Abstract
Objective
Several practice guidelines recommend routine screening for psychological distress in cancer care. The objective was to evaluate the effect of screening cancer patients for psychological distress by assessing the (1) effectiveness of interventions to reduce distress among patients identified as distressed; and (2) effects of screening for distress on distress outcomes.
Methods
CINAHL, Cochrane, EMBASE, ISI, MEDLINE, PsycINFO, and SCOPUS databases were searched through April 6, 2011 with manual searches of 45 relevant journals, reference list review, citation tracking of included articles, and trial registry reviews through June 30, 2012. Articles in any language on cancer patients were included if they (1) compared treatment for patients with psychological distress to placebo or usual care in a randomized controlled trial (RCT); or (2) assessed the effect of screening on psychological distress in a RCT.
Results
There were 14 eligible RCTs for treatment of distress, and 1 RCT on the effects of screening on patient distress. Pharmacological, psychotherapy and collaborative care interventions generally reduced distress with small to moderate effects. One study investigated effects of screening for distress on psychological outcomes, and it found no improvement.
Conclusion
Treatment studies reported modest improvement in distress symptoms, but only a single eligible study was found on the effects of screening cancer patients for distress, and distress did not improve in screened patients versus those receiving usual care. Because of the lack of evidence of beneficial effects of screening cancer patients for distress, it is premature to recommend or mandate implementation of routine screening.
INTRODUCTION
Emotional distress is common among cancer patients as a result of the diagnosis of a life-threatening disease, aggressive medical treatments, changes in lifestyle that occur, and the direct effects of the tumor [1–3]. Increasingly, attention is being paid to the psychological consequences of cancer, with recognition of not only psychiatric disorders such as major depressive disorder (MDD) or anxiety disorders, but also of subsyndromal symptoms of depression and anxiety. In addition, attention is being paid to the broader and more inclusive concept of emotional or psychological distress, as indicated by an elevated score on a one-item distress thermometer or another psychological symptom questionnaire. A number of major cancer organizations have recommended routine screening for distress, broadly defined, and several accrediting agencies mandate routine distress screening on the assumption that identification of distress will result in increased uptake of services and reductions in distress [4–6].
Well-accepted, standard definitions of medical screening define it as an intervention that involves the application of a screening tool to individuals who are not otherwise aware they are at risk, in order to detect a medical condition that can be alleviated through intervention [7,8]. Screening for MDD, for instance, involves the use of depression symptom questionnaires or small sets of questions about depression to identify patients who may have depression, but who have not sought treatment and whose depression has not already been recognized by healthcare providers. Patients identified as possible cases based on a positive screen need to be further assessed to determine if they have depression and, if appropriate, offered treatment [9].
Screening for “distress” is less well-defined since it does not seek to identify patients with a medical condition, and the meaning of a positive screen is less clear. If screening for “distress” is to be done, nonetheless, consistent with well-established definitions of screening [7,8], it would involve using scores above a pre-defined cutoff on a distress screening tool to identify patients to be offered an intervention to reduce psychological distress. Distress screening would be potentially useful if it could improve patient outcomes beyond existing standard care in which patients had access to the same services without being screened.
Three previous reviews [10–12] have sought to evaluate whether there is evidence that routine screening for psychological distress improves psychosocial outcomes among patients with cancer. The reviews have concluded that screening may improve communication between patients and health care providers and may stimulate discussions of psychosocial and mental health issues. The reviews agreed, however, that there is not conclusive evidence that screening for distress improves patient outcomes. One concern about these reviews is that they included studies that would not be considered “screening” based on any standard definition of screening. For example, some included studies used psychosocial questionnaires to inform psycho-oncology consultations that were provided to all patients. This is not screening, however, which, by definition, would involve using the questionnaires to actually determine which patients would receive the psychosocial consultations and potentially be offered psychosocial services [7–9].
In a previous systematic review, we considered the evidence on screening for MDD in cancer patients [13], but did not find evidence to support recommendations of systematic screening for depression. Compared to depression, the target of recommendations for screening for psychological distress is broader in scope, but less clearly defined in terms of targeting a specific medical condition. The objective of the present systematic review was to evaluate the evidence on screening for psychological distress in cancer. Review questions were developed based on the U.S. Preventive Services Task Force (USPSTF) [14,15] analytic framework for evaluating screening programs. The USPSTF framework recognizes the need for RCTs to directly assess links between screening programs and patient outcomes. When direct evidence from screening RCTs is not available or is of low quality, the USPSTF framework assesses key links that are necessary for screening to benefit patients, such as the availability of effective treatments [14,15].
Screening for distress per se differs from other medical screening programs in that there is not a clear, defined medical condition, such as MDD, that screening tools seek to detect. Thus, although reviews of screening usually assess screening tool accuracy compared to a gold standard [14,15], we were not able to do this. Nonetheless, an important prerequisite if screening of psychological distress is to improve patient outcomes is that distress can be reduced through intervention for patients identified as distressed. Thus, consistent with USPSTF methods, Review Question #1 was, “What are the effects of interventions to reduce distress among cancer patients who have been selected for treatment based on a minimum threshold of psychological distress, as would be done in a screening program?” If screening is to be actually recommended as policy, there should be consistent evidence from well-conducted randomized controlled trials (RCTs) [16,17] that screening benefits patients in excess of any possible harms. Thus, Review Question #2 was, “Is routine screening for psychological distress of cancer patients more effective than usual care in reducing symptoms of distress?”
METHODS
Search Strategy
The CINAHL, Cochrane, EMBASE, ISI, MEDLINE, PsycINFO, and SCOPUS databases were searched through April 6, 2011. A search was conducted for studies of interventions designed to reduce psychological distress among cancer patients identified as having distress (Review Question #1) and for studies that assessed outcomes of psychological distress screening interventions (Review Question #2). Search terms are reported in Appendix 1. Manual searches were done on relevant systematic reviews (Appendix 2), reference lists of included articles, and 45 selected journals (March 2011 to May 2012; Appendix 3). We also tracked citations of included articles using Google Scholar [18] and searched the trial registries ClinicalTrials.gov [19] and the International Standard Randomized Controlled Trial Number Register [20] to attempt to identify unpublished treatment or screening RCTs.
Identification of Eligible Studies
Eligible articles included studies in any language on cancer patients with any type of malignancy at any disease stage that reported original data, excluding abstracts, case series, or case reports. Translators assisted reviewers to evaluate titles and abstracts and full-length articles for languages not covered by investigators, who were able to independently review material in English, Dutch, French, and Spanish. Multiple articles from the same cohort were treated as a single study. Studies with mixed populations were included only if cancer data were reported separately.
For Review Question #1, eligible articles were RCTs that compared interventions designed to reduce psychological distress to placebo, usual care, or attention controls in adult cancer patients with elevated distress. Only RCTs that limited inclusion to patients with high levels of distress, rather than all patients with cancer, were included because this is what would occur in a screening program. Indeed, patients with low levels of distress experience only negligible benefits from psychosocial interventions in cancer settings [21]. Small, underpowered studies are often of poor quality, and significant publication bias is a major problem among these studies [22–24]. A number of proposals have been made regarding setting thresholds for minimum number of patients for studies to be included in systematic reviews [23, 24]. In the present review, we included trials that randomized at least 25 patients to each group [25]. Head-to-head comparisons of different interventions without a comparison to usual care or placebo were not eligible. Detailed eligibility criteria that were used for determining study eligibility are shown in Appendix 4.
Eligible articles for Review Question #2 were RCTs that compared outcomes between cancer patients who underwent screening for psychological distress and those who did not. Screening was defined according to the UK National Screening Committee’s definition [7]. Thus, eligible screening trials had to include a strategy to identify patients with high levels of psychological distress based on an a priori-defined cutoff score on a measure of distress. Furthermore, in eligible studies, positive versus negative results of the screening test had to be used to make decisions about further assessment, referral, or treatment. Studies were excluded if questionnaires were used to inform and structure conversations that occurred as part of psychosocial consultations, but not to determine which patients receive services to address distress based on a score above a pre-defined cutoff. Finally, studies that involved administering multiple screening tools for multiple problems were not included, since patients in these studies could have been deemed in need of services due to reasons other than psychological distress (e.g., practical issues related to drug coverage by insurance, transportation and parking, or nutritional needs) [26], and determining whether the psychological distress component of screening influenced distress outcomes would not be possible.
Two investigators independently reviewed articles for eligibility. If either deemed an article potentially eligible based on title and abstract review, then a full-text review was undertaken. Disagreements after full-text review were resolved by consensus.
Evaluation of Eligible Studies
Two investigators independently extracted and entered data into a standardized spreadsheet (see Appendix 5). Discrepancies were resolved by consensus. Risk of bias in studies included for both review questions was assessed with the Cochrane Risk of Bias tool [27] (see Appendix 6), including assessment of financial conflicts of interest as has been recommended [28,29]. Risk of bias was assessed by two investigators, with discrepancies resolved by consensus.
Data Presentation and Synthesis
Psychological distress outcomes reported in each eligible study were classified as primary or secondary for the purposes of the review. For both review questions, when multiple measures of psychological distress were assessed as outcomes, designated primary outcomes for each study were prioritized. If there were no designated primary outcomes, the distress measure that was used to determine eligibility for the trial (Review Question #1) or as the screening tool for psychological distress (Review Question #2) was selected. If multiple instruments were used for distress selection, continuous scores on interview-based observer-rated instruments were prioritized over self-rating instruments. This is because observer-rated instruments, particularly the Hamilton Depression Rating Scale, are used most often as outcome measures in depression trials and considered the gold standard [30]. If there were no observer-rated instruments, and there was more than 1 self-rating instrument, all were reported as secondary outcomes. When outcomes were assessed at multiple time points, the assessment point that followed the end of treatment most closely was reported. Post-intervention effect sizes were reported using the Hedges’s g statistic [31], which represents a standardized difference between 2 means, as well as r2, which is statistically equivalent [32,33], but presents results in terms of percent of variance in distress outcomes due to treatment. Dichotomous outcomes were not extracted since there is no agreed upon gold standard or definition for psychological distress “caseness.”
Eligible studies for each review question were evaluated to determine whether there was sufficient clinical and methodological similarity to support pooling of results. Results from trials with a high degree of clinically heterogeneity in terms of patients, interventions, or study procedures should not be synthesized meta-analytically because the effect estimate that is generated would not be expected to generalize to any given intervention [24]. For Review Question #1 (treatment), studies were heterogeneous in terms of patient samples, therapeutic interventions, outcome measures, and treatment duration. Only 1 eligible study was identified for Review Question #2 (screening). Therefore, results were not pooled quantitatively in a meta-analysis, but were summarized in a systematic review. A review protocol was not published or registered for this systematic review. However, a written protocol was developed and followed for searching, data extraction, and data synthesis with all methods determined a priori.
RESULTS
Review Question #1: Effect of Treatment of Psychological Distress
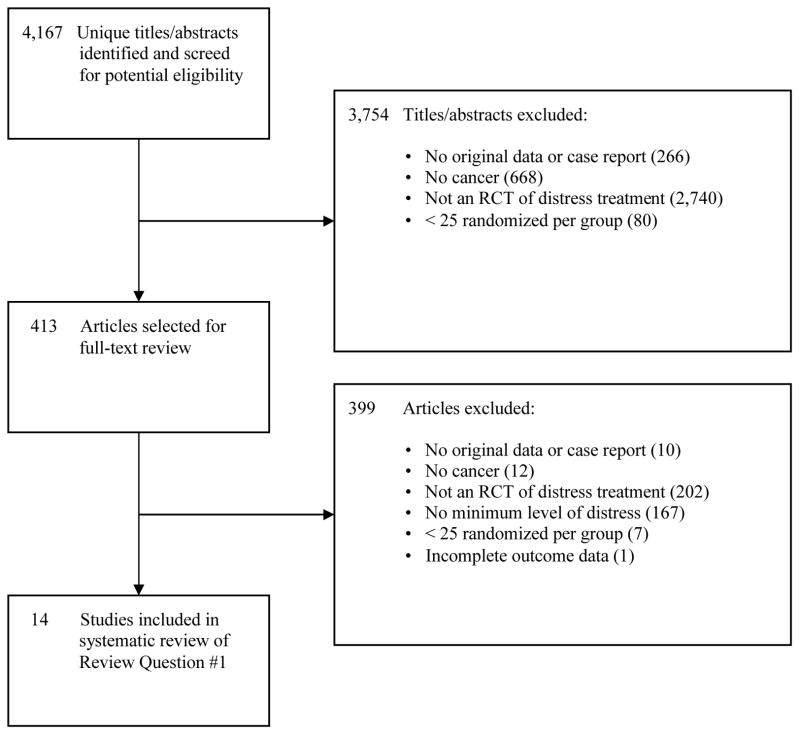
The combined database search for Review Questions #1 (treatment) and #2 (screening) generated 4,167 unique citations. As shown in Figure 1, for Review Question #1 (treatment), 3,754 were excluded after title/abstract review and 399 after full-text review, leaving 14 eligible studies for review. No additional studies were identified through alternative sources, such as hand searching of journals, forward citation of included articles, and review of trial registries.
Figure 1.
PRISMA Flow Diagram of Study Selection Process for Review Question #1
As shown in Table 1, the 14 studies of interventions to reduce psychological distress we reviewed included 12 studies of patients with mixed cancer sites [34–45], 1 study with patients with breast or cervical cancer [46], and 1 study with patients with breast cancer only [47]. Total sample size per study ranged from 55 to 472. Of the 14 studies, 7 randomized at least 64 patients per group [35,37–40,44,45], which would provide adequate (80%) power to detect a medium effect size (standardized mean difference = 0.50) [48].
Table 1.
Characteristics and Outcomes of Randomized Controlled Trials of Distress Treatment
| First Author, Year, Country | Study Funding Source | Cancer Type/Description | Distress Inclusion Criterion | Treatment vs. Control | Number of Patients Randomized | Mean Age (Years) | Males (%) | Treatment Duration | Primary Distress Outcome:a Hedges’s g (95% CI) and r2 | Secondary Distress Outcome(s):a Hedges’s g (95% CI) and r2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Costa, 1985, Romania [34] | NR | Mixed/ Mixed | Depression diagnosis; ZSRDS≥41; and HAMD-17 ≥16 | Mianserin vs. placebo | Total: 73 Tx: 36 Placebo: 37 |
Tx: 49 Placebo: 54 |
Tx: 0% Placebo: 0% |
4 weeks |
HAMD-17: 0.60 (0.13–1.07) r2=.08 |
ZSRDS: 0.64 (0.17–1.11) r2=.10 CGI-S: 0.70 (0.23–1.17) r2=.11 |
| Dwight-Johnson, 2005, United States [46] | Non-industry | Breast or cervical/ Mixed | Symptoms consistent with major depression or dysthymia;b or persistent depressive symptoms at baseline and 1 month later | Collaborative care vs. UC | Total: 55 Tx: 28 UC: 27 |
Tx: 48 UC: 47 |
Tx: 0% UC: 0% |
Minimum of 8 weeksc | NRd | Δ FACT-G emotional:c 0.58 (0.04–1.12) r2 = .08 |
| Ell, 2008, United States [35] | Non-industry | Mixed/ Mixed | Sadness or anhedonia more than half the days, plus PHQ-9 ≥ 10 and/or dysthymia | Collaborative care vs. enhanced UCe | Total: 472 Tx: 242 UC: 230 |
NR | Tx: 16% UC: 14% |
12 months |
PHQ-9:f 0.17 (−0.07–0.42) r2 = .01 |
FACT-G emotional:f 0.29 (0.05–0.54) r2 =.02 SF-12 mental:f 0.21 (−0.04–0.46) r2 = .01i |
| Evans, 1995, United States [36] | Non-industry | Mixed/ Stage II | CES-D ≥ 16 | CBT vs. SSg vs. UC | Total: 78 CBT: 29 SS: 23g UC: 26 |
CBT: 54 UC: 54 |
CBT: 63% UC: 67% |
8 weeks |
CES-D: 0.54 (−0.02–1.10) r2 =.07 |
SCL-90-R GSI: 0.59 (0.03–1.15) r2 = .08 |
| Fann, 2009, United States [37] | Non-industry | Mixed/ NR | MDD or dysthymia diagnosis | Collaborative care vs. UC | Total: 215 Tx: 112 UC: 103 |
Tx: 72 UC: 72 |
Tx: 37% UC: 42% |
12 months |
SCL-20 depression : 0.47 (0.20–0.74) r2 = .05 |
NA |
| Fisch, 2003, United States [38] | Non-industry with drug supplied by industry | Mixed/ Advanced, incurable | TQSS ≥ 2 | Fluoxetine vs. placebo | Total: 163 Tx: 83 Placebo: 80 |
Tx: 61 Placebo: 59 |
Tx: 55% Placebo: 45% |
12 weeksh |
BZSDS:h 0.23 (−0.21–0.66) r2 = .01 |
NA |
| Greer, 1992, United Kingdom [39] | Non-industry | Mixed/ Mixed | HADS-D ≥ 8; HADS-A ≥ 10; or MAC helplessness ≥ 12 and MAC fighting spirit ≤ 47 | CBT vs. UC | Total: 174 Tx: 85 UC: 89 |
Tx: 51 UC: 52 |
Tx: 28% UC: 14% |
8 weeksi | NAj | Δ HADS-A: 0.38 (0.06–0.71) r2 = .04 Δ HADS-D: 0.29 (−0.03–0.61) r2 = .02 Δ MAC helplessness: 0.46 (0.13–0.78) r2 = .05 Δ MAC anxious preoccupation: 0.37 (0.05–0.70) r2 = 0.03 Δ RSCL psychological: 0.33 (0.00–0.66) r2 = .03 Δ PAIS psychological: 0.27 (−0.06–0.59) r2 = .02 |
| Kroenke, 2010, United States [40] | Non-industry | Mixed/ Mixed | PHQ-9 ≥ 10, plus depressed mood and/or anhedoniak | Telecare management vs. UC | Total: 309k Tx: 154 UC: 155 |
Tx: 59l UC: 59l |
Tx: 37%l UC: 28%l |
12 months |
HSCL-20 depression: 0.35 (0.07–0.62) r2 = .03 |
SF-36 MHI depression: 0.32 (0.04–0.60) r2 = .03 |
| Moorey, 2009, United Kingdom [41] | Non-industry | Mixed/ Mixed | HADS-A ≥ 8 or HADS-D ≥ 8 | Nurse-delivered CBT vs. UC | Total: 80 Tx: 45 UC: 35 |
Tx: 65 UC: 62 |
NR | 16 weeksm | NRn | NA |
| Nezu, 2003, United States [42] | Non-industry | Mixed/ Stages I-III |
BSI-GSI ≥ 63 and HAMD-17 ≥ 14 | PST vs. PST-SO vs. WL | Total: 150 PST: 50 PST-SO: 50 WL: 50 |
PST: 49 PST-SO: 46 WL: 47 |
PST: 33% PST-SO: 36% WL: 30% |
Average of 13 weeks | PST vs. WL: HAMD-17: 3.76 (3.07–4.45) r2 = .78 PST-SO vs. WL: HAMD-17: 4.30 (3.54–5.07) r2 = .83 |
PST vs. WL: BSI-GSI: 3.54 (2.87–4.21) r2 = .76 Omega: 2.01 (1.50–2.52) r2 = .51 POMS: 2.15 (1.63–2.67) r2 = .54 PST-SO vs. WL: BSI-GSI: 4.39 (3.62–5.17) r2 = .83 Omega: 1.95 (1.44–2.46) r2 = .49 POMS: 2.01 (1.50–2.53) r2 = .51 |
| Razavi, 1996, Belgium and France [43] | Industry | Mixed/ Mixed | MDD or adjustment disorder diagnosis, plus HADS ≥ 13 | Fluoxetine vs. placebo | Total: 91 Tx: 45 Placebo: 46 |
Tx: 53 Placebo: 53 |
Tx: 18% Placebo: 23% |
5 weeks |
HADS: 0.36 (−0.12–0.84) r2 = .03 |
SCL-90-R GSI: 0.22 (−0.26–0.69) r2 = .01 MADRS: 0.17 (−0.31–0.65) r2 = .01 HAS: 0.21 (−0.26–0.69) r2 = .01 |
| Strong, 2008, United Kingdom [44] | Non-industry | Mixed/ Mixed | HADS ≥ 15; MDD diagnosis; SCL-20 depression ≥ 1.75 | Nurse intervention vs. UC | Total: 200 Tx: 101 UC: 99 |
Tx: 57 UC: 57 |
Tx:31% UC: 28% |
Mean of 7 sessions over 3 months |
SCL-20 depression: 0.37 (0.09–0.65) r2 = .03 |
SCL-10 anxiety: 0.24 (−0.04–0.52) r2 = .01 |
| Van Heeringen, 1996, Belgium, [47] | Industry | Breast/ Stages I-II, non-metastatic | Depression diagnosis and HAMD-21 ≥ 16 | Mianserin vs. placebo | Total: 55 Tx: 28 Placebo: 27 |
Tx: 51 Placebo: 53 |
Tx: 0% Placebo: 0% |
6 weeks | Δ HAMD-21: 0.77 (0.23–1.32) r2 = .13 |
NA |
| Wilkinson, 2007, United Kingdom [45] | Non-industry | Mixed/ Mixed | Depression or anxiety diagnosis | Aromatherapy massage vs. UC | Total: 288 Tx: 144 UC: 144 |
Tx: 52 UC: 53 |
Tx: 14% UC: 13% |
4 weekso | NRj | Δ SAI:o 0.22 (−0.01–0.46) r2 = .01 Δ CES-D:o 0.17 (−0.06–0.41) r2 = .01 |
Abbreviations: BSI-GSI = Global Severity Index of the Brief Symptom Inventory; BZSDS = Brief Zung Self-Rating Depression Scale; CBT = cognitive behavior therapy; CGI-S = Clinical Global Impression Scale for Severity of Illness; CES-D = Center for Epidemiologic Studies Depression Scale; CI = confidence interval; FACT-G emotional = emotional well-being subscale of Functional Assessment of Cancer Therapy - General; HADS = Hospital Anxiety and Depression Scale total score; HADS-A = Anxiety subscale of Hospital Anxiety and Depression scale; HADS-D = Depression subscale of Hospital Anxiety and Depression Scale; HAMD-17 = 17-item Hamilton Depression Rating Scale; HAMD-21 = 21-item Hamilton Depression Rating Scale; HAS = Hamilton Anxiety Scale; HSCL-20 = 20-item Hopkins Symptom Checklist depression scale; MAC anxious preoccupation = anxious preoccupation subscale of Mental Adjustment to Cancer scale; MAC fighting spirit = fighting spirit subscale of Mental Adjustment to Cancer scale; MAC helplessness = helplessness subscale of Mental Adjustment to Cancer scale; MADRS = Montgomery-Asberg Depression Rating Scale; MDD = major depressive disorder; NA = not applicable; NR = not reported; PAIS psychological = psychological distress subscale of Psychosocial Adjustment to Illness Scale; PHQ-9 = 9-item Patient Health Questionnaire; POMS = Profile of Mood States; PST = problem-solving therapy; PST-SO = problem-solving therapy with significant other; RSCL psychological = psychological symptoms subscale of Rotterdam Symptom Checklist; SAI = State Anxiety Inventory; SCL-10 anxiety = anxiety subscale derived from the Symptom Checklist-90; SCL-20 depression = depression subscale derived from the Symptom Checklist-90; SCL-90-R GSI = Global Severity Index of revised Symptom Checklist-90; SF-12 mental = mental component summary of 12-item Short Form Health Survey; SF-36 MHI depression: depression severity subscale of the 36-item Short Form Health Survey Mental Health Inventory; SS = social support; TQSS = Two-Question Screening Survey; Tx = treatment; UC = usual care; WL = waiting list control; ZSRDS = Zung Self-Rating Depression Scale.
Continuous outcomes that favored the treatment group are reported as positive numbers.
Assessed using PHQ-9 and 3 additional questions from PRIME-MD.
Treatment components received varied between study participants. Effects of collaborative intervention were assessed after 8 months.
The PHQ-9 was used to determine eligibility for the trial and was thus classified as the primary outcome, but continuous outcome data were not reported for the PHQ-9.
Enhanced usual care consisted of standard oncology care, educational pamphlets, and a listing of center and community resources.
Results adjusted for sex, race, years in the US, dysthymia, baseline depression severity, baseline anxiety, cancer stage, cancer type, and treatment status.
Results from social support group were not included in this review, as fewer than 25 patients were randomized to this group.
The fourth assessment visit (mean 12.3 weeks post-randomization) was closest to the end of the treatment period. However, only 33/163 patients completed the fourth visit. Outcome data presented here were assessed at the fifth visit (mean 14.9 weeks post-randomization).
Planned treatment duration was 8 weeks, but 28 patients (39%) received additional therapy sessions between 8 weeks and 4 months.
No primary outcome could be identified.
Eligible participants met study criteria for depression, cancer-related pain, or both. Results are reported only for the 309 participants meeting eligibility criteria for depression.
Age and sex were reported for the whole sample (N = 405), and not only the 309 participants enrolled for depression.
Treatment duration was not explicitly stated in the article, but 16 weeks was the last assessment timepoint.
HADS-A and HADS-D were identified in the article as primary outcomes, but insufficient information was provided to extract continuous outcome data. The authors reported that anxiety was significantly reduced in the treatment group at 16 weeks, but not depressive symptoms.
Anxiety and depression outcomes were assessed at 6 weeks.
Four studies were pharmacological interventions designed to treat depression, 2 with mianserin [34,47] and 2 with fluoxetine [38,43]. The other 10 studies included collaborative care interventions [35,37,40,44,46], cognitive behavior therapy [36,39,41], problem solving therapy [42], and aromatherapy massage [45]. Among the drug trials, there was 1 study [38] with at least 64 patients per group, and that study found a small effect size reduction on self-reported depressive symptoms with fluoxetine (Hedges’s g = 0.23). Three other smaller trials [34,43,47] reported somewhat larger effects for fluoxetine (Hedges’s g = 0.36) [43] and mianserin (Hedges’s g = 0.60 to 0.77) [34,47]. Among collaborative care trials, effect sizes were small to moderate for adequately powered trials (Hedges’s g = 0.17 to 0.47) [35,37,40,44]and moderate to large for a smaller study (Hedges’s g = 0.60) [46]. The effect sizes for outcomes reported in a trial of problem-solving therapy trial [42], comparing problem-solving therapy to a wait-list control (Hedges’s g = 3.76) or problem-solving therapy with a significant other to the wait-list control (Hedges’s g = 4.30) were exceedingly large. The effect sizes on 2 outcome measures from aromatherapy with massage [45] were small (Hedges’s g = 0.17 to 0.22) and not statistically significant. Effect sizes for each individual study are shown in Table 1.
Risk of bias ratings are shown in Table 2, and specific explanations for all ratings are available from the authors. Among the 4 trials of antidepressants, all had unclear or high risk of bias for the majority of rating categories [34,38,43,47]. Specifically, all had unclear or high risk related to industry funding and author-industry financial ties, and all were conducted prior to the availability of clinical trial registries. Thus, selective outcome reporting was rated as unclear for all of these trials. Among non-pharmacological treatments, all were rated as high risk for blinding of patients and personnel and for blinding of outcome assessment due to the nature of the interventions and outcome assessments. Generally, quality was mixed in these studies. Not including blinding, only 1 non-pharmacological intervention trial [44] was rated as low risk of bias across all categories, including being registered with sufficiently precise outcome registration to compare to those described in the published trial report. One trial of problem-solving therapy [42] was rated as high risk of bias for Other Sources of Bias. This was due to the unrealistically high effect sizes, approximately 10 times those of other non-pharmacological studies, which were reported for the primary outcome variable. Other meta-analyses have excluded this study as an extreme outlier [49–51].
Table 2.
Assessment of Risk of Bias in Randomized Controlled Trials in Review Question #1 (Treatment) and Review Question #2 (Screening)
| Cochrane Risk of Bias Tool Domainsa
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial, Year, Country | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessmentb | Incomplete Outcome Datab | Selective Outcome Reporting | Author-Industry | ||
| Pharmaceutical Industry Fundingc | Financial Ties and/or Industry Employmentc | Other Sources of Bias | |||||||
|
Review Question #1 (Treatment)
| |||||||||
| Costa, 1985 [34] | Unclear risk | Unclear risk | Low risk | Observer-rated: Low Self-report: Low |
Observer-rated: High Self-report: High |
Unclear risk | Unclear risk | Unclear risk | Low risk |
| Dwight-Johnson, 2005 [46] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: High |
Unclear risk | Low risk | Unclear risk | Low risk |
| Ell, 2008 [35] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: High |
Unclear risk | Low risk | Low risk | Low risk |
| Evans, 1995 [36] | Unclear risk | Unclear risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Unclear risk | Low risk | Low risk | Low risk |
| Fann, 2009 [37] | Unclear risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Unclear risk | Low risk | Low risk | Low risk |
| Fisch, 2003 [38] | Low risk | Low risk | Low risk | Observer-rated: NA Self-report: Low |
Observer-rated: NA Self-report: High |
Unclear risk | Unclear risk | Unclear risk | Unclear riska |
| Greer, 1992 [39] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Unclear risk | Low risk | Low risk | Low risk |
| Kroenke, 2010 [40] | Low risk | Unclear risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Unclear risk | Low risk | Unclear risk | Low risk |
| Moorey, 2009 [41] | Unclear risk | Unclear risk | High risk | Observer-rated: NA Self-report: Unclear |
Observer-rated: NA Self-report: High |
Unclear risk | Low risk | Low risk | Low risk |
| Nezu, 2003 [42] | Low risk | High risk | High risk | Observer-rated: Unclear Self-report: High |
Observer-rated: Low Self-report: Low |
Unclear risk | Low risk | Low risk | High risk |
| Razavi, 1996 [43] | Unclear risk | Unclear risk | Low risk | Observer-rated: Unclear Self-report: Low |
Observer-rated: High Self-report: High |
Unclear risk | Unclear risk | High risk | Low risk |
| Strong, 2008 [44] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Low risk | Low risk | Low risk | Low risk |
| Van Heeringen, 1996 [47] | Unclear risk | Unclear risk | Low risk | Observer-rated: Unclear Self-report: NA |
Observer-rated: High Self-report: NA |
Unclear risk | High risk | Unclear risk | Low risk |
| Wilkinson, 2007 [45] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Unclear risk | Low risk | Low risk | Low risk |
|
| |||||||||
|
Review Question #2 (Screening)
| |||||||||
| Maunsell, 1996 [52] | Low risk | Low risk | High risk | Observer-rated: NA Self-report: High |
Observer-rated: NA Self-report: Low |
Low risk | Low risk | Low risk | Low risk |
Authors of study did not provide data on number of patients who were approached or eligible for the trial, but they did not that the patients enrolled represented a small fraction of eligible patients.
Review Question #2: Effect of Screening for Psychological Distress
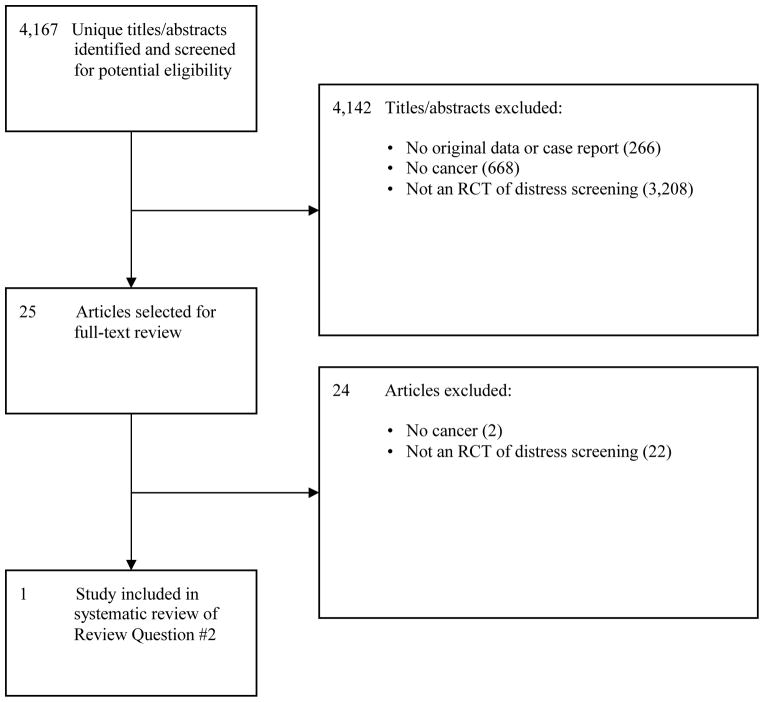
For Review Question #2, 4,142 of the original 4,167 citations were excluded after title and abstract review and 24 after full text review, leaving 1 RCT [52] of screening for psychological distress among newly diagnosed breast cancer patients (Figure 2). In this study, the usual care group (N=127) received a brief psychosocial intervention in the first 2 weeks post-randomization as part of standard care (mean 2.2 social work contacts). The intervention group (N=123) received the same brief intervention (mean 2.4 social work visits) plus telephone screening with the General Health Questionnaire, beginning 21 days post-randomization and continuing monthly for 12 months. Once screening was initiated, 80% of screened patients had at least 1 positive screen, which triggered a social work telephone contact beyond referrals that occurred as part of usual care (mean = 6.1 social work contacts versus 2.4 for usual care). As shown in Table 3, at 12 months post-randomization, Psychiatric Symptom Index scores for the intervention and usual care groups were equivalent. In addition, women in the intervention group were somewhat more likely to have a diagnosis of MDD at 12 months post-randomization (n = 22, 18%) compared to women in the control group (n = 15, 12%), although this was not statistically significant. Risk of bias in this screening RCT was generally low (Table 2).
Figure 2.
PRISMA Flow Diagram of Study Selection Process for Review Question #2
Table 3.
Summary of Randomized Controlled Trial of Screening for Psychological Distress in Cancer
| First Author, Year, Country | Study Funding Source | Cancer Site/ Description | Comparison | Number of Patients Randomized | Mean Age (Years) | Males (%) | Number of Patients in Analysesb | Intervention Duration | Primary Distress Outcome:a Hedges’s g (95% CI) and r2 | Study Funding Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Maunsell, 1996, Canada [52] | Non-Industry | Breast/ Localized or regional |
Intervention: Usual care + telephone screening beginning 21 days post-randomization and repeated at 28-day intervals with the 20-item GHQ. Social workers made follow-up telephone calls to all patients with GHQ ≥ 5 to assess psychological distress and offer psychosocial intervention. Control: Usual care, which included minimal psychosocial intervention as part of initial cancer care. |
Total: 261 Tx: NR UC: NR |
Total: 55 Tx: 55 UC: 56 |
Total: 0% Tx: 0% UC: 0% |
Total: 250 Tx: 123 UC: 127 |
12 months |
Psychiatric Symptom Index: g=0.09 (−0.16 to 0.34) r2 < 0.01 |
Non-Industry |
Abbreviations: CI = confidence interval; GHQ = General Health Questionnaire; NR = not reported; Tx = treatment; UC = usual care.
Reported distress outcome was assessed at the end of the intervention period. A continuous outcome that favored the treatment group is reported in this table as a positive number.
11 patients were eliminated post-randomization, including 1 who was found to have metastatic breast cancer, 1 who did not have breast cancer following re-examination, and 9 with incomplete or unavailable outcome data.
A number of other studies (see Table 4) described by their authors or in other reviews [10–12] as related to screening were excluded from the present systematic review. Several studies were excluded because decisions about whether patients should receive further assessment, referral, or treatment were not based on a pre-specified cutoff score on a measure of distress. In those studies, a range of screening tools was often made available for clinical consultations, but a positive screen on a distress screening tool was not used to determine referral for psychosocial evaluation or treatment. Studies were also excluded because they (1) were not RCTs; (2) included multiple screening tools for many practical or logistical issues, not allowing the effect of screening for psychological distress to be evaluated separately; or (3) did not report distress symptom or diagnosis outcomes.
Table 4.
Excluded Studies for Effect of Screening on Psychological Distress Outcomes (Review Question #2)
| First Author, Year, Country | Cancer site | N consented / randomizeda | Comparison | Distress outcomes | Reason(s) for exclusion |
|---|---|---|---|---|---|
| Berry, 2011, USA [73] | Mixed | 660 |
Intervention: Patients completed a quality of life questionnaire, ESRA-C. A summary of the results, with symptoms above a predetermined threshold flagged, was provided to the clinician prior to visit. Control: Patients completed a quality of life questionnaire, the ESRA-C. No summary of the results was provided to the clinician. |
Only number of PHQ-9 symptoms and EORTC QLQ-C30 emotional function symptoms discussed with clinician, but not distress outcomes were assessed. | A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. Distress symptoms were not an outcome, only the discussion of symptoms. |
| Boyes, 2006, Australia [54] | Mixed | 80 |
Intervention: Results from a computer survey completed prior to each visit were provided to the patient’s oncologist. The survey included and assessment of 12 physical symptoms associated with chemotherapy, symptoms of anxiety and depression (HADS), and perceived supportive care needs (31 items), along with computer-generated suggested strategies to manage each identified issue. Control: Results from computer survey not made available to oncologist. |
No significant difference after 4 visits between groups for change in HADS-D scores and proportion of patients with HADS-D ≥ 11. | Screening of multiple problems and perceived care needs did not allow assessment of the effect of distress screening. In addition, a positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. |
| Bramsen, 2008, The Netherlands [74] | Mixed | 129 |
Intervention: Patients were offered the possibility of psychosocial support by head nurse and information leaflet. Those who accepted were screened using a semi-structured interview with a checklist. Results were discussed in an interview, and patients were asked if they wanted a follow-up contact.. Control: Usual care with no screening. |
No significant difference between groups on EORTC QLQ-C30 emotional functioning subscale, IES total score or GHQ-12 total score. | Not a randomized controlled trial (sequential cohort design). A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment, which was based on whether patients requested it following an interview. |
| Carlson, 2010, Canada [26] | Lung and breast | 1,134 |
Full screening intervention: Results from DT, problem checklist, fatigue and pain thermometers, and PSSCAN, depression and anxiety sections, along with personalized feedback report placed on patient’s electronic medical record at initial visit. Triage intervention: Full screening, as described above, along with an offer to speak to a member of the study psychosocial team about any of the assessed issues. Control: DT completed, but results were not disclosed to patient or placed on electronic medical record. |
No difference between full screening intervention, triage intervention, or usual care groups on PSSCAN depression scores 3 months post-randomization. | Screening of multiple problems did not allow assessment of the effect of distress screening. |
| Detmar, 2002, Netherlands [59] | Mixed | 273b |
Intervention: Patients completed a quality of life questionnaire, the EORTC QLQ-30, at 3 successive outpatient visits with results made available to patient and physician prior to consultation. Control: Usual care. |
No difference between groups in SF-36 Mental Health subscale after 4th visit. | A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. |
| Grassi, 2011, Italy [61] | Mixed | 3,375c |
Intervention: Following a staff educational intervention, the DT was introduced into clinical practice, with referral to psycho-oncology services for assessment and intervention following positive screens (DT >4). Control: Physicians and nurses were able to refer patients to psycho-oncology services based on clinical judgment. |
Only proportion of patients referred to psycho-oncology services and characteristics of referred patients were reported, but not distress outcomes. | Not a randomized controlled trial. In addition, distress was not an outcome. Only proportion of patients referred to psycho-oncology services was reported. |
| Hilarius, 2008, Netherlands [58] | Mixed | 298 |
Intervention: Patients completed a quality of life questionnaire, the EORTC QLQ-30, at 4 outpatient visits, with summaries given to patients and nurses prior to consultation. Control: Standard consultations with physicians and nurses. |
No difference between groups in SF-36 Mental Health subscale at 4th visit. | Not a randomized controlled trial (sequential cohort design). In addition, a positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. |
| Ito, 2011, Japan [64] | Mixed | 998 |
Intervention: The pharmacist administering chemotherapy administered the DIT. Patients scoring above cutoff (≥4 distress; ≥3 impact) were recommended for consultation at psychiatry service. Those who refused were offered detailed information on self-management of mental health and were monitored. Feedback of distress results was provided on patients’ medical charts. Control: Usual care with no screening |
No difference in proportion of patients referred to Psychiatry Service. Shorter period between first chemotherapy visit and visit to Psychiatry Service for intervention group. | Not a randomized controlled trial (retrospective cohort design). In addition, distress was not an outcome. Outcomes were proportion of patients referred to psychiatry service and treated for depression or anxiety, and number of days from first chemotherapy visit to first visit to psychiatry service. |
| McLachlan, 2001, Australia [55] | Mixed | 450 |
Intervention: Patients at their first consultation completed a series of self-report questionnaires via touch-screen computer, including the CNQ, EORTC QLQ-C30, and BDI-SF. A summary of questionnaire results was made available to physicians prior to consultation, which were intended to be used to inform an individualized management plan. Control: Questionnaire responses were not made available to health care team prior to consultation. |
No significant difference between groups at 2 months or 6 months post-randomization for the CNQ psychological domain, BDI-SF scores or EORTC QLQ-C30 Emotional Functioningd | A positive depression screen based on a defined cutoff score was not used to determine who received further assessment or treatment. In addition, screening of multiple problems did not allow assessment of the effect of depression screening. |
| Rosenbloom, 2007, USA [56] | Mixed | 213 |
Assessment, Interview, and Discussion Intervention: At baseline, 1 and 2 months, patients completed FLIC and FACT-G, and FACT-G scores were elaborated through an interview and discussion, the results of which were shared with the treatment nurse prior to visit. Assessment intervention: At baseline, 1 and 2 months, patients completed FLIC and FACT-G, and FACT-G scores were shared with the treatment nurse prior to visit. Control: Patients completed FLIC at baseline. Questionnaire data not shared with the treatment nurse. |
No significant difference between groups at 3 months or 6 months post-randomization for Brief POMS negative mood subscale or FLIC psychological subscale scores. | A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. In addition, screening of multiple problems did not allow assessment of the effect of distress screening. |
| Sarna, 1998, USA [75] | Lung | 48 |
Intervention: Patients completed questionnaires at a number of times, including the SDS and HADS. A summary of results was given to the nurse, who identified problems and proposed interventions. Control: Patients completed questionnaires at a number of times, including the SDS and HADS. No summary of results was given to the nurse |
SDS scores increased for the control group, but did not increase for the intervention group. | A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. |
| Shimizu, 2010, Japan [63] | Mixede | 1,065 |
Intervention: Patients completed 11-point DIT (score range 0–10), and those with a distress score ≥ 4 and an impact score ≥ 3 were referred by their oncologist for a psycho-oncology service consultation. Control: Usual care with referral to psycho-oncology services by physician of patients considered moderately or severely distressed. |
Only number of positive screens and number diagnosed and treated, but not depression outcomes, were assessed. | Not a randomized controlled trial (sequential cohort design). In addition, outcomes included number of positive screens and number treated, but no distress outcomes were assessed. |
| Taenzer, 2000, Canada [60] | Lung | 57 |
Intervention: At a single clinic visit, patients completed the EORTC QLQ-C30, which was provided to clinic staff prior to clinic appointment with no specific instructions for use. Control: Patients completed the EORTC QLQ-C30 |
Only number of quality of life issues addressed in appointment and patients satisfaction, but no depression outcomes, were assessed. | Not a randomized controlled trial (sequential cohort design). In addition, a positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment and no distress outcomes were assessed. |
| Thewes, 2009, Australia [62] | Mixed | 83 |
Intervention: Patients completed the DT, and nursing staff was encouraged to assess problems and discuss psychosocial referral for patients with DT score ≥ 5. Control: Usual care with no screening. |
Contrary to hypothesis, patients in the screened group reported significantly higher level of unmet needs 6 months after initial clinic contact. | Not a randomized controlled trial (sequential cohort design). Outcome of unmet psychosocial needs, but not distress. |
| Velikova, 2004, UK [56] | Mixed | 286 |
Assessment and Feedback Intervention: For a 6 month study period, prior to clinic visits, patients completed the EORTC QLQ-C30 and HADS with results provided to physicians prior to visit. Attention Control: For a 6 month study period, prior to clinic visits, patients completed the EORTC QLQ-C30 and HADS with no results provided to physicians. Usual Care Control: Patients did not complete EORTC QLQ-C30 or HADS. |
Scores on FACT-Emotional Subscale were better in the intervention group than the usual care group, but not different from the attention control group. | A positive distress screen based on a defined cutoff score was not used to determine who received further assessment or treatment. In addition, screening of multiple problems did not allow assessment of the effect of distress screening. |
Abbreviations: BDI-SF = Beck Depression Inventory - Short Form; CNQ = Cancer Needs Questionnaire; DIT = Distress and Impact Thermometer; DT = Distress Thermometer; EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30; ESRA-C = Electronic Self-report Assessment - Cancer; FACT - emotional = Functional Assessment of Cancer Therapy - emotional; FACT-G = Functional Assessment of Cancer Therapy - General; FLIC = Functional Living Index - Cancer; GHQ = General Health Questionnaire; HADS = Hospital Anxiety and Depression Scale; HADS-D = Depression subscale of Hospital Anxiety and Depression scale; IES = Impact of Event Scale; MDD = Major depressive disorder; PHQ = Patient Health Questionnaire; PL = Problem List; POMS = Profile of Mood States; PSI = Psychiatric Symptom Index; PSSCAN = Psychological Screen for Cancer; SDS = Symptom Distress Scale; SF-36 = Short Form - 36 Health Survey Questionnaire.
Number consented for non-randomized controlled trials and number randomized for randomized controlled trials.
Physicians, rather than patients, were randomized. This number is the number of eligible patients who agreed to participate.
Includes 2,268 newly diagnosed cancer patients seen in an oncology department prior to introducing a DT and 1,107 following introduction of the DT.
The authors reported a post-hoc subgroup analysis that found significantly improved BDI-SF scores for the 44 patients in the intervention group with baseline BDI-SF scores ≥ 8 compared to the 19 control patients with BDI SF ≥ 8. However, patients were not randomized based on BDI-SF scores, and the relevance of these results for screening is not clear, since screening is applied to all patients, not only patients identified through screening with high scores.
95% of patients were female.
DISCUSSION
Several clinical recommendations [4–6] have been made for screening for psychological distress to be part of standard cancer care. Guidelines and recommendations, however, vary in the degree to which they are evidence-based [53] and none of these recommendation statements have been based on a systematic review that found benefits from screening, defined according to standard definitions.
There are well-established procedures for evaluating screening programs [8,16,17]. The principal criterion is whether there is evidence from well-conducted RCTs that benefits from screening outweigh possible harms (e.g., economic costs, drug side effects). The main findings of this systematic review are that (1) treatment of distress with pharmacological or behavioral interventions can improve psychological distress in adult cancer patients with psychological distress; and that (2) only 1 RCT of distress screening, with screening defined based on standard definitions of medical screening has been conducted with adult cancer patients. In that study [52] of telephone screening for psychological distress among newly diagnosed breast cancer patients, monthly telephone screening did not improve psychological distress. The authors of that study concluded that a brief psychosocial intervention, which was provided as part of standard care, may have reduced distress and reduced the potential impact of screening. Additionally, the fact that 80% of patients in that study had at least 1 positive screen in a 12-month period suggests that screening may not have effectively identified patients with substantially elevated distress.
Several reviews on screening for distress in cancer patients have been published previously [10–12] and they each concluded that there was not evidence that distress screening improved distress outcomes among cancer patients. Two of these reviews included 7 studies [10,12], and one included 14 studies [11]. The authors of those studies were consistent in arguing that evidence for benefits of screening for distress on patient outcomes in cancer patients is inconclusive and scarce and in calling for high-quality trials to determine if distress screening would improve patient outcomes.
Two of the reviews [10–11] concluded that there is evidence that the use of distress questionnaires may improve communication about psychosocial issues between patients and oncology staff. It is important to keep in mind, however, that using questionnaires to facilitate conversations with patients, while potentially helpful, is not screening and does not inform the question of whether screening with these tools to determine who receives subsequent assessment will benefit patients. Consistent with this, a major shortcoming of previous reviews on distress screening [10–12] is that they all included studies that would not be considered trials of screening interventions in the context of any standard definition of medical screening. Indeed, with the exception of 1 study [52], all of the studies included in these reviews were excluded from the current review for a number of reasons (see Table 4 for excluded distress screening studies). Five studies [26,54–57] screened for multiple problems at the same time (i.e., fatigue, pain, perceived support, and psychological distress), which made it impossible to assess the specific effects of screening for psychological distress. One of those studies [26] screened simultaneously for multiple problems with substantially different possible care responses (e.g., psychological distress, pain, fatigue, weight change, transportation, parking, drug coverage, finances). It was not possible, however, to determine in this study how many patients screened positive for psychological distress versus other practical or logistical issues, such as difficulties with transportation, parking, drug coverage, or finances, none of which would be best managed through psychological intervention. Six studies [55–60] did not use a defined cutoff score to indicate a positive screen for heightened distress or to determine which patients would receive further assessment or treatment. In addition, 6 of the studies [58,60–64] were not RCTs, but were, for example, sequential cohort designs. Finally, 3 of the studies [62–64] did not assess distress as an outcome, but investigated other outcomes, such as referral rates.
Distress screening can benefit patients only to the extent that it identifies patients with significant psychological distress who are not already recognized as distressed or receiving supportive services, successfully engages those patients in treatment, and achieves positive treatment results. In many cancer care settings, however, high numbers of patients are already treated with antidepressants as an attempt to address distress, even though many of these patients do not have depression or a history of depression [65]. Furthermore, as illustrated by one study from Austria [66], the desire for psychosocial support to cope with cancer may not be correlated with distress levels, and nearly as many patients with low levels of distress may desire supportive care as patients above the cutoff criterion on a screening tool. Thus, better patient psychosocial care may be best achieved by providing more information and coordinating care pathways, rather than seeking to automate triage processes through mechanized screening and numerical algorithms.
Beyond screening for distress in cancer care settings, a number of other systematic reviews have concluded that there are no RCTs that have shown that depression screening improves depressive symptoms in cancer [13], cardiovascular disease [67], or perinatal care [68]. A 2008 meta-analysis of depression screening in primary care [69] reviewed 11 trials and found several trials where screening increased identification or treatment of depression, but none where screening improved depression outcomes. The U.S. Preventive Services Task Force has recommended depression screening in primary care [70], but specifies that screening should only occur when integrated depression care systems for evaluation and case management are available. No trials, however, have shown that patients screened and referred for such collaborative care would have better outcomes than patients who are not screened, but who could potentially access collaborative care via other pathways [9]. This was an important reason why the UK National Institute of Clinical Excellence [71] did not recommend routine depression screening in primary care.
Given the current lack of evidence for benefits of distress screening, potential costs from implementing such a program must be carefully considered. An important concern is that routine screening would either take time or consume resources that could be devoted to other patient needs. Some might assume that screening questionnaires are easily and inexpensively implemented. However, this confuses the cost of administering a questionnaire and the cost of screening. The cost of screening includes assessments, consultations, treatment and follow-up services, which is much larger than the cost of administering a questionnaire [7,8].
Another concern is that attention and potentially limited mental health resources could be devoted only to those who screen positive for distress even though many other patients might like to discuss their psychosocial needs or might have self-referred or been referred by their clinicians. It is important that the psychological needs of cancer patients are recognized and addressed, and there are many alternatives to screening to meet this need. As long as there is no evidence that screening leads to improvements in distress, focusing on the availability and implementation of psychosocial support might better benefit cancer patients.
Without high-quality evidence from well-designed RCTs that demonstrate sufficient benefit to justify costs and potential harms from screening, recommendations for implementation of screening programs are premature. Research is needed that compares the benefits and harms of screening for psychological distress in trials in which patients in the screening group may access psychosocial resources via screening or other referral processes and patients in the non-screened group can access the same services via self- or other referral processes. Trials should clearly differentiate psychosocial needs that are best managed in the context of mental health services versus practical or logistical issues that are best addressed via other mechanisms (e.g., parking, insurance). They should also differentiate problems, such as fatigue and pain, which may or may not be related to psychological issues and for which first-line interventions are usually not psychological, from psychological distress.
Acknowledgments
The authors thank Dr. Roy C. Ziegelstein of the Johns Hopkins University School of Medicine, Baltimore, Maryland, USA for helpful comments on an earlier version of this article. He was not compensated for his contribution.
FUNDING/SUPPORT
This research was supported by a grant from the Canadian Institutes for Health Research (KRS 108456; PI Thombs). Ms. Meijer was supported by a VIDI grant from the Dutch Medical Research Council (grant 016.086.397). Ms. Roseman was supported by a Master’s Training Award from the Fonds de la recherche en santé Québec, a McGill University Provost’s Graduate Fellowship and a McGill University Principal’s Graduate Fellowship. Ms. Delisle was supported by a Master’s Training Award from the Fonds de la recherche en santé Québec, and McGill University Graduate Studies Fellowship. Ms. Milette’s work was supported by a Master’s training award from the Fond de la Recherche en Santé du Québec, a McGill University CIBC Fellowship, and a McGill University Maysie MacSporran Graduate Studentship. Dr. Thombs was supported by a New Investigator Award from the Canadian Institutes of Health Research and an Établissement de Jeunes Chercheurs award from the Fonds de la Recherche en Santé Québec. No funding body had any input into any aspect of the study.
Appendix 1. Search strategy for Review Questions #1 and #2
Pubmed
(Depression [MeSH] OR “depressive disorder” [MeSH] OR “major depressive disorder” [MeSH] OR distress [tiab] OR anxiety [MeSH]) OR “quality-of-life” [title]) AND (“mass screening” [MeSH] OR screen* [tiab] OR assess* [tiab] OR “drug therapy” [MeSH] OR “antidepressive agents” [MeSH] OR antidepress* [tiab] OR SSRI [tiab] OR anti-anxiety agents [MeSH] OR psychotherapy [MeSH] OR psychologic [tiab] OR treatment [tiab]OR “treatment outcome” [MeSH]) AND (cancer [MeSH] OR neoplasms [MeSH] OR malignancy [tiab] OR tumor [tiab] OR tumour [tiab] OR oncolog* [tiab])
Humans, clinical trial, randomized controlled trial, all adults: 19+ years
Cochrane
-
#1
MeSH descriptor depressive disorder explode all trees
-
#2
MeSH descriptor depression
-
#3
MeSH descriptor anxiety explode all trees
-
#4
distress: ti,ab,kw
-
#5
anxiety: ti,ab,kw
-
#6
“quality-of-life”: ti,ab,kw
-
#7
(#1 OR #2 OR #3 OR #4 OR #5 OR #6)
-
#8
MeSH descriptor mass screening explode all trees
-
#9
MeSH descriptor psychotherapy explode all trees
-
#10
MeSH descriptor treatment outcome explode all trees
-
#11
MeSH descriptor antidepressive agents explode all trees
-
#12
MeSH descriptor anti-anxiety agents explode all trees
-
#13
assess*: ti,ab,kw
-
#14
screen*: ti,ab,kw
-
#15
antidepress*: ti,ab,kw
-
#16
psychotherapy: ti,ab,kw
-
#17
psychological: ti,ab,kw
-
#18
(#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17)
-
#19
MeSH descriptor neoplasms explode all trees
-
#20
cancer: ti,ab,kw
-
#21
tumor: ti,ab,kw
-
#22
tumour: ti,ab,kw
-
#23
oncol*: ti,ab,kw
-
#24
(# 19 OR #20 OR #21 OR #22 OR #23)
-
#25
(#7 AND #18 AND #24)
-
#26
(randomized AND controlled AND trial): publ.type
-
#27
(#25 AND #26)
PsycINFO
| S1: | 1. “major depression” MM | |
| OR | 2. “depression (emotion)” MM | |
| OR | 3. depress* TI | |
| OR | 4. distress MM | |
| OR | 5. distress TI | |
| OR | 6. anxiety MJ | |
| OR | 7. anxiety TI | |
| OR | 8. quality of life MJ | |
| OR | 9. quality of life TI | |
| S2: | 1. “screening tests” MM | |
| OR | 2. screening MM | |
| OR | 3. screen* TI | |
| OR | 4. screen* AB | |
| OR | 5. assess* TI | |
| OR | 6. treatment MJ | |
| OR | 7. intervention TI | |
| OR | 8. intervention AB | |
| OR | 9. antidepressant drugs MM | |
| OR | 10. antidepress* TI | |
| OR | 11. drug therapy MM | |
| S3: | 1. neoplasms MJ | |
| OR | 2. cancer TI | |
| OR | 3. cancer AB | |
| OR | 4. tumor TI | |
| OR | 5. tumor AB | |
| OR | 6. tumour TI | |
| OR | 7. tumour AB | |
| OR | 8. oncol* TI |
Limit to humans, adulthood (18yrs & older) Treatment outcome / clinical trial
CINAHL
| S1: | 1. depression MM | |
| OR | 2. depress* TI | |
| OR | 3. depress* AB | |
| OR | 4. distress MJ | |
| OR | 5. distress TI | |
| OR | 6. distress AB | |
| OR | 7. anxiety MM | |
| OR | 8. anxiety TI | |
| OR | 9. anxiety AB | |
| OR | 10. “quality-of-life” MM | |
| OR | 11. “quality-of-life” TI | |
| S2: | 1. screening MJ | |
| OR | 2. screen* TI | |
| OR | 3. assess* TI | |
| OR | 4. psychotherapy MJ | |
| OR | 5. treatment TI | |
| OR | 6. therapy TI | |
| OR | 7. intervention TI | |
| S3: | 1. neoplasms MM | |
| OR | 2. cancer TI | |
| OR | 3. cancer AB | |
| OR | 4. tumor TI | |
| OR | 5. tumour TI | |
| OR | 6. oncol* TI |
Limit to humans, exclude Medline, all adult
| S4: | S1 AND S2 AND S3 |
Embase
depression/mj OR “distress syndrome”/mj OR distress:ti,ab OR anxiety/mj OR anxiety:ti,ab OR ‘quality of life’/exp/mj
screening/mj OR screen*:ti,ab OR assess*:ti,ab OR therapy/mj OR “intervention study”/mj OR “antidepressant agent”/mj OR antidepress*:ti,ab OR psychotherapy/mj OR treatment:ti,ab
neoplasm/mj OR cancer:ti,ab OR tumor:ti,ab OR tumour:ti,ab OR oncol*:ti,ab
1 and 2 and 3
Map to preferred terminology, include sub-terms/derivatives (explosion search), search terms must be of major focus in articles found, humans, adult and aged (18 to 64 and 65+ years), controlled clinical trial, randomized controlled trial, Embase only.
ISI
TS=(major depressive disorder) OR TS=depression OR TS=distress OR TI=distress OR TS=anxiety OR TI=anxiety OR TI=(quality of life)
TS=screening OR TI=screen* OR TI=assess* OR TS=drug therapy OR TI=intervention OR TI=treatment OR TI=pharmacological OR TI=psychological OR TI=antidepress* OR TI=psychotherapy OR TI=effect*
TS=neoplasms OR TI=neoplasm* OR TI=malignan* OR TI=cancer OR TI=tumor OR TI=tumour OR TI=oncol*
TS=controlled
#1 AND #2 AND #3 AND #4
Scopus
TITLE-ABS-KEY (“major depressive disorder” OR depress* OR distress OR anxiety OR “quality of life”) AND TITLE-ABS-KEY (screen* OR assess* OR treatment OR “drug therapy” OR intervention OR antidepress* OR psychotherapy OR treatment OR psychologic*)AND TITLE-ABS-KEY (neoplasm* OR cancer OR malignan* OR tumor OR tumour OR oncol*) AND (TITLE-ABS-KEY (randomized OR controlled OR trial))
Appendix 2. Relevant systematic reviews
Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev. 2008(2):CD005537.
Bidstrup PE, Johansen C, Mitchell AJ. Screening for cancer-related distress: Summary of evidence from tools to programmes. Acta Oncol. 2011;50(2):194–204.
Blake-Mortimer J, Gore-Felton C, Kimerling R, Turner-Cobb JM, Spiegel D. Improving the quality and quantity of life among patients with cancer: A review of the effectiveness of group psychotherapy. Eur J Cancer. 1999;35(11):1581–1586.
Bottomley A. Group cognitive behavioural therapy interventions with cancer patients: A review of the literature. European Journal of Cancer Care. 1996;5:143–146.
Bottomley A. Where are we now? evaluating two decades of group interventions with adult cancer patients. J Psychiatr Ment Health Nurs. 1997;4(4):251–265.
Carlson LE, Clifford SS, Groff SL, Maciejewski O, Bultz BD. Screening for depression in cancer care. In: Mitchell AJ, Coyne JC, editors. Screening for Depression in Clinical Practice: An Evidence-Based Guide. 2009. New York, NY: Oxford University Press. pp. 265–295
Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: Review and recommendations. J Clin Oncol. 2012;30(11):1160–1177.
Devine EC, Westlake SK. The effects of psychoeducational care provided to adults with cancer: Meta-analysis of 116 studies. Oncol Nurs Forum. 1995;22(9):1369–1381.
Edwards AG, Hulbert-Williams N, Neal RD. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev. 2008;(3)(3):CD004253.
Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: A review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30(2):112.
Fawzy FI, Fawzy NW, Arndt LA, Pasnau RO. Critical review of psychosocial interventions in cancer care. Arch Gen Psychiatry. 1995;52(2):100–113.
Fawzy FI, Fawzy NW. Group therapy in the cancer setting. J Psychosom Res. 1998;45(3):191–200.
Hart SL, Hoyt MA, Diefenbach M, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. JNCI. [Epub ahead of print].
Irving G, Lloyd-Williams M. Depression in advanced cancer. European Journal of Oncology Nursing. 2010;14(5):395–399.
Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. Ca-a Cancer Journal for Clinicians. 2008;58(4):214–230.
Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: A meta-analysis. Psycho-Oncology. 2009;18(6):571.
Lepore SJ, Coyne JC. Psychological interventions for distress in cancer patients: A review of reviews. Annals of Behavioral Medicine. 2006;32(2):85–92.
Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: A meta-analytical review. Psycho-Oncology. 2001;10(6):490.
Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: A meta-analysis of randomized experiments. Health Psychol. 1995;14(2):101–108.
Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. Journal of the National Cancer Institute. 2002;1994(8):558.
Newport DJ, Nemeroff CB. Assessment and treatment of depression in the cancer patient. J Psychosom Res. 1998;45(3):215–237.
Ng CG, Boks MP, Zainal NZ, de Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord. 2010.
Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. Int J Psychiatry Med. 2006;36(1):13–34.
Appendix 3. Journals Included in Manual Searching
Acta Psychiatrica Scandinavica
American Journal of Medicine
American Journal of Psychiatry
Annals of Behavioral Medicine
Annals of Family Medicine
Annals of Internal Medicine
Archives of General Psychiatry
Archives of Internal Medicine
Australian and New Zealand Journal of Psychiatry
Biological Psychiatry
BMC Psychiatry
British Journal of Psychiatry
British Medical Journal
CA: A Cancer Journal for Clinicians
Cancer
Canadian Journal of Psychiatry
Canadian Medical Association Journal
Depression and Anxiety
European Psychiatry
General Hospital Psychiatry
Health Psychology
Herz
JAMA
Journal of Abnormal Psychology
Journal of Affective Disorders
Journal of Behavioral Medicine
Journal of Cancer Survivorship
Journal of Clinical Oncology
Journal of Clinical Psychiatry
Journal of Clinical Psychology
Journal of the National Comprehensive Cancer Network: JNCCN
Journal of General Internal Medicine
Journal of Psychosomatic Research
Journal of Supportive Oncology
Lancet
New England Journal of Medicine
New Zealand Medical Journal
Psychiatry Research
Psychological Assessment
Psychological Bulletin
Psychological Medicine
Psycho-oncology
Psychosomatic Medicine
Psychosomatics
Psychotherapy and Psychosomatics
Appendix 4. Coding Manual
Review Question #1: Distress treatment
Original data
The article must be an original report of a study, and not, for example, a letter, editorial, systematic review or meta-analysis, or a case series or case report study.
(Adult) cancer
The study sample must consist of cancer patients or survivors of cancer and not, for example, concern partners of cancer patients. When the sample includes cancer patients as well as other patients, data for cancer patients must be separately reported. Only studies on adult patients (≥ 18 years) will be included.
RCT of distress reducing intervention
The study needs to be a randomized controlled trial of treatment designed to reduce general or psychological distress as opposed to medical treatments aimed primarily at treating a physical symptom (e.g., pain, fatigue). Studies can also address treatment-specific distress, such as distress related to chemotherapy or radiation therapy. Treatments can be pharmacological, psychotherapeutic, or other. A distress treatment group has to be compared to a control group. Studies that are head-to-head comparison studies of two active treatments are not included. Only studies with placebo, standard care, or attention control are included. Studies with enhanced standard care (such as providing information to patients and/or physicians) can be included. Studies with control groups in which there is any active intervention, such as getting attention from a provider even if the attention was hypothesized to be inert, are excluded. Distress must be an outcome of the trial. Distress outcome measures can be any measure of general mental health, distress, or depression.
The following paradigm is a guide for deciding whether or not an intervention is intended to reduce distress. If a study meets at least one of the following 3 criteria, we would count it as an intervention designed to reduce psychological distress:
The declared primary outcome is psychological distress (e.g., symptoms of distress, depression, anxiety, mental health function), and the intervention is not a medical treatment aimed primarily at treating the cancer (e.g., chemotherapy). Note: If a study claims that its primary objective/outcome is to improve survival via reducing psychological distress, then count this as an intervention designed to reduce psychological distress.
There are multiple outcomes declared without identification of a primary outcome, some of which are psychological and some of which are not primarily psychological (e.g., physical health or quality of life, fatigue, pain). However, the mechanism of the intervention is known to primarily target cognitions and behaviours related to mood/psychological distress or to target physiological indices of stress that are known to be related to mood/psychological distress. Examples of interventions whose mechanism is known to primarily target cognitions and behaviours related to mood/psychological distress include psychological therapies (e.g., CBT, psychodynamic therapy, behavioural therapy, expressive writing) that can be delivered via a variety of mechanisms (psychotherapy, bibliotherapy, online resources, group delivery). Coping oriented interventions would be included, as well, as coping implies a psychological component. Examples of interventions that target physiological indices of stress that are known to be related to mood/psychological distress include relaxation training, hypnosis, imagery/guided imagery, stress management, breathing training). Examples of interventions that would not meet this definition include exercise, yoga, enhanced nursing care. Note however, that all of these interventions could be included if they meet criterion 1 or 3.
Criteria #1 (primary outcome) and #2 (intervention characteristics) are not met, but entry into the trial depends on meeting a threshold criteria for psychological distress, Examples of interventions in this category might include exercise, yoga, and enhanced nursing care.
Minimum level of distress
In addition, the study must include patients with a minimum level of general, psychological or emotional distress and must exclude patients scoring below that level, or studies must perform separate analyses on patients with distress scores above a cutoff level. Inclusion standards may include a self-report questionnaire or a clinical interview (structured or unstructured) for depression or anxiety disorders. Studies that do not provide separate analyses for patients above a distress cutoff, but, instead, analyze the association between distress and treatment outcome continuously are excluded. Authors will not be contacted for original data if the sample was not dichotomized in the study.
Sample size
There must be at least 25 subjects randomized to each group (distressed vs. non-distressed).
Complete distress outcome data
Outcomes have to be continuous, or a dichotomous response or remission outcome based on defined criteria must be reported.
Review Question #2: Distress screening
Original data
The article must be an original report of a study, and not, for example, a letter, editorial, systematic review or meta-analysis, or a case series or case report study.
(Adult) cancer
The study sample must consist of cancer patients or survivors of cancer and not, for example, concern partners of cancer patients. When the sample includes cancer patients as well as other patients, data for cancer patients must be separately reported. Only studies on adult patients (≥ 18 years) will be included.
RCT of screening for distress
The study needs to be a randomized controlled trial in which the intervention group patients are screened for distress with any measure or screening method and the control group is not screened. A cutoff on a distress screening tool that would be used to identify possible cases and make decisions regarding further assessment or treatment needs to be defined a priori. Studies in which questionnaire results were provided to clinicians without guidance on cutoff scores to determine positive screening status are also excluded. Studies in which both intervention and control groups received the same psychosocial services, but service providers in the intervention group had access to results from psychosocial questionnaires that may have informed their interactions, but did not necessarily determine service allocation decisions, are excluded. Studies that administered multiple screening tools for multiple problems may be included if all of the measures have defined cutoffs for positive screens and all are screens for psychological or general distress. General or psychological distress must be an outcome of the study. Distress outcome measures can be any measure of general mental health, distress, or depression. When distress is measured, but is not an outcome variable of the study (but a predictor or mediator, etc.) studies are excluded.
Appendix 5. Variables included in data extraction form
| First author |
| Year |
| Country |
| Cancer site / description |
| Distress inclusion criterion and cutoff threshold |
| Treatment condition |
| Control condition |
| N randomized, n treatment, n control |
| Mean age |
| Percentage males |
| Number and percentage lost to follow-up |
| Treatment duration |
| Distress outcomes (continuous primary and secondary outcomes): |
| Hedges’s g (95% CI) and r2 |
| Study funding source |
Appendix 6. Cochrane Risk of Bias Tool
Sequence generation
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment
Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Blinding of participants, personnel and outcome assessors
Assessments should be made for each main outcome (or class of outcomes). Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective.
Incomplete outcome data
Assessments should be made for each main outcome (or class of outcomes). Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re-inclusions in analyses performed by the review authors.
Selective outcome reporting
State how the possibility of selective outcome reporting was examined by the review authors, and what was found.
Pharmaceutical industry funding
State the funding source(s) of the trial, or indicate if the trial funding source was not reported.
Author-industry financial ties and/or employment
State whether any trial authors disclosed financial ties and/or employment by the pharmaceutical industry, or if author-industry financial ties or affiliation were not reported.
Other sources of bias
State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre-specified in the review’s protocol, responses should be provided for each question/entry.
Footnotes
FINANCIAL DISCLOSURES
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare that Dr. Stewart is on the Depression Global Advisory Board and Cymbalta Pregnancy Registry Scientific Advisory Committee for Eli Lilly and received support from Ranbaxy Labs in 2012 to travel to Thailand to teach on women’s mental health. No other authors have any conflict of interest disclosures for the past 3-year reporting period.
References
- 1.Holland JC, Bultz BD National Comprehensive Cancer Network (NCCN) The NCCN guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5:3–7. [PubMed] [Google Scholar]
- 2.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Bultz BD, Holland JC. Emotional distress in patients with cancer: the sixth vital sign. Community Oncology. 2006;3:311–314. [Google Scholar]
- 4.National Institute for Clinical Excellence. Guideline on cancer services: Improving supportive and palliative care for adults with cancer. NICE; 2004. [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. NCCN; 2008. Distress management. [Google Scholar]
- 6.Institute of Medicine. Cancer care for the whole patient: Meeting psychosocial health needs. Institute of Medicine; 2007. [Google Scholar]
- 7.UK National Screening Committee. Second report of the UK National Screening Committee. UK: UK National Screening Committee; 2000. [Google Scholar]
- 8.Raffle A, Gray M. Screening: Evidence and practice. UK: Oxford University Press; 2007. [Google Scholar]
- 9.Thombs BD, Coyne JC, Cuijpers P, de Jonge P, Gilbody S, Ioannidis JP, et al. Rethinking recommendations for screening for depression in primary care. CMAJ. 2012;184:413–418. doi: 10.1503/cmaj.111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson LE, Clifford SS, Groff SL, Maciejewski O, Bultz BD. Screening for Depression in Cancer Care. In: Mitchell AJ, Coyne JC, editors. Screening for Depression in Clinical Practice: An Evidence-Based Guide. New York, NY: Oxford University Press; 2009. pp. 265–295. [Google Scholar]
- 11.Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30:1160–1177. doi: 10.1200/JCO.2011.39.5509. [DOI] [PubMed] [Google Scholar]
- 12.Bidstrup PE, Johansen C, Mitchell AJ. Screening for cancer-related distress: Summary of evidence from tools to programmes. Acta Oncol. 2011;50:194–204. doi: 10.3109/0284186X.2010.533192. [DOI] [PubMed] [Google Scholar]
- 13.Meijer A, Roseman M, Milette K, Coyne JC, Stefanek ME, Ziegelstein RC, et al. Depression screening and patient outcomes in cancer: a systematic review. PLoS One. 2011;6:e27181. doi: 10.1371/journal.pone.0027181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U S. Preventive Services Task Force. Screening for depression: recommendations and rationale. Ann Intern Med. 2002;136:760–764. doi: 10.7326/0003-4819-136-10-200205210-00012. [DOI] [PubMed] [Google Scholar]
- 15.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JM, Jungner G. Principles and practices of screening for disease. Geneva: World Health Organization; 1968. [Google Scholar]
- 17.Shakespeare J. Evaluation of screening for postnatal depression against the NSC handbook criteria. 2001. [Google Scholar]
- 18.Bakkalbasi N, Bauer K, Glover J, Wang L. Three options for citation tracking: Google Scholar, Scopus and Web of Science. Biomed Digit Libr. 2006;3:7. doi: 10.1186/1742-5581-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov. [Accessed September 11, 2012]; http://www.clinicaltrials.gov.
- 20. [Accessed February 20, 2011];International Standard Randomised Controlled Trial Number Register. http://www.controlled-trials.com/isrctn.
- 21.Schneider S, Moyer A, Knapp-Oliver S, Sohl S, Cannella D, Targhetta V. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. J Behav Med. 2010;33:1–14. doi: 10.1007/s10865-009-9227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer HC, Gardner C, Brooks JO, III, Yesavage JA. Advantages of excluding underpowered studies in meta-analysis: inclusionist versus exclusionist viewpoints. Psychol Methods. 1998;3:23–31. [Google Scholar]
- 24.Coyne JC, Thombs BD, Hagedoorn M. Ain’t necessarily so: review and critique of recent meta-analyses of behavioral medicine interventions in health psychology. Health Psychol. 2010;29:107–116. doi: 10.1037/a0017633. [DOI] [PubMed] [Google Scholar]
- 25.Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196:173–178. doi: 10.1192/bjp.bp.109.066001. [DOI] [PubMed] [Google Scholar]
- 26.Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28:4884–4891. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Altman DG, Sterne JAC, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Accessed September 10, 2012]. Chapter 8: Assessing risk of bias in included studies. Version 5.1.0 [updated March 2011] Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 28.Roseman M, Milette K, Bero LA, Coyne JC, Lexchin J, Turner EH, et al. Reporting of conflicts of interest in meta-analyses of trials of pharmacological treatments. JAMA. 2011;305:1008–17. doi: 10.1001/jama.2011.257. [DOI] [PubMed] [Google Scholar]
- 29.Roseman M, Turner EH, Lexchin J, Coyne JC, Bero LA, Thombs BD. Reporting of conflict of interest from drug trials in Cochrane reviews: A cross-sectional study. BMJ. 2012;345:e5155. doi: 10.1136/bmj.e5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusin C, Yang H, Yeung A, Fava M. Rating scales for depression. In: Baer L, Blais M, editors. Handbook of clinical rating scales and assessment in psychiatry and mental health. Totowa, NJ: Humana Press; 2010. pp. 7–35. [Google Scholar]
- 31.Hedges LV. Estimation of effect size from a series of independent experiments. Psychol Bull. 1982;92:490–499. [Google Scholar]
- 32.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: A correlational approach. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 33.Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- 34.Costa D, Mogos I, Toma T. Efficacy and safety of mianserin in the treatment of depression of women with cancer. Acta Psychiatr Scand Suppl. 1985;320:85–92. doi: 10.1111/j.1600-0447.1985.tb08081.x. [DOI] [PubMed] [Google Scholar]
- 35.Ell K, Xie B, Quon B, Quinn DI, Dwight-Johnson M, Lee PJ. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans RL, Connis RT. Comparison of brief group therapies for depressed cancer patients receiving radiation treatment. Public Health Rep. 1995;110:306–311. [PMC free article] [PubMed] [Google Scholar]
- 37.Fann JR, Fan MY, Unutzer J. Improving primary care for older adults with cancer and depression. J Gen Intern Med. 2009;24 (Suppl 2):S417–24. doi: 10.1007/s11606-009-0999-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisch MJ, Loehrer PJ, Kristeller J, Passik S, Jung SH, Shen J, et al. Fluoxetine versus placebo in advanced cancer outpatients: a double-blinded trial of the Hoosier Oncology Group. J Clin Oncol. 2003;21:1937–1943. doi: 10.1200/JCO.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Greer S, Moorey S, Baruch JD, Watson M, Robertson BM, Mason A, et al. Adjuvant psychological therapy for patients with cancer: a prospective randomised trial. BMJ. 1992;304:675–680. doi: 10.1136/bmj.304.6828.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroenke K, Theobald D, Wu J, Norton K, Morrison G, Carpenter J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. JAMA. 2010;304:163–171. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moorey S, Cort E, Kapari M, Monroe B, Hansford P, Mannix K, et al. A cluster randomized controlled trial of cognitive behaviour therapy for common mental disorders in patients with advanced cancer. Psychol Med. 2009;39:713–723. doi: 10.1017/S0033291708004169. [DOI] [PubMed] [Google Scholar]
- 42.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 43.Razavi D, Allilaire JF, Smith M, Salimpour A, Verra M, Desclaux B, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatr Scand. 1996;94:205–210. doi: 10.1111/j.1600-0447.1996.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 44.Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372:40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson SM, Love SB, Westcombe AM, Gambles MA, Burgess CC, Cargill A, et al. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:532–539. doi: 10.1200/JCO.2006.08.9987. [DOI] [PubMed] [Google Scholar]
- 46.Dwight-Johnson M, Ell K, Lee PJ. Can collaborative care address the needs of low-income Latinas with comorbid depression and cancer? Results from a randomized pilot study. Psychosomatics. 2005;46:224–232. doi: 10.1176/appi.psy.46.3.224. [DOI] [PubMed] [Google Scholar]
- 47.van Heeringen K, Zivkov M. Pharmacological treatment of depression in cancer patients. A placebo-controlled study of mianserin. Br J Psychiatry. 1996;169:440–443. doi: 10.1192/bjp.169.4.440. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 49.Malouff JM, Thorsteinsson EB, Schutte NS. The efficacy of problem solving therapy in reducing mental and physical health problems: a meta-analysis. Clin Psychol Rev. 2007;27:46–57. doi: 10.1016/j.cpr.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 50.van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res. 2010;69:23–32. doi: 10.1016/j.jpsychores.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104:990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maunsell E, Brisson J, Deschenes L, Frasure-Smith N. Randomized trial of a psychologic distress screening program after breast cancer: effects on quality of life. J Clin Oncol. 1996;14:2747–2755. doi: 10.1200/JCO.1996.14.10.2747. [DOI] [PubMed] [Google Scholar]
- 53.McAlister FA, van Diepen S, Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007;4:e250. doi: 10.1371/journal.pmed.0040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyes A, Newell S, Girgis A, McElduff P, Sanson-Fisher R. Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? Eur J Cancer Care. 2006;15:163–171. doi: 10.1111/j.1365-2354.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 55.McLachlan SA, Allenby A, Matthews J, Wirth A, Kissane D, Bishop M, et al. Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. J Clin Oncol. 2001;19:4117–4125. doi: 10.1200/JCO.2001.19.21.4117. [DOI] [PubMed] [Google Scholar]
- 56.Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology. 2007;16:1069–1079. doi: 10.1002/pon.1184. [DOI] [PubMed] [Google Scholar]
- 57.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 58.Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK. Use of health-related quality-of-life assessments in daily clinical oncology nursing practice: a community hospital-based intervention study. Cancer. 2008;113:628–637. doi: 10.1002/cncr.23623. [DOI] [PubMed] [Google Scholar]
- 59.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 60.Taenzer P, Bultz BD, Carlson LE, Speca M, DeGagne T, Olson K, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology. 2000;9:203–213. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 61.Grassi L, Rossi E, Caruso R, Nanni MG, Pedrazzi S, Sofritti S, et al. Educational intervention in cancer outpatient clinics on routine screening for emotional distress: an observational study. Psychooncology. 2011;20:669–674. doi: 10.1002/pon.1944. [DOI] [PubMed] [Google Scholar]
- 62.Thewes B, Butow P, Stuart-Harris R Greater So Area Hth Serv Screening. Does routine psychological screening of newly diagnosed rural cancer patients lead to better patient outcomes? Results of a pilot study. Aust J Rural Health. 2009;17:298–304. doi: 10.1111/j.1440-1584.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu K, Ishibash Y, Umezawa S, Izumi H, Akizuki N, Ogawa A, et al. Feasibility and usefulness of the ‘Distress Screening Program in Ambulatory Care’ in clinical oncology practice. Psychooncology. 2010;19:718–725. doi: 10.1002/pon.1616. [DOI] [PubMed] [Google Scholar]
- 64.Ito T, Shimizu K, Ichida Y, Ishibashi Y, Akizuki N, Ogawa A, et al. Usefulness of pharmacist-assisted screening and psychiatric referral program for outpatients with cancer undergoing chemotherapy. Psychooncology. 2011;20:647–654. doi: 10.1002/pon.1945. [DOI] [PubMed] [Google Scholar]
- 65.Palmer SC, Taggi A, Demichele A, Coyne JC. Is screening effective in detecting untreated psychiatric disorders among newly diagnosed breast cancer patients? Cancer. 2012;118:2735–2743. doi: 10.1002/cncr.26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Söllner W, Maislinger S, König A, Devries A, Lukas P. Providing psychosocial support for breast cancer patients based on screening for distress within a consultation-liaison service. Psychooncology. 2004;13:893–897. doi: 10.1002/pon.867. [DOI] [PubMed] [Google Scholar]
- 67.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 68.Hewitt CE, Gilbody SM. Is it clinically and cost effective to screen for postnatal depression: a systematic review of controlled clinical trials and economic evidence. BJOG. 2009;116:1019–1027. doi: 10.1111/j.1471-0528.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- 69.Gilbody SD, Sheldon TD, House AD. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178:997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.U S. Preventive Services Task Force. Screening for depression in adults: U.S preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:784–792. doi: 10.7326/0003-4819-151-11-200912010-00006. [DOI] [PubMed] [Google Scholar]
- 71.National Collaborating Center for Mental Health. The NICE guideline on the management and treatment of depression in adults. 2010. (Updated edition) [Google Scholar]
- 72.Söllner W, DeVries A, Steixner E, Lukas P, Sprinzi G, Rumpold G, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84:179–185. doi: 10.1054/bjoc.2000.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berry DL, Blumenstein BA, Halpenny B, Wolpin S, Fann JR, Austin-Seymour M, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011;29:1029–1035. doi: 10.1200/JCO.2010.30.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bramsen I, van der Linden MH, Eskens FJ, Bijvank EM, van Groeningen CJ, Kaufman HJ, et al. Evaluation of a face-to-face psychosocial screening intervention for cancer patients: acceptance and effects on quality of life. Patient Educ Couns. 2008;70:61–68. doi: 10.1016/j.pec.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Sarna L. Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncol Nurs Forum. 1998;25:1041–1048. [PubMed] [Google Scholar]