Abstract
Poor muscle quality and sedentary behavior are risk factors for metabolic dysfunction in children and adolescents. However, because longitudinal data are scarce, relatively little is known about how changes in muscle quality and physical activity influence bone development.
Purpose
In a 2-year longitudinal study, we examined the effects of physical activity and changes in muscle quality on bone parameters in young girls.
Methods
The sample included 248 healthy girls aged 9–12 years at baseline. Peripheral quantitative computed tomography was used to measure calf and thigh muscle density, an indicator of skeletal muscle fat content or muscle quality, as well as bone parameters at diaphyseal and metaphyseal sites of the femur and tibia. Physical activity was assessed using a validated questionnaire specific for youth.
Results
After controlling for covariates in multiple regression models, increased calf muscle density was independently associated with greater gains in cortical (β = 0.13, P < 0.01) and trabecular (β = 0.25, P < 0.001) volumetric bone mineral density (vBMD) and the bone strength index (BSI; β = 0.25, P < 0.001) of the tibia. Importantly, these relationships were generalized, as similar changes were present at the femur. Associations between physical activity and changes in bone parameters were weaker than those observed for muscle density. Nevertheless, physical activity was significantly (all P < 0.05) associated with greater gains in trabecular vBMD and the BSI of the distal femur.
Conclusions
These findings suggest that poor muscle quality may put girls at risk for suboptimal bone development. Physical activity is associated with more optimal gains in weight-bearing bone density and strength in girls, but to a lesser extent than changes in muscle quality.
Keywords: MUSCLE DENSITY, EXERCISE, FEMALE, CHILDREN, BONE, PQCT
INTRODUCTION
Osteoporosis with its concomitant fractures is a major public health concern that will worsen as the population ages (34). Indeed, approximately 30–50% of women will suffer the consequences of fractures related to osteoporosis (34). Once considered only a geriatric condition, osteoporosis is now recognized, in part, as a pediatric disorder manifested later in life (5). Childhood and adolescence are periods of rapid skeletal adaptations when >90% of bone mineral is accrued (2). Furthermore, growth is the most opportune time to modify bone density and structure, the primary determinants of bone strength (31). Thus, identification of modifiable factors that influence bone development may be critical insofar as skeletal adaptations established early in life will likely predict peak bone strength and fracture risk later in life. A better understanding of the modifiable determinants of bone parameters during growth could inform the design of interventions which target lifestyle factors, with the aim of reducing the impact of skeletal deficits on future fracture risk.
One of the prevailing dogmas in the bone field is that bone density, structure and strength adapt to the mechanical loading environment. It is clear that forces from muscle contractions and gravitational loading must be of sufficient magnitude, be imposed at significant rates, and be dynamic in application to provide the necessary stimuli to drive skeletal adaptations (26). Physical activity can be designed to generate forces that meet these criteria; thus, there is considerable interest in the influence of physical activity on bone development. Plausible biological explanations have been proposed, supporting the peri-pubertal years as a “window of opportunity” for maximal skeletal adaptations in response to physical activity that are maintained into adulthood (21). Consistent with this premise, a number of studies in children and adolescents have reported that weight-bearing exercise enhances bone mass, density, and structure at various skeletal sites (14). For example, in a cross-sectional analysis of girls, we showed that physical activity was positively associated with more favorable geometric adaptations of the femur and tibia (6). In addition, physical activity can modify fat and skeletal muscle; soft tissue compartments which may have direct and indirect effects on bone development (28). Thus, physical activity, through its unique ability to subject bones to strains induced by gravitational loading and muscle contractions and its ability to alter muscle quality, has the potential to modify skeletal morphology during growth. However, relatively little is known about the magnitude of the effect of physical activity on changes in bone density, structure and strength during growth because few longitudinal studies exist.
The incidence of childhood obesity has never been higher (24), and the majority of obese adolescents will become obese adults (12). The abrupt increase in childhood obesity has been paralleled by a rising prevalence of type 2 diabetes mellitus (T2DM) (35), which is associated with a number of metabolic complications including hypertension, dyslipidemia, fatty liver disease, and systemic low-grade inflammation (35). Mounting evidence suggests that the pathogenesis of insulin resistance and T2DM is associated with skeletal muscle fat content in adults (35) and children (27), independent of overall adiposity. In addition, the replacement of skeletal muscle fibers by non-contractile components (e.g., lipids) results in a deterioration in muscle quality, which has been linked to muscle weakness (36) and poor physical function (11) in older adults. Furthermore, cross-sectional studies in older adults have demonstrated that this so-called “lipotoxicity” of skeletal muscle is predictive of hip fracture (18) and is negatively associated with volumetric bone mineral density (vBMD) of the tibia (40). Consistent with these observations, we showed that muscle density, an indicator of skeletal muscle fat content or muscle quality (11, 15), was inversely associated with vBMD and indices of bone strength at metaphyseal and diaphyseal sites of the femur and tibia in girls (8). Taken together, these studies suggest that poor muscle quality may be an important risk factor for bone health status, even as early as childhood and adolescence.
In a cohort of girls aged 9–12 years, we previously reported in cross-sectional analyses that physical activity is positively associated with more favorable bone structural parameters (6), whereas skeletal muscle fat content is negatively associated with vBMD and indices of bone strength (8). Furthermore, in the same cohort of girls, we recently showed that lower levels of physical activity were associated with lower muscle density (i.e., higher skeletal muscle fat content) (10). These cross-sectional findings suggest that reduced muscle density and lower levels of physical activity may put girls at risk for suboptimal bone development owing to suboptimal skeletal loading from poor muscle quality and insufficient physical activity. Knowing that longitudinal data on the influences of physical activity and muscle density on bone development are sparse (17, 37, 39), we examined the 2-year effects of physical activity and changes in muscle density on weight-bearing bone development in the same cohort of girls we studied at baseline (6, 8, 10). On the basis of our cross-sectional findings (6, 8, 10), we hypothesized that greater increases in physical activity and muscle density would be associated with greater gains in vBMD, bone structure, and strength of weight-bearing bones in girls over the course of 2 years.
METHODS
Study subjects
The protocol was approved by the University of Arizona Human Subjects Protection Committee and the study was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all guardians and subjects; written informed assent was obtained from all subjects. Details regarding the identification and recruitment of the subjects and the baseline bone, soft tissue, and physical activity characteristics of the study subjects have been published previously (6–8). In brief, we recruited 509 healthy girls in school grade 4 or 6 (age 9–12 years) from 14 elementary and 4 middle schools located in Tucson, Arizona as well as the surrounding area and enrolled them as participants the “Jump-In Building Better Bones study”. The primary aim of Jump-In was to evaluate the effects of a school-based exercise intervention on bone development in girls. The exercise intervention is described in detail in the section that follows. Study exclusion criteria included medications known to affect bone metabolism as well as a medical condition or disability that limited participation in physical exercise. Guardians completed a questionnaire that inquired about subject ethnicity and race. Reflecting the ethnic composition of the population of Tucson, AZ and the surrounding area, 23% of the sample was Hispanic and 77% was non-Hispanic. Sample race was 90% white, 6% Asian, 2% black or African American, 0.5% Native American or Alaska Native, 1% Native Hawaiian or other Pacific Islander, and 0.5% other. Because of motion artifact in 65 baseline pQCT scans, baseline bone and soft tissue composition data were only available on 444 girls (8). Of those girls, 248 returned for 2-year laboratory testing and had acceptable pQCT scans; these subjects were included in the present analysis. Physical activity data were collected on all subjects at baseline and at the 2-year study visit.
Exercise intervention
An exercise intervention, lasting 5–10 minutes/session, was delivered 3 times per week in physical education class and/or recess, depending on school schedules. The intervention continued over the entire 2 years and participants were encouraged to complete the intervention on their own time during school holidays. The intervention employed boxes from which the girls jumped and landed on the ground 40 times per session. Box height increased progressively from 6″ to 12″ to 18″ and ultimately to 24″ over the course of the intervention. Jumping and landing from these heights has been shown to elicit ground reaction forces of ~3–8 times body weight (33). Randomization (intervention/control) was included as a covariate in statistical models.
Clinical assessment
Anthropometric data were collected on all subjects. Weight was measured to the nearest 0.1 kg using a calibrated electronic scale (Seca, Model 881, Hamburg, Germany) and height was measured at full inhalation to the nearest mm using a stadiometer (Shorr Height Measuring Board, Olney, MD). Tibia length (nearest mm) was measured from the proximal end of the medial border of the tibial plateau to the distal edge of the medial malleolus. Femur length (nearest mm) was measured from the base of the patella to the inguinal crease. Baseline coefficients of variation (CVs) for tibia and femur lengths were 0.51% and 0.33%, respectively. Subjects self-reported their date of menarche and Tanner stage using a validated questionnaire (23) that has been shown to agree with physician exam and grading. Recognizing that the ability of Tanner staging to accurately assess maturation is limited (33), we also used an alternate index of maturation (maturity offset) based on estimated years from peak height velocity (PHV) using Mirwald’s equation (22), which was derived from data from a six-year longitudinal study in boys and girls (2). In Mirwald’s sample, the maturity offset equation for girls explained 89% of the variance in years from PHV (22).
Physical activity assessment
As described in detail previously (6, 9), the modified past year physical activity questionnaire (PYPAQ) was used to collect information about the average duration and frequency of physical activity participation. The PYPAQ has been validated in children and adolescents (1). In the present study, we used a modified version of the PYPAQ that has been shown to be positively associated with more favorable geometric adaptations of the femur and tibia in cross-sectional analyses of this cohort (6). We previously showed in this cohort (9), that the modified PYPAQ is a stronger predictor of bone strength than other measures of physical activity [i.e., 3-day physical activity recall questionnaire (3DPAR), pedometer, and the bone-specific physical activity questionnaire (BPAQ)]. Total modified PYPAQ score was computed using the validated equation from Shedd et al. (32): PYPAQ score = Σ1–n [duration (minutes/session) × frequency (days/week) × load (peak strain score (13))], where n was the number of activities a subject reported during the past year. Both change in PYPAQ score from baseline to 2-years and the average of the baseline and 2-year PYPAQ scores were used as continuous variables in statistical models.
Bone and soft tissue composition assessment
Detailed descriptions of the pQCT device and in vivo imaging processing and analysis protocol used in our laboratory have been published (8). Measurements were obtained at the distal 4% and 20% femur and 4% and 66% tibia sites relative to the respective distal growth plates of the non-dominant limb on all subjects using pQCT (XCT 3000; STRATEC Medizintechnik GmbH, Pforzheim, Germany, Division of Orthometrix; White Plains, NY). A scout scan was used to place a reference line at the distal growth plate of the femur and tibia, respectively, with the scanner programmed to subsequently find the sites of interest. All pQCT scans were analyzed using Stratec software, Version 6.0 and operators were trained for pQCT data acquisition and analyses following guidelines provided by Bone Diagnostics, Inc. (Fort Atkinson, WI). At the distal metaphyseal regions of the femur and tibia, Contour mode 3 (169 mg/cm3) was used to measure total bone and Peel mode 4 (650 mg/cm3 with a 10% peel) was used to ensure that only trabecular bone remained. Recognizing the difficulty in interpreting metaphyseal bone density measurements from a single slice (19), we averaged three pQCT slices taken at the 4% femur and 4% tibia regions. At the diaphyseal 20% femur and 66% tibia sites, Contour mode 1 (710 mg/cm3) and Cort mode 2 (710 mg/cm3) were used. Further details on image processing, calculations, and analyses have been described previously (8). Slice thicknesses were 2.3 mm and voxel sizes were set at 0.4 mm. Scanner speed was set at 25 mm/second. Bone strength index (BSI, mg2/mm4), calculated as described by Kontulainen (16), was assessed at the 4% femur and 4% tibia sites; strength-strain index (SSI, mm3) was assessed at the 20% femur and 66% tibia sites using the manufacturer’s software as described previously (8). BSI estimates the bone’s ability to withstand compression forces at metaphyseal sites, while SSI is used to estimate the bone’s ability to resist torsion and bending forces at diaphyseal sites. Reproducibility in our laboratory was <1.1% for vBMD, bone geometry, and indices of bone strength (BSI, SSI) (7).
As described in detail previously (8), at the 20% femur and 66% tibia sites, regional soft tissue composition was assessed using edge detection and threshold techniques to separate adipose, muscle, and bone based on attenuation characteristics, which are directly related to tissue composition and density (11, 15). Images were filtered prior to being analyzed using Contour mode 3 (−101 mg/cm3) and Peel mode 2 (40 mg/cm3) to separate adipose (<40 mg/cm3) and muscle/bone (≥40 mg/cm3), respectively. Images were subsequently filtered with a 7 × 7 image filter that clearly defined the edge of the muscle and eliminated all bone above 120 mg/cm3, ensuring that muscle density was a direct result of the soft tissue within the edge of the muscle. Reproducibility in our lab was <1.4% for calf and thigh muscle density (mg/cm3) and muscle cross-sectional area (MCSA, mm2) (8). Total body mass, total body fat mass, and total body percent fat were obtained from whole-body DXA scans using the GE Lunar Prodigy (software version 5.60.003) fan-beam densitometer (GE Lunar Corp, Madison, WI). Subjects were positioned following standard GE/Lunar protocols.
Statistical analysis
All variables were tested for skewness and kurtosis; plots and multivariable regression models were used to check the data for normality, linearity, outliers, and potential influential observations. Pearson’s correlations were used to examine the relationships between the physical activity variables and the 2-year changes in muscle density of the calf and thigh. Multivariate regression models were used to examine the independent associations of physical activity (average and 2-year change) and 2-year changes in muscle density of the calf and thigh with 2-year changes in bone parameters. The 2-year change in the bone parameter was the dependent variable and each model included the following covariates: baseline bone parameter, ethnicity, randomization, maturity offset (baseline and 2-year change), physical activity (average and 2-year change), as well as the 2-year changes in muscle density, MCSA, subcutaneous fat, and bone length. Thigh soft tissue composition variables were used in all models that included femur bone parameters, whereas calf soft tissue composition variables were included in all models that included tibia bone parameters. All models satisfied the requirements for multiple linear regression analyses. In addition to analyzing the data from all girls combined, we also performed analyses separately in pubertal and randomization groups (data not shown) and explored maturity and randomization interactions with all independent variables in each of the combined models. Given that the patterns were essentially identical for the stratified and combined analyses and that no interactions or effect modifications were present for any of the independent variables, we show the combined results for more straightforward interpretation. A linear mixed effects model that included the same covariates as above, with the exception of muscle density, was used to compare the 2-year changes in bone parameters across quintiles (i.e., fifths) of 2-year change in muscle density of the calf and thigh, respectively. Bone parameters were similar among the middle 3 fifths of 2-year changes in muscle density of the calf and thigh; thus, we collapsed the middle 3 fifths into a single group. In addition, we repeated all analyses substituting maturity offset with Tanner stage. A P value of < 0.05 was considered statistically significant. All analyses were performed using The Statistical Package for the Social Sciences for Windows, Version 20.0 (SPSS, Chicago, IL).
RESULTS
The baseline and 2-year descriptive characteristics of the 248 study subjects are shown in Table 1. As expected, age, maturity, height, body mass, body mass index (BMI), femur length, tibia length, total body lean mass, total body fat mass, total body percent fat, and calf and thigh MCSA increased significantly (all P values < 0.001) from baseline to the 2-year follow-up. In addition, calf and thigh muscle density increased significantly (P < 0.001) over the 2-year period. By contrast, 2-year average physical activity did not change (P = 0.678).
TABLE 1.
Descriptive characteristics of the girls (n = 248) at baseline and 2 years as well as the change in these variables over the 2-year period.
| Baseline | 2 Year | Change | P | |
|---|---|---|---|---|
| Age (yrs) | 10.7 ± 1.1 | 12.8 ± 1.1 | 2.1 ± 0.1 | <0.001 |
| Maturity offset (yrs) | −1.1 ± 1.0 | 0.7 ± 1.0 | 1.8 ± 0.3 | <0.001 |
| Height (cm) | 145 ± 9.9 | 157 ± 9.2 | 12.2 ± 3.8 | <0.001 |
| Body mass (kg) | 38.7 ± 9.5 | 49.8 ± 11.9 | 11.1 ± 4.8 | <0.001 |
| BMI (kg/m2) | 18.3 ± 3.0 | 20.0 ± 3.6 | 1.8 ± 1.6 | <0.001 |
| Femur length (cm) | 34.3 ± 3.1 | 36.8 ± 2.6 | 2.5 ± 1.5 | <0.001 |
| Tibia length (cm) | 33.2 ± 2.9 | 36.3 ± 2.6 | 3.1 ± 1.3 | <0.001 |
| Total body lean mass (kg) | 25.7 ± 5.0 | 32.1 ± 5.6 | 6.4 ± 2.4 | <0.001 |
| Total body fat mass (kg) | 10.9 ± 5.7 | 15.2 ± 7.5 | 4.3 ± 3.5 | <0.001 |
| Total body percent fat (%) | 27.3 ± 8.5 | 29.5 ± 8.2 | 2.1 ± 4.5 | <0.001 |
| Calf muscle density (mg/cm3) | 79.0 ± 1.3 | 79.8 ± 1.2 | 0.8 ± 1.5 | <0.001 |
| Thigh muscle density (mg/cm3) | 76.4 ± 1.6 | 77.5 ± 1.5 | 1.1 ± 1.5 | <0.001 |
| Calf MCSA (mm2) | 3208 ± 583 | 4364 ± 923 | 659 ± 292 | <0.001 |
| Thigh MCSA (mm2) | 3590 ± 711 | 4384 ± 872 | 792 ± 488 | <0.001 |
| Physical activity | 5322 ± 4670 | 5183 ± 4204 | −140 ± 5306 | 0.678 |
Values are presented as mean ± SD. BMI = body mass index; MCSA = muscle cross-sectional area. P values represent paired samples t-Test for difference between the baseline and 2-year study visit.
At baseline, 65% of the girls were early pubertal (Tanner stages II–III, n = 162) and 7% (n = 16) of the girls had reached menarche. By the 2-year follow-up, 48% (n = 118) of the girls were menarcheal. Baseline maturity offset values indicated that girls were on average 1.2 years prior to PHV, with a range from 3.2 years prior to PHV to 1.0 years post PHV. Maturity offset at the 2-year follow-up averaged 0.7 (−1.7 to 3.2) years post PHV. Changes in Tanner stage from baseline to the 2-year follow-up are shown in Table 2.
TABLE 2.
Tanner stage at baseline and 2-year follow-up for the girls (n = 248).
| Baseline to 2-year Tanner stage | No. Girls (%) | |
|---|---|---|
| Prepubertal | 1–1 | 11 (4.4) |
| 1–2 | 24 (9.7) | |
| 1–3 | 40 (16.1) | |
| 1–4 | 11 (4.4) | |
| Early pubertal | 2–2 | 9 (3.6) |
| 2–3 | 39 (15.7) | |
| 2–4 | 33 (13.3) | |
| 2–5 | 1 (0.4) | |
| 3–3 | 14 (5.6) | |
| 3–4 | 48 (19.4) | |
| 3–5 | 18 (7.3) | |
|
| ||
| Total | 248 (100) | |
As expected, bone density, geometry and indices of bone strength at metaphyseal and diaphyseal regions of the tibia and femur improved over the course of the 2-year study in all girls. Comparisons of 2-year percent changes in bone parameters across quintiles of 2-year change in calf muscle density showed that girls in the lowest fifth had significantly less gains in BSI (by −10.6%, P < 0.001, Fig. 1A), trabecular vBMD (by −4.6%, P < 0.01, Fig. 1B), and cortical vBMD (by −1.6%, P < 0.001, Fig. 1C) at the tibia as compared with girls in the highest fifth. Similar trends were observed between the lowest fifth and the middle group (i.e., average of the middle 3 fifths) of 2-year change in calf muscle density, suggesting that girls with higher skeletal muscle fat content (i.e., those in the lowest fifth of calf muscle density) had suboptimal gains in trabecular and cortical vBMD as well as bone strength at the tibia over the 2-year period (Fig. 1A–C).
FIGURE 1.
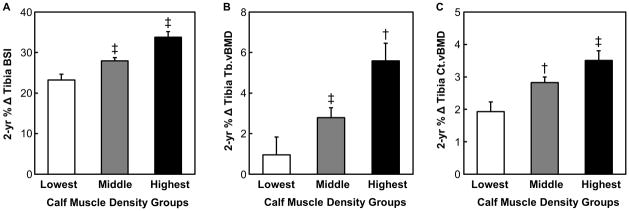
Two year percentage change in A) bone strength index (BSI, mg2/cm4) B) trabecular volumetric bone mineral density (Tb.vBMD, mg/cm3), and C) cortical volumetric bone mineral density (Ct.vBMD, mg/cm3) of the tibia for the lowest, average of the middle 3 (middle), and the highest fifths of 2-year change thigh in muscle density (mg/cm3). A linear mixed effects model was used to evaluate differences in bone parameters among the 3 groups (see “Statistical analysis” section). * P < 0.05 versus the lowest group; † P < 0.01 versus the lowest group; ‡ P < 0.001 versus the lowest group.
Importantly, these changes were not confined to the tibia, but rather were more generalized across the weight-bearing skeleton, as similar trends for 2-year percent changes in femur bone parameters were observed across quintiles of 2-year change in thigh muscle density (Fig. 2 A–C). Specifically, girls in the lowest fifth had reduced gains in BSI (by −6.1%, P < 0.01, Fig. 2A), trabecular vBMD (by −3.1%, P < 0.05, Fig. 2B), and cortical vBMD (by −0.7%, P = 0.075, Fig. 2C) at the femur as compared with girls in the highest fifth of 2-year change in thigh muscle density. Furthermore, similar trends in 2-year percent changes in femoral bone parameters were observed between the lowest fifth and the middle group (i.e., average of the middle 3 fifths) of 2-year change in thigh muscle density (Fig. 2 A–C).
FIGURE 2.
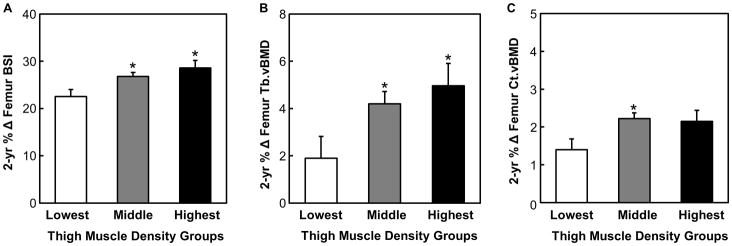
Two year percentage change in A) bone strength index (BSI, mg2/cm4) B) trabecular volumetric bone mineral density (Tb.vBMD, mg/cm3), and C) cortical volumetric bone mineral density (Ct.vBMD, mg/cm3) of the femur for the lowest, average of the middle 3 (middle), and the highest fifths of 2-year change thigh in muscle density (mg/cm3). A linear mixed effects model was used to evaluate differences in bone parameters among the 3 groups (see “Statistical analysis” section). * P < 0.05 versus the lowest group.
We next used multivariable regression models to examine the independent associations between the physical activity (average and 2-year change) and changes in skeletal muscle parameters (i.e., muscle density and MCSA) of the calf with 2-year changes in bone parameters of the tibia, while adjusting for covariates. As shown in Table 3, increased calf muscle density was significantly associated with more optimal gains in trabecular vBMD (β = 0.25, P < 0.001) and BSI (β = 0.25, P < 0.001) at the distal tibia. Furthermore, a greater increase in calf muscle density was significantly associated with a greater gain in cortical vBMD (β = 0.13, P < 0.01) at the diaphyseal tibia site. Change in calf muscle density was not significantly associated with changes in any other bone parameters at the metaphyseal or diaphyseal sites of the tibia (Table 3), although the associations between change in calf muscle density and changes in cortical thickness and SSI approached statistical significance (all P values < 0.10). Greater increase in calf MCSA was significantly (P < 0.001) associated with increases in all bone parameters of the tibia, except cortical vBMD (Table 3). Average physical activity level and 2-year change in physical activity level were not significantly associated with any tibial bone parameters (Table 3), although the association between change in physical activity level and change in cortical thickness of the tibia approached statistical significance (P = 0.059). Correlations between physical activity (average and 2-year change) and 2-year change in calf muscle density were not statistically significant (r = −0.11 to 0.05, P > 0.05).
TABLE 3.
Independent associations between changes in calf skeletal muscle parameters and physical activity with 2-year changes in bone parameters at metaphyseal (4%) and diaphyseal (66%) regions of the tibia in girls.
| Dependent variables | Δ Muscle Density (mg/cm3) | Δ MCSA (mm2) | Δ Physical Activity | Average Physical Activity | Adjusted R2 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β | P | β | P | β | P | β | P | ||
| 4% Tibia | |||||||||
| Δ Tb.vBMD (mg/cm3) | 0.25 | <0.001 | 0.40 | <0.001 | 0.05 | 0.380 | 0.03 | 0.420 | 0.23 |
| Δ Tb.A (mm2) | −0.05 | 0.268 | 0.33 | <0.001 | 0.02 | 0.739 | −0.03 | 0.541 | 0.48 |
| Δ BSI (mg2/cm4) | 0.25 | <0.001 | 0.30 | <0.001 | 0.03 | 0.416 | 0.06 | 0.183 | 0.58 |
| 66% Tibia | |||||||||
| Δ Ct.vBMD (mg/cm3) | 0.13 | 0.003 | 0.08 | 0.192 | 0.02 | 0.583 | 0.01 | 0.895 | 0.58 |
| Δ Ct.A (mm2) | 0.07 | 0.138 | 0.45 | <0.001 | 0.07 | 0.141 | 0.05 | 0.285 | 0.50 |
| Δ EC (mm) | −0.05 | 0.354 | −0.43 | <0.001 | −0.06 | 0.230 | −0.02 | 0.723 | 0.48 |
| Δ PC (mm) | −0.02 | 0.617 | −0.18 | 0.004 | −0.02 | 0.617 | −0.01 | 0.867 | 0.54 |
| Δ Ct.Th (mm) | 0.09 | 0.053 | 0.55 | <0.001 | 0.09 | 0.059 | 0.05 | 0.285 | 0.48 |
| Δ SSI (mm3) | 0.08 | 0.087 | 0.28 | <0.001 | 0.02 | 0.600 | 0.07 | 0.133 | 0.55 |
Standardized β coefficients and P values are presented for Δ calf muscle density, Δ calf MCSA (muscle cross-sectional area) and physical activity (average and 2-year Δ). Model covariates = Δ muscle density, Δ MCSA, Δ physical activity, average physical activity, Δ subcutaneous fat; Δ tibia length, baseline maturity offset, Δ maturity offset, ethnicity, randomization, baseline bone parameter. Statistically significant standardized β coefficients and P values are shown in bold. Tb.vBMD = trabecular volumetric bone mineral density; Tb.A = trabecular area; BSI = bone strength index; Ct.vBMD = cortical volumetric bone mineral density; Ct.A = cortical area; EC = endocortical circumference; PC = periosteal circumference; Ct.Th = cortical thickness; SSI = strength-strain index.
Table 4 shows the results from multivariable regression models examining the independent associations between the physical activity (average and 2-year change) and changes in skeletal muscle parameters (i.e., muscle density and MCSA) of the thigh with 2-year changes in femoral bone parameters, while adjusting for covariates. Similar to the changes observed at the tibia, increased thigh muscle density was significantly associated with greater gains in trabecular vBMD (β = 0.17, P < 0.01) and BSI (β = 0.17, P < 0.01) at the distal femur. Furthermore, increased thigh muscle density was significantly associated with a greater gain in cortical vBMD (β = 0.12, P < 0.05) at the diaphyseal femur site. By contrast, increased thigh muscle density was negatively associated with changes in endocortical/periosteal circumferences (β = −0.13 to −0.15, all P values < 0.01) at the diaphyseal femur site. However, change in thigh muscle density was not significantly associated with any changes in cortical thickness, SSI, or trabecular/cortical area of the femur (Table 4). Overall, the directions of the associations between increased thigh MCSA and changes in femoral bone parameters were similar to those observed between calf MCSA and tibial bone parameters, although the magnitude of the associations at the femur tended to be lower (Table 4). Nevertheless, change in thigh MCSA was significantly associated with greater gains in trabecular vBMD (P = 0.001), BSI (P < 0.001), cortical area (P < 0.001), cortical thickness (P = 0.010) and SSI (P = 0.013) of the femur. Average physical activity level over the 2-year period was significantly associated with increases in trabecular vBMD (P < 0.05) and BSI (P < 0.05) of the distal femur, but was not significantly associated with any of the other changes in femoral bone parameters. In addition, 2-year change in physical activity level was not significantly associated with any femoral bone parameters (Tables 4). Correlations between physical activity (average and 2-year change) and 2-year change in thigh muscle density were not statistically significant (r = −0.05 to −0.01, P > 0.05).
TABLE 4.
Independent associations between changes in thigh skeletal muscle parameters and physical activity with 2-year changes in bone parameters at metaphyseal (4%) and diaphyseal (20%) regions of the femur in girls.
| Dependent variables | Δ Muscle Density (mg/cm3) | Δ MCSA (mm2) | Δ Physical Activity | Average Physical Activity | Adjusted R2 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β | P | β | P | β | P | β | P | ||
| 4% Femur | |||||||||
| Δ Tb.vBMD (mg/cm3) | 0.17 | 0.005 | 0.27 | 0.001 | −0.03 | 0.553 | 0.15 | 0.013 | 0.24 |
| Δ Tb.A (mm2) | −0.08 | 0.170 | −0.03 | 0.704 | 0.04 | 0.409 | 0.09 | 0.111 | 0.34 |
| Δ BSI (mg2/cm4) | 0.17 | 0.002 | 0.37 | <0.001 | −0.04 | 0.399 | 0.10 | 0.048 | 0.41 |
| 20% Femur | |||||||||
| Δ Ct.vBMD (mg/cm3) | 0.12 | 0.021 | −0.02 | 0.757 | −0.02 | 0.725 | −0.01 | 0.924 | 0.48 |
| Δ Ct.A (mm2) | −0.03 | 0.554 | 0.24 | <0.001 | −0.02 | 0.681 | 0.02 | 0.618 | 0.50 |
| Δ EC (mm) | −0.15 | 0.006 | 0.02 | 0.831 | 0.03 | 0.568 | 0.05 | 0.396 | 0.38 |
| Δ PC (mm) | −0.13 | 0.009 | 0.09 | 0.180 | 0.02 | 0.658 | 0.03 | 0.505 | 0.50 |
| Δ Ct.Th (mm) | 0.05 | 0.436 | 0.19 | 0.010 | −0.03 | 0.639 | −0.02 | 0.681 | 0.31 |
| Δ SSI (mm3) | −0.03 | 0.533 | 0.15 | 0.013 | 0.00 | 0.932 | 0.06 | 0.190 | 0.57 |
Standardized β coefficients and P values are presented for Δ thigh muscle density, Δ thigh MCSA (muscle cross-sectional area) and physical activity (average and 2-year Δ). Model covariates = Δ muscle density, Δ MCSA, Δ physical activity, average physical activity, Δ subcutaneous fat; Δ femur length, baseline maturity offset, Δ maturity offset, ethnicity, randomization, baseline bone parameter. Statistically significant standardized β coefficients and P values are shown in bold. Tb.vBMD = trabecular volumetric bone mineral density; Tb.A = trabecular area; BSI = bone strength index; Ct.vBMD = cortical volumetric bone mineral density; Ct.A = cortical area; EC = endocortical circumference; PC = periosteal circumference; Ct.Th = cortical thickness; SSI = strength-strain index.
DISCUSSION
This longitudinal study examined the influences of muscle density and physical activity on bone development in prepubertal and early pubertal girls. The results indicate that reduced muscle density (i.e., increased skeletal muscle fat content) may put girls at risk for suboptimal bone development during growth. We also found that physical activity is linked to greater gains in bone density and strength during puberty, but to a lesser extent than changes in muscle density. These longitudinal findings extend our knowledge of the relationships between physical activity, muscle density, and bone development during growth. The importance of studying peri-pubertal girls is underscored by the observation that over 25% of bone mass is accrued in the two years surrounding peak linear growth (2), which points to the need to identify children who present with low muscle density and skeletal deficits so they can be targeted for lifestyle interventions during this critical period of development when preventative measures are most likely to be successful.
Conventional wisdom has held that the replacement of muscle fibers with non-contractile components such as lipids only occurs under conditions of disuse or with aging (18). However, our data in girls suggest that lesser gains in muscle density are related to suboptimal gains in trabecular vBMD and bone strength (i.e., BSI) at metaphyseal skeletal sites of the distal femur and tibia. Similarly, lesser gains in muscle density are associated with reduced gains in cortical vBMD at diaphyseal sites of the tibia and femur. By contrast, associations between changes in muscle density of the calf and thigh and changes in bone strength (i.e., SSI) at diaphyseal sites of the tibia and femur were not statistically significant. The reason(s) for these observations are not entirely clear, but may reflect the fact that bone strength at diaphyseal skeletal sites is more strongly related to cortical bone geometry than density (16). Furthermore, it remains to be seen as to whether reduced muscle density contributes to muscle weakness and poor physical function in girls as it does in older adults (11, 36). In the present study, physical activity was not associated with changes in muscle density, which is inconsistent with cross-sectional findings in this sample (10). The reason for this disparity is not clear and warrants future investigation.
The inclusion of a relatively large number of prepubertal and early pubertal girls in our study allowed us to show that, even after adjusting for covariates known to influence bone development (20), physical activity was significantly associated with greater gains in trabecular vBMD and bone strength (i.e., BSI) of the distal femur. However, it is important to note that the magnitudes of these associations were relatively weak and that physical activity was not significantly associated with changes in any other bone parameters. Studies in animals have consistently demonstrated that bone strain, induced by activities that involve high-strain magnitudes (i.e., load) and greater loading frequencies (i.e., rate), is the most effective stimulus for optimizing the osteogenic response during growth (3). Consistent with this premise, Deere et al. (4) showed that high impact activity was more strongly associated with hip BMD and geometry than moderate and low impact activities in boys and girls. Therefore, in the present study, it is certainly possible that the impacts (i.e., “load”) encountered during physical activity were not of sufficient magnitude to cause meaningful adaptations in bone. One advantage of the PYPAQ algorithm that we used to quantify physical activity is that it accounts for the duration (defined as average minutes per session), frequency (sessions per week), and load (peak strain score (13)) of physical activity. However, surprisingly, the load component of the PYPAQ score was not significantly associated with any changes in bone parameters in this sample (data not shown). Another component of physical activity is intensity, which can be captured using sophisticated motion sensors (e.g., accelerometers). In a recent cross-sectional study, Sayers et al. (29) found that vigorous, but not moderate or light, physical activity assessed by accelerometry was significantly associated with cortical bone mass in boys and girls. Nevertheless, it is still unknown how well the outputs of these devices relate to bone strain, the predominant stimulus that drives bone formation.
Previous cross-sectional and longitudinal studies have examined the association between MCSA and measures of bone density, structure, and strength (7, 17, 20, 37, 39). These studies have consistently shown that MCSA is significantly related to indices of bone strength in girls. Our longitudinal data are consistent with these findings, and suggest that muscle size, which is strongly related to skeletal muscle force production (30), is an important determinant of bone development in girls. Moreover, these findings also provide support for the ‘Functional Muscle-Bone Unit’ which posits that bone strength is adapted to the routine physiological loads (i.e., forces from muscle contractions) that challenge bone stability (30). Thus, our study provides compelling evidence for encouraging exercise modes in children and adolescents that enhance muscle size and force production.
Our study had both strengths and limitations. The strengths include the relatively large sample size, longitudinal study design, and use of pQCT, which allows assessment of soft tissue and skeletal parameters in growing children. Nevertheless, our results must be interpreted in the context of several limitations. The most apparent limitation is the well-known difficulty in assessing physical activity through self-report questionnaires in children and adolescents. Although we used a validated questionnaire for assessing physical activity in youth (1) and encouraged guardian assistance, we acknowledge that this approach is susceptible to reporting errors, which may have, at least in part, contributed to the relatively low magnitude of the associations between physical activity and the changes in the bone/muscle parameters. Furthermore, it is important to note that pQCT has unavoidable methodological issues with measuring soft tissue composition. For example, this measure cannot distinguish between intra- and extramyocellular fat depots and could be influenced by other factors that have distinct relationships with bone development, such as connective tissue. Indeed, inter- and intramuscular adiposity by magnetic resonance imaging (MRI) does not completely explain pQCT-derived muscle density at the calf, although significant relationships among these variables have been reported in older women (38). Lastly, the long-term reproducibility of pQCT measurements may be influenced by disproportionate growth rates at the distal and proximal ends of long bones (25), although we attempted to reduce the impact of this limitation by averaging three pQCT slices obtained at the distal metaphyseal femur and tibia regions.
These limitations notwithstanding, our finding that reduced muscle density coincides with suboptimal bone development during puberty in girls is concerning. Furthermore, physical activity is significantly linked to greater gains in weight-bearing bone density and strength, but the extent of this effect is less than that of muscle density. These findings suggest that poor muscle quality may not only be an important risk factor for metabolic dysfunction, but also for suboptimal bone development. The long-term consequences of these effects are unknown, but maximizing muscle quality and bone strength during growth is likely to offset the future development of osteoporosis and bone fragility later in life.
Acknowledgments
Grant Support: NIH: HD050775; Clinical Trials #: NCT00729378; Registration Date: 07/17/2008
We appreciate the participation and support of principals, teachers, parents and students from the schools in the Catalina Foothills and Marana School Districts. We also wish to thank the members of the Jump-In Study team for their contributions. The study was supported by Award Number HD-050775 (SG) from the National Institute of Child Health and Human Development. Dr. Farr is supported by T32 DK007352: Diabetes and Metabolism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. Results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Conflict of Interest Statement: None of the authors have a conflict to disclose.
References
- 1.Aaron DJ, Kriska AM, Dearwater SR, et al. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142(2):191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DA, McKay HA, Mirwald RL, et al. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 3.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30(5):781–6. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- 4.Deere K, Sayers A, Rittweger J, et al. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res. 2012;27(9):1887–95. doi: 10.1002/jbmr.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent CE. Keynote address: Problems in metabolic bone disease. In: Frame B, editor. Proceedings of the Henry Ford Hospital Symposium on Metabolic Bone Diseases. Amsterdam: Excerpta Medica; 1973. pp. 1–7. [Google Scholar]
- 6.Farr JN, Blew RM, Lee VR, et al. Associations of physical activity duration, frequency, and load with volumetric BMD, geometry, and bone strength in young girls. Osteoporos Int. 2011;22(5):1419–30. doi: 10.1007/s00198-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr JN, Chen Z, Lisse JR, et al. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46(4):977–84. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26(9):2217–25. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farr JN, Lee VR, Blew RM, et al. Quantifying bone-relevant activity and its relation to bone strength in girls. Med Sci Sports Exerc. 2011;43(3):476–83. doi: 10.1249/MSS.0b013e3181eeb2f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr JN, Van Loan MD, Lohman TG, et al. Lower physical activity is associated with skeletal muscle fat content in girls. Med Sci Sports Exerc. 2012;44(7):1375–81. doi: 10.1249/MSS.0b013e31824749b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 12.Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring) 2010;18(9):1801–4. doi: 10.1038/oby.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groothausen J, Siemer H, Kemper G, et al. Influence of peak strain on lumbar bone mineral density: an analysis of 15-year physical activity in young males and females. Pediatr Exerc Sci. 1997;9:159–73. [Google Scholar]
- 14.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54(3):509–15. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 16.Kontulainen SA, Johnston JD, Liu D, et al. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8(4):401–9. [PubMed] [Google Scholar]
- 17.Kontulainen SA, Macdonald HM, Khan KM, et al. Examining bone surfaces across puberty: a 20-month pQCT trial. J Bone Miner Res. 2005;20:1202–7. doi: 10.1359/JBMR.050214. [DOI] [PubMed] [Google Scholar]
- 18.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DC, Gilsanz V, Wren TA. Limitations of peripheral quantitative computed tomography metaphyseal bone density measurements. J Clin Endocrinol Metab. 2007;92(11):4248–53. doi: 10.1210/jc.2007-0126. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald H, Kontulainen S, Petit M, et al. Bone strength and its determinants in pre-and early pubertal boys and girls. Bone. 2006;39(3):598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 21.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36(4):250–7. doi: 10.1136/bjsm.36.4.250. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirwald RL, Baxter-Jones AD, Bailey DA, et al. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–94. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Morris NM, Udry RJ. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Jama. 2002;288(14):1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 25.Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;(275):274–9. [PubMed] [Google Scholar]
- 26.Robling AG. Is bone’s response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc. 2009;41(11):2044–9. doi: 10.1249/MSS.0b013e3181a8c702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roemmich JN, Clark PA, Walter K, et al. Pubertal alterations in growth and body composition. V. Energy expenditure, adiposity, and fat distribution. Am J Physiol Endocrinol Metab. 2000;279(6):E1426–36. doi: 10.1152/ajpendo.2000.279.6.E1426. [DOI] [PubMed] [Google Scholar]
- 28.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122(5):409–14. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Sayers A, Mattocks C, Deere K, et al. Habitual levels of vigorous, but not moderate or light, physical activity is positively related to cortical bone mass in adolescents. J Clin Endocrinol Metab. 2011;96(5):E793–802. doi: 10.1210/jc.2010-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenau E, Neu CM, Beck B, et al. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17:1095–101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 31.Seeman E, Delmas PD. Bone quality-- the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 32.Shedd KM, Hanson KB, Alekel DL, et al. Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc. 2007;39(12):2189–98. doi: 10.1249/mss.0b013e318155a7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherar LB, Baxter-Jones AD, Mirwald RL. Limitations to the use of secondary sex characteristics for gender comparisons. Ann Hum Biol. 2004;31(5):586–93. doi: 10.1080/03014460400001222. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. Bone health and osteoporosis: a report of the surgeon general. Rockville, MD: United States Department of Health and Human Services, Office of the Surgeon General; 2004. pp. 187–217. [Google Scholar]
- 35.U.S. Department of Health and Human Services. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta (GA): U.S. Department of Health and Human Services Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 36.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 37.Wetzsteon RJ, Petit MA, Macdonald HM, et al. Bone structure and volumetric BMD in overweight children: a longitudinal study. J Bone Miner Res. 2008;23(12):1946–53. doi: 10.1359/jbmr.080810. [DOI] [PubMed] [Google Scholar]
- 38.Wong AK, Beattie K, Bhargava A, et al. Inter and intramuscular adiposity explains only a proportion of the association between muscle density and fractures. J Bone Miner Res. 2012;27(Supplement 1):SA0013. [Google Scholar]
- 39.Xu L, Nicholson P, Wang Q, et al. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24(10):1693–8. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 40.Yerges-Armstrong LM, Miljkovic I, Cauley JA, et al. Adipose tissue and volumetric bone mineral density of older Afro-Caribbean men. J Bone Miner Res. 2010;25(10):2221–8. doi: 10.1002/jbmr.107. [DOI] [PMC free article] [PubMed] [Google Scholar]


