Abstract
Solitary plasmacytoma (SP) is a rare tumor with low incidence. The aim of this study was to investigate the clinical features, treatment strategies, and relative prognostic factors of 66 patients with SP. These patients made up 10.25% of the 644 patients with plasma cell dyscrasias treated at the Tianjin Medical University Cancer Institute and Hospital over the past 12 years. SP always presented with either solitary bone plasmacytoma (SBP) or extramedullary plasmacytoma (EMP), as determined by the location of the lesions. SBP occurred most frequently in the vertebral column and EMP in the upper respiratory tract. In addition to other factors, tumor size, serum M protein level, urinary Bence Jones protein level, and disease progression toward multiple myeloma were significantly different between the two groups (P<0.05). Larger tumor size (≥5 cm) was associated with poor prognosis of local control, multiple myeloma–free survival, overall survival and progression-free survival for SBP patients. Radiotherapy and serum β2 microglobulin <3.5 mg/L were favorable prognostic factors for local control, multiple myeloma-free survival, and progression-free survival in patients with EMP.
Keywords: solitary bone plasmacytoma, extramedullary plasmacytoma, clinical characteristics, prognosis, radiotherapy
Introduction
Solitary plasmacytoma (SP) is an infrequent form of plasma cell dyscrasia. It involves a localized accumulation of neoplastic monoclonal plasma cells and manifests a distinctive osseous or extraosseous growth pattern.1–3 Solitary bone plasmacytoma (SBP) and extramedullary plasmacytoma (EMP) are two clinical subsets of SP reflecting the location of the lesion. The clinical course and prognosis of these two entities are quite different from each other.
Because of the incidence, natural history, and pattern of progression, most studies on this subject have involved relatively small numbers of patients and therefore have had a limited ability to make any robust conclusions regarding the effects of prognostic factors in patients with SP. In the current study, the clinical features, treatment outcomes, and relative prognostic factors of 66 patients with SBP or EMP treated over a 12-year period were analyzed retrospectively at the Tianjin Medical University Cancer Institute and Hospital, one of the largest and most authoritative cancer centers in the People’s Republic of China. To increase understanding of the clinical features and the course of solitary plasmacytoma, a comparison between SBP and EMP was made. Factors that may affect the prognosis of SP were also identified. This information may facilitate the development of appropriate strategies for clinical diagnosis and the treatment of patients with SBP and EMP.
Patients and methods
Patient selection and diagnostic criteria
Between July 2000 and October 2012, 644 patients were diagnosed with pathologically proven plasmacytoma at the Tianjin Medical University Cancer Institute and Hospital. The current study was performed in strict accordance with local ethical guidelines and recommendations of the Declaration of Helsinki (Seoul revision, 2008). Among these patients, and 578 presented with multiple myeloma (MM) at the time of diagnosis. Of these, 66 patients (10.25%) were evaluated as having SP (including 45 having SBP and 21 having EMP). The histological diagnosis was based on the World Health Organization classification system for hematologic malignancies.4 The recommended SBP diagnosis requires a single area of bone damage due to clonal plasma cell hyperplasia; histologically normal marrow aspirate and trephine samples; normal skeletal survey results; no anemia, hypercalcemia, or renal impairment attributable to plasma cell dyscrasia; little or no serum or urinary monoclonal immunoglobulin (level of >20 g/L, possibly indicative of MM); and no additional lesions visible upon magnetic resonance imaging (MRI) scan of the spine.5−7 The EMP cases were selected on the basis of the recommended diagnostic criteria, including single extramedullary masses of clonal plasma cells; histologically normal marrow aspirate and trephine samples; normal skeletal survey results, including radiology of the long bones; no anemia, hypercalcemia, or renal impairment attributable to plasma cell dyscrasia; and little or no serum or urinary monoclonal immunoglobulin.7−9
Clinical investigation and therapeutic evaluation
The clinical assessment and consistent methods used included histological and physical examinations, blood cell counts, blood urea nitrogen, calcium levels, and creatinine, bone marrow biopsy, serum and urine protein immunoelectrophoresis, quantitation of serum immunoglobulins, measurement of the 24-hour Bence Jones protein excretion, and relative radiological examinations, such as X-ray, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography-computed tomography (PET-CT). During and after therapy, local control (LC) was assessed regularly through repeated skeletal survey and bone marrow examination. M protein levels were periodically monitored, as indicated.7
The main therapeutic approaches included radiotherapy, surgery, chemotherapy, and comprehensive therapeutic strategies. The median dose of radiation in the current study was 50 Gy. The chemotherapy regimens mainly included an MP regimen, consisting of melphalan (8 mg/m2, day 1−4) and prednisone (60 mg/m2, day 1−4); a VBMCP regimen, consisting of carmustine (20 mg/m2, day 1), cyclophosph-amide (400 mg/m2, day 1), vincristine (1.2 mg/m2, day 1), melphalan (8 mg/m2, day 1−4), and prednisone (80 mg/m2, day 1−7); and VAD, consisting of vincristine (0.4 g/d, day 1−4), epirubicin (9 mg/m2/d, day 1−4), and dexamethasone (40 mg/d, day 1−4, 9−12, and 17−20).
The therapeutic effects were evaluated. The resolution of soft tissue mass and the absence of a progression of lytic bone lesions were evaluated clinically and radiologically. Normalization of previously elevated serum M protein levels served as a LC. Local relapse was defined using clinical and radiological evidence of local disease progression with a normal skeletal survey and fewer than 10% of the normal number of plasma cells, as indicated by bone marrow examination. The development of multiple lytic lesions or 10% plasma cells in the bone marrow, with or without an increase in M protein or the development of target organ damage, were considered indicative of progression to multiple myeloma (MM). Progression-free survival (PFS) was defined as the duration from the date of diagnosis until the date of either disease relapse or progressive disease after achieving LC, or the development of MM.3
Statistical analysis
LC, MM-free survival (MMFS), PFS, and overall survival (OS) were calculated from the date of diagnosis to the event of interest. Clinical characteristics that impact survival and subsequent failure in patients with SBP and EMP were compared using the Fisher’s exact test and chi-square (χ2) test. The χ2 test was also performed to identify any possible correlations among the progression to MM and other parameters in patients with SP. Univariate analysis was performed to assess the significance of the prognostic factors for LC, MMFS, OS, and PFS. All the statistical tests were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) software. The tests were two-sided, and a P-value<0.05 was considered statistically significant.
Results
The median follow-up time was 48 months (range 3−144 months). Forty-five patients had SBP and 21 had EMP. The median tumor size was 5 cm (range 1.7–21.5 cm), and 35 (53%) patients had tumors larger than 5 cm. Samples were positive for serum M protein, by immunofixation electrophoresis, in 18 patients (27.2%). Among the 45 SBP patients, the mean age was 58.51 years (2–83 years), with 84.4% of patients over the age of 50. The male to female ratio was 1.14:1. Out of the total, 28.9% (13/45) of the tumors presented in the vertebral column, 28.9% in the long tubal bones, 24.4% (11/45) in the sternum and ribs, 8.9% (4/45) in the craniofacial bones, 6.7% (3/45) in the clavicle, and 2.2% (1/45) presented in the mandible (Table 1). Serum M protein was detected in 16 (35.6%) of the 45 cases. The age at diagnosis of the 21 EMP patients ranged from 19 to 83, with a mean age of 56.67 years. Men were more inclined to have EMP, and there was a male to female ratio of 1.63:1. The upper respiratory tract was the most common site of EMP, accounting for 38.1% of the cases, followed by soft tissue (four cases [19%]), pleura/peritonea (three cases [14.3%]), ocular adnexal (two cases [9.5%]), and the tonsils, testes, callosum, and mediastinum (one case each) (Table 1). The tumor sizes were larger in the SBP group than in the EMP group (P=0.019). The proportion of patients with positive serum M protein (P=0.027) and urinary Bence Jones protein (P=0.035) was higher in the SBP group. The detection of progression to MM also showed some discrepancies between the two groups. Some 16 (35.6%) patients in the SBP group and two (9.5%) patients in the EMP group showed evidence of MM (P=0.027) (Table 2). Thirteen SBP patients for whom the vertebral column was affected took an MRI test in our hospital after diagnosis or for the purpose of monitoring therapeutic effect, ten with enhanced MRI and three with routine MRI. Three patients had PET-CT. Eleven patients had cytogenetic examination, which showed no abnormal results.
Table 1.
Sites of localization in the patients with bone or extramedullary plasmacytoma
| Anatomic site | N (%) |
|---|---|
| Solitary plasmacytoma of bone | 45 |
| Vertebral column | 13 (28.9) |
| Long tubal bone | 13 (28.9) |
| Sternum and ribs | 11 (24.4) |
| Craniofacial bone | 4 (8.9) |
| Clavicle | 3 (6.7) |
| Mandible | 1 (2.2) |
| Solitary extramedullary plasmacytoma | 21 |
| Upper respiratory tract | 8 (38.1) |
| Soft tissue | 4 (19.05) |
| Pleura/peritonea | 3 (14.3) |
| Ocular adnexal | 2 (9.5) |
| Other sitesa | 4 (19.05) |
Note:
Tonsil, testis, callosum, mediastinum (one case each).
Table 2.
Clinical characteristics of patients with solitary plasmacytoma
| Characteristics | Histological types
|
P-valuea | |
|---|---|---|---|
| SBP | EMP | ||
| Sex, mean (%) | 0.513 | ||
| Male | 24 (53.3) | 13 (61.9) | |
| Female | 21 (46.7) | 8 (38.1) | |
| Age (years) | 0.216 | ||
| Median (range) | 58.51 (22–83) | 56.67 (19–83) | |
| ≥50 year mean, n (%) | 38 (84.4) | 15 (71.4) | |
| Tumor size at diagnosis, number (%) | 0.019 | ||
| >5 cm | 10 (22.2) | 11 (53.4) | |
| ≥5 cm | 29 (64.4) | 6 (28.6) | |
| N/A | 6 (13.3) | 4 (19) | |
| Serum M protein,b mean (%) | 0.027 | ||
| Negative | 29 (64.4) | 19 (90.5) | |
| Positive | 16 (35.6) | 2 (9.5) | |
| Urinary BJP, mean (%) | 0.035 | ||
| Positive | 17 (37.8) | 2 (9.5) | |
| Negative | 20 (44.4) | 16 (76.2) | |
| N/A | 8 (17.8) | 3 (14.3) | |
| Serum β2 microglobulin, mean (%) | 0.769 | ||
| <3.5 mg/L | 35 (77.8) | 17 (81.0) | |
| ≥3.5 mg/L | 10 (22.2) | 4 (19.0) | |
| Progression to MM, mean (%) | 0.027 | ||
| Present | 16 (35.6) | 2 (9.5) | |
| Absent | 29 (64.4) | 19 (90.5) | |
Notes:
Pearson’s chi-square test
the presence, type, and disappearance of serum M protein were determined by immunofixation electrophoresis.
Abbreviations: BJP, Bence Jones protein; EMP, extramedullary plasmacytoma; Ig, immunoglobulin; LC, light chain; MM, multiple myeloma; N/A, not available; SBP, solitary bone plasmacytoma.
Sixty-two out of the 66 patients were evaluable for treatment response and outcome (among the remaining four patients, one did not receive treatment and the other three did not complete follow up after treatment). The treatments consisted of local radiotherapy (n=25), surgery (n=14), radiotherapy plus surgery (n=19), and combined chemotherapy (n=7, four received VAD, two received MP, and the remaining one received the VBMCP chemotherapy regimen) for the patients who had larger masses or who presented with complicated syndromes (Table 3).
Table 3.
Treatment approaches
| Treatment approaches | SBP | EMP | Patients, n (%) |
|---|---|---|---|
| RT alone | 16 | 9 | 25 (37.9%) |
| RT + CT | 4 | 0 | 4 (6.1%) |
| Surgery alone | 12 | 2 | 14 (2.2%) |
| Surgery + RT | 9 | 10 | 19 (28.8%) |
| Surgery + CT | 3 | 0 | 3 (4.5%) |
| CT regimens | |||
| VAD | 4 | 0 | 4 (6.1%) |
| MP | 2 | 0 | 2 (3.1%) |
| VBMCP | 1 | 0 | 1 (1.5%) |
| Radiation dose | |||
| ≤40 Gy | 8 | 3 | 11 (16.7%) |
| >40 to ≤50 Gy | 13 | 12 | 25 (37.9%) |
| >50 Gy | 8 | 4 | 12 (18.2%) |
Notes: The chemotherapy regimens were: VAD, consisting of vincristine + epirubicin + dexamethasone; MP, consisting of melphalan + prednisone; and VBMCP, consisting of carmustine + cyclophosphamide + vincristine + melphalan + prednisone.
Abbreviations: CT, chemotherapy; EMP, extramedullary plasmacytoma; RT, radiotherapy; SBP, solitary bone plasmacytoma.
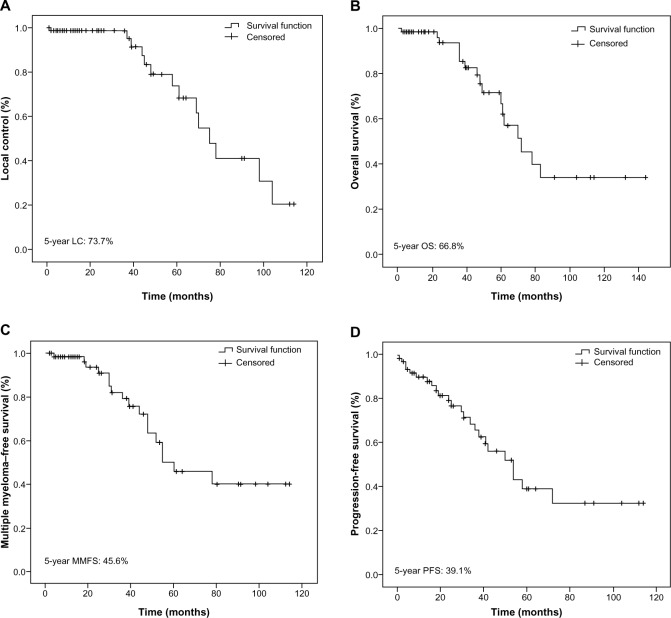
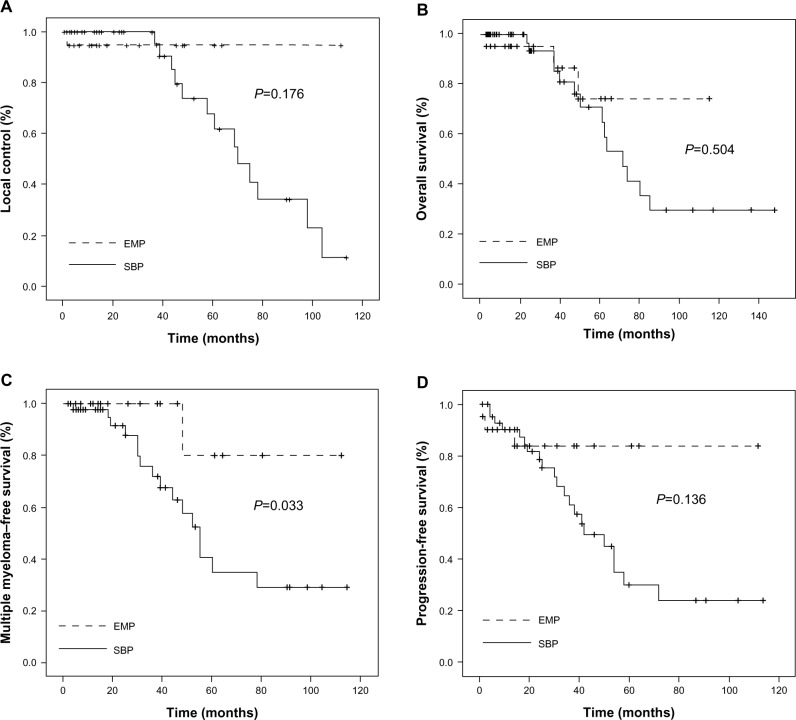
For all 66 SP patients, the 5-year LC rate was 73.7% (Figure 1A). The EMP group showed high LC rates, 95%, which was markedly higher than the 68% seen in the SBP cohort; however, this did not constitute a statistically significant difference (P=0.176) (Figure 2A). Nevertheless, the SP patients treated with radiotherapy showed higher 5-year LC rates than did those without radiotherapy (90.8% vs 53%) (P=0.163), but this difference too, was not found to be statistically significant, due to the small sample size. The 5-year OS rate was 66.8% (Figure 1B). Among the patients with EMP, three patients died during the follow-up period, resulting in the 5-year OS rates of 74.2%. One died from non-cancer-related causes, and the other two died due to the progression to MM. The SBP group had 5-year OS rates of 64.9% (P=0.504) (Figure 2B). In patients with SBP, a total of 14 deaths occurred, eleven from MM and MM-related complications, and three from other causes. The 5-year MMFS rate for all patients was 45.6% (Figure 1C). SBP progressed more frequently to MM than did EMP (P=0.033) (Figure 2C). The median time of progression to MM after diagnosis was 25 (range 3–114) and 15 (range 2–112) months for EMP and SPB, respectively. The 5-year PFS rate for all patients was 39.1% (Figure 1D). The patients with SBP demonstrated worse PFS rates than did the EMP patients (30.0% vs 83.8%); however, this difference was not found to be statistically significant (P=0.136) (Figure 2D).
Figure 1.
Kaplan–Meier plots of local control (A), overall survival (B), multiple myeloma–free survival (C), and progression-free survival (D), for all patients.
Abbreviations: LC, local control; MMFS, multiple myeloma–free survival; OS, overall survival; PRS, progression-free survival.
Figure 2.
Kaplan–Meier plots of local control (A), overall survival (B), multiple myeloma–free survival (C), and progression-free survival (D), according to the disease subsets.
Abbreviations: EMP, extramedullary plasmacytoma; SBP, solitary bone plasmacytoma.
Univariate analysis of the patients with SBP revealed that a large tumor size (≥5 cm) was a significantly adverse prognostic factor affecting LC, MMFS, OS, and PFS. Additionally, positive serum M protein had an adverse effect on LC (P=0.015), MMFS (P=0.005), and OS (P=0.001). Serum β2 microglobulin affected both MMFS (P=0.035) and OS (P=0.018). Bence Jones protein may be a predictive factor for PFS (P=0.017). In patients with EMP, undergoing radiotherapy and lacking elevated β2 microglobulin were favorable prognostic factors for LC and MMFS (P<0.05) (Table 4).
Table 4.
Univariate analysis of the prognostic factorsa
| Type | Factor | Local control
|
MMFS
|
OS
|
PFS
|
||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | P-value | χ2 | P-value | χ2 | P-value | χ2 | P-value | ||
| SBP | Age | 0.032 | 0.858 | 0.543 | 0.461 | 1.398 | 0.237 | 0.840 | 0.360 |
| Sex | 0.410 | 0.552 | 0.009 | 0.923 | 0.023 | 0.879 | 0.034 | 0.853 | |
| Tumor size | 6.221 | 0.045 | 9.273 | 0.010 | 7.196 | 0.027 | 11.624 | 0.003 | |
| Radiotherapy | 1.237 | 0.266 | 0.055 | 0.814 | 0.000 | 0.998 | 0.080 | 0.777 | |
| Serum M protein in IF | 5.878 | 0.015 | 7.708 | 0.005 | 10.183 | 0.001 | 2.515 | 0.113 | |
| Detected BJP | 4.769 | 0.092 | 4.912 | 0.086 | 3.989 | 0.136 | 8.104 | 0.017 | |
| Serum β2 microglobulin | 1.844 | 0.175 | 4.462 | 0.035 | 5.575 | 0.018 | 1.113 | 0.287 | |
| EMP | Age | 0.429 | 0.513 | 0.667 | 0.414 | 1.505 | 0.220 | 1.131 | 0.288 |
| Sex | 1.000 | 0.317 | 1.500 | 0.221 | 0.042 | 0.837 | 0.016 | 0.899 | |
| Tumor size | 1.000 | 0.607 | 1.500 | 0.572 | 0.964 | 0.617 | 0.596 | 0.742 | |
| Radiotherapy | 19 | 0.000 | 4.000 | 0.046 | 0.316 | 0.574 | 0.182 | 0.669 | |
| Serum M protein in IF | 0.111 | 0.739 | 1.500 | 0.221 | 0.435 | 0.509 | 2.149 | 0.143 | |
| Detected BJP | 0.333 | 0.864 | 4.000 | 0.135 | 0.932 | 0.628 | 0.942 | 0.624 | |
| Serum β2 microglobulin | 4.000 | 0.046 | 4.000 | 0.046 | 0.553 | 0.457 | 0.952 | 0.329 | |
Note:
Logrank test.
Abbreviations: BJP, Bence Jones protein; EMP, extramedullary plasmacytoma; IF, immunofixation electrophoresis; MM, multiple myeloma; MMFS, multiple myeloma–free survival; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SBP, solitary bone plasmacytoma.
We also studied possible correlations among the progression to MM and other parameters in SP patients. This was performed with χ2 testing. The results indicated that positive serum M protein in immunofixation was associated with progression to MM for both SBP (P=0.003, contingency coefficient =0.339) and EMP (P=0.002, contingency coefficient =0.362) patients. These results indicated that close attention should be paid to SP patients with positive serum M protein.
Discussion
SP is an independent subtype of plasmacytoma, charac-terized by the proliferation of monoclonal plasma cells, including SBP and EMP. A previous report suggested that SP accounts for 2%−10% of all plasma cell tumors.10 That the ratio of SP to total plasma cell tumors was higher in the present study than in previous studies is related to the fact that the present study was conducted at one of the biggest cancer centers in the People’s Republic of China − many patients with solitary tumors come to this center for treatment, while patients who are believed to be suffering only from anemia or thrombocytopenia tend to go to general hospitals instead. The early symptoms of SBP are not typical. The most common symptom is pain. If the spine is involved, deformities, motor deficits, sensory deficits, and bowel and bladder dysfunction can also occur as a result of epidural spinal cord compression and instability of the vertebra. EMP patients mainly manifest with local masses and relevant symptoms, which are nonspecific and depend on the site and spread of the tumor.11
SBP mostly occurs in the axial skeleton and is always associated with bone pain, pathologic fractures, or nerve involvement, while EMP is observed most frequently in the upper respiratory tract, including the nasal cavity and nasopharynx.12 Other sites of EMP include the digestive tract, skin, lung, breast, eye socket, retroperitoneal area, and testicle.9 In the present study, the vertebral column and upper respiratory tract were found to be the most common site of SBP and EMP, accounting for 28.9% and 38.1% of cases, respectively. So the common sites of occurrence of SBP and EMP in the present study were basically similar to those reported in previous studies. A large-scale American study performed to assess the incidence and survival patterns of plasmacytoma between 1992 and 2004 reported a predominance among male patients, blacks, and older individuals of plasmacytoma and MM.13 The results of the present study were broadly consistent with this previous work: The mean age was 58.51 years (22−83 years), with 84.4% of patients over the age of 50. Also, the male to female ratio was 1.14:1 and 1.63:1, respectively, for the SBP and EMP groups, indicating that men were more likely to experience SP.
According to the current literature,9 skeletal survey to confirm solitary bone lesion, bone marrow biopsy to ensure plasma cell percentage, biopsy to prove plasma cell infiltration, and relative laboratory examinations to exclude myeloma-related organ dysfunction are all necessary for the diagnosis of SP. Patients were enrolled in the present study in accordance with these requirements. Skeletal surveys were found to be beneficial for the detection of local lesions. CT may be more useful in the detection of destroyed bone, and investigators agree that a negative MRI of the thoracic and lumbosacral spine is a prerequisite for the diagnosis of SBP.14 MRI is valuable in the assessment of spinal cord damage, but it appears less accurate in the identification of benign versus malignant vertebral compression fracture than in bone metastasis.15 Kim et al recently claimed that 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has been effective for staging in plasmacytoma and potentially could have a role in response assessment after radiotherapy,16 indicating that novel instruments may be more advantageous to our understanding of the disease and to develop better treatment strategies.
The therapeutic approaches to SP mainly include radiotherapy, surgery, and chemotherapy, though several unanswered questions remain. Because of the excellent LC and the possibility of translating the disease into a long remission or even cure, radiotherapy appears to be an effective treatment for SP.17,18 In the present study, 72.7% (48/66) of SP patients were treated with radiotherapy or with chemotherapy and/or surgery coupled with radiotherapy as the systematic treatment. Among these 48 patients, 79.2% (38/48) had LC. Suh et al reported on 38 SP patients who were treated with radiotherapy over 4 years of observation and drew the conclusion recently that radiotherapy could be used to treat SP effectively and without significant toxicity.10 Radiation levels of at least 40 Gy in limited fields are recommended for the management of solitary extramedullary plasmacytoma.19 This is consistent with the results of the present study, in which the median dose of radiation was 50 Gy. The current National Comprehensive Cancer Network guidelines listed radiotherapy to the involved field and/or surgery as the primary therapy for EMP.20 If complete surgical tumor resection is unfeasible or impossible, if the lymph nodes are affected, or if the patient is high risk, then combined therapy (surgery and radiation) is recommended.21,22 Here, 26 SP patients were treated with combined therapy, and the LC rate was 84.6% (22/26), which was higher than the LC rate found among the patients treated with radiotherapy alone (75%) or surgery alone (71.4%). The role of surgery in treating SP should also be discussed. One recent study demonstrated that radiotherapy combined with surgery produced better OS in patients with solitary EMP of the head and neck.23 Although some scholars maintain that radiotherapy is associated with good survival, surgery should be required when pathologic fractures are present in an involved bone, when immediate decompensation of a tumor is necessary, or when moderate doses of radiotherapy are problematic due to the presence of adjacent critical organs.10 The function of chemotherapy remains unclear to date, and there is no consensus on chemotherapy use for patients with SBP. Encouragingly, a recent study found that the use of chemotherapy showed a tendency toward better MMFS in patients with SBP.10 In summary, most scholars advocate radiation therapy for SP, but surgery and postoperative radiotherapy are helpful for patients with local recurrence.
Due to the rarity and the scattered reports of SP, determination of the prognostic factors can be difficult, but it is certain that the prognosis is poor once the diagnosis is made that SP is concurrent with MM. Concomitant with the present study, Kilciksiz et al also explained that SBP has a signifi-cantly higher risk of progression to MM than does EMP.1 For patients treated with radiotherapy, progression to MM remains the main problem.24,25 Investigations should focus on adjuvant chemotherapy and novel therapeutic agents. Ozsahin et al also pointed out that therapeutic strategies should be developed for bone or bulky extramedullary SP.26 Recently, Suh et al reported that in patients with EMP, large tumor size (>5 cm) and elevated β2 microglobulin (>3.5 mg/L) were significant adverse factors, affecting survival. In patients with SBP, radiation doses ≥40 Gy were found to be favorable prognostic factors for LC.10 Bettini et al have confirmed the prognostic value of the β2 microglobulin in MM.27 In the present study, large tumor size (≥5 cm), positive serum M protein and serum β2 microglobulin were found to affect the prognosis of SBP patients. Radiotherapy and serum β2 microglobulin <3.5 mg/L were found to be favorable prognostic factors for EMP patients.
An additional novel finding of this study was the correlation between positive serum M protein in immunofixation electrophoresis and progression to MM, which has been shown to be correlated to disease progression. This suggests that attention should be paid to local bone involvement in routine clinical work. Patients with risk factors for MM require close follow up, and more investigations into the use of novel therapeutics should be performed to show how the progression to MM could be prevented or delayed.
Conclusion
A total of 644 cases of plasma cell dyscrasia were registered at the Tianjin Medical University Cancer Institute and Hospital between 2000 and 2012. Of these, 10.25% were diagnosed with SP, including 45 cases of SBP and 21 cases of EMP. The vertebral column and upper respiratory tract were the most common sites of SBP and EMP, respectively. SBP was more inclined to progress to MM than was EMP, and the statistical analysis indicated that SBP had a poorer prognosis. Because of the high sensitivity to radiation, an effective modality of treatment of SP appears to be radiotherapy. In the present study, we found that for SBP patients, larger tumor sizes (≥5 cm), positive serum M protein, and serum β2 microglobulin were factors affecting prognosis. Radiotherapy and serum β2 microglobulin levels were also found to have an influence on LC and MMFS for EMP patients. For this reason, patients with risk factors require close follow up, and more investigation into the use of novel therapeutics should be used to prevent disease progression. More large clinical studies must be performed to improve the understanding of SP.
Acknowledgments
We gratefully acknowledge the support of grants from the National Natural Science Foundation of China (grant numbers 30672208 and 81270603).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. Scientific World Journal. 2012;2012:895765. doi: 10.1100/2012/895765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorsbach RB, Hsi ED, Dogan A, Fend F. Plasma cell myeloma and related neoplasms. Am J Clin Pathol. 2011;136(2):168–182. doi: 10.1309/AJCPENJ68FFBRIYB. [DOI] [PubMed] [Google Scholar]
- 3.Baghmar S, Mohanti BK, Sharma A, et al. Solitary plasmacytoma: 10 years’ experience at All India Institute of Medical Sciences, New Delhi. Leuk Lymphoma. 2013;54(8):1665–1670. doi: 10.3109/10428194.2012.750725. [DOI] [PubMed] [Google Scholar]
- 4.SH SH Swerdlow, E Campo, Harris NL, et al., editors. WHO Classifi-cation of Tumours of Haematopoietic and Lymphoid Tissues. Lyons: International Agency for Research on Cancer (IARC); 2008. [Google Scholar]
- 5.Hughes M, Soutar R, Lucraft H, Owen R, Bird J.Guidelines on the Diagnosis and Management of Solitary Plasmacytoma of Bone, Extramedullary Plasmacytoma and Multiple Solitary Plasmacytomas: 2009 Update London: British Committee for Standards in Haematology; 2009Available from: http://www.guideline.gov/content.aspx?id=15514Accessed October 14, 2013 [Google Scholar]
- 6.Xiao J, Huang W, Yang X, Teng H.Solitary plasmacytoma of bone Gupta A.Multiple Myeloma-An Overview Rijeka: InTech; 2012Available from: http://www.intechopen.com/books/multiple-myeloma-an-overview/solitary-plasmacytoma-of-boneAccessed October 14, 2013 [Google Scholar]
- 7.Soutar R, Lucraft H, Jackson G, et al. Working Group of the UK Myeloma Forum. British Committee for Standards in Haematology. British Society for Haematology Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol) 2004;16(6):405–413. doi: 10.1016/j.clon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47–51. [PubMed] [Google Scholar]
- 9.Salogub C, Lokhmatova E, Sozin S.Solitary bone and extramedullary plasmacytoma Gupta A.Multiple Myeloma-An Overview Rijeka: InTech; 2012Available from: http://www.intechopen.com/books/multiple-myeloma-an-overview/plasmocytomaAccessed October 14, 2013 [Google Scholar]
- 10.Suh YG, Suh CO, Kim JS, Kim SJ, Pyun HO, Cho J. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol. 2012;91(11):1785–1793. doi: 10.1007/s00277-012-1510-6. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol. 2002;3(3):255–259. doi: 10.1007/s11864-002-0015-2. [DOI] [PubMed] [Google Scholar]
- 12.Straetmans J, Stokroos R. Extramedullary plasmacytomas in the head and neck region. Eur Arch Otorhinolaryngol. 2008;265(11):1417–1423. doi: 10.1007/s00405-008-0613-0. [DOI] [PubMed] [Google Scholar]
- 13.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992–2004. Br J Haematol. 2009;144(1):86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology AM Soc Hematol Educ Program. 2005;2005(1):373–376. doi: 10.1182/asheducation-2005.1.373. [DOI] [PubMed] [Google Scholar]
- 15.Lafforgue P, Clairet D, Chagnaud C, et al. Aspects and role of spinal MRI in the assessment of solitary plasmacytoma and multiple myeloma. Apropos of 11 cases. Rev Rhum Mal Osteoartic. 1992;59(5):317–326. French. [PubMed] [Google Scholar]
- 16.Kim PJ, Hicks RJ, Wirth A, et al. Impact of 18F-fluorodeoxyglucose positron emission tomography before and after definitive radiation therapy in patients with apparently solitary plasmacytoma. Int J Radiat Oncol Biol Phys. 2009;74(3):740–746. doi: 10.1016/j.ijrobp.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park) 2000;14(1):101–108. discussion 111–112, 115. [PubMed] [Google Scholar]
- 18.Jyothirmayi R, Gangadharan VP, Nair MK, Rajan B. Radiotherapy in the treatment of solitary plasmacytoma. Br J Radiol. 1997;70(833):511–516. doi: 10.1259/bjr.70.833.9227234. [DOI] [PubMed] [Google Scholar]
- 19.Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211–215. doi: 10.1111/j.1445-5994.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network (NCCN) NCCN Multiple Myeloma Guideline. Version 1.2012 NCCN; 2012Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#myelomaAccessed July 26, 2012 [Google Scholar]
- 21.Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: Tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305–2314. [PubMed] [Google Scholar]
- 22.Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacy-toma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys. 2001;50(1):113–120. doi: 10.1016/s0360-3016(00)01572-8. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki R, Yasuda K, Abe E, et al. Multi-institutional analysis of solitary extramedullary plasmacytoma of the head and neck treated with curative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):626–634. doi: 10.1016/j.ijrobp.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Kilciksiz S, Celik OK, Pak Y, et al. Turkish Oncology Group-Sarcoma Working Party Clinical and prognostic features of plasmacytomas: a multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol. 2008;83(9):702–707. doi: 10.1002/ajh.21211. [DOI] [PubMed] [Google Scholar]
- 25.Knobel D, Zouhair A, Tsang RW, et al. Rare Cancer Network Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118. doi: 10.1186/1471-2407-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006;64(1):210–217. doi: 10.1016/j.ijrobp.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Bettini R, Redaelli S, Maino C, et al. Prognostic value of serum beta2-microglobulin in multiple myeloma. Recenti Prog Med. 2005;96(2):81–86. Italian. [PubMed] [Google Scholar]