Abstract
Purpose
Enzalutamide, a second-generation androgen antagonist, was approved by the FDA for castration-resistant prostate cancer (CRPC) treatment. Immunotherapy has been shown to be a promising strategy for prostate cancer. This study is performed to provide data to support the combination of enzalutamide and immunotherapy for CRPC treatment.
Experimental Design
Male C57BL/6 or TRAMP prostate cancer model mice were exposed to enzalutamide and/or a therapeutic vaccine targeting Twist, an antigen involved in epithelial-to-mesenchymal transition and metastasis. The physiological and immunological effects of enzalutamide were characterized. The generation of Twist-specific immunity by Twist-vaccine was evaluated. Finally, the combination of enzalutamide and Twist-vaccine to improve TRAMP mice overall survival was evaluated.
Results
Enzalutamide mediated immunogenic modulation in TRAMP-C2 cells. In vivo, enzalutamide mediated reduced genitourinary tissue weight, enlargement of the thymus, and increased levels of T-cell excision circles. Because no changes were seen in T-cell function, as determined by CD4+ T-cell proliferation and Treg functional assays, enzalutamide was determined to be immune inert. Enzalutamide did not diminish the Twist-vaccine’s ability to generate Twist-specific immunity. Twist was confirmed as a valid tumor antigen in TRAMP mice by immunohistochemistry. The combination of enzalutamide and Twist-vaccine resulted in significantly increased overall survival of TRAMP mice compared to other treatment groups (27.5 vs. 10.3 weeks). Notably, the effectiveness of the combination therapy increased with disease stage, i.e., the greatest survival benefit was seen in mice with advanced-stage prostate tumors.
Conclusions
These data support the combination of enzalutamide and immunotherapy as a promising treatment strategy for CRPC.
Keywords: enzalutamide, cancer vaccine, ADT, immunotherapy, combination therapy, prostate cancer, immunogenic modulation
Introduction
Localized prostate cancer is treated with surgery, radiotherapy, or watchful waiting, while recurrent disease is further treated with androgen-deprivation therapy (ADT) (1). Most patients on ADT eventually develop castration-resistant prostate cancer (CRPC), characterized by a rise in prostate-specific antigen and subsequent progression of disease despite castrate blood levels of testosterone (2). However, emerging evidence suggests that CRPC remains dependent on androgen-receptor signaling for growth. Indeed, CRPC is commonly associated with increased expression of androgen receptor, arising from amplification or mutation of the androgen-receptor gene or other mechanisms (3). Enzalutamide is an androgen-receptor antagonist that blocks androgens from binding to the androgen receptor and prevents nuclear translocation and coactivator recruitment of the ligand-receptor complex. Enzalutamide has been evaluated in clinical trials (4, 5), including the AFFIRM trial, which demonstrated a 4.8-month advantage in overall survival with enzalutamide compared to placebo (6). Prostate cancer immunotherapy recently achieved significant milestones with the approval of vaccines sipuleucel-T (7) and the successful clinical trials of PROSTVAC-VF (8). Systemic androgen ablation is known to activate thymic regeneration, and androgens are known to regulate a variety of immune responses (9–12). Recently, a phenomenon called immunogenic modulation; exposure of tumor cells to therapies that consequently alter tumor phenotype to render the tumors more susceptible to immune-mediated attack, has been studied and reviewed (13, 14). The immunogenic modulation property of enzalutamide is currently unknown. Our hypothesis is that enzalutamide 1) induces thymic regeneration, and 2) mediates immunogenic modulation in prostate tumor cells rendering them more sensitive to immune-mediated attack. We further hypothesize that the immunogenic modulation property of enzalutamide could be harnessed in combination with immunotherapy to improve treatment for CRPC.
Twist is a member of a highly conserved BHLH transcription factor that has been implicated in metastasis. Its overexpression is associated with poor prognosis in many cancer types (15–19). In a preclinical study, suppressing Twist expression in highly metastatic murine mammary carcinoma cells prevented the cells from metastasizing from the mammary gland to the lung (20). The same study also showed that activation of Twist led to loss of epithelial cell markers, activation of mesenchymal markers, and induction of cell motility, all of which suggest Twist’s important role in epithelial-to-mesenchymal transition (EMT), and therefore the process of metastasis (20).
In this study we evaluated the efficacy of combination therapy with enzalutamide plus a yeast-based (Saccharomyces cerevisiae) vaccine engineered to express Twist antigen in the TRAMP model of spontaneous prostate cancer, wherein tumor development resembles disease progression in humans, from prostatic intraepithelial neoplasia (PIN) to metastatic CRPC (21–23). Immunohistochemistry confirmed Twist as a valid tumor antigen in the TRAMP model, and our data suggest that Twist expression increased as tumors progressed. Here, the combination of enzalutamide and Twist-vaccine significantly improved overall survival in TRAMP mice, particularly those with advanced disease, while either modality alone failed.
To our knowledge, this is the first study to demonstrate 1) the immunogenic modulation property of enzalutamide; 2) the physiological effect of enzalutamide in C57BL/6 and TRAMP mice, i.e., reduced genitourinary (GU) tissue weight, enlarged thymus, and increased levels of T-cell excision circles (TREC); 3) the minimal effects of enzalutamide on T-cell activity; 4) the use of Twist, a driver of EMT, as a therapeutic vaccine target, and the ability of Twist-vaccine to generate Twist-specific immunity in C57BL/6 and TRAMP mice; and 5) significantly improved overall survival in TRAMP mice with the combination of enzalutamide and Twist-vaccine. These data support the combination of enzalutamide and immunotherapy as a promising treatment for CRPC.
Materials and Methods
Animals
The National Cancer Institute’s Frederick National Laboratory for Cancer Research (Frederick, MD) supplied 8- to 12-week-old male C57BL/6 mice. TRAMP mice on the C57BL/6 background were bred and maintained at the National Institutes of Health (Bethesda, MD) (24). TRAMP mice were sorted into 4 age groups, which in this model represent different stages of prostate cancer development: 8–12 weeks old = PIN, 12–20 weeks old = well-differentiated adenocarcinoma, 20–28 weeks old = moderately-differentiated adenocarcinoma, and 28 weeks or older = poorly-differentiated adenocarcinoma (23, 24).
Tumor cells
TRAMP-C2 murine prostate adenocarcinoma cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in the recommended medium.
Vaccine constructs
Recombinant S. cerevisiae yeast constructs without antigen (control-vaccine) or expressing Twist (Twist-vaccine) were engineered by methods similar to those previously described (25) (GlobeImmune, Inc., Louisville, CO).
Enzalutamide diet preparation
Enzalutamide (Medivation, San Francisco, CA) was admixed with Research Diet (New Brunswick, NJ), which was fed to animals as indicated in each experiment and as previously described (26).
Physiological and immunological effects of enzalutamide
C57BL/6 mice (n=3/group) were not treated or treated with enzalutamide at targeted daily doses of 0, 1, 10, 50, or 100 mg for 14 days. Peripheral blood was collected from the retro-orbital cavity and analyzed for CBC and enzalutamide concentration in plasma by HPLC. Spleens were harvested and a mixed lymphocyte (H-2d vs. H-2b) assay and an anti-CD3 proliferation assay were performed as previously described (27, 28). GU tissues and thymuses were harvested and weighed. Immune-cell population subsets from splenocytes were analyzed by flow cytometry. In a separate study, TRAMP mice (n=2–9/group) were sorted into 4 groups as previously described and randomized to receive no treatment or enzalutamide 10 mg/day for 4 or 12 weeks. Mice were sacrificed and their GU tissues and thymuses were harvested and weighed.
Physiological effects of Enzalutamide vs. castration
C57BL/6 mice (n=4/group) were not treated or subjected to castration 14 days prior sacrifice. GU tissues and thymuses were harvested and weighed.
Phenotypic analysis by flow cytometry
Cells were stained and fixed as previously described (26). Multicolor cytometric analyses were performed using a Becton Dickinson LSRII and analyzed using FACSDiva software or using a FACS can flow cytometer using CellQuest software (BD Biosciences, San Jose, CA).
TREC RT-PCR
C57BL/6 mice (n=7/group) were not treated or treated with enzalutamide 10 mg/day for 14 days. Blood was collected and DNA was purified using a QIAamp-DNA mini kit (Qiagen, Valencia, CA). PCR was performed using the previously described sjTREC primers and probe for C57BL/6 mice (29). In a separate study, TRAMP mice (n=2–8/group) were not treated or treated with enzalutamide for 4 weeks, after which TREC levels were analyzed as described.
Immunological assays
C57BL/6 mice (n=6/group) were not treated or were vaccinated with control-vaccine or Twist-vaccine at 4 yeast units (YU)/animal (1 YU=107 yeast particles) on days 0, 7, 14, and 21. On day 35 mice were sacrificed and splenocytes were analyzed for Twist-specific CD4+ T-cell proliferation using Twist peptide (FSVWRMEGAWSMSAS) (CPC Scientific, Sunnyvale, CA), as previously described (30). LCMV peptide (RPQASGVYMGNLTAQ) and ConA were used as negative and positive control of CD4+ T-cell proliferation, respectively. In a subsequent study, C57BL/6 mice (n=6/group) were not treated, vaccinated 4 times with Twist-vaccine at 4 YU/animal on days 0, 7, 14, and 21, and/or treated with enzalutamide 10 mg/day starting on day 0. On day 35, spleens were harvested and analyzed for Twist-specific CD4+ T-cell proliferation as described above. In another independent study (n=5/group), C57BL/6 and TRAMP mice harboring well differentiated tumors were not treated or vaccinated three times with Twist-vaccine at 4 YU/animal weekly. On day 28, spleens were harvested and analyzed for Twist-specific CD4+ T-cell proliferation and IFN-γ production. In a separate study, TRAMP mice at various stages of tumor development were vaccinated with Twist-vaccine at 4 YU/animal and treated with enzalutamide 10 mg/day for 12 months. Three mice that received the combination of enzalutamide and Twist-vaccine survived and were analyzed for immunological responses. Pooled splenic T cells from these mice were analyzed for Twist-specific CD4+ T-cell proliferation, as described above, and for peptide-specific IFN-γ and TNF-α production. To evaluate CD8+ T-cell responses, spleens were harvested and coincubated for 7 days with Twist peptide (1 µg/mL, TQSLNEAFL), prostate stem-cell antigen (PSCA) peptide (1 µg/mL, NITCCYSDL), survivin peptide (1 µg/mL, CFFCFKEL), and p15E peptide (1 µg/mL, KSPWFTTL, referred to as gp70 peptide). Supernatants from these cultures were collected and analyzed for murine IFN-γ and TNF-α by cytometric bead array according to the manufacturer’s instructions (BD Biosciences).
RNA interference (siRNA)
siRNA duplexes targeting Twist sequences and control were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). TRAMP-C2 cells were transfected with Twist siRNA and control siRNA according to the manufacturer’s instructions. The interference of Twist expression was confirmed by RT-PCR analysis using TaqMan probes for Twist (Mm00442036_m1) (Applied Biosystems, Foster City, CA). All values are expressed as a ratio to the endogenous control GAPDH as previously described (31). A migration assay was performed as previously described (32).
Cytotoxicity T-cell Assays (CTL)
The H-2Kb-restricted gp70-specific CD8+-cytotoxic T-cells recognizes the peptide p15e604 have been previously described (33). The CTL were performed as previously described (34).
Twist immunohistochemistry
At each stage of prostate cancer development in the TRAMP mice, Twist expression was detected using rabbit-polyclonal antibody to Twist (Abcam, Cambridge, MA) according to the manufacturer’s instructions. Entire slides were digitally scanned by an Aperio ScanScope CS scanning system and analyzed by Aperio ImageScope Viewer software (Aperio Technologies Inc., Vista, CA). Twist-positive tumor regions were measured using the Positive Pixel Count v9 algorithm.
Treg functional assay
TRAMP mice (n=2–8/group) were not treated or treated with enzalutamide for 4 weeks. Spleens were harvested from sacrificed mice, and CD4+CD25+FoxP3+ Tregs were purified using a Treg isolation kit according to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC). Tregs were found to be > 90% pure by flow cytometry. Treg functional assays were performed as previously described (27, 35). CD8+ T-cell from naïve untreated C57BL/6 mice were used as responders.
Survival Study
TRAMP mice (n=25–27/group) were not treated or randomized to receive enzalutamide 10 mg/day, Twist-vaccine 4 YU/animal, or a combination of enzalutamide and Twist-vaccine. At initiation of treatments, average mouse ages were 11 weeks (PIN), 17 weeks (well-differentiated adenocarcinoma), 25 weeks (moderately-differentiated adenocarcinoma), and 34 weeks (poorly-differentiated adenocarcinoma). Vaccinations were given weekly for 3 months, then twice a month for 3 months, and monthly thereafter.
Statistical analysis
GraphPad Prism 5® statistical software (GraphPad Software, La Jolla, CA) was used to measure 2-tailed unpaired t-tests for differences between groups, one-way ANOVA for differences among groups with Bonferroni's multiple comparison test and Wilcoxon tests of survival.
Results
Enzalutamide has minimal effects on T-cell activity
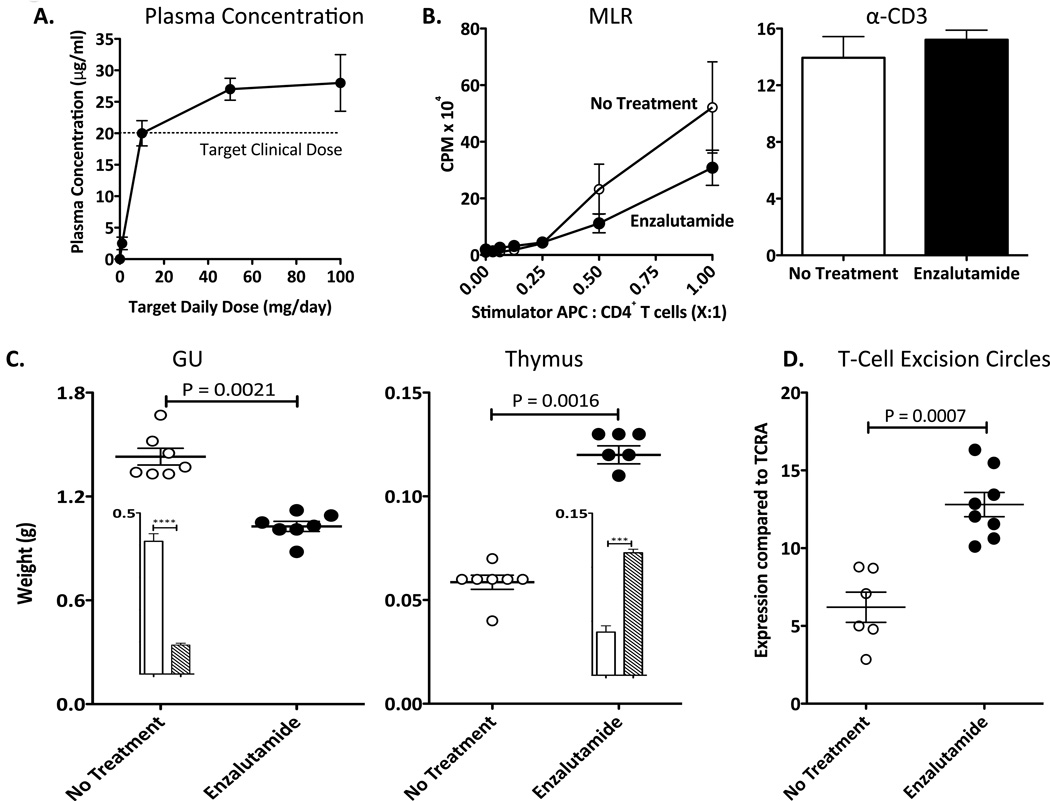
To facilitate preclinical animal modeling, enzalutamide, which is administered orally to humans, was formulated into rodent diet at different concentrations to achieve target daily doses of 0, 1, 10, 50, and 100 mg. After 14 days of treatment, dose-dependent levels of enzalutamide were detected in mouse plasma (Fig. 1A). The dose level in animals that achieved the equivalent therapeutic levels observed in humans (4) was 10 mg/day, and thus was the dose used in all subsequent studies. Untreated and enzalutamide-treated mice had comparable food intake, body weight, and CBCs. Flow cytometry analysis of splenocytes revealed no differences between untreated and enzalutamide-treated mice in the number of immune-cell population subsets (CD4+ and CD8+ T cells, Tregs, and myeloid-derived suppressor cells [MDSCs]) (P > 0.05). The functional activity of CD4+ T-cell was determined by mixed lymphocyte reaction (MLR) and anti-CD3 proliferation. By MLR, no differences were observed in allogeneic CD4+ T-cell proliferative responses between untreated and enzalutamide-treated mice (P > 0.05) (Fig. 1B). Additionally, CD4+ T-cell proliferation, stimulated with plate-bound anti-CD3, was similar between untreated and enzalutamide-treated mice (P = 0.48) (Fig. 1B). These findings indicate that enzalutamide has minimal effects on T-cell activity and is immune inert.
Figure 1.
Enzalutamide does not affect T-cell activity in male C57BL/6 mice, but does mediate a reduction in GU tissue weight, enlargement of the thymus, and increased TREC levels. A, enzalutamide concentration in plasma of mice on daily enzalutamide diet. Mice (n=3) were treated with enzalutamide at target daily doses of 0, 1, 10, 50, and 100 mg for 14 days. Collected blood was tested for enzalutamide concentration in plasma. B, effect of enzalutamide on CD4+ T-cell activity. Mice (H-2b, n=3) were not treated (open circles) or treated with enzalutamide (closed circles) for 14 days. CD4+ T cells were collected and co-cultured with allogeneic splenocytes (stimulator; H-2d) for 5 days (left panel) at different stimulator to CD4+ T cells responder ratios. Effect of enzalutamide on anti-CD3-induced proliferation of CD4+ T cells was evaluated by culturing purified CD4+ T cells with 2 µg/mL plate-bound anti-CD3 (right panel). Proliferation in response to stimuli was measured by incorporation of 3H-thymidine, which was added during the final 18 h. C, exposure to enzalutamide reduced GU tissue weight and enlarged thymus in mice (n=7) not treated (open circles) or treated with enzalutamide (closed circles) for 14 days. Mice (n=4) were left untreated (open bar) and subjected to castration 2 weeks (hashed bar) prior sacrifice. Mice subjected to castration showed significant reduction of GU tissues (P =0.0001) and enlarged thymus (P=0.0004) when compared to control mice (insert). F, enzalutamide significantly increased TREC levels in mice (n=7) not treated (open circles) or treated with enzalutamide (closed circles) for 14 days. 100 ng of DNA from blood was collected and TREC levels were quantified by RT-PCR. Results were normalized against the constant gene segment of TCRA, which serves as an endogenous reference gene. All experiments were done 3 times with similar results. Statistical analyses were done by Student’s t-test. Error bars indicate mean ± S.E.M. from triplicate measurements.
Enzalutamide reduces GU tissue weight, enlarges the thymus, and increases TREC levels
Clinically, ADT reduces prostate size and weight and increases thymus weight (10). In this study, male mice were castrated or sham-castrated, as previously described (24), then sacrificed 2 weeks later. Harvested GU tissue and thymuses from untreated and sham-castrated mice were similar in weight, but castrated mice showed significantly reduced GU tissue weight (P = 0.0001) and increased thymus size (P = 0.0004) compared to untreated mice (Fig 1C inserts). To determine whether enzalutamide creates similar effects, mice were not treated or treated with enzalutamide for 14 days. Enzalutamide-treated mice had significant reductions (P = 0.0021) in GU tissue weight and significant increases (P = 0.0016) in thymus weight (Fig. 1C) compared to untreated mice. To determine if increased thymus size corresponds with increased thymic function, we evaluated changes in levels of TREC, an episomal DNA by-product generated when gene segments encoding the T-cell receptor are rearranged (29). We collected blood from enzalutamide-treated and untreated mice and performed RT-PCR to detect the presence of TREC. We observed a significant increase (P = 0.0007) in TREC levels in peripheral blood from enzalutamide-treated mice compared to untreated mice (Fig. 1D). These data indicate that enzalutamide mediates significant physiological changes in mice, including reduction in GU tissue weight, enlargement of the thymus, and increased levels of TREC in peripheral blood.
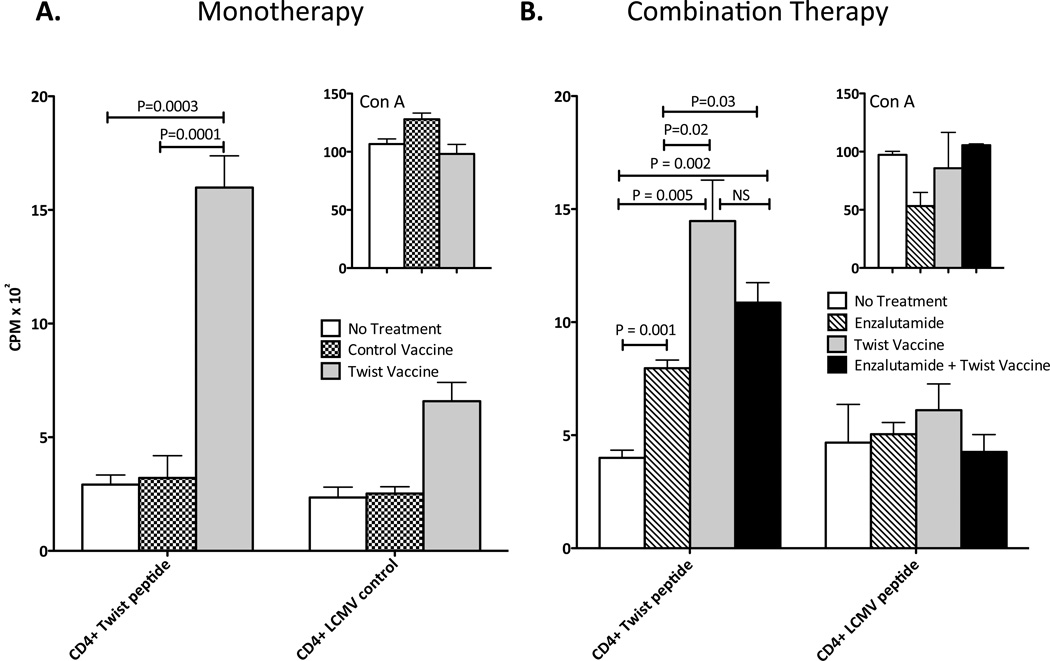
Vaccination with Twist vaccine generates Twist-specific immune responses
Twist, a highly conserved transcription factor, plays an essential role in metastatic processes and is highly expressed in prostate cancer tissue, making it a potential target for vaccine immunotherapy (15, 20). To determine if a Twist-vaccine could elicit Twist-specific immune responses, groups of mice were not treated or were vaccinated with 1 YU of control-vaccine or Twist-vaccine on days 0, 7, 14 and 21 and sacrificed 14 days later. Untreated mice and mice vaccinated with control-vaccine showed negligible CD4+-T cell proliferation (Fig. 2A). In contrast, vaccination with Twist resulted in a 5-fold increase in antigen-specific CD4+-T cell proliferation compared to untreated (P = 0.0003) or control-vaccine (P = 0.0001). To determine if the addition of enzalutamide beneficially affected the generation of Twist-specific immunity, mice were not treated, or were given Twist-vaccine alone, enzalutamide alone, or enzalutamide and Twist-vaccine on days 0, 7, 14 and 21 and sacrificed 14 days later. Untreated mice generated minimal Twist-specific CD4+-T-cell proliferation (Fig. 2B). Enzalutamide treatment resulted in a 2-fold increase in CD4+ Twist proliferation (P = 0.001) compared to untreated mice. Vaccination with Twist resulted in a 3.6-fold and 1.8-fold increase in CD4+ Twist proliferation compared to untreated (P = 0.005) and enzalutamide-treated mice (P = 0.02), respectively. Combination treatment with Twist-vaccine and enzalutamide resulted in a 3-fold and 1.5-fold increase in CD4+ Twist proliferation compared to untreated (P = 0.002) and enzalutamide-treated mice (P = 0.03), respectively. There was no significant difference in CD4+ Twist proliferation between mice receiving Twist-vaccine and mice receiving the combination treatment. All groups demonstrated similar CD4+-T cell proliferation against LCMV peptide (negative control, Fig. 2) and ConA (positive control, Fig. 2 inserts). These data demonstrate that the combination of enzalutamide and Twist-vaccine does not negatively affect the production of Twist-specific immunity.
Figure 2.
Vaccination with Twist induces antigen-specific T-cell responses in male C57BL/6 mice. A, mice (n=6) were not treated or were vaccinated with 1 YU of control-vaccine or Twist-vaccine on days 0, 7, 14, and 21. On day 35, mice were sacrificed, spleens were harvested, and CD4+ T cells were purified and tested for proliferation by culturing with irradiated APCs and Twist peptide (5 µg/mL) or LCMV control peptide (5 µg/mL) or ConA positive control (10 µg/mL) for 5 days. Proliferation in response to stimuli was measured by incorporation of 3H-thymidine, which was added during the final 18 h. B, mice (n=6) were not treated, or were treated with enzalutamide alone, 1YU of Twist-vaccine alone, or a combination of enzalutamide and Twist-vaccine. Mice were vaccinated on days 0, 7, 14, and 21 and sacrificed on day 35. Spleens were harvested and CD4+ T cells were purified and tested for proliferation as described above. All experiments were done twice with similar results. Statistical analyses were done by Student’s t-test. Error bars indicate mean ± S.E.M. from triplicate measurements.
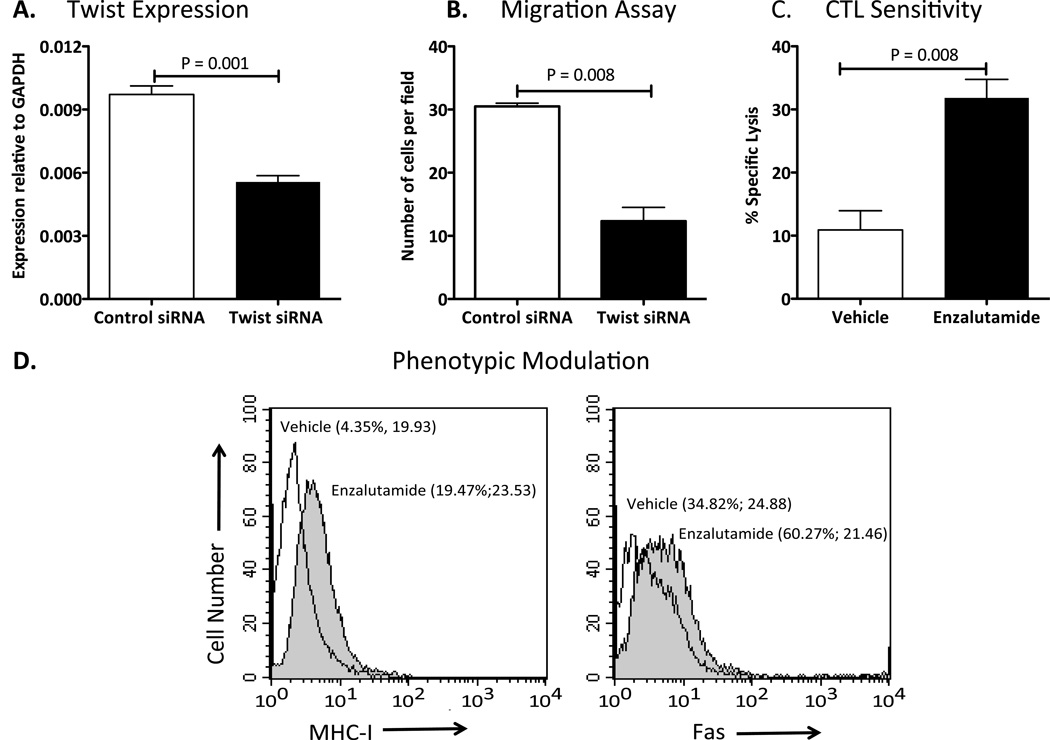
Silencing Twist reduces the migratory capacity of TRAMP-C2 cells
Twist plays a critical role in the metastasis of murine mammary carcinoma, as previously demonstrated (20). To investigate whether Twist also plays a critical role in the migratory capacity of TRAMP-C2 cells, we used Twist-siRNA to transiently down-regulate the expression of Twist. When levels of Twist mRNA expression were analyzed by RT-PCR (Fig. 3A), we observed a 45% reduction in the levels of Twist mRNA (P = 0.001). In vitro migration assay demonstrated that the partial reduction of Twist expression reduced the migratory capacity of TRAMP-C2 cells by 60% (P = 0.008) compared to control cells (Fig. 3B).
Figure 3.
Twist plays an important role in the migratory function of TRAMP-C2 cells and enzalutamide mediates immunogenic modulation. A, expression of Twist was analyzed by RT-PCR in TRAMP-C2 cells transiently transfected with Twist or control siRNAs. B, In vitro cell migration assay was performed using the transiently transfected cells. C, enzalutamide improved TRAMP-C2 cells sensitivity to gp70-specific CD8+-cytotoxic T-cell lysis. TRAMP-C2 cells were exposed in vitro to either vehicle (DMSO) or 10 µM enzalutamide for 48 h. Cells were harvested, washed, and labeled with 111In. The sensitivity of TRAMP-C2 target cells to gp70-specific killing was determined after cells were incubated at an effector:target ratio of 50:1. D, enzalutamide mediated increase expression of cell surface Fas and MHC-I molecules on TRAMP-C2 cells. TRAMP-C2 tumor cells were treated in vitro for 48 hr with vehicle (open histogram) or 10 µM enzalutamide (shaded histogram) and were analyzed for surface expression Fas and MHC-I by flow cytometry. Numbers in parentheses indicate percentage of positive cells and Mean Fluorescence Intensity. Data is representative of two independent experiments. Statistical analyses were done by Student’s t-test. Error bars indicate mean ± S.E.M. from triplicate measurements.
Enzalutamide mediates immunogenic modulation in TRAMP-C2 cells
Immunogenic modulation is exposure of tumor cells to therapies that sensitizes them to immune-mediated attack. To determine whether enzalutamide mediates immunogenic modulation, TRAMP-C2 cells were treated with enzalutamide in vitro and used as target cells in gp70-mediated killing, which is specific CTL line that recognizes the endogenous retrovirus env peptide p15e604. Exposing TRAMP-C2 cells to enzalutamide significantly enhanced (P = 0.008) p15e604-specific CTL-mediated lysis relative to tumor cells exposed to vehicle (Fig. 3C). Increase expression of cell surface expression of Fas and MHC class I molecules has been implicated in enhancing antitumor T-cell responses through diverse mechanisms (36). Flow cytometry analyses demonstrated that enzalutamide exposure increased the expression of Fas and MHC class I molecules on the surface of TRAMP-C2 cells (Fig. 3D).
Twist expression increases along with tumor progression in TRAMP mice
TRAMP is an ideal animal model for prostate cancer because tumor progression in TRAMP mice closely mimics prostate cancer progression in humans (22, 23). When TRAMP mice of different ages (representing different stages of disease) were sacrificed and their prostates were harvested, we observed increases in Twist expression as mice ages (i.e. tumors progressed from PIN to poorly-differentiated adenocarcinomas) by immunohistochemistry (Fig. 4). Digital quantification of tissue slides revealed the following percentages of Twist+ cells: 47% in mice with PIN; 57% in mice with well-differentiated adenocarcinoma; 64% in mice with moderately-differentiated adenocarcinoma; and 74% in mice with poorly-differentiated adenocarcinoma. This accords with findings in human prostate cancer, where elevated Twist protein levels are positively associated with Gleason score, suggesting that increased Twist expression is associated with poor prognosis in prostate cancer (15).
Figure 4.
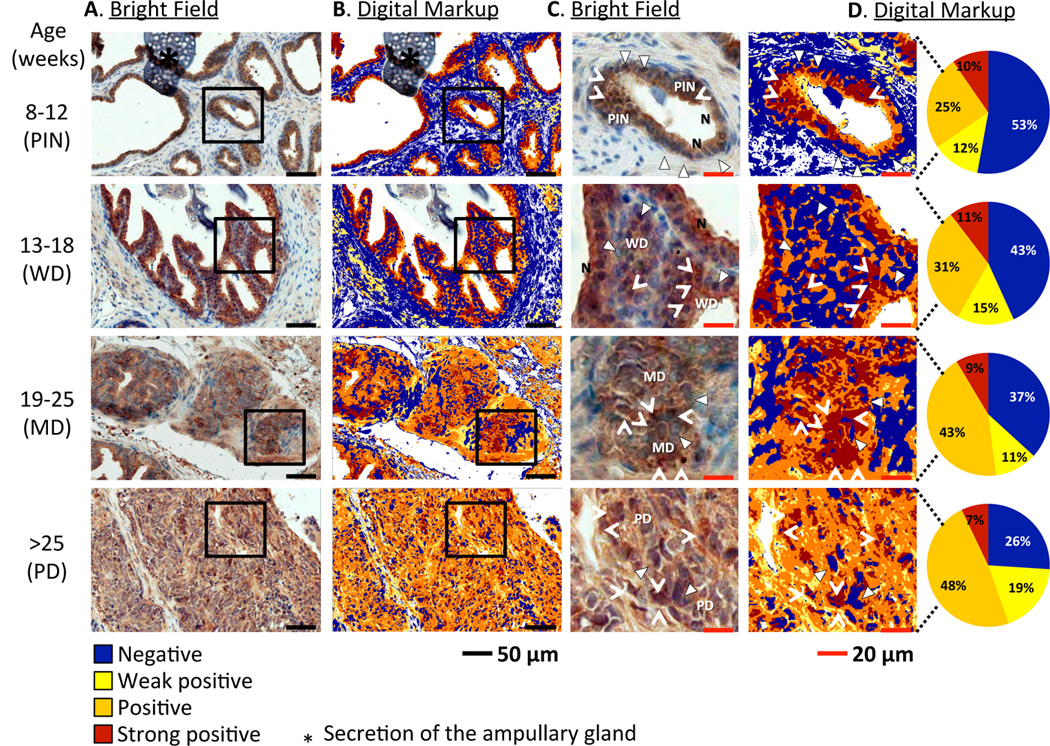
Twist expression in prostate tissues from TRAMP mice varies at different stages of tumor development. A, immunohistochemical analysis of prostate sections from TRAMP mice (n=3/group) of different ages (representing different stages of tumor development). Sections were stained with Twist-DAB and counterstained with hematoxylin. B, digital markup of sections depicted in column A, elaborated by Positive Pixel Count v9 algorithm using Aperio ImageScope image analysis software. C, magnification of tissues within squares in column A. D, magnification of tissues within squares in column and digital quantification of pixels indicating Twist expression: blue = negative, yellow = weak positive, orange = positive, brown = strong positive. Triangles: blue nuclei, negative for Twist. Arrowheads: brown nuclei, positive for Twist. N: normal epithelium. PIN: prostatic intraepithelial neoplasia. WD: well-differentiated adenocarcinoma. MD: moderately-differentiated adenocarcinoma. PD: poorly-differentiated adenocarcinoma. Note absence of Twist expression in nuclei of normal epithelia compared to neoplasias. *: secretion of the ampullary gland.
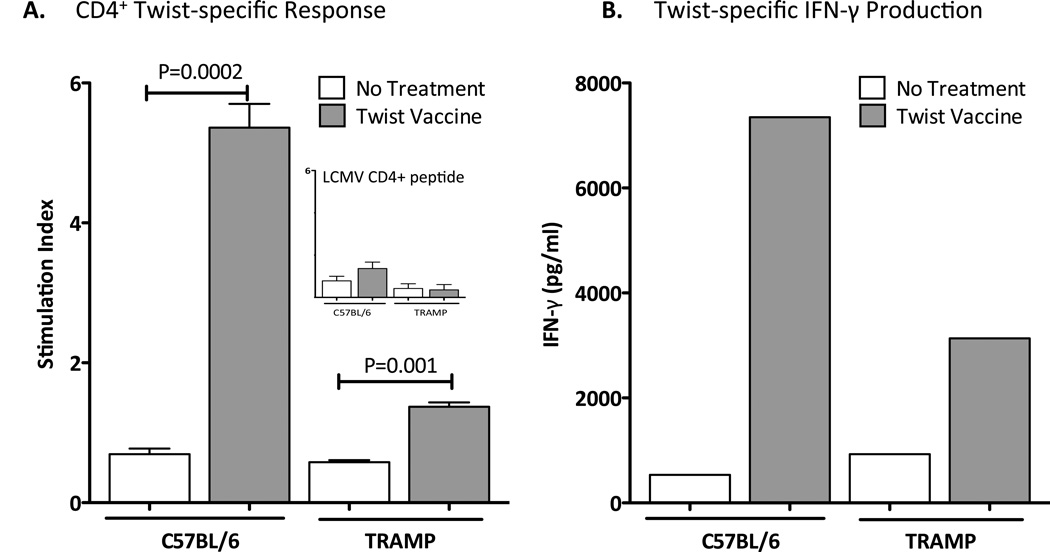
Vaccination with Twist vaccine generates Twist-specific immune responses in TRAMP mice
To determine if the Twist-vaccine could elicit Twist-specific immune responses in TRAMP mice, groups of C57BL/6 and TRAMP mice were not treated or vaccinated 3 times weekly with 1 YU of Twist-vaccine. Untreated C57BL/6 and TRAMP mice showed similar negligible CD4+-T cell proliferation (Fig. 5A) and Twist-specific IFN-γ production (Fig. 5B). In contrast, vaccination with Twist resulted in an 8-fold and 2.5-fold increase in Twist-specific CD4+-T cell proliferation compared to no treatment group in C57BL/6 (P = 0.0002) and in TRAMP mice (P = 0.001), respectively (Fig. 5A). All groups showed similar insignificant proliferation towards LCMV negative control (Fig. 5A insert). Additionally, vaccination with Twist generated a 14-fold and 3.5-fold increase in Twist-specific IFN-γ production compared to no treatment group in C57BL/6 and TRAMP mice respectively (Fig. 5B).
Figure 5.
Vaccination with Twist induces antigen-specific T-cell responses in TRAMP mice. A, C57BL/6 and TRAMP mice (n=5) harboring moderately-differentiated adenocarcinomas at average age of 17 weeks were not treated or were vaccinated with 1 YU of Twist-vaccine on days 0, 7, and 14. On day 28, mice were sacrificed, spleens were harvested, and CD4+ T cells were purified and tested for proliferation by culturing with irradiated APCs and Twist peptide (5 µg/mL) or LCMV control peptide (5 µg/mL) or ConA positive control (10 µg/mL) for 5 days. Proliferation in response to stimuli was measured by incorporation of 3H-thymidine, which was added during the final 18 h. Statistical analyses were done by Student’s t-test. Error bars indicate mean ± S.E.M. from triplicate measurements. B, to evaluate CD8+ T-cell responses, spleens were harvested and coincubated for 7 days with Twist peptide (1 µg/mL). Lymphocytes were restimulated with fresh irradiated naive splenocytes and each peptide: 1 µg/mL of Twist peptide or 1 µg/mL or HIV-gag peptide. Twenty-four hours later, the supernatant fluid was collected and analyzed for murine IFN-γ using the Cytometric Bead Array kit. Nonspecific IFN-γ production in response to HIV-gag control peptide was subtracted from that induced by Twist peptide. Data are from two separate independent experiments.
In TRAMP mice, enzalutamide mediates reduction of GU tissue weight and thymic enlargement while remaining immune inert
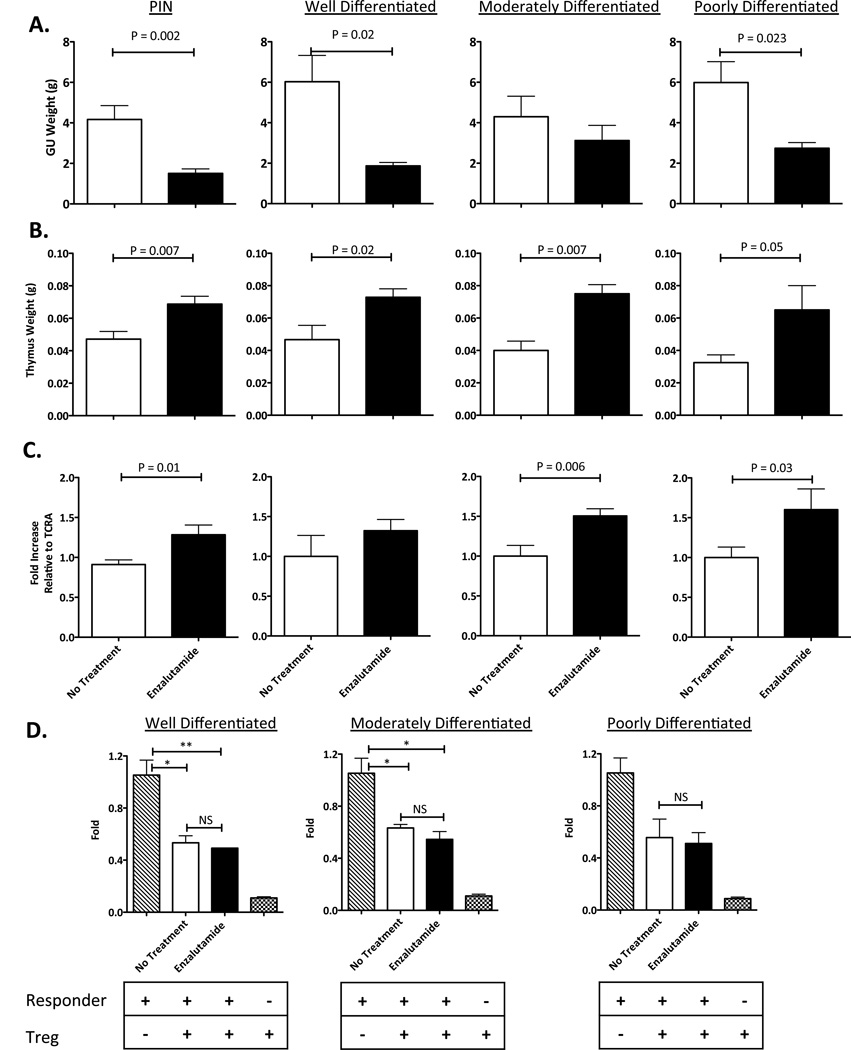
TRAMP mice of different ages (representing different tumor stages) were randomized to receive no treatment or enzalutamide for 4 or 12 weeks. Mice were sacrificed at the end of treatment and their GU tissues and thymuses were harvested and weighed. Treatment with enzalutamide mediated reduced GU tissue weight in all tumor stages (Fig. 6A). Significantly reduced GU tissue weight was observed in mice with PIN (P = 0.002), well-differentiated adenocarcinomas (P = 0.02), and poorly-differentiated adenocarcinomas (P = 0.023). In enzalutamide-treated mice, significant increases in thymus weight were observed in all tumor stages (PIN: P = 0.007, well-differentiated: P = 0.02, moderately-differentiated: P = 0.007, and poorly-differentiated: P = 0.05) compared to untreated mice (Fig. 6B). Enzalutamide-treated mice also showed increases in TREC levels in all tumor stages (PIN: P = 0.01, moderately-differentiated: P = 0.006, and poorly-differentiated: P = 0.03) compared to untreated mice (Fig. 6C). Immunohistochemistry analyses of tumor tissues demonstrated that there was a 4-fold increase in CD3+ tumor-infiltrating lymphocytes in testis (P = 0.006) of enzalutamide-treated when compared to untreated mice. Flow cytometry analyses of splenocytes and thymocytes, however, revealed no significant differences in the number of subsets of immune-cell populations (CD4+ and CD8+ T cells, Tregs, and MDSCs) between untreated and enzalutamide-treated mice (P > 0.05). There was no difference in Treg suppressive function between enzalutamide-treated and untreated mice. In both groups, Tregs were able to suppress CD8+ T-cell proliferation by 50% (Fig. 6D). By MLR, no differences were observed in allogeneic CD4+ T-cell proliferative responses between untreated and enzalutamide-treated mice (data not shown).
Figure 6.
In TRAMP mice, enzalutamide reduces GU tissue weight, enlarges the thymus, and is immune inert. A, enzalutamide mediates reduction of GU tissue weight. TRAMP mice (n=4–9) of different ages (representing different stages of tumor development) were not treated (open circles) or treated with enzalutamide 10 mg/day(closed circles) for 4 weeks. Mice were sacrificed and their GU tissues harvested and weighed. B, enzalutamide induces thymic enlargement. TRAMP mice (n=2–8) were not treated (open circles) or treated with enzalutamide 10 mg/day (closed circles) for 4 weeks. Mice were sacrificed and their thymic tissues harvested and weighed. C, enzalutamide significantly increases TREC levels in TRAMP mice. TRAMP mice (n=4–9) at different ages were not treated (open circles) or treated with enzalutamide 10 mg/day (closed circles) for 4 weeks and then sacrificed. 100ng of DNA from blood was collected and TREC levels were quantified by RT-PCR. Results were normalized against the constant gene segment of TCRA, which serves as an endogenous reference gene. D, enzalutamide does not alter Treg suppressive function. TRAMP mice (n=2–8) were not treated (open circles) or treated with enzalutamide 10 mg/day (closed circles) for 4 weeks. Spleens were harvested and CD4+CD25+FoxP3+ Tregs were purified and cultured with irradiated APCs, responder (purified CD8+ T cells from naïve C57BL/6 mice), and 0.1 µg/mL soluble anti-CD3 cross-linking antibody for 72 h. 3H-thymidine was added to the wells for the last 18 h. Error bars indicate mean ± S.E.M. from triplicate measurements. All experiments were done twice with similar results. Statistical analyses were done by Student’s t-test.
Combination treatment with enzalutamide and Twist-vaccine significantly improved overall survival in TRAMP mice
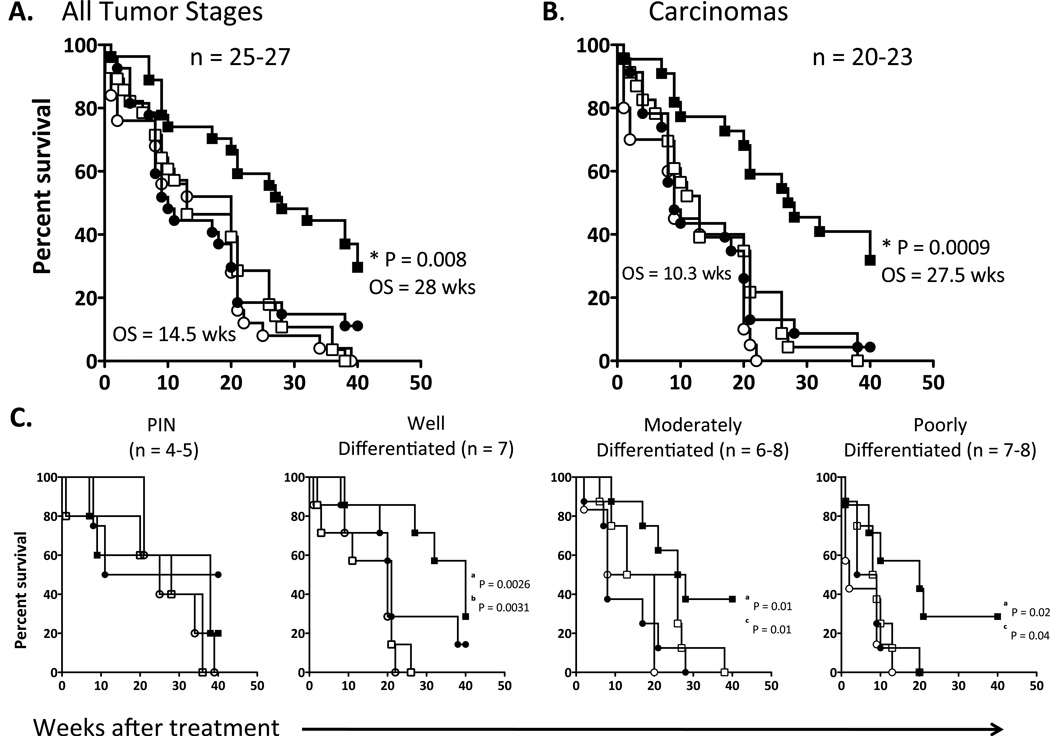
To determine whether the combination of enzalutamide and Twist-vaccine would beneficially affect overall survival in TRAMP mice, mice were randomized to receive no treatment, enzalutamide, Twist-vaccine, or the combination of enzalutamide and Twist-vaccine. No differences were observed among untreated mice and mice receiving Twist-vaccine or enzalutamide alone (Fig. 7A). However, mice receiving the combination treatment showed a significant increase in overall survival (28 vs. 14.5 weeks post-treatment) compared to untreated mice (P = 0.0001) or mice receiving Twist-vaccine (P = 0.0003) or enzalutamide (P = 0.008) alone. This represents a 75% reduction in death rate (HR: 3.9) for mice receiving combination therapy compared to untreated mice.
Figure 7.
Combination therapy with enzalutamide and Twist-vaccine increases overall survival in TRAMP mice. TRAMP mice (n=25–27) were randomized to receive no treatment (open circles), Twist-vaccine alone (open squares), enzalutamide alone (closed circles), or enzalutamide and Twist-vaccine (closed squares). Vaccine was given weekly for 3 months, then twice a month for 3 months, and monthly thereafter. Survival curve analyses are indicated for: A, mice with all tumor stages; B, mice with all tumor stages except PIN (* = P value of comparison between mice receiving combination treatment and mice receiving enzalutamide alone); C, mice from each stage of tumor progression. a represents the P value between untreated and mice receiving combination treatment. b represents the P value between mice receiving Twist-vaccine and combination treatment. c represents the P value between mice that receiving Enzalutamide and combination treatment.
Similar results were observed in mice with all tumor stages except PIN (Fig. 7B). Mice receiving Twist-vaccine or enzalutamide alone showed no improvement in overall survival over untreated mice. In contrast, mice receiving the combination treatment showed a statistically significant increase in overall survival (27.5 vs. 10.3 weeks post-treatment) compared to untreated mice (P ≤ 0.0001) or mice receiving Twist-vaccine (P = 0.0003) or enzalutamide (P = 0.0009) alone. Subset analyses based on different ages of mice (representing different stages of tumor development) showed no significant differences in mice with PIN among all treatment groups (Fig. 7C). Mice with well-differentiated adenocarcinomas treated with the combination therapy had improved survival compared to untreated mice (P = 0.0026) or mice receiving Twist-vaccine alone (P = 0.0031). Mice with moderately-differentiated adenocarcinomas treated with the combination therapy had significantly improved survival compared to untreated mice (P = 0.01) or mice receiving enzalutamide alone (P = 0.01). Mice with poorly differentiated adenocarcinomas treated with the combination therapy had improved survival compared to untreated mice (P = 0.02) or mice receiving enzalutamide alone (P = 0.04). Data from these subset analyses suggest that tumor-bearing mice treated with the combination of enzalutamide and Twist-vaccine received the greatest survival benefit.
Evidence of antigen cascade and tumor-infiltrating lymphocytes in TRAMP-mice received combination therapy
To detect the presence of Twist-specific and antigen cascade to other tumor antigens, the three mice that survived and received combination therapy from the aforementioned survival study were sacrificed and their spleens were analyzed. Mice receiving the combination therapy generated a 4-fold increase in Twist-specific CD4+-T cell proliferation compared to controls (Supplemental Table 1). CD8+ T cells from mice receiving the combination therapy generated 4-to 20-fold increases in Twist-specific IFN-γ and TNF-α production compared to age-matched controls. Combination-treated mice also showed 4 - to 32-fold increases in CD8+ T-cell responses against PSCA, survivin, and gp70 antigens compared to age-matched controls (Supplemental Table 1). Analyses of CD3+ tumor infiltrating lymphocytes of tumor tissues from these mice revealed similar number in CD3+ infiltrates compared to controls (data not shown).
Discussion
Androgen deprivation therapy’s ability to initially reduce tumor burden makes it a cornerstone of prostate cancer treatment (1, 37). However, most patients eventually become refractory to ADT and develop CRPC. While it was initially thought that CRPC was completely resistant to ADT, it has since been demonstrated that CRPC remains dependent on androgen signaling for growth and that CRPC is sensitive to further manipulation of androgen signaling (38). ADT continues to play a major role in the management of CRPC. Androgens regulate a variety of immune responses, and the effects of ADT on the immune system are well documented (10, 11, 39). In aged male mice, androgen ablation regenerates the thymus, enhances the T-cell repertoire, restores peripheral T-cell phenotype, and abrogates immune tolerance of prostate tumor cells (9, 10). In humans, ADT also induces T-cell infiltration of the prostate (40). Immunogenic modulation is exposure of tumor cells to conventional therapies that consequently alters tumor phenotype to render the tumors more susceptible to immune-mediated attack (13, 36). The immunomodulatory effects of the conventional therapies include upregulation of tumor antigens, costimulatory molecules, Fas, and MHC moieties. The immunogenic modulation property of ADT, enzalutamide in particular, has not been described. We thus hypothesized that enzalutamide induces thymic regeneration and mediates immunogenic modulation and therefore renders prostate tumor cells sensitivity to immune-mediated attack. Because of the immunomodulatory properties, we further hypothesized that enzalutamide could be exploited in combination with immunotherapy in the form of a therapeutic vaccine to create a more robust treatment option for CRPC.
This study demonstrated that enzalutamide mediates a reduction in GU tissue weight and enlargement of the thymus in both C57BL/6 (Fig. 1) and TRAMP mice (Fig. 6). In TRAMP mice, enzalutamide treatment also significantly increased the numbers of CD3+ tumor infiltrating lymphocytes in tumor tissues when compared to untreated mice. Quantification of TREC levels in peripheral blood provides an estimate of recent thymic emigrant levels and thus indirectly indicates the magnitude of thymic function (41). Here, enzalutamide-induced enlargement of the thymus was indeed accompanied by increased TREC levels, indicating a potential improvement in thymic function. However, there were no differences in the phenotype or function of T cells isolated from splenocytes from enzalutamide-treated mice compared to untreated mice, suggesting that enzalutamide can be administered in combination with immunotherapy without affecting immune response.
Twist, a member of a highly conserved BHLH transcription factor, has been implicated in the metastatic process, and its overexpression is associated with poor prognosis in many cancer types (15–19). Twist was shown to play a major role in metastasis in a 4T1 murine mammary tumor model (20). Here we describe the significant role of Twist in the TRAMP-C2 model. When Twist expression was partially silenced, the migratory potential of TRAMP-C2 cells significantly diminished (Fig. 3B). Twist was confirmed as a valid target antigen in the TRAMP model, and its expression increased as tumors progressed (Fig. 4). These data accord with previous studies implicating Twist in EMT and tumor-cell invasion and metastasis, and showing that increased Twist protein levels correlate positively with Gleason score (15, 20). Data presented in this study further suggest that the Twist-vaccine is able to elicit Twist-specific immunity in C57BL/6 (Fig. 2) and in TRAMP mice (Fig. 5) that persists for an extended period of time. Our first hypothesis was that the increased thymic function induced by enzalutamide generated new pools of naïve T cells, thus creating an ideal environment for vaccination and induction of Twist-specific immunity. Our data, however, suggest that the combination of enzalutamide and Twist-vaccine did not yield any additional benefit in the generation of Twist-specific immunity (Fig. 2). Our second hypothesis was that enzalutamide mediated immunogenic modulation. Indeed, our data demonstrate that treatment of enzalutamide increased cell surface expression of Fas and MHC-I moieties and consequently improved sensitivity of TRAMP-C2 cells to immune-mediated attack (Figs. 3C and 3D).
Although enzalutamide mediated a reduction in GU tissue weight in TRAMP mice at different ages (representing different stages of tumor development) (Fig. 6), the reduction in tumor burden did not translate to improved overall survival when enzalutamide was used as monotherapy (Fig. 7). This observation contrasts with the efficacy of enzalutamide as monotherapy for prostate cancer in humans, where enzalutamide treatment resulted in a 4.8-month advantage in overall survival compared to placebo (6). When Twist-vaccine was used as monotherapy, there was no improvement in survival compared to untreated mice. When enzalutamide and Twist-vaccine were used in combination, however, increased survival was achieved in mice at various tumor stages (Fig. 7). A subset analysis of survival revealed that older mice with more advanced tumors received the most benefit from the combination therapy. Mice with PIN did not benefit from the combination therapy, perhaps due to negligible Twist expression in this population (Fig. 4). Because Twist is associated with metastasis, Twist may not be highly expressed in precancerous lesions such as PIN (Fig. 4) (15), where vaccination against other target antigens may be more effective. This observation is supported by Hernandez et al., who used PSCA vaccine to treat young TRAMP mice with PIN (42). The PSCA vaccine’s successful induction of long-term protection against prostate cancer was due to the fact that the vaccine was delivered in the early stages of tumor development. This highlights the fact that tumor antigen must be expressed at high levels to elicit a robust and effective immune-mediated response. Additionally, mice that survived and received combination treatment with enzalutamide and Twist-vaccine for 12 months experienced antigen cascade (Supplemental Table 1), a host response not only to the antigen in the vaccine but also to other tumor antigens associated with a given tumor type (33, 43–45).
Our data demonstrate that enzalutamide is able to reduce tumor burden and mediates immunogenic modulation rendering tumor cells to be sensitive to immune-mediated attack. The antigen-specific immunity generated by a cancer vaccine may add further negative pressure. Tumor-specific immunity induced by a therapeutic vaccine is active, dynamic, and, more importantly, able to persist long after vaccination, potentially conferring protection against tumor recurrence. Findings from this study could provide a rationale for the combination of enzalutamide with a clinically active therapeutic vaccine such as PROSTVAC-VF for the treatment of CRPC. Published data from phase II trials of PROSTVAC-VF in metastatic CRPC (n = 125) demonstrate that the vaccine is well tolerated, and is associated with a 44% reduction in death rate and an 8.5-month improvement in median overall survival compared to placebo (8). Clinical trials evaluating the efficacy of combination of PROSTAC-VF and enzalutamide in patients with CRPC (NCT01867333) and in non-metastatic castration-sensitive prostate cancer patients (NCT01875250) are currently underway (www.clinicaltrials.gov).
To our knowledge, this is the first study to demonstrate: (a) the previously unknown immunogenic modulation property of enzalutamide; b) the physiological effect of enzalutamide in the C57BL/6 mice and in TRAMP model, including reduced GU tissue weight, enlarged thymus, and increased TREC levels; (c) the minimal effects of enzalutamide on T-cell activity; (d) the use of Twist, a driver of EMT/metastasis, as a target for therapeutic vaccine; (e) the ability of Twist-vaccine to generate Twist-specific immunity; and (f) significantly improved overall survival in TRAMP mice treated with the combination of enzalutamide and a therapeutic vaccine, especially in mice with advanced prostate adenocarcinomas. These data support the combination of enzalutamide and immunotherapy as a promising treatment for CRPC.
Supplementary Material
Translational Relevance.
This study examined whether the combination of anti-androgen therapy enzalutamide, plus a therapeutic vaccine targeting Twist, an antigen involved in epithelial-to-mesenchymal transition and metastasis, could improve survival in TRAMP (transgenic adenocarcinoma of the mouse prostate) mice, a spontaneous prostate cancer model. Enzalutamide mediated immunogenic modulation in vitro, reduced genitourinary tissue weight, enlargement of the thymus, increased levels of T-cell excision circles in vivo. Because no changes were seen in T-cell function, as determined by CD4+ T-cell proliferation and Treg functional assays, enzalutamide was determined to be immune inert. Furthermore, enzalutamide did not diminish the Twist-vaccine’s ability to generate Twist-specific immunity. The combination of enzalutamide and Twist-vaccine resulted in significantly increased overall survival of TRAMP mice compared to other treatment groups (27.5 vs. 10.3 weeks). Notably, the effectiveness of the combination therapy increased with disease stage, i.e., the greatest survival benefit was seen in mice with advanced-stage prostate tumors. These findings establish a rationale for the combined use of immunotherapy and anti-androgen therapy enzalutamide as a promising treatment strategy for CRPC.
Acknowledgements
The authors thank Dr. Jeffrey Schlom for his helpful suggestions, Dr. Alfredo Molinolo for assistance in interpreting immunohistochemistry and pathology data, Marion Taylor for technical assistance, and Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Grant Support
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of Interest: The authors have declared that no competing interests exist.
Authors' Contributions
Conception and design: A. Ardiani, B. Farsaci, C.J. Rogers, J.W. Hodge
Development of methodology: A. Ardiani, B. Farsaci, J.W Hodge
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Ardiani, B. Farsaci
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Ardiani, B. Farsaci, J.W. Hodge
Writing, review, and/or revision of the manuscript: A. Ardiani, J.W. Hodge
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Ardiani, B. Farsaci, C.J. Rogers, A. Protter, D. Apelian, Z. Guo, T.H. King, J.W. Hodge
Study supervision: J.W. Hodge
References
- 1.Huggins C. Endocrine-induced regression of cancers. Cancer Res. 1967;27:1925–1930. [PubMed] [Google Scholar]
- 2.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2009;10:981–991. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg C, Miller K, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(s5) abstr LBA 1. [Google Scholar]
- 7.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(Suppl 2):B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 11.Asirvatham AJ, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology. 2006;147:257–271. doi: 10.1210/en.2005-0942. [DOI] [PubMed] [Google Scholar]
- 12.Desai KV, Michalowska AM, Kondaiah P, Ward JM, Shih JH, Green JE. Gene expression profiling identifies a unique androgen-mediated inflammatory/immune signature and a PTEN (phosphatase and tensin homolog deleted on chromosome 10)-mediated apoptotic response specific to the rat ventral prostate. Mol Endocrinol. 2004;18:2895–2907. doi: 10.1210/me.2004-0033. [DOI] [PubMed] [Google Scholar]
- 13.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 16.Kyo S, Sakaguchi J, Ohno S, Mizumoto Y, Maida Y, Hashimoto M, et al. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37:431–438. doi: 10.1016/j.humpath.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, Kajiyama H, Ino K, Terauchi M, Yamamoto E, Nawa A, et al. Twist expression in patients with cervical cancer is associated with poor disease outcome. Ann Oncol. 2008;19:81–85. doi: 10.1093/annonc/mdm344. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP, Morgan M. Breast cancer progression with a Twist. Nat Med. 2004;10:777–778. doi: 10.1038/nm0804-777. [DOI] [PubMed] [Google Scholar]
- 19.Wallerand H, Robert G, Pasticier G, Ravaud A, Ballanger P, Reiter RE, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 23.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 24.Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. The TRAMP mouse as a model for prostate cancer. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 20. Chapter 20. 2001. p. 5. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, et al. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26:509–521. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130:1948–1959. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodge JW, Rad AN, Grosenbach DW, Sabzevari H, Yafal AG, Gritz L, et al. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92:1228–1239. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 29.Broers AE, Meijerink JP, van Dongen JJ, Posthumus SJ, Lowenberg B, Braakman E, et al. Quantification of newly developed T cells in mice by real-time quantitative PCR of T-cell receptor rearrangement excision circles. Exp Hematol. 2002;30:745–750. doi: 10.1016/s0301-472x(02)00825-1. [DOI] [PubMed] [Google Scholar]
- 30.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14:4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton DH, Litzinger MT, Fernando RI, Huang B, Palena C. Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Semin Oncol. 2012;39:358–366. doi: 10.1053/j.seminoncol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11:2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 34.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8:e70417. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39:323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huggins C, Stevens R, Hodges C. Studies on prostate cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg (Chicago) 1941;43:209–223. [Google Scholar]
- 38.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 39.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 40.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geenen V, Poulin JF, Dion ML, Martens H, Castermans E, Hansenne I, et al. Quantification of T cell receptor rearrangement excision circles to estimate thymic function: an important new tool for endocrine-immune physiology. J Endocrinol. 2003;176:305–311. doi: 10.1677/joe.0.1760305. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 43.Arlen PM, Madan RA, Hodge JW, Schlom J, Gulley JL. Combining Vaccines with Conventional Therapies for Cancer. Update Cancer Ther. 2007;2:33–39. doi: 10.1016/j.uct.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.