Abstract
Background
Dietary fiber reduces the intestinal absorption of nutrients and the blood concentrations of cholesterol and triglycerides.
Aim
We wished to test the hypothesis that high-viscosity (HV) and low-viscosity preparations of barley and oat β-glucan modify the expression of selected genes of lipid-binding proteins in the intestinal mucosa and reduce the intestinal in vitro uptake of lipids.
Methods
Five different β-glucan extracts were separately added to test solutions at concentrations of 0.1–0.5% (wt/wt), and the in vitro intestinal uptake of lipids into the intestine of rats was assessed. An intestinal cell line was used to determine the effect of β-glucan extracts on the expression of intestinal genes involved in lipid metabolism and fatty acid transport.
Results
All extracts reduced the uptake of 18:2 when the effective resistance of the unstirred water layer was high. When the unstirred layer resistance was low, the HV oat β-glucan extract reduced jejunal 18:2 uptake, while most extracts reduced ileal 18:2 uptake. Ileal 18:0 uptake was reduced by the HV barley extract, while both jejunal and ileal cholesterol uptakes were reduced by the medium-purity HV barley extract. The inhibitory effect of HV barley β-glucan on 18:0 and 18:2 uptake was more pronounced at higher fatty acid concentrations. The expression of genes involved in fatty acid synthesis and cholesterol metabolism was down-regulated with the HV β-glucan extracts. β-Glucan extracts also reduced intestinal fatty-acid-binding protein and fatty acid transport protein 4 mRNA.
Conclusions
The reduced intestinal fatty acid uptake observed with β-glucan is associated with inhibition of genes regulating intestinal uptake and synthesis of lipids. The inhibitory effect of β-glucan on intestinal lipid uptake raises the possibility of their selective use to reduce their intestinal absorption.
Keywords: β-Glucan, Cholesterol, Fatty acids, Intestinal lipid uptake, Sterol regulatory element-binding protein, Fatty acid synthesis
1. Introduction
It is of nutritional and medical interest that dietary fibers may be used to reduce the intestinal absorption of nutrients and the blood concentrations of cholesterol and triglycerides, and thereby be of therapeutic benefit. For example, feeding fiber supplements to diabetic animals reduces nutrient absorption [1,2]. In humans, dietary fiber has been used to reduce the blood concentrations of cholesterol and triglycerides [3,4], presumably acting to reduce the intestinal absorption of lipids. Similarly, long-term feeding studies incorporating viscous soluble fiber in the form of β-glucan have demonstrated reductions in plasma cholesterol concentrations in hypercholesterolemic men [5,6]. The inhibitory effect of viscous soluble fiber on postprandial rise in glucose and insulin [7] is diminished when the viscosity of the fiber preparation is reduced by acid treatment [8].
β-Glucan is a type of soluble fiber that is found in cereal grains such as oats and barley [8,9]. Molecular weight, solubility and viscosity are important physicochemical properties of β-glucan that are affected by the genetic attributes of oat/barley grains (waxy vs. nonwaxy), as well as processing conditions [10]. These physicochemical properties influence the functionality of β-glucan in the body [11–13] (e.g., as demonstrated by Wood et al. [14] in terms of the relationship between β-glucan viscosity and glycemic response). High β-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes [15]. It has been suggested that cereals that are high in β-glucan increase the viscosity of meal bolus in the small intestine, thereby delaying nutrient absorption [16].
An increase in the effective resistance or viscosity of the unstirred water layer (UWL) adjacent to the intestinal brush border membrane (BBM) reduces the uptake of lipids and sugars [17–19]. It is not known whether the inhibitory effect of viscous dietary fibers on the uptake of lipids is due to an increase in the viscosity — and therefore the resistance — of the UWL, a direct effect on the BBM or some other yet unidentified mechanism. Thus, it is important to examine the effect of these extracts on lipid uptake under experimental conditions, where UWL resistance is both high and low. Previous studies have validated the use of this in vitro method in which the thickness and resistance of the UWL are high when the bulk phase is unstirred (0 rpm). When the bulk phase is stirred at 600 rpm, the thickness and resistance of the UWL are lower. The high-resistance situation applies to the in vivo situation and largely reflects the viscosity of the UWL. When the resistance of the UWL is low, the potential effects of the β-glucan on the function of the intestinal BBM may be better assessed.
The uptake of cholesterol and long-chain fatty acids across the BBM is mediated both by passive transport and by BBM transporters [20,21]. Once lipids are in the enterocytes, they may be metabolized or transported out of the cell. Three key transcription factors designated as sterol regulatory element-binding proteins (SREBPs) 1a, 1c and 2 regulate the transcription of genes involved in fatty acid and cholesterol syntheses [22]. Dietary polyunsaturated fatty acids suppress intestinal SREBP-1c mRNA without altering the expression of its target gene fatty acid synthase (FAS) or acetyl-CoA carboxylase (ACC) [23], but the effects of dietary fiber extracts on these pathways are not known. In addition, beyond the observation that intestinal fatty-acid-binding protein (i-FABP) mRNA is lower in exfoliated colonocytes in the feces of rats fed oat bran versus rats fed wheat bran [24], our understanding of the regulation of genes involved in fatty acid uptake in the intestine [fatty acid transport protein 4 (FATP4), ileal lipid-binding protein (ILBP) and i-FABP] is limited. These fatty-acid-binding proteins may play a role in the intestinal absorption of lipids [25,26]. Accordingly, the objective of this study was to test the hypothesis that the β-glucan in barley and oats has an antiabsorptive effect and that the inhibitory effect is influenced by the physico-chemical properties of the β-glucan and may also be associated with down-regulation of i-FABP and FATP4 genes involved in fatty acid uptake in rats.
2. Materials and methods
2.1. Preparation of β-glucan extracts
Derby and CDC Candle varieties of regular oats and waxy barley, respectively, were used for the extraction of β-glucan. High-purity (HP) and high-viscosity (HV) β-glucan fractions were prepared by alkali extraction (using sodium bicarbonate at pH 9 and 55°C for 1 h) of β-glucan from oat/barley flour (from grains pearled to 20% and pin milled), followed by its precipitation, washing with ethanol and air drying, as previously described by Thomson and Dietschy [18]. Low-viscosity (LV) β-glucan fractions were obtained by exposing the HV β-glucan solutions to excess shear by pumping the solution through a Microfluidizer Processor (M-110 EH; Microfluidics, Newton, MA) at a pressure between 15,000 and 20,000 psi. Medium-purity (MP) barley (CDC Candle) β-glucan extract was prepared in accordance with Vasanthan and Temelli [27].
The β-glucan content of the extracts was determined according to the enzymatic assay procedure of McCleary and Glennie-Holmes [28] using the β-glucan determination kit obtained from Megazyme International Ireland, Ltd. (Wicklow, Ireland). Solutions of different gum concentrations (0.2–1.0%, wt/wt) were prepared by mixing the powder into water, heating it up to 85°C and holding it at that temperature for 1 h with continuous stirring to ensure complete solubilization of β-glucan.
The viscosity of β-glucan gum solutions was determined using a UDS 200 Dynamic Spectrometer (rheometer) (PAAR Physica, Glen Allen, VA) in control shear rate mode equipped with a DG27 double-gap cup and bob and a Peltier temperature control unit. Viscosity was determined in duplicate at a shear rate of 129 s−1 (100 rpm). The instrument was calibrated with S3 standard oil (3.408 mPa s at 25°C; Cannon Instrument Co., State College, PA). Tests were performed at 20°C (±0.03°C) using a 7-ml sample size, which was measured by weight, not by volume, for increased accuracy.
2.2. Animals
The principles for the care and use of laboratory animals, approved by the Canadian Council on Animal Care and the Council of the American Physiological Society, were observed in the conduct of these studies. The protocol was approved by the Animal Ethics Committee of the University of Alberta. Forty-eight adult male and female BioBreeding rats were housed in pairs at a temperature of 21°C, with 12 h of light and 12 h of darkness. Water and food were supplied ad libitum. Animals were fed a standard laboratory chow diet.
2.3. Lipid uptake studies
2.3.1. Tissue preparation and incubation
The animals (seven to eight in each group) were sacrificed by an intraperitoneal injection of Euthanyl (sodium pentobarbital, 240 mg/100 g body weight). The whole length of the small intestine was rapidly removed and rinsed with cold saline. The intestine was opened along its mesenteric border. The proximal third (“jejunum”) and the distal third (“ileum”) of the intestine were cut and mounted as flat sheets in transport chambers. The chambers were placed in preincubation beakers containing oxygenated Krebs–bicarbonate buffer (pH 7.2) at 37°C, and tissue discs were preincubated for 15 min to allow the tissue to equilibrate at this temperature. The rate of uptake of nutrients was determined from the timed transfer of the transport chambers to incubation beakers containing [3H]inulin and 14C-labeled nutrients in oxygenated Krebs–bicarbonate buffer (pH 7.2, 37°C).
The 14C-labeled probes included stearic acid (18:0), linoleic acid (18:2) and cholesterol. The labeled probes were supplied by Amersham Biosciences, Inc. (Baie d’Urfe, QC), and unlabeled probes were supplied by Sigma (St. Louis, MO).
In the first experiment, five freshly prepared β-glucan extracts (E1, E2, E3, E4 and E5) from barley or oat sources (Table 1) were added directly to Krebs–bicarbonate buffer at a concentration of 0.5% (wt/wt). No extract was added to the control solution. The probe concentrations used were 0.10 mM long-chain fatty acids and 0.05 mM cholesterol.
Table 1.
Properties and sources of β-glucan extracts
| Extract | Source | β-Glucan content of extracts (% wt/wt) | Viscosity of 0.5% (wt/wt) extract solution (mPa s measured at 129 s−1 and 20°C) |
|---|---|---|---|
| E1 | Barley (Candle variety) | High (74.1%) | High (37.2) |
| E2 | Barley (Candle variety) | High (74.1%) | Low (5.0) |
| E3 | Oat (Derby variety) | High (78.5%) | High (17.8) |
| E4 | Oat (Derby variety) | High (78.5%) | Low (6.0) |
| E5 | Barley (Candle variety) | Medium (50%) | Higha |
Unfortunately, the data on E5 viscosity have been lost.
For “stirred” solutions, preincubation and incubation beakers were mixed with circular magnetic bars at identical stirring rates, which were precisely adjusted using a strobe light. Stirring rates were reported as revolutions per minute. A stirring rate of 600 rpm was selected to achieve a low effective resistance of the intestinal UWL [15,29,30]. For “unstirred” solutions, preincubation beakers were stirred at 600 rpm, but the test solution in the incubation beakers was unstirred (0 rpm).
In the second uptake experiment, we examined the effect of 0.5% E1 (HP, HV barley β-glucan) on the in vitro intestinal uptake of varying concentrations (0.05–0.2 mM) of 18:0, 18:2 and cholesterol when the bulk phase was stirred at 600 rpm.
In all of these experiments, the lipids were solubilized in 20 mM taurodeoxycholic acid (Sigma), and [3H]inulin was used as a nonabsorbable marker to correct for adherent mucosal fluid volume.
2.3.2. Determination of uptake rates
After the incubation of the discs in labeled solutions for 6 min, the experiment was terminated by removing the chamber and by rinsing the tissue in cold saline for approximately 5 s. The exposed mucosal tissue was then cut out of the chamber with a circular steel punch, placed on a glass slide and dried overnight in an oven at 55°C. The dry weight of the tissue was determined, and the tissue was transferred to scintillation counting vials. The samples were saponified with 0.75 M NaOH, scintillation fluid was added and radioactivity was determined with an external standardization technique to correct for variable quenching of the two isotopes.
The rates of uptake of nutrients were determined as nanomoles per 100 mg of tissue per minute. Two-way analysis of variance (ANOVA) was performed, and individual differences were determined using the Student–Neuman–Keuls (SNK) multiple-range test. Statistical significance was accepted as P≤.05.
2.4. Gene expression studies
2.4.1. NCI-H716 intestinal cell culture
The methods used for intestinal cell culture have been published [31,32]. Human NCI-H716 cells were obtained from the American Type Culture Collection (Rockville, MD). For proliferation, the cells were grown in a suspension in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. For the experiments, a total of 2×106 cells were seeded in six-well culture plates coated with Matrigel (Becton Dickinson, Bedford, MA) and grown in Dulbecco’s modified Eagle’s medium high glucose, 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. After 24 h, a fresh medium without serum but containing 0.2% delipidated bovine serum albumin and test agents were added. After 42 h, the cells were washed with Hank’s balanced salt solution and stored at −80°C until RNA extraction.
2.4.2. Analysis of mRNA expression
Total RNA was extracted using the RNeasy total RNA purification system, as described by the manufacturer (Qiagen). Reverse transcription was performed with an input of 1 μg of total RNA using the First Strand cDNA Synthesis Kit for RT-PCR (AMV) (Boehringer Mannheim) with oligod(T)15 as primer. Primers used for the amplification of cDNAs of interest were synthesized by Sigma Genosys (Ontario, Canada) and are described in Table 2. Polymerase chain reactions (PCRs) were heated for two cycles to 98°C for 1 min, 60°C for 2 min and 72°C for 2 min, and then cycled 28 times through a 1-min denaturation step at 94°C, a 1-min annealing step at 60°C and a 2-min extension step at 72°C in a DNA thermal cycler (Eppendorf USA). Actin primers were included in the reaction as internal controls for all genes. To ensure the quality of semiquantification PCR, both the control sequence and the sequence of interest were found to be in their log phase at 28 cycles, and the efficiencies of amplification of both sequences were similar. The reverse transcriptase step was equally efficient on both sequences. PCR products (10 μl) were separated on a 2% agarose gel and visualized by ethidium bromide staining. Quantification of the PCR products was performed using the densitometric NIH Image Program.
Table 2.
Primer sequences for reverse transcription PCR
| Gene | Forward primer | Reverse primer | PCR size (bp) |
|---|---|---|---|
| FAS | GGTCTTGAGAGATGGCTTGC | CAGGTTGACAGCAGCCAAGT | 519 |
| ACC | TACATGCCCAAGAGCGTACA | GCCATCCACAATGTAAGCAC | 510 |
| SREBP-1a | GCTTTGGAGCAGGCGCTG | GGGCTGGGGTAGCCTAAC | 476 |
| SREBP-1c | CGCGGAGCCATGGATTGC | GGGCTGGGGTAGCCTAAC | 440 |
| SREBP-2 | CTGGCTGCTCAAGAAAGTCT | AAGGACTCTATAGCTCGCTC | 540 |
| i-FABP | GGCTGGAATGTAGTGGAGAG | CTGGGCACTGTCGAATGTAC | 128 |
| ILBP | TGGGATCGCCAGCGATGTAAT | CGGAGTAGTGCTGGGACCAA | 101 |
| FATP4 | CTGCCTGAGCTGCACAAAAC | GTAGATAGAACAGCGGGTCTTCAC | 102 |
| Actin | GTTGCTATCCAGGCTGTG | CATAGTCCGCCTAGAAGC | 739 |
Results are presented as the average of a minimum of three independent experiments. Values for mRNA expression are expressed as average densitometric units±S.E.M. Statistical significance is represented as P≤.05 and determined using ANOVA.
3. Results
3.1. Effect of β-glucan extracts on lipid uptake
In the first experiment, five freshly prepared β-glucan extracts (E1, E2, E3, E4 and E5) from barley or oat sources (Table 1) were added to the test solutions at a concentration of 0.5%. In order to mimic the high UWL resistance observed in the intestine in vivo, we examined the in vitro uptake of nutrients when the bulk phase was unstirred (0 rpm) and, therefore, the UWL resistance was high. Under these conditions, the β-glucan extract preparations in a concentration of 0.5% had no effect on the jejunal or ileal uptake of 0.1 mM 18:0 or 0.05 mM cholesterol (Table 3). In contrast, each of the extracts significantly reduced both the jejunal uptake and the ileal uptake of 0.1 mM 18:2.
Table 3.
The effect of β-glucan extracts on the in vitro intestinal uptake of 18:0, 18:2 and cholesterol under conditions where the UWL resistance was high (bulk phase was unstirred)
|
In vitro intestinal uptake (nmol 100 mg−1 min−1)
|
||||||
|---|---|---|---|---|---|---|
| Control | Extract 1 (HP, HV barley) | Extract 2 (HP, LV barley) | Extract 3 (HP, HV oat) | Extract 4 (HP, LV oat) | Extract 5 (MP, HV barley) | |
| Jejunum | ||||||
| 18:0 | 0.34±0.06 | 0.19±0.04 | 0.21±0.04 | 0.27±0.07 | 0.23±0.03 | 0.18±0.04 |
| 18:2 | 0.42±0.07 | 0.20±0.04* | 0.19±0.03* | 0.10±0.02* | 0.27±0.06* | 0.06±0.02* |
| Cholesterol | 0.35±0.02 | 0.30±0.04 | 0.31±0.04 | 0.32±0.03 | 0.25±0.02 | 0.34±0.02 |
| Ileum | ||||||
| 18:0 | 0.28±0.04 | 0.25±0.05 | 0.24±0.04 | 0.24±0.04 | 0.29±0.04 | 0.27±0.06 |
| 18:2 | 0.66±0.09 | 0.02±0.03* | 0.33±0.11* | 0.27±0.05* | 0.14±0.03* | 0.12±0.03* |
| Cholesterol | 0.38±0.03 | 0.23±0.08 | 0.37±0.03 | 0.29±0.03 | 0.30±0.03 | 0.39±0.03 |
The lipids were solubilized in 20 mM taurodeoxycholic acid. The number of animals in each group was 8 (mean±S.E.M.).
Extract 1=HP, HV barley β-glucan; Extract 2=HP, LV barley β-glucan; Extract 3=HP, HV oat β-glucan; Extract 4=HP, LV oat β-glucan; Extract 5=MP, HV barley β-glucan.
Significantly different from control (ANOVA, SNK multiple-range test: P<.05). Comparisons were made within each row.
When the bulk phase was stirred at 600 rpm and the resistance of the UWL was low, the jejunal uptake of 0.1 mM 18:0 was not significantly affected by any of the extracts (Table 4). E3 (HP, HV oat) and E5 (MP, HV barley) significantly reduced the jejunal uptake of 0.1 mM 18:2. E5 (MP, HV barley) reduced jejunal cholesterol uptake. In the ileum, E1 (HP, HV barley) and E5 (MP, HV barley) reduced 18:0 uptake. Uptake of 18:2 was significantly reduced by E1 (HP, HV barley), E3 (HP, HV oat), E4 (HP, LV oat) and E5 (MP, HV barley). Ileal cholesterol uptake was reduced by E5 (MP, HV barley).
Table 4.
The effect of β-glucan extracts on the in vitro intestinal uptake of 18:0, 18:2 and cholesterol under conditions where the UWL resistance was low (bulk phase was stirred at 600 rpm)
|
In vitro intestinal uptake (nmol 100 mg−1 min−1)
|
||||||
|---|---|---|---|---|---|---|
| Control | Extract 1 (HP, HV barley) | Extract 2 (HP, LV barley) | Extract 3 (HP, HV oat) | Extract 4 (HP, LV oat) | Extract 5 (MP, HV barley) | |
| Jejunum | ||||||
| 18:0 | 80±0.14 | 0.35±0.06 | 1.00±0.23 | 0.75±0.11 | 0.76±0.15 | 0.29±0.06 |
| 18:2 | 1.49±0.13 | 1.09±0.14 | 1.41±0.28 | 0.74±0.11* | 0.95±0.20 | 0.74±0.08* |
| Cholesterol | 0.80±0.06 | 0.65±0.08 | 0.71±0.07 | 0.64±0.04 | 0.87±0.09 | 0.37±0.05* |
| Ileum | ||||||
| 18:0 | 0.69±0.07 | 0.29±0.04* | 0.51±0.08 | 0.50±0.07 | 0.62±0.07 | 0.14±0.05* |
| 18:2 | 1.03±0.09 | 0.63±0.08* | 0.82±0.09 | 0.59±0.05* | 0.53±0.04* | 0.63±0.07* |
| Cholesterol | 0.73±0.05 | 0.57±0.11 | 0.63±0.06 | 0.63±0.04 | 0.66±0.10 | 0.34±0.05* |
The lipids were solubilized in 20 mM taurodeoxycholic acid. The number of animals in each group was 7–8 (mean±S.E.M.).
Extract 1=HP, HV barley β-glucan; Extract 2=HP, LV barley β-glucan; Extract 3=HP, HV oat β-glucan; Extract 4=HP, LV oat β-glucan; Extract 5=MP, HV barley β-glucan.
Significantly different from control (ANOVA, SNK multiple-range test: P<.05). Comparisons were made within each row.
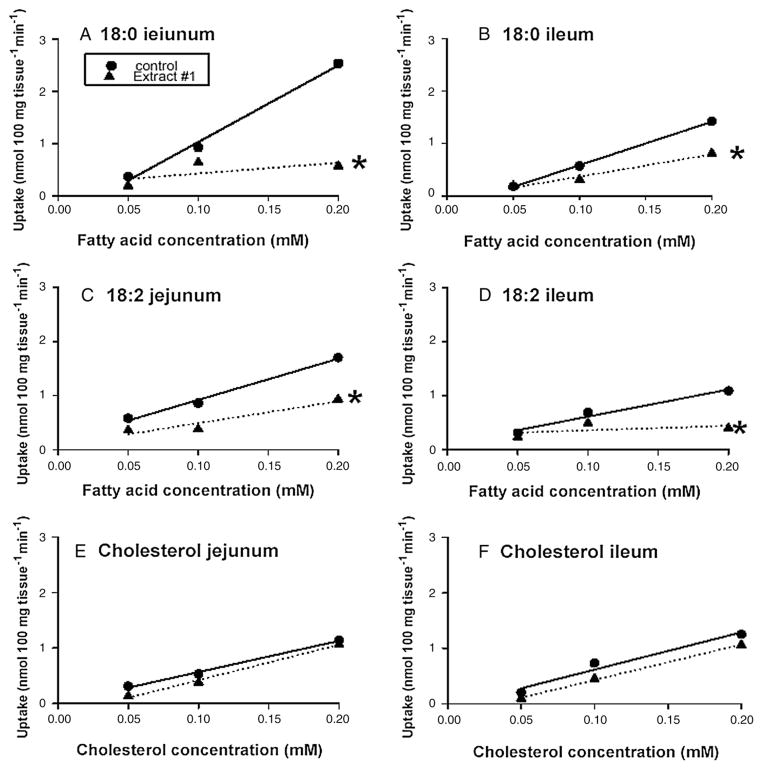
In the second experiment, we examined the effect of 0.5% E1 on the in vitro jejunal and ileal uptakes of varying concentrations (0.05–0.2 mM) of 18:0,18:2 and cholesterol when the bulk phase was stirred at 600 rpm (Fig. 1). The inhibitory effect of 0.5% E1 on the jejunal and ileal uptakes of varying concentrations of 18:0 and 18:2 became greater with higher concentrations of these fatty acids. The inhibitory effect of E1 became statistically significant only at higher concentrations of 18:0 (Fig. 1). Similarly, at higher concentrations of 18:2, E1 significantly inhibited jejunal uptake (Fig. 1). In contrast, even when the concentration of cholesterol was varied from 0.05 to 0.20 mM, E1 had no effect on cholesterol uptake (Fig. 1). Thus, the lack of effect of E1 on the uptake of cholesterol at a concentration of 0.05 mM (Table 4) cannot be regarded as a result of the concentration of cholesterol and likely represents a true lack of effect of E1 on cholesterol uptake.
Fig. 1.
The effect of 0.5% Extract #1 on the jejunal and ileal uptake of varying concentrations of 18:0,18:2 and chlolesterol. * Indicates a significant difference (p<0.05) between the slopes of the lines. The bulk phase was stirred at 600 rpm. The lipids were solubized in 20 mM taurodeoxycholic acid. Extract #1 = high purity (~80%), high viscosity (HV), barley (candle variety) beta glucan (n=8).
3.2. Effect of β-glucan on mRNA expression
Five freshly prepared β-glucan extracts (E1, E2, E3, E4 and E5) from barley or oat sources (Table 1) were added to the culture media at a concentration of 0.5% (wt/wt). Expression of the lipogenic gene FAS was significantly reduced by all five β-glucan extracts (Fig. 1). However, a greater inhibition of FAS mRNA was demonstrated with the HV extracts (E1, E3 and E5). Only the HV barley (E1) and oat (E3) extracts inhibited the expression of ACC (Fig. 2). The HV extracts E1, E3 and E5 significantly reduced the abundance of the transcription factor SREBP-1a involved in fatty acid synthesis (Fig. 3). The LV extracts (E2 and E4) significantly reduced SREBP-1a mRNA abundance, but to a lesser extent than the HV extracts. While SREBP-1c mRNA abundance was also reduced by all three HV extracts, only barley (E1) was significant (P≤.05), with E3 (P=.07) and E5 (P=.06) showing a trend toward decreased expression. The expression of the SREBP-1c transcription factor was not affected by the LV extracts. Similarly, only the HV extracts (E1, E3 and E5) reduced the mRNA levels of SREBP-2.
Fig. 2.
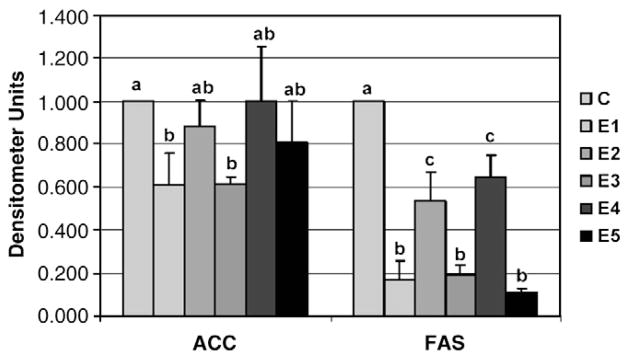
The effect of β-glucan extracts on the in vitro intestinal mRNA expression of acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS). NCl-H716 cell (1 × 106) were incubated for 42 hr with DMEM + 0.2% BSA with or without 0.5% (wt/vol) of one of the 5 β-glucan extracts. PCR products for genes of interest were normalized to actin mRNA to control for any loading discrepancies. Densitometer values are presented as mean ± SEM. Treatments with different letters are significantly different (P<0.05).
Fig. 3.
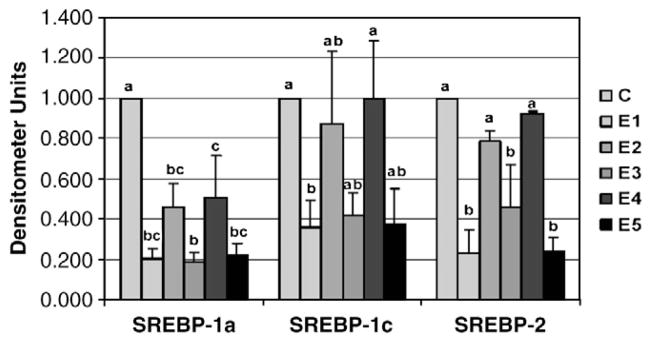
The effect of β-glucan extracts on the in vitro intestinal mRNA expression of SREBP-1a, SREBP-1c and SREBP-2.
All five β-glucan extracts reduced i-FABP and FATP4 mRNA levels (Fig. 4). Although mRNA levels of ILBP appeared to increase with the higher-viscosity extracts, this difference was not statistically significant.
Fig. 4.
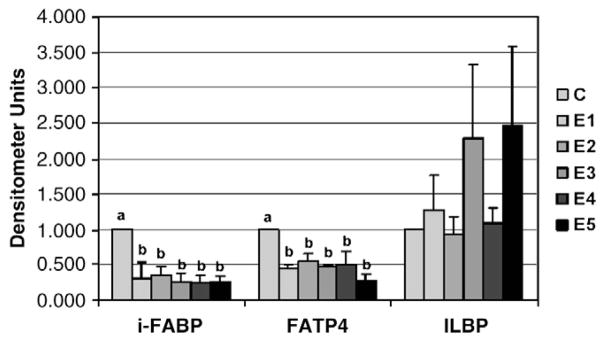
The effect of β-glucan extracts on the in vitro intestinal mRNA expression of i-FABP, FATP4 and ILBP.
4. Discussion
4.1. Lipid uptake
In the natural setting in the intestine, the effective resistance of the intestinal UWL is high, and this is best mimicked in the experimental setting where the bulk phase is unstirred [16]. Under these circumstances, each of the extracts reduced the jejunal uptake of 18:2, but not 18:0 or cholesterol (Table 3). The uptake of these lipids is sensitive to UWL resistance, and this resistance can be enhanced further by an increase in the viscosity of the UWL [17]. As well, the thickness of UWL has been shown to increase in the presence of β-glucan solution [33]. There was no influence of either the HV β-glucan extracts or the LV β-glucan extracts on the uptake of either 18:0 or cholesterol, suggesting that the effect of UWL resistance on the uptake of these lipids had already reached a maximum and could not be increased further by the addition of β-glucan.
Each of the extracts inhibited the jejunal and ileal uptakes of 18:2 when unstirred layer resistance was high (Table 3), suggesting the possibility that the inhibitory effect of these extracts was not due only to their viscosity but due also to a direct effect of the extracts on the BBM passive or carrier-mediated processes of lipid uptake. To better examine the effect of the extracts on BBM-mediated events, experiments were performed when the bulk phase was stirred at 600 rpm to reduce the effective UWL resistance. Indeed, a direct effect of the β-glucan on the BBM uptake of 18:2 can be demonstrated when the bulk phase is stirred (600 rpm) and UWL resistance is low (Table 4). In addition, some — but not all — of the extracts inhibited 18:0 and cholesterol uptakes under these conditions (Table 4).
β-Glucan had no effect on cholesterol uptake (Table 3) under conditions where the bulk phase was unstirred. However, E5 significantly reduced jejunal and ileal cholesterol uptakes when the bulk phase was stirred (Table 4), suggesting possible effects on the BBM. Of interest, long-term feeding studies incorporating viscous soluble fiber in the form of β-glucan have demonstrated reductions in plasma concentrations in hypercholesterolemic men [5,6]. Since these in vitro studies enable us to look only at the influence of β-glucan on the diffusion of cholesterol across the UWL and permeation through the UWL, it needs to be emphasized that it is possible that the β-glucan may also modify cholesterol absorption by an influence on some other step in the cholesterol absorption process, such as enterocyte metabolism or exit of cholesterol from the enterocyte across the basolateral membrane.
Under stirred conditions, the inhibitory effect on the uptake of two long-chain fatty acids was even greater at higher concentrations of 18:0 and 18:2 (Fig. 1). This inhibitory effect was unlikely an effect on bile acid micelles, since inhibitory effects on cholesterol uptake were not seen at higher concentrations (Fig. 1). Still, it is possible that β-glucan inhibits the uptake of other nutrients such as cholesterol at concentrations higher than those used in this study.
This study did not demonstrate a marked difference between the effects of β-glucan derived from barley and the effects of β-glucan derived from oats on in vitro intestinal transport. In fact, there is now sufficient evidence to suggest that four major water-soluble fiber types, β-glucan, psyllium, pectin and guar gum, are effective in lowering total and low-density lipoprotein levels in humans. Given the similarity in responses reported for the four fiber types, it is possible that a common hypocholesterolemic mechanism may exist. However, one difference that has been noted between fiber types is the relative production of acetate, propionate, butyrate and total short-chain fatty acids (SCFAs) — the fermentation byproducts of dietary fiber utilization by bacteria in the gut. Wood et al. demonstrated that oat β-glucan fermented more rapidly than guar gum, and this was reflected in higher concentrations of total SCFA and, particularly, acetate and butyrate. Butyrate production was greater with oat β-glucan than with guar gum at all time points assessed over a 24-h incubation period. While data remain controversial, it has been suggested that SCFA production and the propionate/acetate ratio may influence lipid metabolism.
While there appears to be a certain commonality between the effects of various dietary fiber types on lipid metabolism/cardiovascular risk, the differences noted by varying the viscosity of dietary fibers is more pronounced, particularly in terms of glycemia. Jenkins et al. have demonstrated that a necessary property of dietary fiber that maximizes benefits on blood glucose control is HV, which likely acts via changes in absorption and transit time. Reducing the viscosity of guar gum via hydrolysis results in concurrent loss of clinical efficacy. In our work, although it appears that HV β-glucan may have had a more pronounced effect on lipid uptake (under stirred conditions) than did the lower-viscosity extracts (Table 4), further study is required to determine the optimal extract composition for use as an antiabsorptive agent.
4.2. Gene expression
We examined the direct effects of the five β-glucan extracts on the expression of selected genes involved in intestinal fatty acid and cholesterol uptake and metabolism. Solutions containing the barley and oat extracts at 0.5% were incubated with the NCI-H716 intestinal cell line. Consistently, the HV extracts were able to down-regulate the expression of FAS, ACC, SREBP-1a and SREBP-1c, genes known to play critical roles in fatty acid synthesis. Similarly, the expression of SREBP-2, which is involved in cholesterol synthesis, was specifically down-regulated by the HV extracts.
In contrast to the extensive evidence for the role of dietary lipids in regulating fatty acid synthesis in the liver and the involvement of SREBPs, little is known regarding this regulation in the intestine. This lack of understanding should not undermine the importance of the intestine to overall lipid metabolism in the body. While the liver is the most important site of cholesterol synthesis under normal lipidemic conditions, the intestine is also an important contributor, and both tissues play a pivotal role in the overall balance of cholesterol in the body. Of the total amount of cholesterol synthesized each day in the body, the liver and intestine account for 15% and 10%, respectively. In the hypercholesterolemic state, while most organs reduce cholesterol biosynthesis in response to fasting, the intestine becomes a major production site and can increase its contribution by up to 50%.
Our findings in the intestinal cell line showing a down-regulation of SREBP-2, a key transcription factor involved in cholesterol synthesis, indicate that changes at the level of transcription may be occurring within the cells to modify cholesterol metabolism. Field et al. [34,35] have shown that mRNA levels of SREBP-2 were increased in the intestines of hamsters on a cholesterol-depletion diet and were minimally suppressed by a diet enriched in cholesterol. In the CaCo-2 intestinal cell line, these same investigators showed that cholesterol influx decreased mRNA levels of SREBP-2 and that depleting cells of cholesterol increased SREBP-2 mRNA levels [36]. Given that only the E5 extract was able to inhibit cholesterol uptake in the absorption studies, the down-regulation of SREBP-2 mRNA seen most prominently with the HV barley extract is likely due to mechanisms other than cholesterol influx. Whether the cellular changes we have demonstrated will result in reduced cholesterol output from intestinal cells remains to be elucidated.
In addition to regulating cholesterol metabolism, the intestine also plays an integral role in fatty acid uptake and synthesis. Only recently have Field et al. [23] shown that ingestion of fat by hamsters decreases fatty acid synthesis in the proximal small intestine. Specifically, feeding n–3 polyunsaturated fatty acids suppressed intestinal SREBP-1c mRNA levels. Independently in our in vitro experiments, we showed that the uptake of long-chain fatty acids is inhibited and that FAS, ACC, SREBP-1a and SREBP-1c mRNA levels are decreased with β-glucan extracts. While our work expands the understanding of the regulation of genes involved in intestinal fatty acid synthesis by demonstrating that β-glucan, particularly HV extracts, down-regulates FAS, ACC, SREBP-1a and SREBP-1c, much more work is needed to fully understand the pathways involved in fatty acid synthesis in the intestine and its implications for cardiovascular health.
While our understanding of the function of i-FABPs remains far from complete, we do know that increases in the amount of lipid in the diet coordinately increase the expression of lipid-binding proteins [37]. Our labeled uptake studies demonstrate an antiabsorptive effect of barley β-glucan extracts on stearic and linoleic acids in the rat intestine. This would support the reduced i-FABP and FATP4 mRNA independently observed in the expression studies. Chapkin et al. [24] have also shown that i-FABP mRNA in exfoliated colonocytes in the feces of rats fed oat bran is lower than that in the feces of rats fed wheat bran. More recently, blocking of FATP4 function has been targeted as an alternative treatment to pancreatic lipase inhibitors for inhibition of fat absorption. Using targeted deletion, Gimeno et al. [38] demonstrated that the loss of one allele of FATP4 in mice reduced fatty acid uptake but did not alter fat absorption on either a normal-fat diet or a high-fat diet. Deletion of both FATP4 alleles, however, resulted in embryonic lethality. Given the rising incidence of obesity and insulin resistance, treatments that inhibit intestinal fat absorption are important targets and may involve cereal β-glucans.
In summary, this study demonstrates that β-glucan inhibits the in vitro uptake of the long-chain fatty acids 18:0 and 18:2, particularly at higher fatty acid concentrations. Under certain experimental conditions, cholesterol uptake was also reduced. The expression of intestinal genes associated with fatty acid and cholesterol syntheses and fatty acid transport was also down-regulated. The potential use of β-glucan in the reduction of nutrient absorption in humans warrants further study.
Acknowledgments
We are grateful to the Alberta Barley Commission and the Alberta Agricultural Research Institute for financial support.
We would like to acknowledge the technical assistance of Elizabeth Wierzbicki and the excellent editing of Jill Sparling.
Abbreviations
- ACC
acetyl-CoA carboxylase
- ANOVA
analysis of variance
- BBM
brush border membrane
- FAS
fatty acid synthase
- FATP4
fatty acid transport protein 4
- HP
high purity
- HV
high viscosity
- i-FABP
intestinal fatty-acid-binding protein
- ILBP
ileal lipid-binding protein
- LV
low viscosity
- MP
medium purity
- SNK
Student–Neuman–Keuls
- SREBP
sterol regulatory element-binding protein
- UWL
unstirred water layer
Footnotes
Laurie A. Drozdowski and Raylene A. Reimer contributed equally to this publication.
References
- 1.Gee JM, Blackburn NA, Johnson IT. The influence of guar gum on intestinal cholesterol transport in the rat. Br J Nutr. 1983;50:215–24. doi: 10.1079/bjn19830091. [DOI] [PubMed] [Google Scholar]
- 2.Johnson IT, Gee JM. Effect of gel-forming gums on the intestinal unstirred water layer and sugar transport in vitro. Gut. 1981;22:398–403. doi: 10.1136/gut.22.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behall KM, Schoffield DJ, Hallfrisch J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am J Clin Nutr. 2004;80:1185–93. doi: 10.1093/ajcn/80.5.1185. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh GH, Whyte J, McCarthur R, Nestel PJ. Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr. 1991;53:1205–9. doi: 10.1093/ajcn/53.5.1205. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Story L, Sieling B, Chen WJ, Petro MS, Story J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am J Clin Nutr. 1984;40:1146–55. doi: 10.1093/ajcn/40.6.1146. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JW, Gustafson NJ. Hypocholesterolemic effects of oat and bean products. Am J Clin Nutr. 1988;48:749–53. doi: 10.1093/ajcn/48.3.749. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJA, Wolever TMS, Leeds AR, Gassull MA, Haisman P, Dilawari J, et al. Dietary fibers, fiber analogues and glucose tolerance: importance of viscosity. Br Med J. 1978;1:1392–4. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood PJ, Braaten JT, Scott FW, Riedel KD, Wolynetz MS, Collins MW. Effect of dose and modifications of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br J Nutr. 1994;72:731–43. doi: 10.1079/bjn19940075. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The hypocholesterolemic effects of beta-glucan in oatmeal and oat bran. A dose-controlled study. JAMA. 1991;265:1833–9. [PubMed] [Google Scholar]
- 10.Burkus Z, Temelli F. Effect of extraction conditions on the yield, composition and viscosity stability of barley β-glucan gum. Cereal Chem. 1998;75:805–9. [Google Scholar]
- 11.Tappy L, Gugolz E, Wursch P. Effects of breakfast cereals containing various amounts of β-glucan fibers on plasma glucose and insulin response in NIDDM subjects. Diabetes Care. 1996;19:831–4. doi: 10.2337/diacare.19.8.831. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins AL, Jenkins DJA, Zdravkovic U, Wursch P, Vuksan V. Depression of the glycemic index by high levels of β-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr. 2002;56:622–8. doi: 10.1038/sj.ejcn.1601367. [DOI] [PubMed] [Google Scholar]
- 13.Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, et al. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with β-glucan. Am J Clin Nutr. 1999;69:55–63. doi: 10.1093/ajcn/69.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Wood PJ, Beer MU, Butler G. Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br J Nutr. 2000;84:19–23. [PubMed] [Google Scholar]
- 15.Braaten JT, Scott FW, Wood PJ, Riedel KD, Wolynetz MS, Brule D, et al. High β-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabetes Med. 1994;11:312–8. doi: 10.1111/j.1464-5491.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 16.Wursch P, Pi-Sunyer FX. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care. 1997;20:1774–80. doi: 10.2337/diacare.20.11.1774. [DOI] [PubMed] [Google Scholar]
- 17.Thomson AB, Dietschy JM. Derivation of the equations that describe the effects of unstirred water layers on the kinetic parameters of active transport processes in the intestine. J Theor Biol. 1977;64:277–94. doi: 10.1016/0022-5193(77)90357-5. [DOI] [PubMed] [Google Scholar]
- 18.Thomson AB, Dietschy JM. Experimental demonstration of the effect of the unstirred water layer on the kinetic constants of the membrane transport of D-glucose in rabbit jejunum. J Membr Biol. 1980;54:221–9. doi: 10.1007/BF01870238. [DOI] [PubMed] [Google Scholar]
- 19.Westergaard H, Dietschy JM. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974;54:718–32. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansbach CM, Gorelick F. Development and physiological regulation of intestinal lipid absorption: II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645–50. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 21.Black D. Development and physiological regulation of intestinal lipid absorption: I. Development of intestinal lipid absorption: cellular events in chylomicrons assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–24. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 22.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–40. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 23.Field FJ, Born E, Mathur SN. Fatty acid flux suppresses fatty acid synthesis in hamster intestine independently of SREBP-1 expression. J Lipid Res. 2003;44:1199–208. doi: 10.1194/jlr.M300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Chapkin RS, Clark AE, Davidson LA, Schroeder F, Zoran DL, Lupton JR. Dietary fiber differentially alters cellular fatty acid-binding protein expression in exfoliated colonocytes during tumor development. Nutr Cancer. 1998;32:107–12. doi: 10.1080/01635589809514727. [DOI] [PubMed] [Google Scholar]
- 25.Atshaves BP, Foxworth WB, Frolov A, Roths JB, Kier AB, Oetama BK, et al. Cellular differentiation and i-FABP protein expression modulates fatty acid uptake and diffusion. Am J Physiol. 1998;274(3 Pt 1):C633–44. doi: 10.1152/ajpcell.1998.274.3.C633. [DOI] [PubMed] [Google Scholar]
- 26.Luxon BA. Inhibition of binding to fatty acid binding proteins reduces the intracellular transport of fatty acids. Am J Physiol. 1996;271(1 Pt 1):G113–20. doi: 10.1152/ajpgi.1996.271.1.G113. [DOI] [PubMed] [Google Scholar]
- 27.Vasanthan T, Temelli F. International Patent WO 02/27011 A2 Grain fractionation methods and products. 2002
- 28.McCleary BV, Glennie-Holmes M. Enzymatic quantification of (1→3)(1→4)-β-D-glucan in barley and malt. J Inst Brew. 1985;91:285–95. [Google Scholar]
- 29.Lukie BE, Westergaard H, Dietschy JM. Validation of a chamber that allows measurement of both tissue uptake rates and unstirred layer thicknesses in the intestine under conditions of controlled stirring. Gastroenterology. 1974;67:652–61. [PubMed] [Google Scholar]
- 30.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake in the intestinal mucosal cell. J Clin Invest. 1976;58:97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries JE, Dinjens WN, De Bruyne GK, Verspaget HW, van der Linden EP, de Bruine AP, et al. In vivo and in vitro invasion in relation to phenotypic characteristics of human colorectal carcinoma cells. Br J Cancer. 1995;71(2):271–7. doi: 10.1038/bjc.1995.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobi M, Chintalapani S, Goo R, Maliakkal B, Reddy J, Lundqvist M, et al. Omperazole inhibits growth of cancer line of colonic origin. Dig Dis Sci. 1995;40(7):1526–30. doi: 10.1007/BF02285203. [DOI] [PubMed] [Google Scholar]
- 33.Lund BEK, Gee JM, Brown JC, Wood PJ, Johnson IT. Effect of gum on the physical properties of the gastrointestinal contents and on the uptake of D-galactose and cholesterol by rat small intestine in vitro. Br J Nutr. 1989;62:91–101. doi: 10.1079/bjn19890010. [DOI] [PubMed] [Google Scholar]
- 34.Field FJ, Born E, Murthy S, Mathur SN. Gene expression of sterol regulatory element-binding proteins in hamster small intestine. J Lipid Res. 2001;42:1–8. [PubMed] [Google Scholar]
- 35.Field FJ, Born E, Murthy S, Mathur SN. Regulation of sterol regulatory element-binding proteins in hamster intestine by changes in cholesterol flux. J Biol Chem. 2001;276(20):17576–83. doi: 10.1074/jbc.M010917200. [DOI] [PubMed] [Google Scholar]
- 36.Field FJ, Born E, Murthy S, Mathur SN. Regulation of sterol regulatory element-binding proteins by cholesterol flux in CaCo-2 cells. J Lipid Res. 2001;42:1687–98. [PubMed] [Google Scholar]
- 37.Bernlohr DA, Simpson MA, Hertzel AV, Banaszak LJ. Intracellular lipid-binding proteins and their genes. Annu Rev Nutr. 1997;17:277–303. doi: 10.1146/annurev.nutr.17.1.277. [DOI] [PubMed] [Google Scholar]
- 38.Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, et al. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem. 2003;278:49512–6. doi: 10.1074/jbc.M309759200. [DOI] [PubMed] [Google Scholar]