Abstract
Objective
First, we review the development and plasticity of the central auditory pathways in infants and children with hearing loss who are fitted with cochlear implants (CIs). Second, we describe case studies demonstrating the clinical utility of the P1 central auditory evoked potential (CAEP) for evaluating cortical auditory maturation in the rapidly increasing number of cochlear-implanted children who have multiple disabilities.
Study Design
Children who receive CIs provide a platform to examine the trajectories of deprivation-induced and experience-dependent plasticity in the central auditory system. We review the evidence for, and time limits of sensitive periods for cortical auditory maturation framing an optimal period for cochlear implantation. Finally, we evaluate the use of the P1 biomarker as an objective assessment tool in the special case of children with multiple disabilities.
Results
The P1 response was useful in assessing central auditory maturation in patients with CHARGE association, ANSD, and Pallister-Killian Syndrome concomitant with hearing loss.
Conclusion
The presence of co-existing disabilities in addition to hearing loss poses unique challenges regarding both pre-intervention evaluation and post-intervention rehabilitation for children with multiple disabilities. When combined with a standard audiological test battery, the P1 CAEP biomarker has a useful role in objectively evaluating the maturation of central auditory pathways to determine the effectiveness of various intervention strategies in hearing-impaired children with multiple disabilities.
Keywords: P1 Cortical auditory evoked potential, cochlear implant, sensitive period, multiple disabilities
Introduction
I. Sensitive periods for central auditory development in cochlear implanted children
Cortical auditory evoked potentials (CAEPs) are acoustically evoked and non-invasively recorded components of the electroencephalogram (EEG) that reflect long-term changes in auditory cortical maturation. Normal development of the central auditory pathways is likely a necessary but not sufficient condition for development of speech and oral language skills. The P1 component of the CAEP response reflects function of the thalamo-cortical pathways and auditory cortex (1). The latency of the P1 response has been shown to systematically vary as a function of age and, as such, can be used as a biomarker of cortical auditory development (2–7). The P1 biomarker has been utilized extensively to assess the development and plasticity of the central auditory system in normal hearing children and children with hearing loss (6, 8–10). Our laboratory has established 95% confidence intervals for the developmental trajectory of P1 latency in normal hearing subjects 0.1 years to 20 years (11). These confidence intervals provide normative data useful for assessing cortical auditory maturation in hearing-impaired children (11).
Large scale studies of congenitally deaf children fit with CIs have allowed us to establish the existence and time limits for a sensitive period for central auditory development in humans. Research from our laboratory demonstrated that from a total of 245 children who received stimulation via CIs, children who were implanted prior to age 3.5 showed P1 CAEP responses with normal latency and morphology while children implanted after age 7 demonstrated abnormal P1 latency and morphology (12–14). Children receiving stimulation between ages 3.5 and age 7 demonstrated highly variable cortical responses, with only 50% of these children having normal P1 latencies regardless of age of implantation within that range. Another study of 231 implanted children from our laboratory showed that when stimulation via the implant was delivered by age 3.5 years, then latency and morphology of the P1 reached age-appropriate values within 3–6 months following the onset of stimulation, on the other hand, implantation after age 7 years resulted in abnormal P1 responses even when measured years after years of experience with the implant (13, 15). Taken together, outcomes from these studies indicate a sensitive period of approximately 3.5 years in which central auditory pathways are maximally plastic. These findings also converge with demonstration of a brief, sensitive period in animal models (16–19).
The sensitive period coincides with the peak of intrinsically regulated synaptogenesis which occurs at about 3–4 years in human children (20). Since this period of synaptic overshoot is independent of auditory experiences it appears to have a protective effect on the central auditory pathways. Implantation within this sensitive period provides the external stimulation needed for synaptic pruning and refinement of the central auditory pathways (26). Language outcomes for congenitally deaf children implanted within the sensitive period for development demonstrate significantly better speech perception and language skills in comparison to late-implanted children (21, 22). In a large scale study of 117 cochlear implanted children, Tajudeen and colleagues (2010) compared speech performance of children implanted in the first year, second year and third year of life. When evaluated as a function of experience rather than chronological age at implantation, average speech performance was almost equivalent between groups of children implanted at various times under the age of 3 years (21).
Following long-term auditory deprivation there is evidence of a partial or total decoupling of higher-order areas from primary auditory cortex (18, 23). Because higher-order cortex fundamentally involves multimodal processing, such a decoupling of higher order areas from primary auditory cortex may allow recruitment of other sensory modalities or may allow other sensory input to take over in the higher-order cortex (27). In late-implanted children, absence of the N1 cortical auditory evoked potential, reflecting input from higher order cortex, indicates improper activation of auditory cortex and restricted development of inter- and intra- cortical connections, consistent with the decoupling hypothesis proposed by Kral and colleagues (18, 28). Hence, when cochlear implantation occurs after the end of the sensitive period, the stimulation may be introduced into a re-organized central auditory system such that the bottom up auditory information delivered by the implant to primary auditory cortex may not receive appropriate top-down modulation from higher order cortex necessary for attaching the meaning and context needed for oral language learning (12, 18, 29). PET studies in prelingually deaf children implanted after the sensitive period also demonstrate increased blood flow and glucose hypometabolism correlated with poorer auditory and language outcomes, indicative of cross-modal reorganization in auditory cortex (30). Overall, the research on typically developing children with cochlear implants clearly indicates the benefits of early implantation, under age 3 years, but best by age 1 year, to take advantage of the highly plastic central auditory system in early childhood.
II. Cochlear implantation in children with multiple disabilities
Children with multiple disabilities account for a significant portion of hearing-impaired children (31). The most prevalent co-morbid disabilities among children with hearing loss include learning difficulties (11.2%), visual impairment (7.45%), developmental delay (2.31%), cerebral palsy (3.62%), speech and language problems (2.46%), neuromotor issues (2.35%), psychosocial issues (2.19%), cognitive problems (2.16%), brain abnormalities (2.0%), and other disabilities (4.56%) (31). While children with significant disabilities concomitant with hearing loss have been traditionally excluded from CI candidacy, the rate of implantation for this population has steadily increased in recent years (32, 33). Today, children with additional co-existing disabilities are estimated to account for 30% to 40% of pediatric implanted patients (34, 35). Interestingly, significantly fewer profoundly deaf children with a cochlear implant have multiple disabilities compared to profoundly impaired children who do not have an implant (31, 36). This may indicate lower incidence of cochlear implantation among children with multiple handicaps (31, 36).
Because many children with additional disabilities who receive implants are not likely to perform as well as typically developing CI children, candidacy procedures in the special case of multiple disabilities varies widely between clinics (36). The amount of benefit received and the developmental trajectory of speech and language skills in an implanted child with multiple disabilities may not be as favorable as typically developing hearing-impaired children with CIs (36). For example, in a study by Birman and colleagues (2012), the majority of typically developing cochlear-implanted children were able to achieve age-appropriate language levels at normal rates of language acquisition, whereas children with developmental delay were only able to achieve limited speech sound discrimination and no verbal language after twelve months of CI use (35). That said, nearly half of the children with additional disabilities in the study were implanted after the sensitive period of approximately 3.5 years. Anecdotally, we have observed that children with additional handicaps may be implanted at later ages (compared with typically developing deaf children), likely due to the focus on more serious medical conditions than deafness at conditions and likely due to difficulty in evaluating CI candidacy. Delayed implantation represents an additional contributing factor to the poorer outcomes observed cochlear implanted children with multiple disabilities. Clearly, the determination of whether a child with multiple disabilities will benefit from a cochlear implant is a multifaceted decision that must be based upon realistic expectations and close examination of the complex needs of the child.
The specific associated disability and its influence on the regulation of sensitive periods may also contribute to the variability in speech and language outcomes post-implantation among children with multiple disabilities, though there is little research regarding this topic (36). In fact, there is little or no information regarding central auditory development in children whose hearing loss is concomitant with additional disabilities. It is likely that aspects of developmental plasticity such as sensitive periods will be influenced differently depending on the nature of the disability. For example, genetic disorders that affect regulation of synaptic development, like Fragile X syndrome or Rett syndrome, may result in an alteration of the sensitive period time frame due to a disruption of the underlying synaptic growth/development processes (37, 38). Disorders affecting neural aspects of central auditory development may similarly alter central auditory plasticity (37, 38). For example, Auditory Neuropathy Spectrum Disorder (ANSD) is a disorder of neural dys-synchrony in the auditory nerve, brainstem, and central pathways which is concomitant with sensorineural hearing loss and has been found to adversely affect the sensitive period for cochlear implantation. In a recent study, we examined P1 latencies in 24 children with ANSD who received CIs (39). Children with ANSD who received CIs under age 2 years showed a greater distribution of normal P1 latencies compared with children who received implants after age 2 years. These findings provide preliminary evidence for a sensitive period of approximately 2 years for central auditory development in ANSD. Thus, Cardon and Sharma’s results suggest that the sensitive period for central auditory plasticity in deafness may be decreased for children with ANSD. In future studies, children who have deafness concomitant with other disorders might provide a unique population in which to observe different various outcomes of sensitive period regulation in deafness, given that these children have inherently different genetic and extrinsic variables mediating their auditory development.
Evaluating and monitoring progress pre- and post-intervention proves to be a particular challenge for clinicians since behavioral audiometric results are often variable and difficult to measure objectively (40). In many cases, obtaining reliable auditory threshold information for children with multiple conditions is difficult or impossible to obtain using visual reinforcement audiometry (VRA) when the child cannot condition to the stimulus and assessing speech perception proves difficult given that many of these children never develop closed set or open set speech discrimination (40). Additionally, for a portion of these children with multiple disabilities, serious health conditions prevent the children from sedation under anesthesia, making Auditory Brainstem Response (ABR) thresholds difficult to obtain (41). Since the P1 CAEP biomarker is easy to record, non-invasive (no anesthesia), it provides an objective measurement of cortical maturation and can serve as a useful clinical tool for assessing efficacy of intervention with hearing aids or CIs and/or establishing CI candidacy in this special population. In the next section we describe case studies demonstrating the clinical utility of the P1 CAEP biomarker in hearing impaired children without additional disabilities and in children for whom hearing loss was concomitant with other handicaps.
Material and Methods
Subjects
CAEPs recorded in 5 hearing-impaired children at various ages are described in this study. Two of the subjects had no disabilities in addition to their hearing loss. We describe results from these children to demonstrate the efficacy of the P1 CAEP biomarker in a typical clinical setting. Three of subjects had additional disabilities including a child with Auditory Neuropathy Spectrum Disorder (ANSD), CHARGE syndrome, and Pallister-Killan Syndrome.
Procedures
Standard electrophysiological and/or behavioral audiometric techniques were used to evaluate hearing losses by each subject’s managing audiologist. Hearing aids or CIs were implemented based on the standard criteria and fitting protocols at the clinics in which each child was fitted.
CAEPs were recorded in response to the speech syllable /ba/. The stimuli used in this study are identical to those described in previous studies in our laboratory (4, 8, 12). The stimulus was delivered via a loudspeaker located at 45 degrees azimuth or through insert earphones. The stimulus was presented at a comfortable loudness level for implanted subjects and at least 10–20 dB above threshold for children wearing hearing aids.
Evoked responses were recorded with Cz as the active electrode, an electrode placed on the mastoid as the reference, and a ground electrode placed on the forehead. Eye movements were monitored using a bipolar electrode montage placed on lateral outer canthus referenced to superior outer canthus. For children with cochlear implants, in order to minimize the electrical artifact produced by CIs, we recorded responses along the isopotential contour and minimized the artifact using common mode rejection cancellation techniques. An active electrode was placed at Cz and several reference electrodes were placed at locations around the forehead, nasion, orbits and mastoids. A ground electrode was placed on the forehead. Details of this procedure are discussed in detail in a previous publication by our group (42).
CAEPs were collected using a 100 ms pre-stimulus interval and a 600 ms post-stimulus recording window and an analog filter setting of 0.1 to 1000 Hz. At least 300 sweeps were collected for each subject. Sweeps greater than ±100 uV were rejected offline, after which remaining sweeps were averaged. Grand average waveforms for each subject are shown in the figures in the results section below. The P1 was defined as the first robust positive component in the waveform. P1 latency was computed at the peak of P1 response.
Testing for all subjects took place in an electromagnetically shielded sound booth. Subjects were seated on a parent or caretaker’s lap in a comfortable reclining chair during the recordings. Children were allowed to watch a video or cartoon of their choice on a TV monitor that was placed in front of them in the sound booth with audio levels were muted. The typical test session lasted for approximately 1 hour. For all testing, the subjects’ hearing aids or CIs were set at their usual settings and functioning appropriately.
Results
Case 1
Patient 1 was a male child born full-term, without complication, and who failed his newborn hearing screening. A severe-profound sensorineural hearing loss was confirmed with ABR within 2 months after birth (FIG 1A). The etiology of the hearing loss was unknown. No family history of congenital hearing loss was noted. The patient was fit with hearing aids bilaterally at age 4 months.
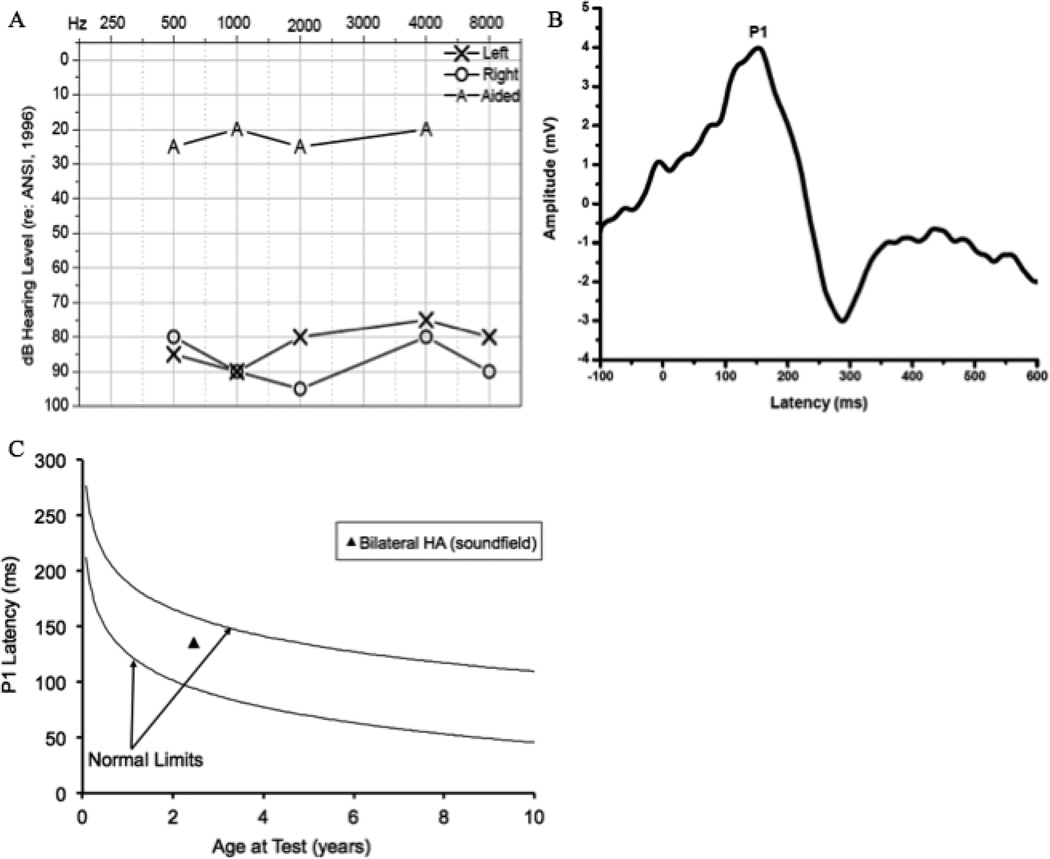
Figure 1.
Audiogram of behavioral unaided and aided thresholds for Case 1 (1A), Grand average CAEP response for Case 1 obtained after intervention via bilateral hearing aids (1B), and average P1 latency as a function of child’s age plotted against the 95% confidence limits for normal-hearing children for Case 1 (1C).
In this case of a child with hearing loss and no additional disabilities, P1 responses were used to assess whether the child’s hearing aids were providing adequate amplification for normal cortical auditory maturation. CAEP responses using the P1 biomarker were assessed at age 2.5 years, roughly 26 months after hearing aid fitting. As seen in Figure 1B, the P1 response was robust and normal latency and morphology are evident. Figure 1C shows the P1 latency as a function of chronological age relative to the 95% confidence intervals for normal development of the P1 response. P1 latencies fell within normal limits for the child’s age, clearly demonstrating that the hearing aids were providing auditory stimulation sufficient for normal central auditory development. The presence of the P1 with normal latency and morphology was consistent with the patient’s aided pure-tone average threshold (PTA) of 15 dB HL, suggesting that the hearing aids were providing appropriate audibility necessary for the development of speech and oral language (Fig. 1A). Behavioral reports that the child continues to perform well with hearing aids and is acquiring oral language are consistent with these findings.
Case 2
Subject 2 was a child identified with a severe-profound sensorineural hearing loss bilaterally using ABR. The hearing loss was secondary to Connexin 26. Subject 2 was fitted with hearing aids at 11 weeks. Behavioral audiological testing revealed unaided pure-tone averages (PTA) greater than 100 dB HL bilaterally (Fig. 2A). CAEP waveforms were recorded 2 months following hearing aid fitting. We saw absent P1 responses (plotted in Fig 2C) indicating a lack of proper maturation of the central auditory pathways consistent with the child’s diagnosis of bilateral severe-profound sensorineural hearing loss. P1 responses indicated that that the hearing aids were not providing adequate amplification for normal auditory development.
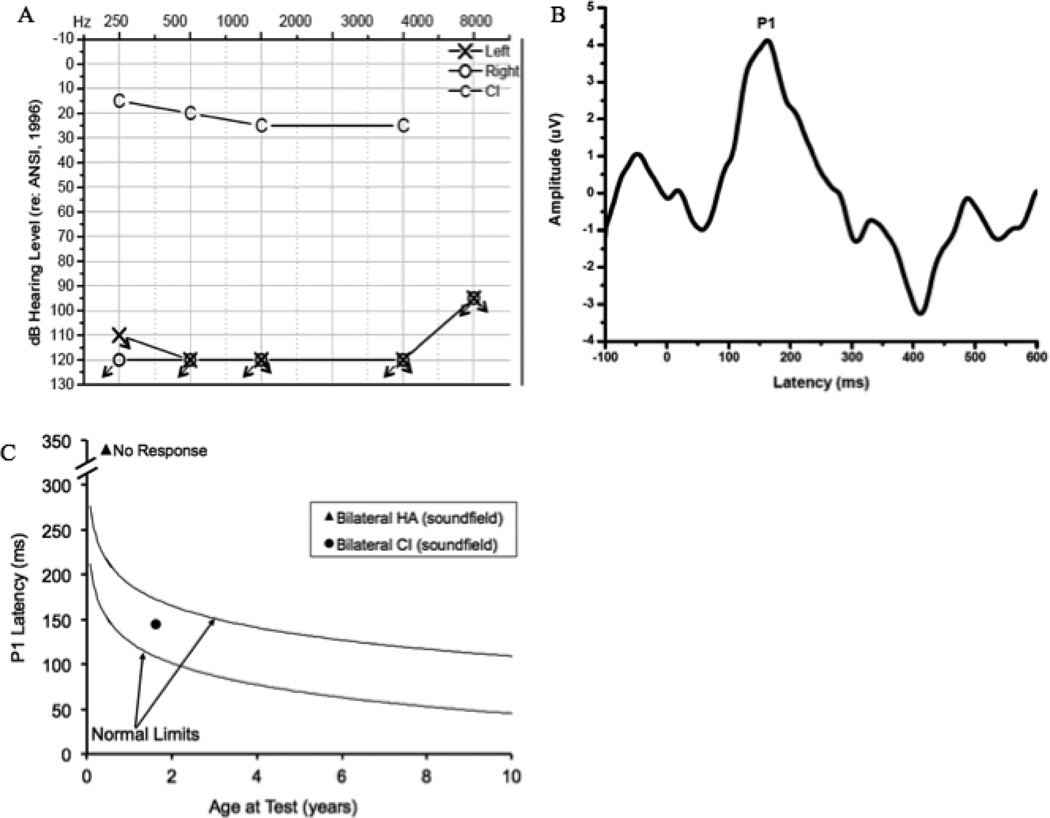
Figure 2.
Audiogram of behavioral unaided thresholds and aided thresholds post-bilateral cochlear implantation for Case 2 (2A), Grand average CAEP response for Case 2 obtained post-bilateral cochlear implantation (2B), and average P1 latency as a function of child’s age plotted against the 95% confidence limits for normal-hearing children pre- and post-implantation for Case 2 (2C).
Due to lack of benefit during the hearing aid trial, Subject 1 met the standard audiologic criteria of candidacy for cochlear implantation and was fit with bilateral CIs at age 7 months. The patient’s implants were activated at age 8 months. CAEP testing was repeated 11 months post-activation. A replicable CAEP P1 response with age-appropriate morphology was present when both ears were stimulated bilaterally through CIs (Fig 2B). The latency of the P1 response was within normal limits according to the patient’s age (Fig 2C). If the P1 latency with hearing aids is compared to the P1 latency following cochlear implantation (Fig 2C), it is evident that the latency of this response decreased rapidly, occurring outside normal limits prior to implantation and falling within normal limits after 11 months of CI use. These results are consistent with age-appropriate expressive language skills and nearly age-appropriate receptive language skills observed 5 months post- cochlear implantation, and are congruent with a pure tone average of approximately 20 dB HL. Case 2 is representative of the typical development and high degree of plasticity shown for hearing-impaired who receive CIs within the sensitive period.
Case 3
Subject 3 is a female child born 24 weeks gestational age with a complex medical history. Multiple high-risk factors for hearing loss were evident at birth including low birth weight, low Apgar scores, high frequency oscillatory ventilation, ototoxic medication, family history of congenital hearing loss, and other medical complications. The patient was in the neonatal intensive care unit for 5.5 months after birth. Subject 3 initially passed her newborn hearing screening in the right ear. She was referred for failing the newborn screening in her left ear. ABR test results conducted shortly after birth indicated a profound bilateral sensorineural hearing loss. Results from polarity reversal of the ABR showed a cochlear microphonic, consistent with a diagnosis of Auditory Neuropathy Spectrum Disorder (ANSD) in both ears. An intact VIII nerve was present bilaterally. A hearing aid trial was initiated at age 1 month. Behavioral testing with hearing aids indicated that the child was receiving some benefit from conventional amplification but that the hearing aids were likely not providing sufficient access to speech sounds required for auditory discrimination and normal development of age-appropriate language skills (FIG 3). The child met the standard audiological candidacy criteria for cochlear implantation and was fit with a CI in her left ear at age 20 months.
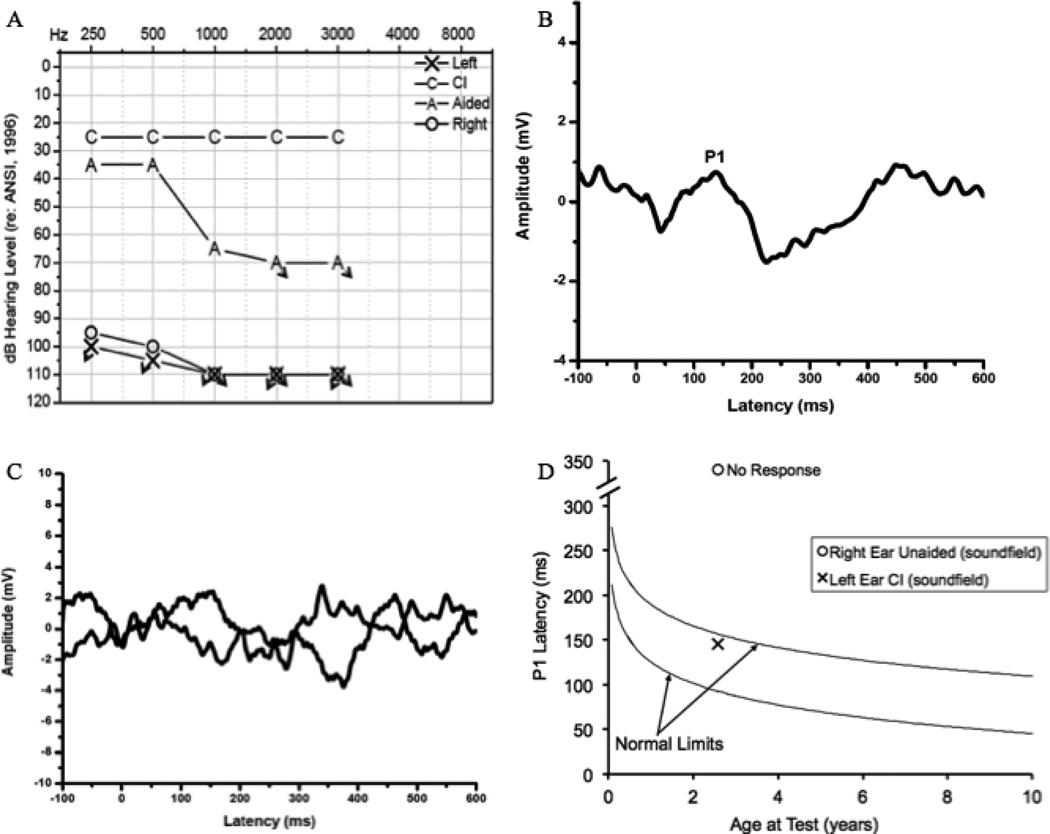
Figure 3.
Audiogram of behavioral unaided thresholds, aided thresholds, and thresholds post-left ear cochlear implantation for Case 3 (3A), Grand average CAEP response for Case 3 obtained in the left ear post- unilateral cochlear implantation (3B), non-replicable grand average CAEP responses obtained in the unaided right ear (3C), and average P1 latency for the implanted left ear and unaided right ear as a function of child’s age plotted against the 95% confidence limits for normal-hearing children both pre- and post-implantation in Case 3 (3D).
P1 responses were recorded at age 31 months, approximately 11 months after left-ear implantation. The subject’s parents were interested in pursuing future implantation of the right ear; therefore, P1 responses were evaluated in both the implanted left ear and the unaided right ear. Responses were recorded first with the patient’s left CI turned on. As shown in Figure 3B, a low-amplitude P1 response was present with a latency that fell within normal limits given the child’s developmental age, indicating that the CI is providing adequate stimulation of the auditory pathways. This is consistent with the child’s PTA of 25 dB HL and speech awareness thresholds of 10 dB HL 6 months post-CI activation (Fig. 3A). The smaller than typical amplitude of the P1 response has been described previously for children with ANSD (43). Normal P1 latency is also congruent with reports of progression in spoken language by the child’s parents and speech-language pathologist as well as the considerable improvement of the child’s score on the Meaningful Auditory Integration Scale (from 15% pre-implantation to 95% post-implantation) and a score of 25% for one-syllable words on the open-set Glendonald Auditory Screening Procedure (GASP) at 6 months post-implantation. CAEPs were then recorded with the CI turned off and the level of the stimulus increased to the maximum possible intensity for the unaided right ear in order to assess central auditory maturation in the right ear. No replicable P1 response was recorded for the right ear (Fig 3C). This lack of replicability of the CAEP response is consistent with the child’s profound hearing loss in this ear, suggesting that central auditory pathways ascending from the unaided right ear were receiving no stimulation and subsequently showed a lack of maturation.
In this case of hearing loss concomitant with ANSD, CAEP results indicated that early implantation (i.e., before the 2 year sensitive period cut-off for children with ANSD) and 11 months of CI use in the left ear provided sufficient stimulation for normal development of the central auditory pathways (39). The absence of a replicable P1 response in the right ear was consistent with the lack of adequate stimulation to the central auditory pathways on this side. Subject 3’s parents are now in the process of pursuing a second implant for her right ear, in part, based on the P1 CAEP results.
Case 4
Subject 4 was born 4.5 weeks premature with no complications during pregnancy. The patient displayed a history of cleft lip and palate, middle ear infections, and was diagnosed with CHARGE association. She failed her newborn hearing screening bilaterally. ABR waveforms obtained at age 3 months revealed replicable responses at 70 to 80 dB HL in the left ear and at 60 to 65 dB HL in the right ear, suggesting the presence of a moderately-severe to severe sensorineural hearing loss bilaterally. Subject 4 was fit with hearing aids bilaterally at age 5 months. Behavioral audiometric thresholds obtained using visual reinforcement audiometry (VRA) displayed aided PTA of 45 dB, however, the reliability of these results was questionable at best (Fig. 4A).
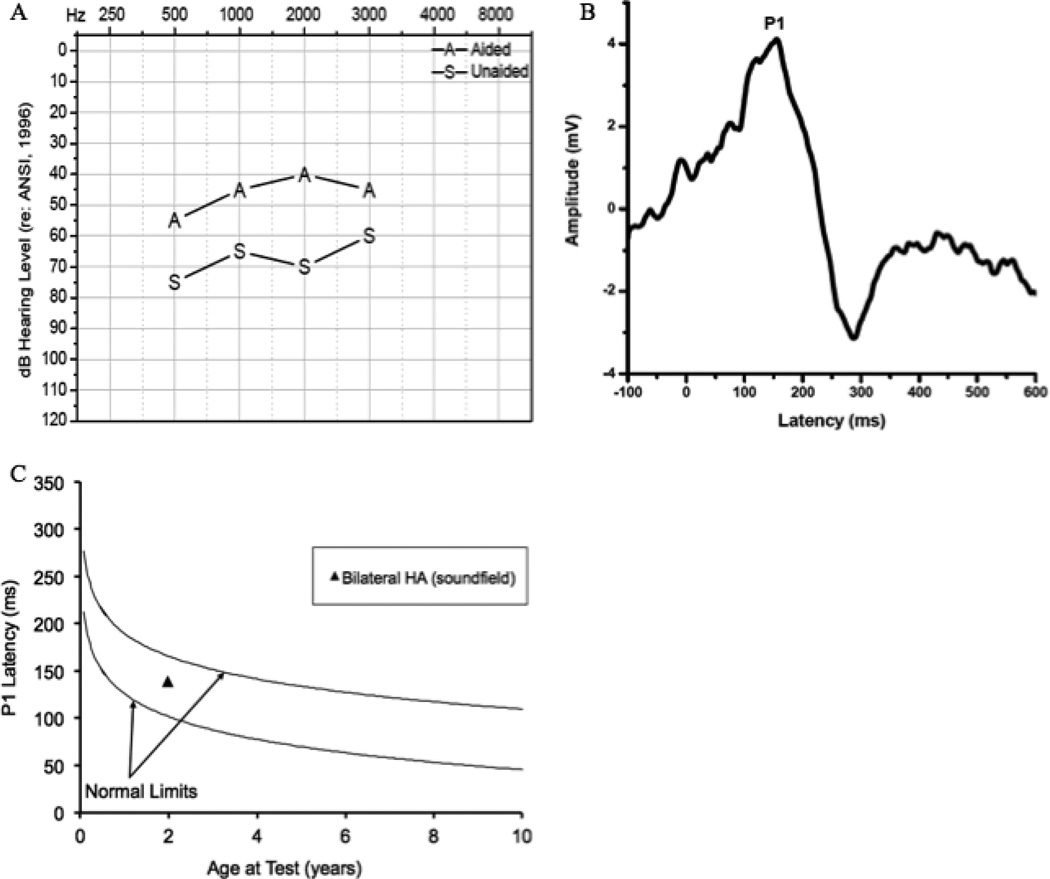
Figure 4.
Audiogram of behavioral unaided and bilateral aided thresholds for Case 4 (4A), Grand average CAEP response for Case 4 obtained after bilateral hearing aid intervention (4B), and average P1 latency as a function of child’s age plotted against the 95% confidence limits for normal-hearing children after hearing aid intervention for Case 4 (4C).
CAEP responses were assessed at age 23 months to examine central auditory maturation with conventional amplification (Fig. 4B). Stimuli were presented bilaterally via soundfield. A robust, replicable P1 response with age-appropriate morphology was present. As shown in Figure 3C, the latency of the P1 response was within normal limits according to the child’s developmental age. The presence of the P1 response occurring within normal limits indicates that the patient’s hearing aids were providing adequate benefit to allow age-appropriate maturation of the central auditory pathways. No pre- or post-intervention speech perception testing was possible for this child.
In this case, CAEP P1 responses were obtained to directly assess central auditory maturation for a child diagnosed with CHARGE and fit with hearing aids. In the face of additional handicaps demonstrated by this child preventing reliable behavioral assessment of audiometric thresholds or speech perception performance, P1 results provided useful information on the age-appropriate development of the central auditory pathways.
Case 5
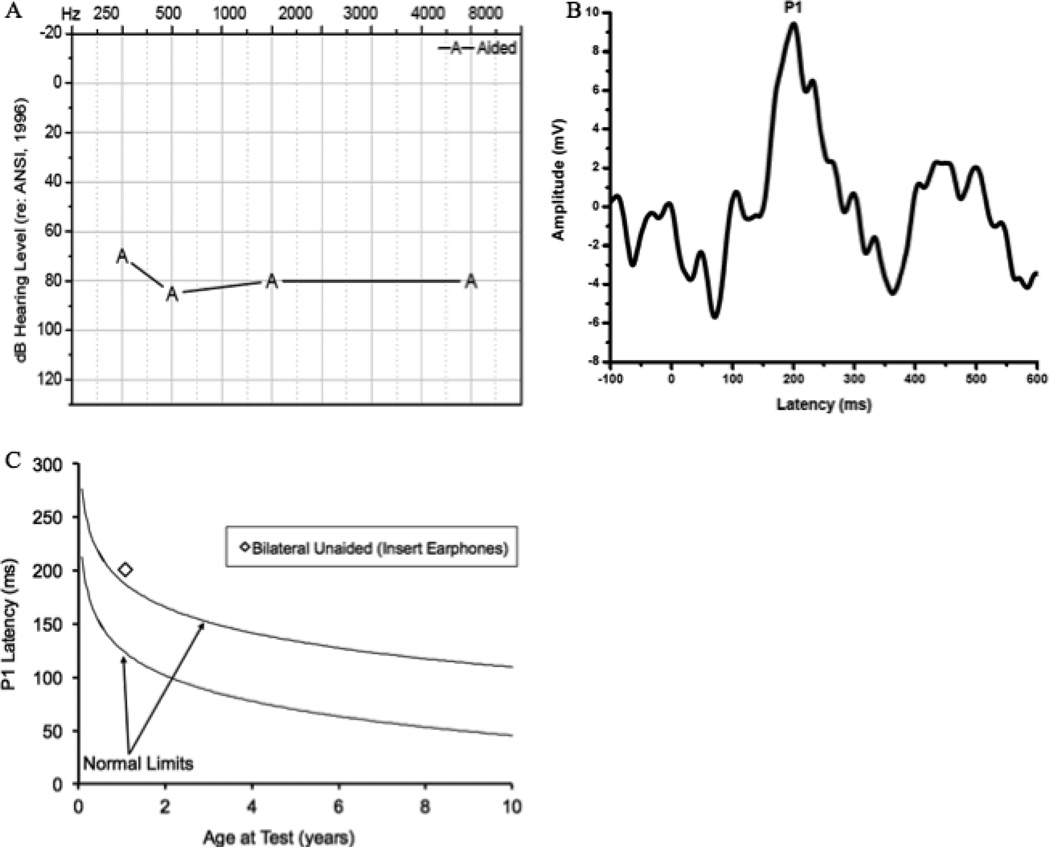
Subject 5 was a male child born full-term with Pallister-Killan Syndrome characterized by hydrocephalus, severe hypotonia, and vision problems. The child failed his newborn hearing screening. ABR testing exhibited a profound hearing loss in the left ear and severe-to-profound hearing loss in the right ear. Subject 5 was fit with bilateral hearing aids at age 8 months. Aided thresholds were attempted using VRA beginning at age 8 months, but the reliability of these responses for this child were poor due to inadequate muscle control for head support. Behavioral testing with hearing aids resulted in pure tone thresholds of approximately 70 dB HL, indicating that the child was receiving little benefit from conventional amplification necessary for the development of age-appropriate auditory skills (Fig. 5A). Speech perception testing was attempted but was not attainable for this child. Due to lack of availability of reliable behavioral test results and in order to establish candidacy for cochlear implantation, CAEP testing was recommended by the audiologist to assess central auditory maturation with conventional amplification.
Figure 5.
Audiogram of aided thresholds for Case 5 (5A), Grand average CAEP response for Case 5 obtained after bilateral hearing aid intervention (4B), and average P1 latency as a function of child’s age plotted against the 95% confidence limits for normal-hearing children after hearing aid intervention for Case 5 (4C).
The patient was tested via bilateral insert earphones due to significant feedback from the powerful hearing aids. As shown in Figure 5B, a P1 response was clearly present. However, the latency of the P1 response fell outside of normal limits according to the child’s age (Fig. 5C). The delayed latency of the P1 response falling outside normal limits indicates that the hearing aids were not providing adequate stimulation for the central auditory pathways to develop normally.
In this case of multiple disabilities, P1 responses were used to assess cortical maturation and function for which conventional audiometric techniques could not reliably assess hearing aid benefit. While a P1 CAEP response was present, the latency of this response fell outside of normal limits according to the child’s chronological age, suggesting that the child may be a candidate for cochlear implantation, given that the child had already received a hearing aid trial using powerful hearing aids.
Discussion
The case studies in this review illustrate the use of the CAEP P1 latency as a biomarker for central auditory development in children with hearing loss with and without additional disabilities who received intervention through conventional hearing aids and CIs. In Cases 1 and 2, we demonstrate the use of the P1 CAEP in the typical clinical setting where amplification was initiated using traditional hearing aids. In Case 1, the latency of the P1 response fell within normal limits, providing evidence that the hearing aids were providing adequate amplification required for normal auditory development. On the other hand, in Case 2, the P1 latency was outside of normal limits with amplification, but rapidly decreased to normal limits within a short period after cochlear implantation. This outcome is consistent with our previous results (4) and with animal studies (23), demonstrating that children implanted within the sensitive period tend to display age-appropriate cortical latencies within 6–12 months after implantation. The P1 latency in Cases 1 and 2 provided objective evidence that was consistent with successful use of amplification and/or electrical stimulation.
The P1 biomarker also provides an objective way to assess the development of central auditory pathways in children with multiple disabilities for whom behavioral audiometric and speech measures may not reliably predict or assess benefit with intervention. Cases 3, 4, and 5 demonstrate the utility of the P1 biomarker in the clinical decision-making process for children with multiple disabilities. For Case 3 who had co-morbid ANSD, the P1 response verified that the unilateral CI was providing sufficient benefit to allow normal development of the central auditory pathways, and that the child was a reasonable candidate for bilateral implantation since the unaided ear showed absent P1 responses. In ANSD, audiometric thresholds are often not consistent with behavioral outcomes, and therefore objective verification of benefit from the CI in the left ear and lack of benefit in the unaided right ear was useful in this case.
In case 4, we reported on a child with CHARGE association who had received intervention with amplification. In this instance, the child showed age-appropriate central auditory maturation (indicated by normal P1 latency). On the other hand, in Case 5, we reported on a child with Pallister- Killian Syndrome who showed delayed cortical maturation with amplification (indicated by delayed P1 latency), consistent with his evaluation for CI candidacy.
It is of interest to compare the use of the P1 CAEP for the hearing-impaired children with and without additional disabilities. Cases 1 and Case 4 were both fitted with hearing aids at similar ages, i.e., 4 months and 5 months, respectively, and CAEP P1 responses were recorded at comparable ages, i.e., 26 months and 23 months respectively. Although Case 4’s hearing loss was part of the CHARGE association, and Case 1 had no additional disabilities, the P1 responses were similarly robust with comparable morphology and age-appropriate latencies for both children (Figs 1B and Fig 4B respectively). As Children with CHARGE syndrome often show involvement of the auditory nerve and/or deficits in the central pathways, the robust P1 responses were a particularly promising result for this patient in whom no reliable speech perception testing was possible.
We recorded P1 responses 11 months post-cochlear implantation for Cases 2 and 3. Both children, Case 2 whose hearing loss was secondary to Connexin 26 (and in whom there was no additional disability), and Case 3 whose hearing loss was co-morbid with ANSD showed age-appropriate P1 latencies (although as is typical with some children with ANSD, P1 amplitude was lower for Case 3). Taken together, results for Cases 1–4 suggest that for at least some children with multiple disabilities, if appropriate intervention is provided early, then central auditory development can proceed in a manner comparable to that seen for hearing-impaired children without multiple disabilities. Given previous reports of less than favorable behavioral outcomes for CI children with multiple disabilities (31, 35, 36), the CAEP P1 provides the ability to separate the effects of sensory stimulation from the cognitive/intellectual deficits associated with the disability. For example, in Cases 3 and 4, normal P1 responses provided information that the implant was appropriately fit, allowing central auditory development to proceed normally, in turn providing the rehabilitative team with the information they needed to focus on the more cognitive aspects of language acquisition.
Overall, despite the fact that the rate of cochlear implantation for children with multiple disabilities is increasing rapidly, there is very little information on cortical development and plasticity specific to disabilities that are co-morbid with hearing loss. It is possible that there are clinically relevant differences in central auditory system plasticity in hearing impaired children depending on the etiology of the disorder. As previously mentioned, our team has documented preliminary evidence of a significantly shorter sensitive period (of 2 years) for children with ANSD, indicating a much shorter window of plasticity which could affect clinical decision-making for children with ANSD. Future studies should attempt to understand central auditory plasticity in children as a function of the diverse genetic or environmental causes for their hearing loss. This evidence may help guide decisions regarding cochlear implant candidacy for children with specific additional disabilities and better understand variable outcomes within this special population.
In summary, given the high cochlear implantation rate worldwide for children with deafness and co-morbid disabilities, the cases in this study highlight the clinical use of the P1 response latency as a biomarker for central auditory development in children with multiple disabilities. When combined with a standard audiological test battery, the CAEP P1 biomarker has a useful role in objectively evaluating the maturation of central auditory pathways to determine the effectiveness of various intervention strategies in hearing impaired children who demonstrate additional handicaps.
Acknowledgments
This research was supported by NIH NIDCD R01 DC0625 to A.S.
List of References
- 1.Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6(6):363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- 2.Eggermont JJ. On the rate of maturation of sensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1988 Oct;70(4):293–305. doi: 10.1016/0013-4694(88)90048-x. [DOI] [PubMed] [Google Scholar]
- 3.Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006 Feb;212(1–2):185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Dorman M, Spahr A, Todd NW. Early cochlear implantation in children allows normal development of central auditory pathways. Ann Otol, Rhinol Laryngol Suppl. 2002 May;189:38–41. doi: 10.1177/00034894021110s508. (May) [DOI] [PubMed] [Google Scholar]
- 5.Pang EW, Taylor MJ. Tracking the development of the N1 from age 3 to adulthood: an examination of speech and non-speech stimuli. Clin neurophysiol. 2000 Mar;111(3):388–397. doi: 10.1016/s1388-2457(99)00259-x. [DOI] [PubMed] [Google Scholar]
- 6.Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000 Feb;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 7.Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clinical Neurophysiol. 2005 Mar;116(3):648–657. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Sharma a, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol. 1997 Nov;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont JJ, Ponton CW. The neurophysiology of auditory perception: from single units to evoked potentials. Audiology Neurootol. 2002;7(2):71–99. doi: 10.1159/000057656. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Martin K, Roland P, Bauer P, Sweeney MH, Gilley P, et al. P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad of Audiol. 2005 Sep;16(8):564–573. doi: 10.3766/jaaa.16.8.5. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Dorman MF, Spahr AJ. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002 Jul 19;13(10):1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. [cited 2013 Mar 6];Ear Hear. 2002 Dec;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Dorman M. Central auditory development in children with cochlear implants: clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Gilley P, Dorman MF, Baldwin R. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46(9):494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- 16.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb Cortex. 2000 Jul;10(7):714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- 17.Kral a, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurootol. 2001;6(6):346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- 18.Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012 Feb;35(2):111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J Comp Neurol. 1997 Aug 25;385(2):230–244. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal Comp Neurol. 1997 Oct 20;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Tajudeen Ba, Waltzman SB, Jethanamest D, Svirsky M a. Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otology Neurotol. 2010 Oct;31(8):1254–1260. doi: 10.1097/MAO.0b013e3181f2f475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh S, Kim C, Kang EJ, Lee DS, Lee HJ, Chang SO, et al. Speech Perception after Cochlear Implantation over a 4-Year Time Period. Acta Otolaryngol. 2003 Jan;123(2):148–153. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- 23.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002 Aug;12(8):797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- 24.Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005 May;15(5):552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- 25.Kral A, Tillein J. Brain plasticity under cochlear implant stimulation. Adv Otorhinolaryngol. 2006 Jan;64:89–108. doi: 10.1159/000094647. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Dorman M. Central auditory development, plasticity and re-organization after cochlear implantation: Evidence from evoked potential and brain imaging studies. In: Zeng F-G, Popper AN, Fay RR, editors. Auditory Prostheses: New Horizons. New York: Springer Science+Business Media, LLC; 2011. [Google Scholar]
- 27.Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nature Neurosci. 2010 Nov;13(11):1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- 28.Kral A, Eggermont JJ. What’s to lose and what's to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Res Rev. 2007 Nov;56(1):259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Kral A, Eggermont JJ. What’s to lose and what's to learn: development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Research Rev. 2007 Nov;56(1):259–269. doi: 10.1016/j.brainresrev.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Lee D, Lee J, Oh S, Kim S, Kim J, Chung J, Lee M, Kim C. Cross-modal plasticity and cochlear implants. Nature. 2001 Jan 11;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- 31.Fortnum HM, Marshall, DH SA. Epidemiology of the UK population of hearing-impaired children, including characteristics of those with and without cochlear implants - audiology, aetiology, cormobiditiy and affluence. Int J Audiol. 2002;41:170–179. doi: 10.3109/14992020209077181. [DOI] [PubMed] [Google Scholar]
- 32.Berrettini S, Forli F, Genovese E, Santarelli R, Arslan E, Chilosi AM, et al. Cochlear implantation in deaf children with associated disabilities: challenges and outcomes. Int J Audiol. 2008 Apr;47(4):199–208. doi: 10.1080/14992020701870197. [DOI] [PubMed] [Google Scholar]
- 33.Waltzman SB, Scalchunes V, Cohen NL. Performance of multiply handicapped children using cochlear implants. Am J Otol. 2000 May;21(3):329–335. doi: 10.1016/s0196-0709(00)80040-x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, KC WS. Clinical Management of Children With Cochlear Implantation. San Diego, CA: Plural Publishing; 2009. Cochlear implantation in children with multiple disabilities; pp. 573–562. [Google Scholar]
- 35.Birman CS, Elliott EJ, Gibson WPR. Pediatric cochlear implants: additional disabilities prevalence, risk factors, and effect on language outcomes. Otology Neurotol. 2012 Oct;33(8):1347–1352. doi: 10.1097/MAO.0b013e31826939cc. [DOI] [PubMed] [Google Scholar]
- 36.Pyman B, Blamey P, Lacy P, Clark G DR. The development of speech perception in children using cochlear implants: effects of etiologic factors and delayed milestones. Am J of Otol. 2000;21(1):57–61. [PubMed] [Google Scholar]
- 37.Martin BS HM. Pathological Plasticity in Fragile X Syndrome. Neural Plast. 2012 doi: 10.1155/2012/275630. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGraw CM, Samaco RC ZH. Adult neural function requires MeCP2. Science. 2011;8(333):186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardon G, Sharma A. Central Auditory Maturation and Behavioral Outcome in Children with Auditory Neuropathy Spectrum Disorder who Use Cochlear Implants. Int J Audiol. 2013 doi: 10.3109/14992027.2013.799786. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trimble K, Rosella LC, Propst E, Gordon KA, Papaioannou V, Papsin BC. Speech Perception Outcome in Multiply Disabled Children Following Cochlear Implantation: Investigating a Predictive Score. J Am Acad Audiol. 2008 Sep 1;19(8):602–611. doi: 10.3766/jaaa.19.8.4. [DOI] [PubMed] [Google Scholar]
- 41.Edwards LC. Children with cochlear implants and complex needs: a review of outcome research and psychological practice. J Deaf Stud Deaf Ed. 2007 Jan;12(3):258–268. doi: 10.1093/deafed/enm007. [DOI] [PubMed] [Google Scholar]
- 42.Gilley PM, Sharma A, Dorman M, Finley CC, Panch AS, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006 Aug;117(8):1772–1782. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Sharma A, Cardon G, Henion K, Roland P. Cortical maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder. Int J Audiol. 2011 Feb;50(2):98–106. doi: 10.3109/14992027.2010.542492. [DOI] [PMC free article] [PubMed] [Google Scholar]