagen mixture (100μl of cells in normal growth media mixed with 200μl of collagen mix as described above for growth assays) per well of 24-well plates pre-coated with 0.5% BSA and allowed to set at 37°C. Gels were incubated in normal growth media for 24 hours. Growth media was removed and SFM or 4T1 CM containing specific antibodies or recombinant proteins at concentrations mentioned previously was added to the wells and gels released. Gels were photographed at various time-points and ImageJ used to measure gel area and assess contraction.
Western blotting
Lysates were prepared from cell pellets in 1% NP40 lysis buffer. Proteins from lysates were separated on NuPAGE® Novex® Bis-Tris 10% gels (Invitrogen). Gels were transferred to PVDF membranes (Millipore) and probed with antibodies specific to beta-actin, α-SMA, p-FAK (Y397) (Abcam), Akt (9272), p-Akt (S473), (Cell signaling), FAK (610087) (BD Biosciences).,
ELISA
ELISA plates were incubated with CM overnight, washed and blocked before incubating with antibody specific to LOXL2 (N-15, Santa Cruz; 1:100), washing and incubating with goat secondary antibody (Santa Cruz). Wells were washed and incubated with europium labelled antibody before developing with DELFIA enhancement solution (PerkinElmer, Inc.). Plates were read at 615nm on a SpectroMax 5 plate reader (Molecular Devices).
LOXL2 activity assay
Fluorescence-based enzymatic activity assays were carried out as previously described (24).
MTS assays
Cells were plated in triplicate in 96-well plates, either on a layer of 50% collagen or within a layer of 33% collagen (as above for growth and contraction assays), and allowed to set at 37°C. SFM alone or containing TGFβ or recombinant LOXL2 protein was added to the wells and cell proliferation assay performed on days 0, 1, 2, 3. For measurements, 20 μl of CellTiter 96® AQueous One Solution reagent (Promega) was directly added to media in culture wells, plates incubated for 2 hr at 37°C, then absorbance measured at 490 nm using a SpectroMax5 plate reader (Molecular Devices).
Quantitative real-time PCR
Total RNA was isolated from cell pellets using RNeasy Mini Kits (Qiagen). DNase treatment and cDNA synthesis were performed using QuantiTect Reverse Transcription Kits (Qiagen). Quantitative real-time PCRs for mouse beta-actin, Loxl2 and acta2 were performed using TaqMan® Gene Expression Assays (Applied Biosystems). Quantitative real-time PCR for mouse SDF1 and FN was performed using SYBR green mix (Promega) and the following primers:
FN: AGGCATAAGGTTCGGGAAGAGGT (forward)
GCAGTTGTCACAGCGCCAGCC (reverse)
SDF1: CTTCATCCCCATTCTCCT (forward)
GACTCTGCTCTGGTGGAAGG (reverse)
In vivo assays
For orthotopic models, control and shLOXL2 4T1 cells (1×105) were injected into the fourth mammary fat pad of 6-8 week old female syngeneic Balb/c (Harlan) mice. All experiments were approved by the Home Office and performed following UKCCCR Guidelines for the welfare and use of animals in cancer research. Treated mice received twice weekly intraperitoneal injections of anti-LOXL2 antibody (N-15; Santa Cruz Biotechnology Inc.) or IgG from goat serum (Sigma) at 0.5mg/kg for 4 weeks. Mice were culled when tumors reached maximum size. Tumors were removed and either fixed in 4% paraformaldehyde or frozen in optimal cutting temperature (OCT) compound (Tissue-Tek®).
Immunohistochemistry/Immunofluorescence
Paraffin embedded tissue sections were deparaffinized, rehydrated and incubated with 3% hydrogen peroxidase in methanol for 15 minutes to quench endogenous peroxidase activity. Antigen retrieval was carried out in a target retrieval solution (Dako) at 95°C for 15 minutes. Non-specific binding was blocked by incubation with 5% goat serum for 1 hour. Primary antibodies were applied overnight at 4°C, and biotinylated secondary antibodies for 45 minutes at room temperature, followed by a final 30 minute incubation with streptavidin-biotin peroxidase solution. Visualization was carried out using 3,3′-diaminobenzine (DAB) and counterstaining using Mayer’s haemalum. Flash frozen tissue sections were fixed in methanol and stained using Alexa Fluor® secondary antibodies (Molecular Probes, Invitrogen). Methods as previously described (29, 30). Antibodies are described above plus an antibody that detects the ER-TR7 antigen (Santa Cruz). Samples were visualized using an Olympus BX51 and a 10x or 20x magnification lens. Images were analyzed using Cell D software (Olympus). Brightness and contrast were adjusted equally in all images presented.
Statistical Analysis
Data was analyzed using the Student t-test unless otherwise stated, and considered significant when the p value was <0.05. All statistical tests were two-sided. Bar graphs represent the mean and standard error across multiple independent experimental repeats unless otherwise stated. Statistical significance representations: *p<0.05, **p<0.01, ***p<0.001.
RESULTS
Inhibition of LOXL2 reduces α-SMA expression in 4T1 orthotopic tumors
We have previously shown that LOXL2 expression is clinically correlated with metastasis and that 4T1 mouse mammary cancer cells metastasize in a LOXL2-dependent manner in vivo (24). We sought to better understand how tumor-derived LOXL2 could enhance tumor progression, with a focus on fibroblast activation. We first manipulated LOXL2 expression in 4T1 cells. 4T1 cells were infected with a scrambled control or mouse LOXL2-specific shRNA viruses to generate knockdown (4T1 shLOXL2) and control (4T1 control) cell lines. The shLOXL2 cell lines had significantly decreased expression of LOXL2 mRNA and protein compared with controls (24). ELISA and activity assays were carried out to further confirm that shLOXL2 cells expressed less LOXL2 protein and had lower LOXL2 activity (Supplementary Figures 1A + B, respectively).
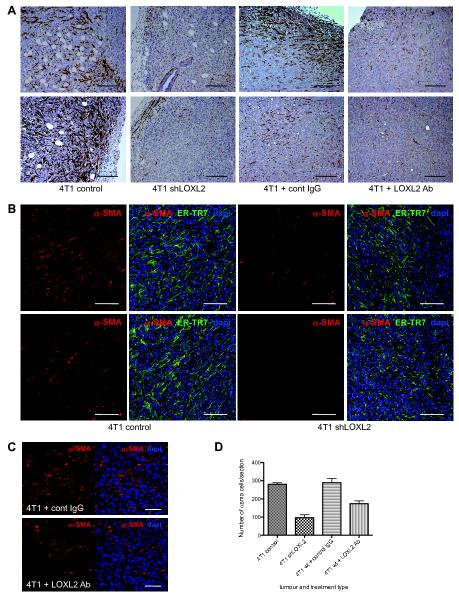
To assess the role of tumor-derived LOXL2 on host stromal cells, control and shLOXL2 cells were implanted into the mammary fat pad of Balb/c mice. 4T1 wild-type (4T1 wt) cells were also implanted into Balb/c mice and these mice received twice weekly doses of either a control IgG or a LOXL2-specific targeting antibody (24), to block secreted LOXL2 function. Tumors were harvested when they reached maximum size. Tumor sections were stained with antibodies specific for α-SMA, a marker of fibroblast activation. Inhibition of LOXL2 both by genetic means as well as antibody means led to a reduction in α-SMA-positive activated fibroblasts in the tumors (Figure 1A).
Figure 1. Inhibition of LOXL2 in vivo decreases fibroblast activation and production of a dense ECM structure.
(A) Immunofluorescent images of 4T1 control and shLOXL2 tumor sections as well as sections from wild-type tumors (4T1 wt) grown in mice treated with control antibody (cont IgG) or LOXL2-specific antibody (LOXL2 Ab) stained with an α-SMA-specific antibody and counterstained with haematoxylin. n = 8 and 8 for mice harboring 4T1 control and shLOXL2 tumors respectively. n = 3 for mice harboring 4T1 wt tumors treated with control IgG and LOXL2 antibody. 2-3 sections were stained for each tumour per mouse. Scale bar, 200μm. (B) Immunofluorescent images of 4T1 control and shLOXL2 tumor sections stained with antibodies to detect α-SMA (red) and the ER-TR7 antigen (green). Sections were counterstained with dapi (blue). n = 4 and 7 for mice harboring 4T1 control and shLOXL2 tumors respectively. 2-3 sections were stained for each tumour per mouse. Scale bar, 100μm. (C) Immunofluorescent images of 4T1 wt tumor sections taken from mice treated with control antibody (cont IgG) or LOXL2-specific antibody (LOXL2 Ab) stained with an α-SMA-specific antibody (red) and counterstained with dapi (blue). n = 3 for each treatment. Scale bar, 50μm. (D) Quantification of α-SMA positive cells in 4T1 control and shLOXL2 tumors (n = 4 and 7 respectively) as well as 4T1 wt tumors treated with control IgG or LOXL2 antibody (n = 3). **P < 0.01 and ***P < 0.001.
ER-TR7 is a marker for reticular fibroblasts and is also an antigen present on the ECM secreted by these fibroblasts, therefore using an antibody that detects the ER-TR7 antigen provides a means to identify fibroblasts and also visualize the fibrillar mesh-like ECM structure present in a tissue (31). To analyze the activated fibroblasts embedded in the ECM of the 4T1 tumors, sections were co-stained with antibodies specific for ER-TR7 and α-SMA. Control tumors contained many cells expressing α-SMA (red) embedded within an ECM matrix consisting of dense fibres lined up in a parallel fashion (green) (Figure 1B, left panels). In comparison, shLOXL2 tumors expressed very low levels of α-SMA and exhibited a scattered ECM structure (Figure 1B, right panels). Expression of α-SMA (red) was also analyzed by immunofluorescence in tumor sections taken from mice treated with control IgG or LOXL2-specific antibody (Figure 1C). Quantification of α-SMA cells in these tumor sections confirmed that inhibition of LOXL2 either genetically or by using a LOXL2-specific antibody resulted in significantly fewer α-SMA-positive cells (P < 0.0001 and P = 0.008, respectively; Figure 1D). 4T1 shLOXL2 tumors also possessed significantly lower levels of ER-TR7 staining compared to 4T1 control tumours (P = 0.003; Supplementary Figure 1C). These findings suggest that LOXL2 facilitates fibroblast activation and production of a strong fibrous ECM structure in 4T1 tumors, both of which are known to enhance tumor cell invasion and metastasis (32-36).
Inhibition of tumor-secreted LOXL2 reduces the branching of fibroblasts grown on collagen
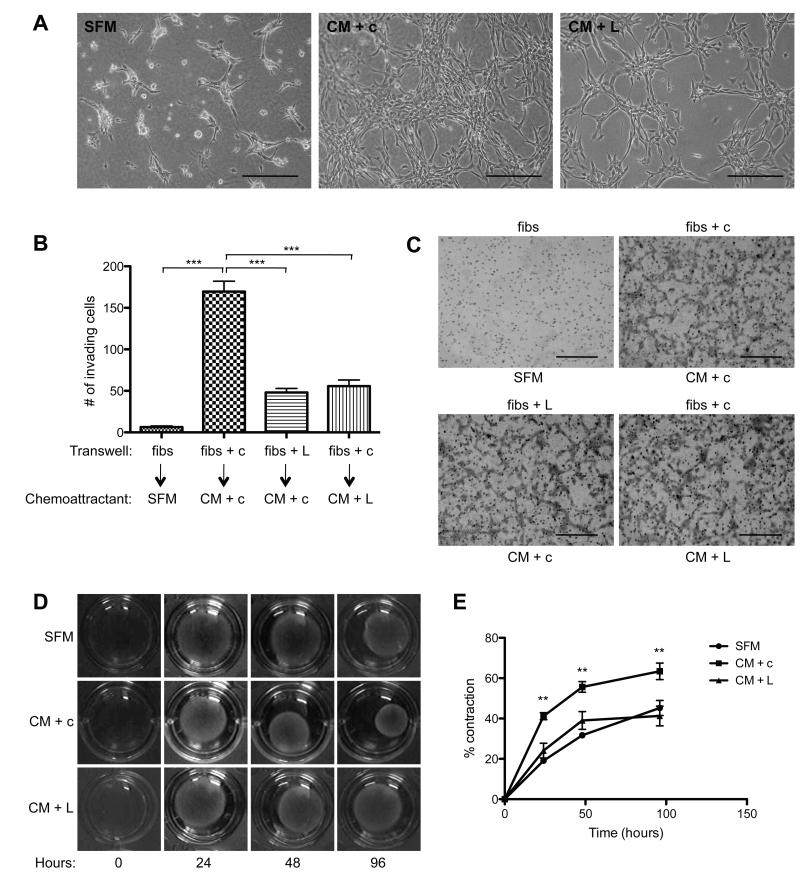
Following the observation that 4T1 tumors expressing high levels of LOXL2 exhibited extensive α-SMA expression compared with 4T1 shLOXL2 tumors, we sought to determine whether LOXL2 could drive fibroblast activation. Not all CAFs express α-SMA and once they do begin to express α-SMA they are not truly functional myofibroblasts until they have gained contractile properties by incorporating α-SMA into stress fibres (37), therefore, we used a number of in vitro models to assay LOXL2-mediated phenotypic changes in the fibroblasts. Initially, the morphology of fibroblasts grown on collagen matrices was assessed. Conditioned media (CM) from 4T1 cells was used as a source of tumor secreted factors, including high levels of LOXL2, in the following experiments. Immortalized mouse dermal fibroblasts, referred to simply as fibroblasts from hereon, plated on a layer of collagen initially formed round structures in the presence of serum-free media (SFM) and started to develop a branched phenotype after 48 hours. Fibroblasts were plated on top of a layer of collagen in normal growth media and allowed to adhere for 24 hours. The cells were then washed with SFM and incubated for 48 hours in 4T1 CM in the presence or absence of a LOXL2-specific targeting antibody. A goat isotype IgG was used as a control. Cells in adjacent wells were incubated with SFM alone. The fibroblasts formed branched structures within 24 hours in the presence of 4T1 CM (Figure 2A, centre panel). Inhibition of LOXL2 using a LOXL2-specific antibody dramatically reduced the ability of the fibroblasts to form branched structures (Figure 2A, right panel), suggesting tumor-secreted LOXL2 can activate fibroblasts in culture.
Figure 2. Tumor-derived LOXL2 facilitates fibroblast branching on collagen, fibroblast invasion and collagen contraction.
(A) Fibroblasts were plated on 1.5mg/ml collagen matrices and stimulated with serum-free media (SFM) or 4T1 conditioned media (CM) in the presence of control (c) or LOXL2-specific (L) antibodies. Scale bar, 200μm. (B) Quantification of fibroblasts (fibs) alone or in the presence of control (c) or LOXL2-specific antibody (L) invading through Matrigel (or migrating through uncoated control membranes (C)) towards SFM or CM in the presence of control or LOXL2-specific antibodies. ***P < 0.001. Scale bar, 500μm. (D) Contraction of collagen matrices by fibroblasts after incubating in SFM or CM in the presence of control (c) or LOXL2-specific (L) antibodies over 96 hours. (E) Quantification of collagen contraction seen in (D). **P < 0.01.
Inhibition of tumor-secreted LOXL2 reduces the ability of fibroblasts to invade through ECM
To investigate if tumor-secreted LOXL2 could influence fibroblast invasion we performed transwell invasion assays. Fibroblasts were plated on top of a layer of recombinant basement membrane (Matrigel) in transwell inserts. 4T1 CM was used as the ‘chemoattractant’ and SFM as a control. Uncoated (i.e. ECM-free) control transwell inserts were used to assess cell migration. The LOXL2-targeting antibody was used to inhibit fibroblast- or tumor-derived LOXL2 by adding it to either the fibroblasts or the CM chemoattractant, respectively. A goat isotype IgG was added to adjacent control wells. After incubation for 24 hours the number of fibroblasts that had invaded through the ECM towards the CM was quantified. Limited invasion of fibroblasts was observed when SFM was used as a control chemoattractant. In contrast, the fibroblasts became highly invasive when CM was used as a chemoattractant (P < 0.0001; Figure 2B). Inhibition of fibroblast- or tumor-derived LOXL2 significantly reduced the ability of the fibroblasts to invade through the ECM towards CM (P < 0.0001; Figure 2B). Importantly, inhibition of LOXL2 had no effect on non-invasive cell migration in the control transwell inserts (Figure 2C). These findings suggest that LOXL2 secreted from the fibroblasts as well as the tumor cells is required to facilitate fibroblast invasion through ECM.
LOXL2 is required for fibroblast-mediated collagen contraction
When surrounded by matrix in the presence of tumour-secreted factors, fibroblast-matrix adhesions increase as a result of fibroblast activation, leading to contraction of the collagen matrices. We therefore investigated the effect of tumor-secreted LOXL2 on fibroblast-mediated collagen contraction. Fibroblasts were suspended in collagen matrices and incubated in 4T1 CM in the presence or absence of a LOXL2-targeting antibody. A goat isotype IgG was used as a control. Addition of the CM induced the fibroblasts to contract the collagen gels by 63.43 ± 4.10% over 96 hours (Figure 2D + E). In contrast, fibroblasts suspended in collagen gels exposed to CM and a LOXL2-specific antibody were only able to induce 41.28 ± 4.91% contraction over the same period, similar to the rate of contraction observed for the control gels, where fibroblasts were suspended in collagen incubated in SFM alone (Figure 2D + E). Quantitative analysis revealed that the LOXL2-specific antibody significantly inhibited contraction at 24, 48 and 96 hours (P = 0.0018, 0.0089 and 0.0061, respectively; Figure 2E). To confirm that LOXL2 facilitates fibroblast branching on collagen and contraction of collagen these assays were performed with 4T1 shLOXL2 CM. Similarly to fibroblasts incubated with 4T1 CM in the presence of a LOXL2-specific antibody, fibroblasts incubated with 4T1 shLOXL2 CM formed fewer branches on collagen and had decreased collagen contraction ability (Supplementary Figure 2).
Recombinant LOXL2 protein induces fibroblast branching, contraction and invasion
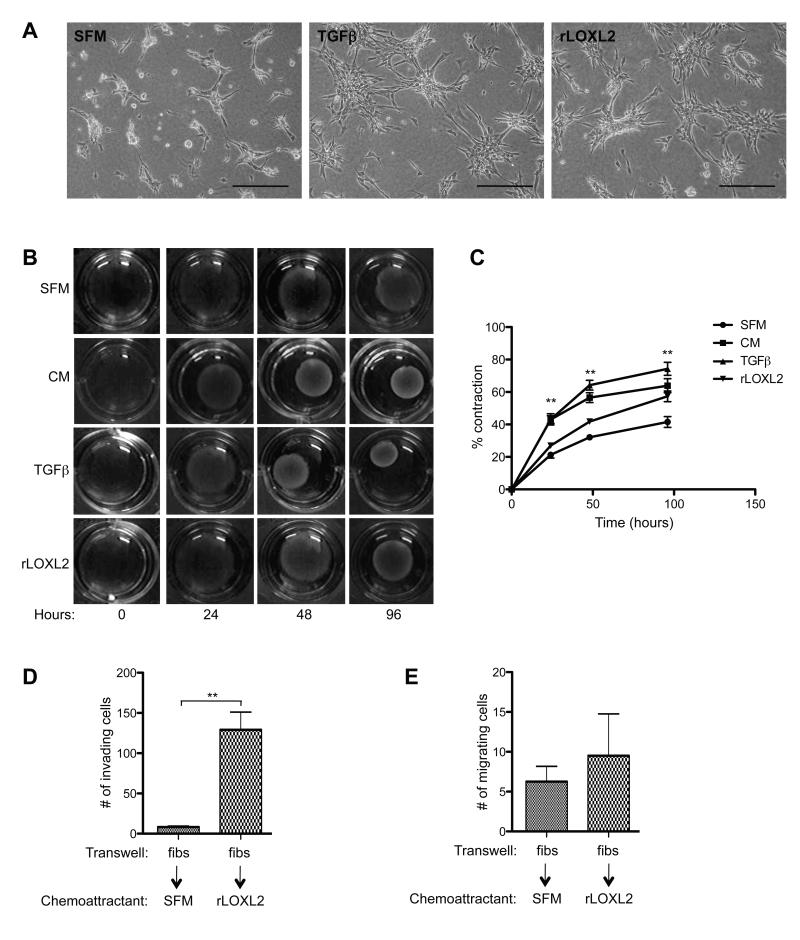
To confirm that LOXL2 in the CM was driving fibroblast activation we assessed the response of fibroblasts to recombinant LOXL2 protein (rLOXL2). TGFβ is the most potent myofibrogenic growth factor in wound healing and fibrosis (38-40) and provided a positive control for fibroblast activation. Fibroblasts formed more extensive branched structures after 24 hours in the presence of TGFβ compared to SFM alone (Figure 3A). Importantly, addition of rLOXL2 induced the fibroblasts to form extensive branched structures within 24 hours, comparable to the rate of branching observed in response to TGFβ, suggesting that LOXL2 alone is able to activate fibroblasts (Figure 3A, right panel).
Figure 3. Recombinant LOXL2 stimulates fibroblast branching on collagen, collagen contraction and invasion of fibroblasts through ECM.
(A) Fibroblasts were plated on 1.5mg/ml collagen matrices and stimulated with serum-free media (SFM), TGFβ or recombinant LOXL2 (rLOXL2) protein. Scale bar, 200μm. (B) Contraction of collagen matrices by fibroblasts after incubating in SFM, 4T1 conditioned media (CM), TGFβ or rLOXL2 protein over 96 hours. (C) Quantification of collagen contraction seen in (B). (D) Quantification of fibroblasts (fibs) invading through Matrigel (or migrating through uncoated control membranes (E)) towards SFM or rLOXL2. **P < 0.01.
Recombinant LOXL2 was also able to induce 57.34 ± 3.28% fibroblast-mediated collagen contraction over 96 hours compared with 41.52 ± 3.32% contraction observed for control fibroblasts incubated in SFM alone (Figure 3B + C). Quantitative analysis revealed that rLOXL2 significantly enhanced contraction above what was observed in SFM alone at 24, 48 and 96 hours (P = 0.035, <0.0001 and 0.0069, respectively; Figure 3C).
To determine whether rLOXL2 could increase fibroblast invasion directly, transwell invasion assays were carried out. Significantly increased invasion through ECM was observed when rLOXL2 was provided as a chemoattractant (P = 0.0016; Figure 3D). Of note, rLOXL2 was unable to induce fibroblast migration through uncoated control membranes (Figure 3E). These findings further support the direct effects of LOXL2 on fibroblast activation as measured by increased branching on collagen matrices, collagen contraction and invasion.
Fibroblasts exposed to rLOXL2 express increased α-SMA
To determine whether fibroblasts incubated with rLOXL2 were indeed activated at a molecular level, fibroblasts were grown on collagen and harvested for protein and RNA analysis 48 hours after the addition of rLOXL2. Western blotting revealed that exogenous LOXL2 induced the expression of α-SMA to similar levels observed in fibroblasts incubated with TGFβ (Figure 4A). Quantitative real time-PCR (QRT-PCR) analysis confirmed a 2.58-fold (± 0.20) increase in the expression of the gene encoding α-SMA (acta2) following incubation with rLOXL2 (P < 0.0001; Figure 4B). These results support a role for LOXL2 in directly activating fibroblasts.
Figure 4. Recombinant LOXL2 mediates fibroblast activation.
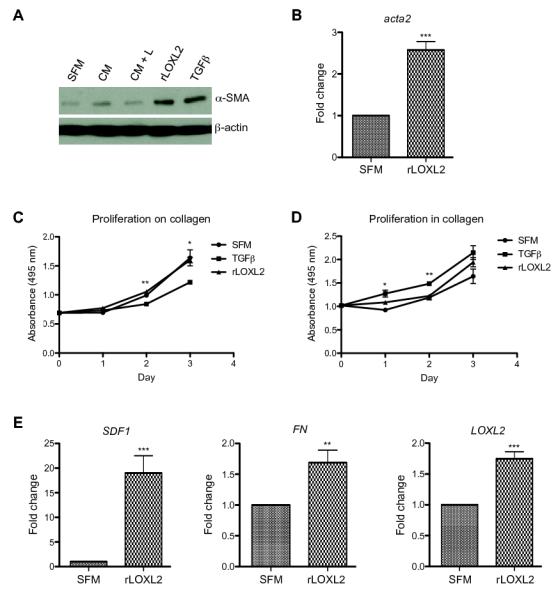
(A) Western blot analysis of α-SMA expression in fibroblasts plated on collagen and stimulated with 4T1 conditioned media (CM) in the presence of control (c) or LOXL2-specific (L) antibodies or SFM in the presence of TGFβ or rLOXL2 protein. Fibroblasts grown in SFM provided a control. Probing with an antibody to detect β-actin was used as a loading control. (B) QRT-PCR analysis of acta2 mRNA expression in fibroblasts grown on collagen in the presence of rLOXL2. Gene expression levels are shown as fold-changes over the expression measured in fibroblasts grown in the presence of SFM alone. ***P < 0.001. (C) Proliferation of fibroblasts grown over 3 days on top of collagen (or within collagen (D)) in the presence of TGFβ or rLOXL2 protein assessed using an MTS assay. Proliferation rates were compared with those of fibroblasts grown in SFM alone. *P < 0.05 and **P < 0.01. (E) QRT-PCR analysis of SDF1, FN and LOXL2 mRNA expression in fibroblasts grown on collagen in the presence of rLOXL2 protein. Gene expression levels are shown as fold-changes over the expression measured in fibroblasts grown in the presence of SFM alone. **P < 0.01 and ***P < 0.001
Recombinant LOXL2 does not induce fibroblast proliferation
To confirm that LOXL2-mediated phenotypic changes were not due to altered proliferation, we carried out MTS assays. Fibroblasts were plated on a layer of collagen, to mimic branching assays, or mixed within a collagen matrix, to mimic collagen contraction assays, and stimulated with TGFβ or rLOXL2 for 3 days. Cell growth in separate wells was measured each day. No significant differences in proliferation of fibroblasts plated either on collagen or in collagen after incubation with rLOXL2 were observed (Figure 4C + D), supporting the effects of LOXL2 on fibroblast phenotype being due to fibroblast activation alone. Interestingly, addition of TGFβ significantly decreased the proliferation of fibroblasts grown on collagen (P = 0.0013 and 0.042 on day 2 and 3, respectively; Figure 4C) and increased the proliferation of fibroblasts grown in collagen (P = 0.011 and 0.0062 on day 1 and 2, respectively; Figure 4D). This highlights the complexity of TGFβ action on cell proliferation and differentiation and suggests that LOXL2 may activate fibroblasts in a TGFβ-independent manner.
Fibroblasts exposed to rLOXL2 exhibit increased expression of SDF1 and fibronectin
Activated CAFs secrete elevated levels of extracellular matrix proteins and tumor-promoting factors such as fibronectin (FN) and stromal cell-derived factor 1 (SDF1) (4, 12, 13). To determine whether fibroblasts activated in the presence of exogenous LOXL2 express more FN and SDF1, cells were plated on collagen, stimulated with rLOXL2 for 48 hours and gene expression assessed by QRT-PCR. Unstimulated fibroblasts provided a control. Fibroblasts stimulated with rLOXL2 exhibited a 19.00-fold (± 3.51) increase in the expression of SDF1 (P = 0.0006; Figure 4E, left panel). In addition, rLOXL2-stimulated fibroblasts exhibited a significant but less dramatic 1.69-fold (± 0.20) increase in the expression of FN (P = 0.0037; Figure 4E, centre panel). Interestingly, these fibroblasts also expressed 1.75-fold (± 0.11) increased levels of LOXL2 (P < 0.0001; Figure 4E, right panel), suggesting a positive feed forward loop has been established which maintains high levels of secreted LOXL2 in the tumor microenvironment.
LOXL2 mediates fibroblast activation through β3 integrins
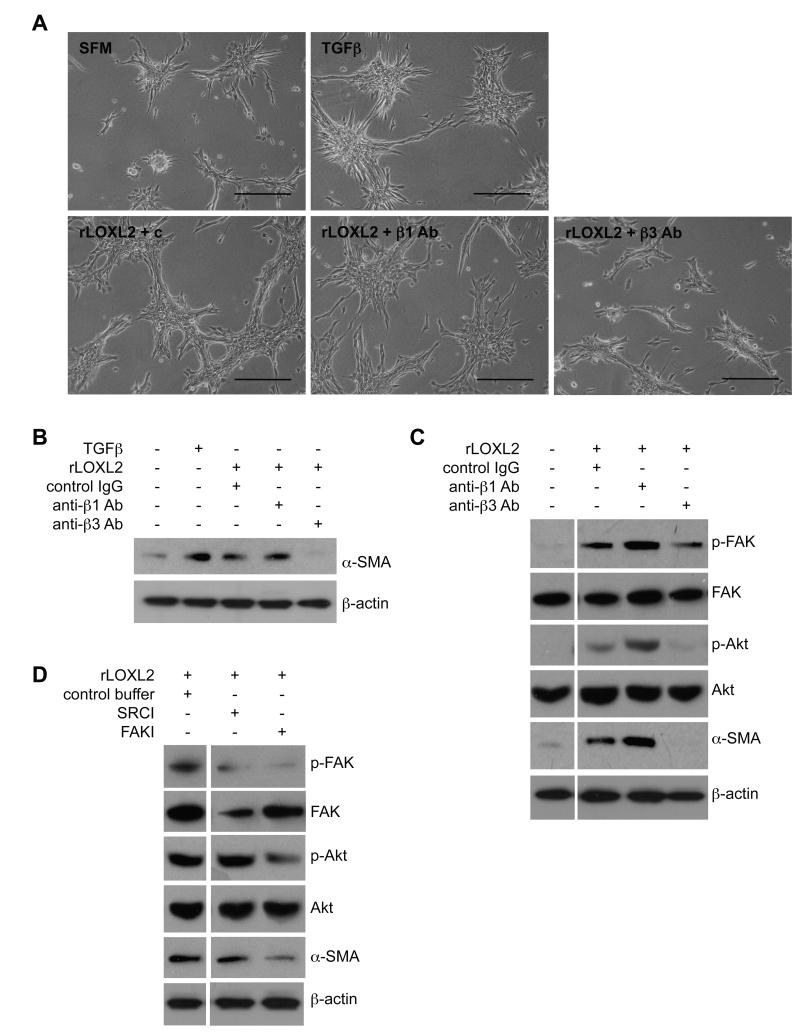
Our findings suggested a role for secreted LOXL2 in fibroblast activation. One method by which fibroblasts respond to external signals is through integrins (41) and the most common integrins found in focal adhesions contain the β1 and β3 subunits (42, 43). To determine whether these integrin subunits mediate fibroblast activation in response to exogenous LOXL2 we used β1 and β3 function-blocking antibodies. Fibroblasts were plated on collagen matrices and allowed to adhere for 24 hours. Cells were then washed and incubated with rLOXL2 in the presence or absence of integrin-specific blocking antibodies. A hamster isotype IgG was used as a control. LOXL2-mediated formation of branches was reduced in the presence of integrin β3-specific antibody but not in the presence of integrin β1-specific antibody (Figure 5A). To confirm these findings, cells were harvested for western blotting analysis. Consistent with the phenotypic studies, integrin β3-specific antibody but not integrin β1-specific antibody could block LOXL2-mediated activation of fibroblasts as determined by the expression of α-SMA (Figure 5B).
Figure 5. LOXL2-mediated matrix remodeling activates fibroblasts via FAK and Akt signaling.
(A) Fibroblasts were plated on 1.5mg/ml collagen matrices and stimulated with serum-free media (SFM), TGFβ or recombinant LOXL2 (rLOXL2) protein in the presence of control (c) or blocking antibodies specific to β1 or β3 integrins (β1 Ab and β3 Ab, respectively). Scale bar, 200μm. (B) Western blot analysis of α-SMA expression in fibroblasts plated on collagen and stimulated with rLOXL2 in the presence of control antibody or blocking antibodies specific to β1 or β3 integrins. Probing with an antibody to detect β-actin was used as a loading control. (C) Western blot analysis of FAK activation (p-FAK) and Akt activation (p-Akt) in fibroblasts plated on collagen and stimulated with rLOXL2 in the presence of control antibody or blocking antibodies specific to β1 or β3 integrins. Expression of α-SMA was also determined. Probing with antibodies to detect total FAK, Akt and β-actin were used as loading controls. (D) Western blot analysis of FAK activation (p-FAK), Akt activation (p-Akt) and α-SMA expression in fibroblasts plated on collagen and stimulated with rLOXL2 in the presence of SRC or FAK inhibitors (SRCI and FAKI, respectively).
LOXL2 activates fibroblasts through FAK and Akt signaling
Focal adhesion kinase (FAK) is a critical component of focal adhesions that associates with integrins and relays signals from extracellular stimuli (20, 44, 45). We investigated whether LOXL2-driven fibroblast activation was mediated via FAK phosphorylation. Firstly, fibroblasts plated on collagen matrices were incubated with rLOXL2 in the presence or absence of integrin-specific blocking antibodies. Western blot analysis revealed that phosphorylation of FAK was increased in cells exposed to rLOXL2 (Figure 5C). Incubation with an integrin β3-specific antibody reduced FAK phosphorylation as well as phosphorylation of Akt downstream of FAK (Figure 5C). This inhibition was specific for integrin β3, as integrin β1-blocking antibodies did not reduce FAK or Akt activation mediated by rLOXL2 (Figure 5C). Indeed, blocking with the integrin β1-blocking antibody appeared to increase phosphorylation of Akt. Therefore, we tested another integrin β1-blocking antibody, which targets integrin α5β1. In addition, we tested all the antibodies on 3T3 fibroblasts to confirm the previous results were not just a characteristic of C57Bl/6 fibroblasts. Once again, inhibition of rLOXL2-mediated activation of FAK, Akt and α-SMA in fibroblasts grown on collagen was specific for integrin β3 and was not affected by blocking integrin β1 (Supplementary Figure 3A). Furthermore, neither of the two integrin β1-blocking antibodies was able to inhibit rLOXL2-mediated collagen contraction by 3T3 fibroblasts, whereas a reduction in collagen contraction was observed following treatment with integrin β3-blocking antibody (Supplementary Figure 3B).
To confirm that LOXL2 mediates activation of fibroblasts through FAK signaling, a FAK inhibitor (FAKI) was added to fibroblasts growing on collagen in the presence of rLOXL2. Phosphorylation of FAK was reduced following incubation with the FAKI and this led to decreased Akt phosphorylation and reduced expression of α-SMA (Figure 5D). We also tested the involvement of SRC, another important component of focal adhesion complexes, by adding a SRC inhibitor (SRCI) to fibroblasts incubated with rLOXL2. This did not result in decreased phosphorylation of FAK (when compared to total FAK levels) or Akt, nor was a decrease in the expression of α-SMA observed (Figure 5D), demonstrating that LOXL2 activates fibroblasts specifically through engaging the FAK/Akt signaling pathway.
DISCUSSION
The influence of the tumor microenvironment on the proliferation, invasion and metastatic potential of the tumor cells is now widely appreciated, however, the underlying molecular mechanisms have remained unclear. In particular, the tumor-promoting ability of α-SMA-positive CAFs, the myofibroblasts, has been demonstrated in breast, prostate, pancreatic and skin cancer mouse models (9-14). Following the observation that 4T1 tumors expressing very high levels of LOXL2 exhibited extensive α-SMA expression compared with 4T1 shLOXL2 tumors, we sought to determine whether LOXL2 could facilitate fibroblast activation and hence play a role in formation of the myofibroblast population of CAFs present in the tumor microenvironment. By assaying for α-SMA expression as well as phenotypic changes we were able to investigate the role of LOXL2 in fibroblast activation.
Fibroblasts exposed to tumor-derived LOXL2 or recombinant LOXL2 protein exhibited increased rates of branching on collagen matrices and increased contraction of collagen. In addition, tumor-derived LOXL2 as well as rLOXL2 behaved as chemoattractants in transwell assays, inducing fibroblasts to invade through ECM. Finally, fibroblasts incubated with rLOXL2 expressed elevated levels of α-SMA, SDF1, FN and LOXL2, further confirming the activated state of these cells. We hypothesized that exogenous LOXL2 was inducing fibroblast activation through engaging integrins and activating components of focal adhesion complexes. Indeed, rLOXL2 induced α-SMA expression in the fibroblasts through β3 integrin, FAK and Akt activation. In contrast to the decreased Akt activation seen in the presence of integrin β3-blocking antibodies, we noted a slight increase in Akt activation following inhibition of integrin β1 with two separate blocking antibodies. The reason for this apparent increased Akt activation is unknown and will be subject to future investigations. Finally, the fibroblasts used in all of these studies were of C57Bl/6 origin. We validated many of the results in 3T3/Swiss fibroblasts demonstrating that LOXL2-mediated fibroblast activation was not strain specific.
LOXL2 was first identified due to its overexpression in normal senescent human fibroblasts (46). Interestingly, it has been shown that many genes overexpressed in senescent fibroblasts are also upregulated in CAFs (47). Moreover, LOXL2 belongs to a gene expression signature in fibroblasts that is upregulated in response to serum and has the ability to predict cancer progression (48) and outcome in patients with breast cancer (49). Together with the data we have presented here it is clear that LOXL2 plays an important role in fibroblasts and their activation in the tumor microenvironment.
We have previously shown that inhibiting LOXL2 in mammary cancer cells reduces tumor cell invasion and metastasis, without reducing primary tumor growth (24). The data we have presented here suggest that the decreased metastasis observed following LOXL2 inhibition is due not only to decreased invasion of the tumor cells themselves but also to reduced fibroblast activation in the tumor stroma. Importantly, we have additionally shown that activated fibroblasts then express more LOXL2, driving a feed-forward loop to further enhance cancer progression. Hence, treatment of the mice with LOXL2-specific inhibitors will inhibit fibroblast-derived LOXL2 both directly and indirectly. Therefore, the functional significance of inhibiting LOXL2-mediated activation of fibroblasts is the reduction of tumor cell invasion and metastasis rather than decreased growth of the primary tumor.
In summary, our findings uncover a novel mechanism of fibroblast activation, a novel function for LOXL2, and strongly support the targeting of tumor-derived LOXL2 to prevent cancer progression.
Supplementary Material
Acknowledgements
We would like to thanks the staff of the BSU and histopathology departments at the Institute of Cancer Research for assistance with in vivo work and processing of tissue samples. The study was designed and developed by HEB and JTE. Experiments were performed by HEB with the assistance of DB and GL. The manuscript was written by HEB with assistance from JTE.
Grant Support We are grateful to the Association for International Cancer Research [#09-0796] (HEB), the Institute of Cancer Research (HEB, JTE), and Cancer Research UK [#C107/A10433] (DB, GL and JTE) for supporting the research.
Abbreviations
- LOXL2
lysyl oxidase-like 2
- shRNA
short hairpin RNA
- α-SMA
alpha smooth muscle actin
- FAK
focal adhesion kinase
- CAF
cancer associated fibroblast
- SFM
serum-free media
- CM
conditioned media
- rLOXL2
recombinant LOXL2
- QRT-PCR
quantitative real-time PCR
- SDF1
stromal-cell derived factor 1
- FN
fibronectin
- FAKI
FAK inhibitor
- SRCI
SRC inhibitor
Footnotes
CONFLICTS OF INTEREST: The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Tarin D, Croft CB. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105:189–90. [PubMed] [Google Scholar]
- 4.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 7.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–12. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 8.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–83. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 9.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42. [PubMed] [Google Scholar]
- 16.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 18.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 19.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 20.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89:8487–91. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 22.Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–31. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- 23.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 24.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–72. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 26.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103:407–24. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 27.Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2012 doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 28.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–16. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 30.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem. 1986;34:883–90. doi: 10.1177/34.7.3519751. [DOI] [PubMed] [Google Scholar]
- 32.Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–15. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 35.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G, Desmouliere A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6 doi: 10.4161/cam.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–6. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- 39.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–44. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 41.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–86. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 44.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller MD, Parsons JT. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993;3:258–62. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 46.Saito H, Papaconstantinou J, Sato H, Goldstein S. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272:8157–60. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 47.Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98:245–9. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102:3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.