Abstract
Purpose
We performed a retrospective meta-analysis of adolescents and young adults (AYAs) with AML to determine if differences in outcome exist following treatment on pediatric versus adult oncology treatment regimens.
Patients and Methods
We compared the outcomes of 517 AYAs with AML aged 16 to 21 years (yrs) who were treated on Children's Oncology Group (COG), Cancer and Leukemia Group B (CALGB) and Southwest Oncology Group (SWOG) frontline AML trials from 1986 to 2008.
Results
There was a significant age difference between AYA cohorts in the COG, CALGB and SWOG trials (median, 17.2 vs 20.1 vs 19.8 years, p<0.001). The 10 year event-free survival (EFS) of the COG cohort was superior to the combined adult cohorts, 38±6% vs 23±6%, log-rank p=0.006; as was overall survival, (OS) 45±6% vs 34±7%, with a 10 year estimate comparison of p=0.026. However, the younger age of the COG cohort is confounding, with all patients aged 16-18 years doing better than those 19-21 years. Although the 10 year relapse rate was lower for the COG patients, 29±6% vs 57±8% (Gray's p<0.001), this was offset by a higher post-remission treatment-related mortality (TRM) 26±6% vs 12±6% (Gray's p<0.001). Significant improvements in 10 year EFS and OS were observed for the entire cohort in later studies.
Conclusion
Patients treated on pediatric trials had better outcomes than those treated on adult trials, but age is a major confounding variable, making it difficult to compare outcomes by cooperative group.
Keywords: Acute myeloid leukemia, adolescents, young adults, antineoplastic combined chemotherapy protocols
Introduction
Acute myeloid leukemia (AML) affects patients of all ages, and survival rates in general decrease with advancing age; many factors might contribute to this fact. Adolescents and young adults (AYAs) with AML are cared for by both pediatric and adult oncologists, with dose intensity higher in pediatrics. AYAs with acute lymphoblastic leukemia (ALL) have improved survival when treated on pediatric treatment regimens compared to those designed for older adults (1,2).
Two small preliminary studies have addressed whether AYAs have better outcomes on pediatric or adult AML trials. AYAs treated on one Children's Cancer Group (CCG) trial (3) did betterthan AYAs treated with adult therapies at MD Anderson Cancer Center (MDACC) (4), but the CCG patients were younger than at MDACC. Patients less than 21 years treated at St. Jude Children's Research Hospital and MDACC demonstrated that survival decreased with advancing age, with minimum examination of results by treatment regimens (5).
The current report compares the relative effectiveness of adult and pediatric AML therapy in AYA patients utilizing data from COG, CALGB, and SWOG frontline studies.
Patients and Methods
All patients provided informed consent according to federal and institutional guidelines and in accordance with the Declaration of Helsinki. All COG, CALGB and SWOG studies included in this analysis were performed using IRB-approved protocols. The later studies were registered at www.ClinicalTrials.gov since its inception in 2000.
Childhood Trials
Two hundred eighty-one patients age 16 - 21 years were enrolled on CCG-2861(6), 2891(3,7), 2941 (8), 2961(9), and COG AAML03P1(10) studies from 1986-2008, with details in the references and Table 1. The first four trials each used an “intensive-timing” induction, and for post remission therapy compared outcomes of patients receiving aggressive high-dose cytarabine, and in two cases autologous transplantation, to those assigned to allogeneic blood or marrow transplantation (BMT). In COG AAML03P1, induction was intensified utilizing a Medical Research Council (MRC) approach (11).
Table 1. Summary of Treatment for all protocols [see References 3,6-19 for full protocol].
| Protocol/Courses | Induction Courses, Drugs and Timing | Post-Remission Courses, Drugs and Timing | BMT |
|---|---|---|---|
| COG 2861 + 2891 (3,8-9) Intensive timing, 6 Courses | Dexamethasone, AraC*, 6 thioguanine, VP*, and Rubidomycin (DNM*) (DCTER), given over 4 days (cycle 1) followed by a 6 Day rest, then repeated over another 4 days (cycle 2), ×2 (i.e., 2nd Induction course repeated in an identical fashion after CBC recovery) | 1st Course: timing intensive HiDAC, “Capizzi II”, bid total 8 doses day 1,2 and 8,9, with L'asparginase 2nd and 3rd Courses: 28 day cycles of 6 thioguanine, vincristine, AraC, cyclophosphamide and 5 Azacytidine 4th Course: DCTER 1 cycle (4days) only |
Allogeneic if Match Family Donor (MFD); Others Allocated to Autologous BMT [2861] or randomized to chemo vs autologous BMT [2891] |
| COG 2941 + 2961 (10-11) 3 Courses | Intensively timed DCTER as above except first cycle in each of the 2 courses substituted idarubicin for DNM | Capizzi II as per 2861/2891 [patients were then randomized to no further therapy or IV IL-2, with no differences in outcome]. | Allogeneic if MFD available |
| COG 03P1 (12) 5 Courses | AraC, DNM and VP as a 10 day Course [ADE10] plus GO* 2nd course ADE over only 8 Days |
1st Course: HiDAC bidx5 days, and etoposide ×5 days 2nd Course: HiDAC bid days 1-4, mitoxantrone days 4-7 and GO 3rd Course: Capizzi II as per 2861/2891 |
Allogeneic if MFD available |
| CALGB 8525 (14) 5-6 Courses | AraC and DNM (“7+3”)×1 If patients had morphologic residual disease after Course #1, a second Course of “5+2” was given on day 14 | 4 Courses of 3 Randomized arms of AraC: 100mg/m2/dx5, 400mg/m2/d×5, or 3 grams/m2 bid ×6 given day 1, 3, and 5 | None |
| CALGB 9022 (15) 5-6 Courses | Same as 8525 | 3 Courses:
|
None |
| CALGB 9222 (16) 5-6 Courses | Same as 8525 | 2 Randomized Arms:
|
None |
| CALGB 9621 (17) 2-5 Courses | AraC (7 days), DNM (3 days), VP (3 days), or ADE randomized to receive or not receive PSC-8333 (Valspodar) as an MDR1 inhibitor If residual disease, a second Course of AraC (5), DNM (2), and VP was given on day 14 | 2 Arms
|
Autologous except CBF + AML |
| CALGB 19808 (18) 3-5 Courses | Same as 9621, randomized to receive or not receive PSC-833 | 2 Arms
|
Same as 9621 |
| SWOG S58600 (19) 2-4 Courses | Randomized Arms
If residual AML: Repeat same regimen ×1 |
Induction 1) Randomized Arms
Induction 2) Post-remission Arm
|
None |
| SWOG S9500 (20) 2-9 Courses | “7+3” followed by HiDAC bid days 8-10, ×1 | HiDAC bid days 1, 3, and 5 as tolerated to 4 courses, followed by “5+1” as tolerated to 4 courses | None |
| SWOG S0106 (21) 4-7 Courses | Randomized Arms
If residual AML on day 14: “7+3” |
HiDAC bid days 1, 3, and 5, ×3 followed by randomization: GO ×3 vs none | Allogeneic, only for patients with adverse cytogentics and MSD |
AraC: Cytarabine DNM: Daunomycin VP: Etoposide GO: Gemtuzumab Ozogamycin
Adult Trials
During 1986 to 2008, 149 patients aged 16 – 21 years were enrolled on sequential CALGB trials for newly diagnosed AML, including CALGB 8525 (12), 9022 (13), 9222 (14), 9621 (15), and 19808 (16). All utilized daunorubicin/cytarabine based induction and high-dose cytarabine based intensification. In the 9621 and 19808 trials, autologous transplantation was performed in patients without core-binding factor cytogenetics, with no allogeneic transplants offered. SWOG enrolled 87 AYAs on three front-line trials for AML from 1986 to 2008, SWOG 8600 (17), 9500 (18), and S0106 (19). All studies utilized daunorubicin and cytarabine, in one case this therapy plus gemtuzumab ozogamicin. Neither autologous nor allogeneic transplantation were offered except that allogeneic transplantation be considered for patients with high-risk cytogenetics and a matched sibling donor in S0106.
Statistical Analysis
CCG-2861 was current as of September 21, 2001, CCG-2891, January 14, 2004; CCG-2961, November 6, 2009; CCG-2941, April 14, 2005; and AAML03P1, May 12, 2010. Data from CALGB and SWOG studies were current as of June 28, 2010 and May 12, 2010, respectively. The significance of observed differences in proportions was tested using the Chi-squared test and Fisher's exact test when data were sparse. The Mann-Whitney test was used to determine the significance between differences in median values. Study entry characteristics analyzed included sex, white blood cell count (WBC), bone marrow blasts percentage, FAB classification, and weight-related groups defined by Body Mass Index (BMI) percentage. Median times from diagnosis to study entry for COG, CALGB and SWOG were 1, 1 and 2 days, respectively. Race and ethnicity were not analyzed due to differences in data collection among the groups. Weight groups were defined as either underweight (BMI < 11%), middleweight (11%-94%) or overweight (BMI ≥95%) using accepted American standards. For cytogenetics, patients were classified as per the Byrd Classification system (20) as favorable [t(8;21), inv(16) or t(16;16)], adverse [complex karyotype ≥3 abnormalities, inv(3) or t(3;3), t(6;9), t(6;11), -7, +8 sole or with one other abnormality not favorable, or t(11;19)], or intermediate [all others].
Overall survival (OS) was defined as time from study entry to death. Event-free survival (EFS) was defined as time from study entry until death, relapse, or failure to achieve complete remission (CR) after receiving up to two courses of induction therapy, except for patients who enrolled on SWOG S9500 who received only one course of induction. Relapse free survival (RFS) was defined as time from end of induction (EOI) for patients in CR, censoring patients who died without an intervening relapse. Relapse risk (RR) was measured from end of induction for patients in CR to relapse where deaths without a relapse were considered competing events. Post-remission treatment related mortality (TRM) was recorded from EOI for patients in CR to death without a relapse, censoring relapses. AYAs lost to follow-up were censored at their date of last known contact. Patients who received allogeneic BMTs were not censored in these analyses, with the exception of the indicated RR and TRM, and some OS and EFS analyses in which COG patients were censored at the time of study transplant because few patients on the adult trials received an allogeneic transplant and were unidentified.
The Kaplan-Meier method was used to estimate OS and EFS. Estimates include 95% confidence intervals (CIs) using Greenwood's estimate of the standard error. Cumulative incidence of TRM and RR were estimated using the method of Kalbfleisch and Prentice (21). The significance of predictor variables was tested with the log-rank statistic for OS and EFS. Gray's statistic was used to compare cumulative incidence curves for RR and TRM (22). Cox proportional hazard models were used to estimate hazard ratios (HR) for cohorts of patients defined by age and cooperative group and for analyzing age as a continuous variable for univariate and multivariate analyses of OS, EFS, and RFS. The proportional hazards assumption was tested for all co-variates. Analyses of OS that compared 16 to 18 year old vs 19 to 21 year old patients or patients from COG vs CALGB and SWOG studies violated the proportional hazards assumption, and therefore a direct comparison between the 10-year estimates of OS were summarized instead of the log-rank statistic.
Results
Patient Characteristics
Patient characteristics are shown in Table 2. Overall, there were 517 patients included in the analysis, with a median age of 18 years, with the range in all three groups from 16 to 21 years. However, the COG cohort was significantly younger with a median age of 17.2 years, p<0.001, with 94% of the COG patients younger than 19 and only one at 21 years. The median ages of the CALGB (20.1) and SWOG (19.8) patients were comparable; only four were 16 years old.
Table 2. Patient Characteristics at Study Entry.
| ALL patients (N=517) | COG studies (N=281) | CALGB studies (N=149) | SWOG studies (N=87) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | p-value | ||
| Studies | ||||||||||
| CCG-2861 | 13 | 3% | 13 | 5% | ||||||
| CCG-2891 STD | 29 | 6% | 29 | 10% | ||||||
| CCG-2891 INT | 56 | 11% | 56 | 20% | ||||||
| CCG-2941 | 6 | 1% | 6 | 2% | ||||||
| CCG-2961 | 123 | 24% | 123 | 44% | ||||||
| AAML03P1 | 54 | 10% | 54 | 19% | ||||||
| CALGB 8525 | 44 | 9% | 44 | 30% | ||||||
| CALGB 9022 | 19 | 4% | 19 | 13% | ||||||
| CALGB 9222 | 30 | 6% | 30 | 20% | ||||||
| CALGB 9621 | 17 | 3% | 17 | 11% | ||||||
| CALGB 19808 | 39 | 8% | 39 | 26% | ||||||
| SWOG S8600 | 51 | 10% | 51 | 59% | ||||||
| SWOG S9500 | 8 | 2% | 8 | 9% | ||||||
| SWOG S0106 | 28 | 5% | 28 | 32% | ||||||
| Gender | ||||||||||
| Male | 275 | 53% | 156 | 56% | 70 | 47% | 49 | 56% | 0.196 | |
| Female | 242 | 47% | 125 | 44% | 79 | 53% | 38 | 44% | ||
| Age (yrs) | ||||||||||
| 16 | 128 | 25% | 124 | 44% | 3 | 2% | 1 | 1% | ||
| 17 | 132 | 26% | 111 | 40% | 14 | 9% | 7 | 8% | ||
| 18 | 81 | 16% | 28 | 10% | 34 | 23% | 19 | 22% | ||
| 19 | 51 | 10% | 12 | 4% | 21 | 14% | 18 | 21% | ||
| 20 | 64 | 12% | 5 | 2% | 38 | 26% | 20 | 23% | ||
| 21 | 61 | 12% | 1 | 0% | 39 | 26% | 22 | 25% | ||
| Median | Range | Median | Range | Median | Range | Median | Range | p-value | ||
| Age - median (range) | 18.0 | (16.0 - 21.99) | 17.2 | (16.0 - 21.6) | 20.1 | (16.5 - 21.98) | 19.8 | (16.9 - 21.99) | <0.001 | |
| WBC (×103/μL) - median (range) | 19.1 | (0.5 - 860) | 20 | (0.5 - 860) | 18.1 | (0.5 - 453) | 16.8 | (0.6 - 415.8) | 0.669 | |
| BM Blasts % - median (range) | 73 | (0 - 100) | 74 | (1 - 100) | 71.5 | (0 - 99) | 70.5 | (0 - 100) | 0.570 | |
| FAB Classification | ||||||||||
| M0 | 17 | 3% | 12 | 4% | 4 | 3% | 1 | 1% | 0.348 | |
| M1 | 96 | 19% | 54 | 19% | 23 | 16% | 19 | 23% | 0.413 | |
| M2 | 179 | 35% | 94 | 34% | 57 | 39% | 28 | 34% | 0.490 | |
| M4 | 137 | 27% | 73 | 26% | 40 | 28% | 24 | 29% | 0.870 | |
| M5 | 49 | 10% | 28 | 10% | 12 | 8% | 9 | 11% | 0.780 | |
| M6 | 11 | 2% | 5 | 2% | 4 | 3% | 2 | 2% | 0.806 | |
| M7 | 5 | 1% | 4 | 1% | 1 | 1% | 0 | 0% | 0.462 | |
| Other | 13 | 3% | 9 | 3% | 4 | 3% | 0 | 0% | ||
| Unknown | 10 | 2 | 4 | 4 | ||||||
| Weight group (Body Mass Index) | ||||||||||
| Underweight (BMI ≤ 11%) | 52 | 10% | 24 | 9% | 20 | 13% | 8 | 9% | 0.103 | |
| Middleweight (BMI 11 - 94%) | 346 | 67% | 201 | 72% | 90 | 60% | 55 | 63% | 0.806 | |
| Obese (BMI ≥ 95%) | 117 | 23% | 54 | 19% | 39 | 26% | 24 | 28% | 0.233 | |
| Unknown | 2 | 2 | 0 | 0 | ||||||
The median WBC count and blast percentage at diagnosis were 19.1 × 109/L and 73%, respectively, with no differences by cooperative group in these or in the FAB classification distribution. Cytogenetic data are reported in Table 3. There was an even distribution across the three cooperative groups in the proportion of patients in each risk group for all endpoints except for a paucity of patients in the adverse risk group from SWOG. Cytogenetic results were unknown on almost half the patients. Molecular data were incomplete during the study period, especially in the early years, and were not analyzed.
Table 3. Cytogenetic Risk Groups based on the Byrd classification for 3 different endpoints (20).
| All Patients N=517 | COG N=281 | CALGB N=149 | SWOG N=87 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Group for CR | N | % | N | % | N | % | N | % | p-value |
| Favorable | 79 | 27% | 42 | 26% | 27 | 28% | 10 | 32% | 0.889 |
| Intermediate | 176 | 62% | 101 | 63% | 54 | 57% | 21 | 68% | 0.459 |
| Adverse | 31 | 11% | 17 | 11% | 14 | 15% | 0 | 0% | 0.050 |
| Unknown | 231 | 121 | 54 | 56 | |||||
| Risk Group for Relapse | |||||||||
| Favorable | 79 | 29% | 42 | 28% | 27 | 30% | 10 | 33% | 0.797 |
| Intermediate | 156 | 58% | 87 | 57% | 50 | 56% | 19 | 63% | 0.756 |
| Adverse | 37 | 14% | 23 | 15% | 13 | 14% | 1 | 3% | 0.225 |
| Unknown | 245 | 129 | 59 | 57 | |||||
| Risk Group for Overall Survival | |||||||||
| Favorable | 80 | 28% | 42 | 26% | 28 | 29% | 10 | 32% | 0.732 |
| Intermediate | 141 | 50% | 80 | 50% | 45 | 47% | 16 | 52% | 0.887 |
| Adverse | 65 | 22% | 38 | 24% | 22 | 23% | 5 | 16% | 0.646 |
| Unknown | 231 | 121 | 54 | 56 | |||||
Remission Induction
The CR rate after receiving up to two courses of induction was 79% for the entire cohort. These rates were significantly different among all three groups (COG, 82%; CALGB, 76%; SWOG, 71%, p=0.045); and there were no differences in actuarial survival at 60 days (92%, 95%, and 95%, respectively).
Overall Survival (OS) and Event-Free Survival (EFS)
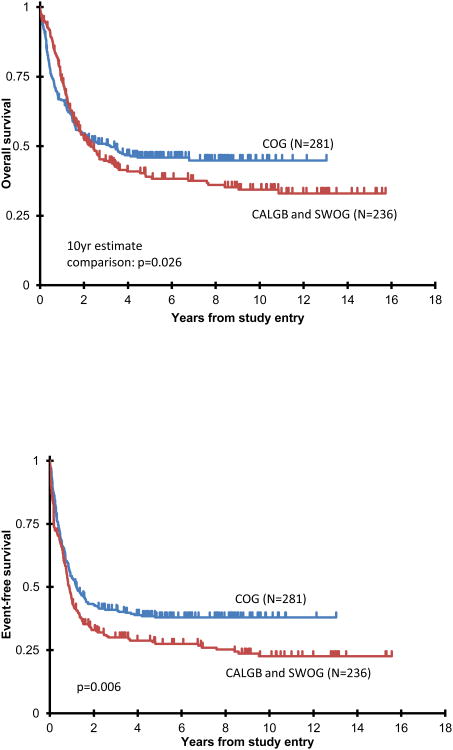
Ten year OS was higher for the COG cohort (Figure 1A) than for the two adult cohorts, 45±6% vs 34±7% with a 10 year estimate comparison of p=0.026. The adult trials had similar OS, 35 ± 8% for CALGB and 33 ± 12% for SWOG, p=1.00. Similarly, the 10 year EFS was 38±6% for the 281 AYAs on COG trials compared to 23±6% for the patients on the adult trials, log-rank p=0.006, Figure 1B. Results from CALGB and SWOG were comparable, 24±8% and 21±10%, respectively; hence, for all other analyses, the adult groups were combined for a single comparison to COG. When we repeated the OS and EFS analyses censoring the COG transplant recipients, N=77, the overall results were similar. EFS for COG chemotherapy only patients was 36±7%, p=0.023 vs 23% on adult trials; and OS 44±7%, p=0.053 vs 34% on adult trials.
Figure: 1.
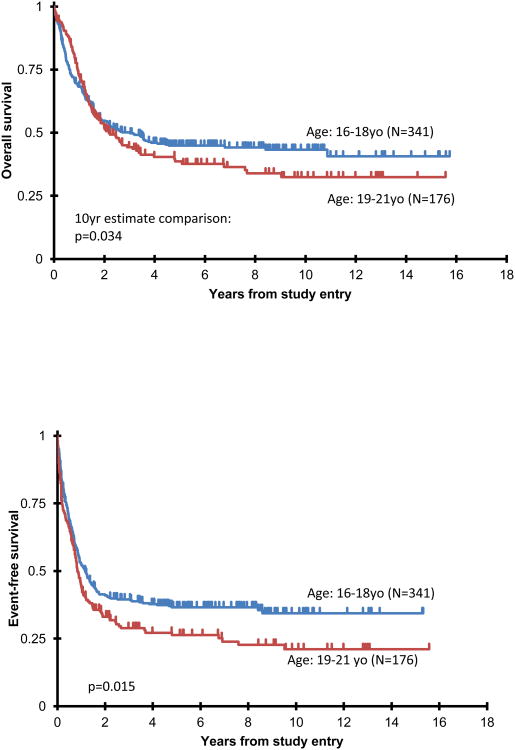
Actuarial OS (1A) and EFS (1B) from study entry comparing patients studied on COG vs adult trials (CALGB/SWOG); and comparing patients 16-18 vs 19-21 years (1C, 1D). Note that in Figures 1A and 1C, proportional hazards were violated: p value represents patient estimates at 10 years.
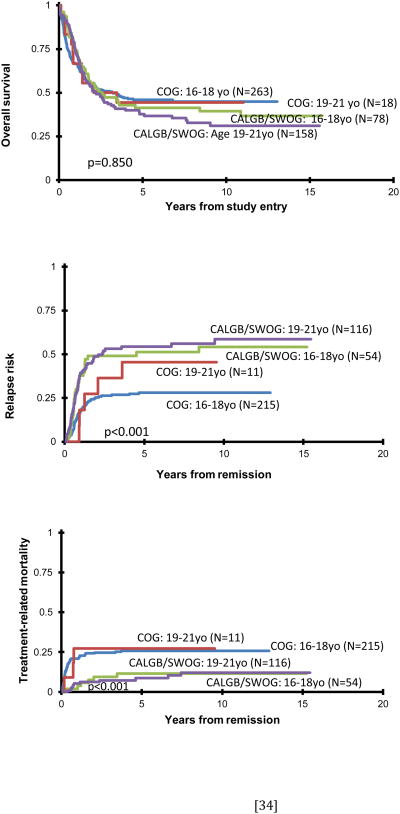
In examining age, overall survival at 10 years for the 16-18 year old patients was 43±6% compared to 32±8% for those 19-21 years, p=0.034 when comparing 10 year estimates (Figure 1C). Figure 1D shows a significant difference in EFS between the two age cohorts irrespective of treatment, patients 16-18 years (N=341) having a 10 year EFS of 34±6% compared to 21±7% for those 19-21 years (N=176), log-rank p=0.015. When one stratifies the entire cohort based on pediatric vs adult protocols and age (Figure 2A) for OS, there were no significant differences among the four curves.
Figure: 2.
Actuarial OS from study entry (2A), risk of relapse (2B) and treatment mortality (2C) stratifying patients by COG vs adult (CALGB/SWOG) protocols and age.
Relapse Risks (RR)
AYAs treated on the COG protocols had a markedly reduced incidence of relapse at 10 years, 29±6%, and 35±8% censoring BMT patients, compared to those treated on adult trials, 57±8% (Gray's p<0.001 for both comparisons). Younger patients on both the pediatric and adult trials had fewer relapses, 34±6% vs 58±10% for the older AYAs (Gray's p<0.001). Both age groups treated on COG trials had much lower relapse rates than patients on the adult trials irrespective of age (Figure 2B).
Treatment-Related Mortality (TRM)
Due to differences in the definition of TRM among the cooperative groups during induction therapy, we analyzed TRM only from EOI. AYAs treated on the more myelosuppressive COG protocols had a higher degree of TRM (26±6%) than those treated on the adult protocols (12±6%, Gray's p<0.001). Censoring BMT patients on COG trials only reduced the TRM to 22±7%, still p<0.001 compared to adult trials. Figure 2C shows the TRM stratified by both cooperative groups and age (Gray's p<0.001 overall). Age played less of a role in TRM compared to the dramatic differences seen in RR.
Role of Confounding Variables, Especially Age
Patient characteristics were examined as potential confounders to the superior outcomes of patients on pediatric trials. Cox linear regression analyses were performed using age as a continuous variable examining endpoints (Table 4). No significant differences were seen for OS. However, increasing age was a significant risk factor for EFS and RFS, despite a lower TRM associated with increasing age. Looking at the adult and pediatric groups individually, only EFS in the COG studies demonstrated poorer outcomes with increasing age (HR 1.16, p=0.038). No other patient characteristics examined were prognostic except for adverse cytogenetics, which was highly significant (p<0.001). There was a lower incidence of TRM for patients with favorable cytogenetics on both pediatric and adult trials (HR 0.53,p=0.014).
Table 4. Impact of older age (continuous variable) on outcome endpoints.
| For all patients (N=517) | |||
|---|---|---|---|
| Age in years: continuous variable | Cox analyses | ||
| HR | 95% CI | p-value | |
| OS from study entry | |||
| Age | 1.04 | 0.97 – 1.11 | 0.247 |
| EFS from study entry | |||
| Age | 1.09 | 1.03 – 1.16 | 0.005 |
| TRM from remission (N=396) | |||
| Age | 0.84 | 0.72 – 0.97 | 0.018 |
| RFS from remission (N=396) | |||
| Age | 1.18 | 1.08-1.29 | <0.001 |
Univariate and multivariate analyses were done for EFS from study entry looking at age in two discrete cohorts as above; and pediatric vs adult studies (Table 5). Increased age and being on adult studies were risk factors for outcome. However, in the multivariate analyses neither age nor studies utilized showed a significant difference, because there was such a strong correlation between age and pediatric vs adult trials. Multivariate analysis of OS was not appropriate due to non-proportional hazards. Finally, we looked at just the 16 to 18 year old cohort comparing patients treated on either COG or CALGB/SWOG trials. Overall survival for those treated on the COG protocols, N=263, was 44.9±6.6% at ten years versus 39.5±11.6% for the 78 patients treated on the adult trials (p=0.417). There remained a significant reduction in relapse risk for patients treated on the COG protocols, while the treatment related mortality was significantly higher for the same patients on COG trials.
Table 5. Cox univariate and multivariate analyses of event-free survival.
| Cox analyses | ||||
|---|---|---|---|---|
| EFS from study entry | N | HR | 95% CI | p-value |
| univariate | ||||
| age: 16-18 yr | 341 | 1.00 | ||
| age: 19-21 yr | 176 | 1.31 | 1.05 - 1.63 | 0.015 |
| COG studies | 281 | 1.00 | ||
| Adult studies | 236 | 1.35 | 1.09 - 1.66 | 0.006 |
| multivariate | ||||
| age in years (continuous) | 1.06 | 0.97 - 1.15 | 0.238 | |
| COG studies | 1.00 | |||
| Adult studies | 1.17 | 0.86 - 1.60 | 0.319 | |
| age: 16-18 yr | 1.00 | |||
| age: 19-21 yr | 1.13 | 0.85 - 1.50 | 0.408 | |
| COG studies | 1.00 | |||
| Adult studies | 1.25 | 0.95 - 1.65 | 0.119 | |
Multivariate analyses were run to determine if the period of study (early, 1986-1995; late, 1996-2008) or cytogenetics played any confounding role. Only adverse cytogenetics was confounding in the limited subset of patients having known cytogenetics. After adjusting for cytogenetics in multivariate models, 19-21 year olds (HR=1.24, 95% CI: 0.92 – 1.68, p=0.165) and patients on adult studies (HR=1.32, 95% CI: 0.99 – 1.77, p=0.062) had non-significantly worse EFS.
Effect of Trial Era on Outcome
Finally, we compared the outcomes of studies before 1996 to studies from 1996 forward. OS at 10 years increased from 34±6% to 48±6% (p=0.045); and EFS from 26±6% to 33±8% (p=0.039) in later studies. Unfortunately, TRM doubled from 12±5% to 26±6% (Gray's p< 0.001), but the relapse rate markedly declined, from 51±8% to 36±10% in more recent studies, Gray's p<0.001.
Discussion
There has been increasing attention given to the outcome of AYAs with cancer. In the United States, lesser improvements in survival have been found in younger adults compared to either children or older adults. This fact may be driven in part by a much lower participation rate in clinical trials, as noted for some cancers (23). However, treatment effects probably play the largest role. As noted, ALL AYA patients will do substantially better if treated on pediatric protocols (1,2). Patients in this age group with common pediatric tumors such as rhabdomyosarcoma (24) and Ewing's sarcoma (25) also seem to fare better when treated on pediatric protocols. However, for cancers common in both age groups, e.g. Hodgkin lymphoma, results are comparable (26). AYAs with adult type cancers may actually fare better in the hands of adult oncologists (27).
Small preliminary studies in de novo AML were inconclusive regarding the optimum approach of treating AYAs on childhood or adult protocols (4,5). Age was found to be an important prognostic factor in patients 0-55 years treated on a common MRC protocol (28). When pediatric-like therapy was administered to adult patients <50 years, increased death from toxicity counterbalanced an improvement in leukemia-free survival (29).
We investigated AML outcomes of 517 AYAs from three large cooperative groups (COG, CALGB, and SWOG) making this the largest attempt at comparing pediatric and adult therapy. We took into account all potential measurable variables that could skew results. With the exception of age, none of the other characteristics seemed to play a role in the results obtained. In the limited subset of patients with adequate cytogenetics, multivariate analyses revealed that adverse karyotypes were an important prognostic factor, but hazard ratios for older age (1.24) and adult studies (1.32) remained high, albeit with limited power. The OS and EFS were superior for patients treated on COG protocols, but significantly more patients on the adult trials were older, and those patients did worse than younger adolescents irrespective of protocol. Even in this small age range of six years, the influence of increasing age on lowering survival rates was noteworthy. We found this fact most surprising, but appeared to be from both higher relapse rates and higher TRM among the 19 to 21 year olds. Our best explanation is that this six year period is a microcosm of overall results in AML between children and older adults.
However, we noted striking differences between causes for mortality. The more myelosuppressive pediatric protocols had more anti-leukemia efficacy than the adult trials, with halving of relapse rates; but the marked toxicity that ensued was also clearly apparent with TRM of 26% compared to 12% for the adult trials (p<0.001).
Aggressive pediatric AML protocols are better tolerated in young children, with better survival than adults enrolled on trials designed for middle-aged adults, which must modify therapy for tolerability. Pediatric trials would be superior for AYAs if the TRM with current therapy could be lowered. Molecular markers and inhibitors are allowing treatment to be further stratified, sometimes with greatly improved outcome without major myelosuppression (30). But for the vast majority of patients with AML, it is not yet feasible to reduce profoundly myelosuppressive therapy and obtain optimal cure rates. In one of the pediatric trials cited herein, CCG-2961, a dramatic improvement in overall TRM resulted when an amendment requiring specific mandatory supportive care measures was implemented (9). Furthermore, the improvement in TRM specifically among the AYAs on CCG-2961 was even more dramatic, 43% pre vs 22% post-amendment (p=0.03) (31). Overall OS improved from 43% to 57%, and the results also documented a “learning curve”, reflected by a lowering of TRM with time, even before the supportive care amendment was implemented. This has been noted in adult trials (32), where traditionally supportive care is left to institutional guidelines, with results generalizable to community/standard practice. It is recommended that adult AML patients be cared for by physicians who are experienced in treating leukemia. The pediatric protocols have in general been more specific in outlining such guidelines.
Until more highly effective molecular inhibitors of specific AML subtypes are discovered, pediatric and adult oncologists taking care of these patients should focus their attention on supportive care measures to lower TRM. Perhaps an intergroup trial of AML in 16 to 30 year olds could be implemented to better understand age related differences in outcome, laying the groundwork for future AYA trials.
Acknowledgments
This study was presented in part at the 2010 American Society of Hematology Meeting in Orlando, FL, December 3, 2010.
Generously funded by a grant from the Young Adult Alliance of the LIVESTRONG Foundation, The Coleman Leukemia Research Foundation; and Grants U10_CA98543, CA98413, CA101140, CA77658, CA31946, CA41287, CA32102, and CA38926 from the National Cancer Institute
Footnotes
Precis: AYAs with AML had equivalent outcomes whether treated on pediatric or adult trials when factoring in age. Pediatric trials led to significantly lower relapse fates as well as higher treatment related mortality.
Disclaimers: no conflicts of interest
None: Woods, Franklin, Alonzo, Gerbing, Donohue, Othus, Horan, Applebaum, Estey, Bloomfield, Larson
References
- 1.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 3.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation and aggressive chemotherapy in children with AML in remission: A report from the Children's Cancer Group. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 4.Woods WG, Alonzo T, Lange B, Jeha S, Estey EH. Acute Myeloid Leukemia (AML) in Adolescents and Young Adults (AYAs): A comparison of outcomes between patients treated on childhood or adult protocols. Blood. 2001;98:462a–463a. [Google Scholar]
- 5.Razzouk BI, Estey E, Pounds S, et al. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106:2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- 6.Woods WG, Kobrinsky N, Buckley J, et al. Intensively timed induction therapy followed by autologous or allogeneic bone marrow transplantation for children with acute myeloid leukemia or myelodysplastic syndrome: A Children's Cancer Group Pilot Study. J Clin Oncol. 1993;11:1448–1457. doi: 10.1200/JCO.1993.11.8.1448. [DOI] [PubMed] [Google Scholar]
- 7.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: A report from the Children's Cancer Group. Blood. 1996;87:4979–4989. [PubMed] [Google Scholar]
- 8.Lange BJ, Dinndorf P, Smith FO, et al. Pilot Study of Idarubicin-Based Intensive-Timing Induction Therapy for Children with Previously Untreated Acute Myeloid Leukemia: Children's Cancer Group Study 2941. J Clin Oncol. 2004;22:150–156. doi: 10.1200/JCO.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group Phase 3 Trial for untreated pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a Pilot Study of the Safety of Gemtuzumab Ozogamicin in Combination with Chemotherapy for Newly Diagnosed Childhood Acute Myeloid Leukemia: A Report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 11.Gibson BES, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 12.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 13.Moore JO, Dodge RK, Amrein PC, et al. Granulocyte-colony stimulating factor (filgrastim) accelerates granulocyte recovery after intensive postremission chemotherapy for acute myeloid leukemia with aziridinyl benzoquinone and mitoxantrone: Cancer and Leukemia Group B Study 9022. Blood. 1997;89:780–788. [PubMed] [Google Scholar]
- 14.Moore JO, George SL, Dodge RK, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005;105:3420–3427. doi: 10.1182/blood-2004-08-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia <60 years old: Final induction results of CALGB Study 9621. J Clin Oncol. 2004;22:4290–4301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 16.Kolitz JE, George SL, Marcucci G, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B Study 19808. Blood. 2010;116:1413–1421. doi: 10.1182/blood-2009-07-229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: A Southwest Oncology Group Study. Blood. 1996;88:2841–2851. [PubMed] [Google Scholar]
- 18.Petersdorf SH, Rankin C, Head DR, et al. Phase II evaluation of an intensified induction therapy with standard daunomycin and cytarabine followed by high dose cytarabine for adults with previously untreated acute myeloid leukemia: a Southwest Oncology Group Study (SWOG-9500) Am J Hematol. 2007;82:1056–1062. doi: 10.1002/ajh.20994. [DOI] [PubMed] [Google Scholar]
- 19.Petersdorf S, Kopecky K, Stuart RK, et al. Preliminary results of Southwest Oncology Group Study S0106: an International Intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:326a. [Google Scholar]
- 20.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, New York: John Wiley and Sons; 1980. [Google Scholar]
- 22.Gray RJ. Class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 23.Bleyer A, Montello M, Budd T, Saxman S. National Survival Trends of Young Adults with Sarcoma; Lack of Progress is Associated with Lack of Clinical Trial Participation. Cancer. 2005;103:1891–1897. doi: 10.1002/cncr.20995. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari A, Dileo P, Casanova M, et al. Rhabdomyosarcoma in adults A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98:571–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 25.Paulussen MRA, Dirksen U, Jurgens H. Ewing tumours: Outcome in children, adolescents and adult patients. Euro J Cancer (suppl) 2007;5:209–215. [Google Scholar]
- 26.Eichenauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin's Lymphoma in Adolescents Treated with Adult Protocols: A Report from the German Hodgkin Study Group. J Clin Oncol. 2009;27:6079–6085. doi: 10.1200/JCO.2008.20.2655. [DOI] [PubMed] [Google Scholar]
- 27.Howell DL, Ward KC, Austin HD, Young JL, Woods WG. Access to Pediatric Cancer Care by Age, Race, and Diagnosis, and outcomes of Cancer Treatment in Pediatric and adolescent Patients in the State of Georgia. J Clin Oncol. 2007;25:4610–4615. doi: 10.1200/JCO.2006.07.6992. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. Brit J Haem. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 29.Rytting M, Ravandi F, Estey E, et al. Intensively timed combination chemotherapy for the induction of adult patients with acute myeloid leukemia: long-term follow-up of a phase 2 study. Cancer. 2010;116:5272–5278. doi: 10.1002/cncr.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravandi F, Estey E, Jones D, et al. Effective Treatment of Acute Promyelocytic Leukemia with All-Trans-Retinoic Acid, Arsenic Trioxide, and Gemtuzumab Ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canner J, Alonzo TA, Franklin J, et al. Differences in Outcomes of Newly Diagnosed Acute Myeloid Leukemia for Adolescent/Young Adult and Younger Patients: A Report form the Children's Oncology Group. Submitted to. Cancer. :2013. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Othus M, Kantarjian H, Petersdorf S, et al. Declining Rates of Treatment-Related Mortality Recent in Patients With Newly-diagnosed AML Given “Intense” Induction Regimens: A report from SWOG and MD Anderson. Blood. 2012 doi: 10.1038/leu.2013.176. Abstract #129, ASH. [DOI] [PMC free article] [PubMed] [Google Scholar]