Abstract
Background
Patients with chronic rhinosinusitis (CRS) exhibit centrally mediated behavioral changes commonly referred to as sickness behavior. Sleep alteration is a component of sickness behavior which is estimated to affect up to 70 million patients annually. Patients with CRS have poor sleep quality, and little is known about the underlying etiology and pathophysiology. This narrative review aims to further organize and present the current knowledge associating sleep and CRS.
Methods
A literature search was conducted of the OVID MEDLINE database using key search words including: “chronic rhinosinusitis”, “sleep”, “sleep disorders”, and “sleep dysfunction”. Additional keywords “nasal obstruction”, “nasal polyp”, and “fatigue” were identified and utilized to further delineate relevant articles.
Results
The articles that specifically addressed sleep and CRS were dissected and presented as follows; 1) chronic rhinosinusitis and sleep, 2) chronic rhinosinusitis and fatigue 3) chronic rhinosinusitis, nasal obstruction and sleep, 4) pathophysiology of sleep in chronic rhinosinusitis (cytokines in both sleep and chronic rhinosinusitis and their association to the neuroimmune biology of chronic rhinosinusitis).
Conclusions
Patients with CRS have sleep dysfunction that is associated with their disease severity and overall quality of life. The etiology of sleep dysfunction in CRS is most likely multifactorial. Increasing evidence suggests sleep dysfunction in patients with CRS is partly due to the inflammatory disease process, and sleep physiology in patients with CRS may be actively regulated by the inflammatory component of the disease.
Keywords: Sinusitis, sleep, fatigue, rhinology, review
INTRODUCTION
As the sun descends, we enter another state of consciousness. This phenomenon, sleep, occupies a third of our lives, yet most of us do not ponder sleeps’ origin or function until it is disturbed in disease states such as chronic rhinosinusitis (CRS). Throughout history, philosophers and physicians have struggled to understand sleep in both health and disease. Hippocrates hypothesized that sleep occurred due to heat loss from extremities into the core of the body while Aristotle suggested that stomach vapors produced during digestion are transported throughout the body to bring about sleep. It is now established that sleep is a component of sickness behavior which is an actively regulated process that may become maladaptive leading to sleep dysfunction.
As a component of health and disease, poor sleep is now widely investigated and estimated to affect up to 70 million patients annually.1 In addition, many chronic diseases, such as CRS, have a high prevalence of pathological sleep dysfunction greater than that typically identified in the general population.2 Sleep dysfunction has staggering effects on daily performance, quality-of-life (QOL), disease severity, healthcare costs, and mortality.3 Given that up to 16% of the population of the United States has CRS, sleep dysfunction in CRS is an important concept for patients, physicians, and policymakers alike.
Patients with CRS have reduced sleep quality, although the underlying etiology or pathophysiology has received little attention.2 The mechanism of sleep impairment in CRS is most likely multi-factorial including, but not limited to, nasal obstruction,4 efferent and/or afferent neural signaling, or brain-immune signaling via immune mediators such as interleukin-1 (IL-1) and tumor necrosis factor (TNF).5–7 Accumulating evidence is elucidating the sophisticated and intertwined communication between the central nervous system (CNS) and the immune system. This bidirectional communication elicits “sickness behavior” commonly seen in animals and is thought to explain, in part, the adaptive responses to infection such as increased sleepiness.7–9 Furthermore, sleep disruption and altered inflammatory cytokine levels are associated with CRS and other chronic inflammatory diseases 10–20, providing further insight into the biochemical regulation of sleep. This review aims to organize and present our current understanding of the association between CRS and sleep dysfunction. We further explore the physiology of sleep in disease as it relates to CRS.
METHODS
There are very few published studies that have specifically addressed sleep disturbance in patients with CRS. An OVID MEDLINE database search (1946–2013) was performed limiting the articles to the English language and using the keywords “chronic sinusitis” OR “rhinosinusitis” OR “chronic rhinosinusitis” AND “sleep” OR “sleep disorders” OR “sleep dysfunction”. This search revealed 377 articles. Titles and abstracts were reviewed for relevance. Those studies investigating sleep and CRS were deemed appropriate for this narrative review. An additional Ovid MEDLINE search was performed adding the keywords “nasal obstruction” OR “nasal polyp” OR “fatigue” to our original query which revealed 2 additional articles.
Literature findings were organized and discussed as associations between CRS, sleep, fatigue, and nasal obstruction. The biochemical/humoral regulation of sleep and CRS was reviewed separately followed by discussion of those immune mediators known to have dual roles both in the pathophysiology of CRS and their associated somnogenic activity. Therefore, this review article is organized into the following sections:
Chronic Rhinosinusitis and Sleep
Chronic Rhinosinusitis and Fatigue
Chronic Rhinosinusitis, Nasal Obstruction, and Sleep
-
Pathophysiology of Sleep in Chronic Rhinosinusitis
Cytokines and Sleep Mechanisms
Cytokines Related to Chronic Rhinosinusitis and Sleep
Chronic Rhinosinusitis and the Neuroimmune Biology of Sleep
RESULTS
Chronic Rhinosinusitis and Sleep
Patients with CRS commonly describe sleep abnormalities, although this has not been thoroughly investigated. The vast majority of studies evaluating sleep and its role in CRS have been accomplished through the use of CRS disease-specific instruments, such as the Rhinosinusitis Disability Index (RSDI),10,11 the Sinonasal Outcomes Test (SNOT-22),12 and the Rhinosinusitis Outcome Measure-31 (RSOM-31).13 Sleep was found to be one of the most severely affected domains in the RSOM-31 in patients with CRS.13 Additional insight into sleep dysfunction has come from use of the RSDI and SNOT-22 surveys, which include sleep-specific survey items. For instance, early work by Benninger et al. using the RSDI demonstrated sleep improvement in patients with CRS following sinus surgery.10 These CRS disease-specific studies demonstrate that sleep impairment is a substantial concern in patients with CRS.
There are limited studies prospectively evaluating sleep as it relates to CRS using sleep validated instruments such as the Epworth Sleepiness Scale (ESS)14, Calgary Sleep Apnea Quality of Life Index15 or the Pittsburgh Sleep Quality Index (PSQI).16 A recent prospective multi-institutional investigation demonstrated that patients with CRS have impaired quality of sleep as measured by the PSQI survey. Patients reporting poor sleep were more likely to be female, have comorbid depression and abuse tobacco compared to patients reporting good sleep quality. Poor sleep quality did not correlate to disease severity as measured by endoscopy or CT staging, but was significantly correlated with disease-specific QOL, even after eliminating sleep-related questions from these instruments.2 It is not yet known if improving CRS disease-specific QOL or disease severity can improve sleep in patients with CRS.
In summary, patients with symptomatic CRS have a high prevalence of sleep pathology. The relationship between sleep dysfunction and QOL in CRS is likely bi-directional, whereby disability predicts worse sleep and worse sleep may influence QOL. Further investigation into this relationship may provide insight into the function of sleep in both health and disease and ultimately result in treatment options for patients with CRS reporting sleep dysfunction.
Chronic Rhinosinusitis and Fatigue
Often used in a similar sense by both patients and physicians alike, the terms “sleepiness” (sleep dysfunction) and “fatigue” can clearly be distinguished, as sleep dysfunction does not always correlate with a subjective feeling of fatigue.17 Likewise, fatigue is a state of sustained exhaustion resulting in difficulty performing physical and mental tasks, which sleep alone cannot necessarily alleviate.
Fatigue and sleep dysfunction are two components of sickness behavior that can present simultaneously in the same patient, resulting in significant detriment to QOL.18 Therefore, the inter-relationship between sleep and fatigue may be significant in patients with CRS. Sleep complaints and fatigue are two of the most debilitating symptoms reported by patients with CRS.19 Both sleep quality and fatigue are associated with disease-specific QOL in patients with CRS.2 A systematic review and meta-analysis demonstrated significant improvement in fatigue in patients with CRS following endoscopic sinus surgery.20,21 It is not known whether the improvement in fatigue following endoscopic sinus surgery improves patients’ sleep dysfunction. However, it is likely that improvement in fatigue would result in improvement of sleep dysfunction, as higher levels of fatigue are associated with poor sleep quality,22 disturbed circadian rhythms, and immune activation.23,24 Further studies need to characterize both sleep and fatigue in patients with CRS, as fatigue is likely a confounding factor in regards to sleep disturbance and needs to be evaluated by systematically investigating both conditions.25
Chronic Rhinosinusitis, Nasal Obstruction, and Sleep
The nose accounts for greater than 50% of the total resistance of the upper airway. Early studies evaluated the effects of nasal obstruction on sleep by artificially inducing occlusion and found significantly increased number of apneas and cortical arousals.26,27 These early studies cemented the theory that nasal obstruction may predict sleep disordered breathing (SDB) and microarousals. SDB subsequently was thought to reduce sleep quality, thereby affecting daytime sleepiness and performance. However, the relationship between nasal obstruction, sleep quality and SDB in the literature is sometimes conflicted and continues to lack clarity.28–30
Nasal obstruction may play a role in sleep dysfunction in patients with CRS. For instance, patients with nasal obstruction related to nasal polyposis have a 2-fold higher risk of sleep dysfunction.4 As such, patients with CRS and nasal obstruction due to nasal polyposis had significant improvement in nasal resistance and a mean reduction in excessive daytime sleepiness following endoscopic sinus surgery. However, improving nasal resistance did not significantly improve the apnea hypopnea index (AHI) in patients with CRS with nasal polyps.31 This data suggests that subjective measures of sleep dysfunction do not correlate with objective measures of sleep dysfunction. In addition, these studies imply that nasal obstruction is only partially contributing to sleep dysfunction in patients with CRS.
Nasal congestion and obstruction are commonly reported symptoms in patients with CRS and allergic rhinitis (AR).32,33 There is a paucity of literature evaluating nasal obstruction and sleep as it specifically pertains to CRS. In contrast, a larger body of evidence exists linking nasal obstruction with sleep dysfunction in AR, such that patients with AR and nasal obstruction have increased periodic breathing during sleep, snoring, microarousals and chronic non-restorative sleep.34,35 Although higher levels of nasal resistance in AR appear to increase the risk for moderate to severe SDB by 1.8 times, it did not significantly correlate with increasing levels of AHI.29 Nasal obstruction and/or nasal congestion in AR appear to be playing a role in sleep disruption that has not been systematically evaluated in patients with CRS. In summary, AR may partially contribute to and exacerbate both nasal obstruction and sleep dysfunction in patients with CRS.
Therapies aimed at relieving nasal obstruction have been shown to improve nasal resistance and sleep quality. However, reported outcomes and definitions of surgical success are inconsistent in the literature making it difficult to draw definitive conclusions. Evidence does suggest that nasal surgery can improve postoperative sleep quality in some patients.36 For example, septoplasty and inferior turbinate reduction has been shown to improve snoring in up to 86% of patients37 with associated improvement in nasal breathing by increasing nasal air temperature and humidity 4–6 months after surgery.38 However, a recent meta-analysis assessed the effects of nasal surgery on SDB and found that nasal surgery does not improve objective sleep indices.39
In contrast to objective measures, treatment to address structural nasal defects has been shown to improve subjective measures of sleep quality. For example, correction of an obstructed nasal airway significantly improves disease-specific and general QOL in adult patients with obstructive sleep apnea 3 months after surgery.40 Medical management with nasal corticosteroids has been shown to improve subjective sleep41,42 by reducing daytime sleepiness and fatigue,43,44 significantly lowering AHI,45 improving sleep quality,46 but insufficiently improving objective sleep quality41,45 or daytime sleepiness in chronic fatigue syndrome.47 In contrast to these studies, one prospective multicenter study demonstrated that the use of intranasal steroids was not associated with improved sleep quality.48
In summary, the cumulative evidence suggests that nasal surgery is unreliable in improving objective measures of sleep quality but may improve subjective, patient-reported measures of sleep quality. Even less clear is whether nasal obstruction, addressed by surgery or medical management can improve sleep function and disease-specific QOL in patients with CRS.
The association between nasal obstruction and sleep dysfunction in patients with CRS remains unclear. Nasal obstruction has been shown to play a role in sleep disruption in AR and conclusions have been inferred to patients with CRS. We acknowledge that AR is a separate disease process with differing etiology, cytokine profile and possibly very different sleep mechanisms. Future studies are needed to further evaluate the role of nasal obstruction and sleep dysfunction in patients with CRS.
Pathophysiology of Chronic Rhinosinusitis and Sleep
Cytokines and Sleep Mechanisms
The concept of sleep regulator substances (SRSs) influencing sleep at the molecular level was developed over 100 years ago when Ishimori transferred cerebral spinal fluid (CSF) from sleep - deprived dogs to normal dogs, thereby inducing sleep.49 The isolation and characterization came many years later when the first substance was isolated in the 1970’s and identified in the 1980’s as a muramyl peptide. Muramyl peptides are components of microbial cell walls and contribute to our current knowledge of the biochemical regulation of sleep through what are termed SRSs.
Substances considered SRSs must meet criteria that have been previously published.7,50 Briefly: 1) the substance and or its receptor oscillates with sleep propensity; 2) sleep is increased or decreased with administration of the substance; 3) blocking the action or inhibiting the production of the substance changes sleep; 4) disease states, such as infection associated with altered sleep, also change levels of the putative SRS; and finally, 5) the substance acts on known sleep regulatory circuits. Many substances that have been linked to CRS meet some of these criteria including pro-inflammatory cytokines, hormones, and bacterial cell wall products, but only a few meet all the required characteristics to be considered a SRS. Some of the key substances involved in both the biochemical regulation of sleep and CRS are summarized in Table 1. The best characterized substances involved in regulating non-rapid eye movement sleep (NREMS) include IL-Iβ, TNF-α, and growth hormone releasing hormone (GHRH). Nitric oxide (NO) and prolactin meet criteria for regulating rapid eye movement sleep (REMS). The ensuing discussion will primarily focus on IL- Iβ and TNF-α both linked to the physiological and pathological humoral regulation of sleep and the inflammatory cascade in CRS, followed by a limited discussion on other mediators linked to the biochemical regulation of sleep and CRS.
Table 1.
Mediators implicated in non-rapid eye movement sleep, rapid eye movement sleep, and chronic rhinosinusitis.
| Somnogenic Substances/Immune Mediators | Effects NREMS | Effects REMS | CRS | References |
|---|---|---|---|---|
| Interleukin-1 | ↑↔↓ | + | Krueger7, Lennard,62 Mullol95 | |
| Interleukin-1 Receptor Antagonist | ↓ | Opp 96 | ||
| Interleukin-2 | ↑ | + | Kubota97, Selezn’ov 98 | |
| Interleukin-4 | ↓ | + | Krueger7, Kushikata90, | |
| Interleukin-8 | ↑ | + | Selezn’ov,98 Garcia-Garcia99, Mullol95, | |
| Interleukin-6 | ↑↔↓ | + | Bauer100, Vgontzas94, Hogan101, Ghaffar102 | |
| Interleukin-10 | ↓ | + | Krueger7, Kushikata89, Opp103, Toth104, Jyonouchi 105 | |
| Interleukin-13 | ↓ | + | Kubota91, al Ghamdi,106 Reh107 | |
| NF-kβ | ↑ | + | Kubota,108 (inhibitor attenuates IL1 sleep), Xu109 | |
| Interleukin-15 | ↑ | Kubota97 | ||
| Interleukin-18 | ↑ | + | Kubota,110 Okano111 | |
| Inferferon alpha | ↑↔↓ | ↓ | Bohnet112 | |
| Interferon gamma | ↑ | + | Kubota,113 Shin114 | |
| Tumor Necrosis Factor | ↑ | + | Krueger,7 Kubota,63,80 Lennard62 | |
| Soluble tumor necrosis factor receptor | ↓ | Kubota113 | ||
| Tissue Growth Factor Beta | ↓ | + | Kubota,91 Kuo115 | |
| Histamine | ↓ | + | Tashiro,82 | |
| CystLT | ↑ | + | Okuda,83 Sri-Kantha,85 Perez-Novo 116 | |
| TLR | ↑ | ↓ | + | Wisor,117 Sartorius,118 Lane119 |
| Prostaglandin | ↑↔↓ | ↑ | Sri Kantha,85 Hayaishi120, Urade121, Krueger122 | |
| Prolactin | ↑ | + | Zhang,123 Obal,124 Platt125 (prolactin- induced protein) | |
| Nitric Oxide | ↑ | ↑ | + | Chen,126 Cespuglio,127 Zhang128 |
NREMS, non-rapid eye movement sleep; REMS, rapid eye movement sleep; CRS, chronic rhinosinusitis; NF-kB, nuclear factor kappa beta; CystLT, cysteinyl-leukotriene; TLR, toll-like receptor; relative mean changes in sleep increase (↑), decrease (↓) or varied effect (↔)
Peripherally produced cytokines or growth factors provide a signal to the brain that an infection is occurring, and thereby stimulate sleep through five main pathways including: 1) stimulation or alteration of afferent transmission (e.g., through the vagus) with consequential signaling to the brain; 2) transport across the blood brain barrier (BBB) through the circumventricular organs; 3) altering the level or activity of another substance that signals the brain; 4) altering the blood brain barrier; 5) direct passage across the BBB (Figure 1).51,52 The fact that cytokines act in the brain to induce physiological adaptations may begin to help explain sickness behavior in patients with CRS.
Figure 1.
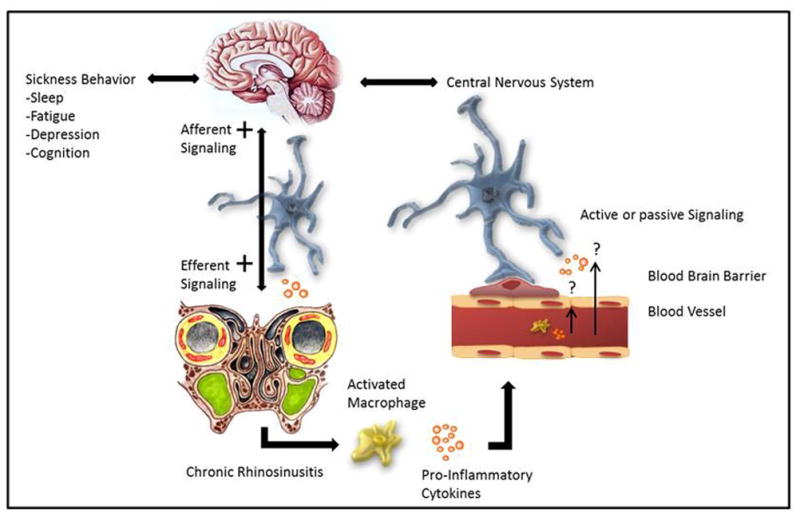
Increased somnolence is an important component of sickness behavior and evidence demonstrates that inflammatory mediators are instrumental in eliciting these symptoms via central neuronal signaling. The pro-inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)- α released in chronic rhinosinusitis (CRS) are involved in the normal physiological regulation of sleep. The mechanisms by which local inflammatory cytokines might signal the central nervous system in patients with CRS to induce sickness behavior is unknown. However, preliminary evidence suggests they may: 1) Access or signal the brain via transport across the blood brain barrier (BBB), through the circumventricular organs (subfornical organ, vascular organ of the laminar terminalis, median eminence, intermediate and posterior lobes of the pituitary, pineal gland, subcommissural organ and the area postrema), 2) Act locally, as opposed to systemically, through the stimulation or alteration of afferent neuronal transmission, 3) Alter the level or activity of another substance that signals the brain, and/or 4) Act through direct passage across the BBB through molecular transporters.
IL-1β, Interleukin-1 beta; TNF-α, tumor necrosis factor-alpha.
Cytokines related to Chronic Rhinosinusitis and Sleep
We are gaining a better understanding of the molecular crosstalk that occurs between cytokines released by the immune system and the CNS. Cytokines up-regulated in response to infection and inflammation mediate CNS responses, including excess sleep via activation of the central nervous system possibly through the somatotropic and hypothalamic-pituitary-adrenal (HPA) axes.9 Two of the most studied inflammatory cytokines involved in CRS include IL-Iβ and TNF-α, which are well known SRSs.
IL-1β and TNF-α are up-regulated in patients with CRS and regulate the inflammatory cascade in chronic inflammation.6 TNF-α and IL-1β are produced by macrophages in response to stimuli such as bacterial lipopolysaccharide (LPS) 53–55 while TNF-α antagonist reduce inflammatory activity and nasal mucus hypersecretion.56 Single nucleotide polymorphisms (SNPs) in IL-1β and TNF-α have been associated with CRS and nasal polyps.57,58 In addition, the TNF-α induced protein 3 superfamily gene was found to be associated with severe CRS.59 Karosi et al. investigated the inflammatory changes in patients with CRSwNP by measuring TNF-α receptors via immunofluorescent assays. These patients had increased expression of type I TNF-α receptor (TNFR-I) and type II TNF-α receptor (TNFR-II).60 TNF mRNA and protein levels are elevated in nasal polyp patients, which subsequently up-regulates CC chemokine ligand 2 (CCL2). The up-regulation of CCL2, represents one pathway in which TNF-α stimulates monocytes, thereby, regulating the pathogenesis of CRSwNP.61 In patients with CRS, mucosal levels of IL-1β, IL-6, IL-8, and TNF-α were all found to be elevated compared to controls.62 Systemic steroid treatment significantly reduces levels of TNF-α, IL-1β and corresponding inflammation.62
Chronic Rhinosinusitis and the Neuroimmune Biology of Sleep
There is a considerable amount of literature implicating the pro-inflammatory cytokines TNF-α and IL1-β in sleep regulation.63 TNF-α and IL1-β can signal via juxtacrine, autocrine, paracrine and even endocrine signaling pathways. Pro-inflammatory cytokines act within the CNS to induce sickness behavior (Figure 1). In humans, sleep loss and altered pro-inflammatory cytokine levels are associated with fatigue,64,65 pain,66 depression,67 impaired cognition68 and memory loss, 69 all of which are comorbidities of CRS.
Cytokines can exert their actions only if they are biologically active and if their receptors are present on target cells. For instance, current data shows: (1) IL-1 protein is present in normal brain and found primarily in neurons and glia; (2) IL-1 converting enzyme is found in normal brain, implicating biologically active IL-1; (3) IL-1 receptor proteins have a localized distribution in the brain primarily in the hypothalamus and hippocampus; (4) induction of NREMS by IL-1 and inhibition of NREMS by IL-1 antagonists; and (5) changes in IL-1 and its mRNA is associated with sleep.
TNF and IL-1 induce sleep when administered centrally or systemically.7 IL-1β increases NREMS in non-primates.5,70–72 At lower doses IL-1β increases sleep, whereas high-dose inhibits sleep.5 The higher doses of IL-1β are thought to activate negative feedback mechanisms including corticotrophin releasing hormone (CRH). Antagonists (e.g., antibodies and soluble receptors) of IL-1β and TNF-α decrease sleep and rebound after sleep deprivation, whereas substances that activate or up-regulate IL-1 (e.g., murayml dipeptide) increase sleep.
IL-1β and TNF-α mRNA in the brain are elevated during sleep and lower during wakefulness, and correlate with sleep propensity. These brain levels also increase during sleep deprivation. TNF and IL-1 proteins also vary with the sleep-wake cycle and correlate to sleep propensity.73,74 Additionally, plasma levels of TNF in humans correlate with EEG delta power (a measure of sleep intensity).75 Mice lacking the IL-1 Type 1 receptor sleep less during dark hours than do controls,76 whereas mice lacking the TNF-55-kD receptor have attenuated NREMS during daylight hours.77
TNF and IL-1 exert their sleep-promoting actions on sleep-active neurons. IL-1 receptors are expressed in the pre-optic area/anterior hypothalamus.76 Thus, IL-1β stimulates sleep-active neurons and inhibits wake-active neurons in the POA/anterior hypothalamus.78,79 Furthermore, microinjection of TNF-α into the POA and IL-1 into the subarachnoid space underlying the ventral surface of the rostral basal forebrain enhances NREMS.80,81
Other cytokines and growth factors linked to CRS have also been connected to sleep regulation (Table 1). Histamine has well known central effects on sleep regulation, although it may also play a role locally. Histamine and the H-1 receptor have been localized to the CNS and regulate the sleep-wake-cycle and arousal.82 One of the most common side effects of the first generation antihistamines includes drowsiness due to binding H-1 receptors centrally. Locally, histamine causes vasodilatation, vascular permeability and mucus production, which may impair sleep through nasal obstruction.
Cysteinyl leukotrienes (CystLTs) are well known for their effects locally on vascular permeability, mucus secretion and rhinorrhea, thereby causing nasal obstruction. Interestingly, nasal challenge studies with leukotrienes show at least a 10-fold greater potency of inducing nasal obstruction than histamine.83,84 Leukotrienes directly injected into the CNS have been shown to increase slow wave sleep85 and their expression has a diurnal variation.86
Cytokines, such as IL-1, IL-4, IL-6, IL-10 and IL-13 are released locally in CRS and have been linked to sleep regulation through their central effects. Of these IL-1 is the only interleukin that has met the published criteria of a somnogenic substance. We found that elevated expression of IL-4 and IL-13 was associated with worse sleep quality in patients with CRS.87 Likewise, increased expression IL-4 and IL-10 are correlated with sleep dysfunction in CRS.88 Intracerebral injections of IL- 4, IL-10 and IL-13 all decrease NREM sleep.89–91 Interestingly, IL-4 has been shown to play a critical role in higher brain functions including sleep, memory and learning. Caregivers with high peripheral circulating levels of IL-6 perceived their sleep poorer than caregivers with low levels as measured by the PSQI.92 Low-grade chronic systemic inflammation as measured by elevated IL-6 levels is associated with lower nighttime sleep93 and its expression has diurnal variation.94
CONCLUSION
Patients with CRS routinely demonstrate poor disease-specific and general QOL which may, in part, be secondary to sleep dysfunction. Nasal obstruction may be playing a role in sleep disordered breathing and/or overall sleep quality. Fatigue is a possible cofounder to sleep dysfunction in patients with CRS and needs to be independently evaluated and addressed. Immune mediators or “cytokines” convey to the brain that inflammation or infection has occurred in the periphery, although the mechanism of this communication is still unknown. Clinical evidence is accumulating to further illuminate the possibility that the subjective complaints of patients with CRS are either due to disease itself or the release of inflammatory mediators that are acting in the brain. Further investigation into this complex interaction will make it possible to design treatment strategies by blocking immune mediators, their downstream signaling molecules, or directly targeting these mediators.
SUMMARY.
Patients with CRS commonly report poor sleep quality that significantly correlates with poor QOL. Until recently, few studies have prospectively evaluated sleep in patients with CRS. Future work needs to specifically evaluate sleep in patients with CRS both utilizing patient-based and objective measures in a prospective manner.
Patients with CRS report increased levels of fatigue that have profound effects on QOL. Fatigue can be considered a confounder when evaluating sleep function in CRS and needs to be considered and controlled.
Patients with CRS commonly report nasal obstruction which may be playing a role in sleep dysfunction. It is not yet known whether improving nasal obstruction improves dysfunctional sleep in CRS, however data are accumulating that suggest improving nasal obstruction improves patient-perceived quality of sleep.
IL-1 and TNF expression and actions in the CNS have been implicated in the regulation of sleep and have been strongly associated with the pathophysiology of CRS. Injection of IL-1 increases sleep, antagonists decrease sleep, substances that activate or upregulate IL-1 increase sleep, substances that inhibit IL-1 in turn decrease NREM sleep, levels of protein and mRNA are diurnal and correlate to sleep propensity, mice with mutated IL1-R sleep less and sleep deprivation increases IL-1.
There are many other cytokines which have the capacity to enhance NREMS; the list includes IL1-α, IL2, IL15, IL18, acidic fibroblast growth factor (aFGF), nerve growth factor (NGF), TNF-β, IFN-gamma, EGF, and BDNF.
The mechanisms by which cytokines signal the CNS to cause sickness behavior in patients with CRS are unknown. Prospective studies examining systemic and local cytokine profiles and their association to sickness behavior in patients with CRS will be instrumental in further delineating the complex neuro-immune interactions.
Footnotes
Conflict of Interest: None
Financial Disclosures: Timothy L. Smith, MD, MPH is supported by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD. (2R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (http://www.clinicaltrials.gov) ID# NCT01332136. Timothy L. Smith, MD is also a consultant for Intersect ENT (Palo Alto, CA.) which is not affiliated in any way with this investigation.
References
- 1.Policy ACoSMaRBoHS. Sleep Disorders and Sleep Deprivation An Unmet Public Health Problem. National Academic Press; 2006. [PubMed] [Google Scholar]
- 2.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep Quality and Disease Severity in Patients with Chronic Rhinosinusitis. Laryngoscope. 2013 doi: 10.1002/lary.24040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells ME, Vaughn BV. Poor sleep challenging the health of a Nation. Neurodiagn J. 2012;52:233–249. [PubMed] [Google Scholar]
- 4.Serrano E, Neukirch F, Pribil C, et al. Nasal polyposis in France: impact on sleep and quality of life. J Laryngol Otol. 2005;119:543–549. doi: 10.1258/0022215054352108. [DOI] [PubMed] [Google Scholar]
- 5.Opp MR, Obal F, Jr, Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991;260:R52–58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- 6.Otto BA, Wenzel SE. The role of cytokines in chronic rhinosinusitis with nasal polyps. Curr Opin Otolaryngol Head Neck Surg. 2008;16:270–274. doi: 10.1097/MOO.0b013e3282fb2885. [DOI] [PubMed] [Google Scholar]
- 7.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 9.Alt JA, Obal F, Jr, Traynor TR, Gardi J, Majde JA, Krueger JM. Alterations in EEG activity and sleep after influenza viral infection in GHRH receptor-deficient mice. J Appl Physiol. 2003;95:460–468. doi: 10.1152/japplphysiol.01190.2002. [DOI] [PubMed] [Google Scholar]
- 10.Benninger MS, Benninger RM. The impact of allergic rhinitis on sexual activity, sleep, and fatigue. Allergy Asthma Proc. 2009;30:358–365. doi: 10.2500/aap.2009.30.3244. [DOI] [PubMed] [Google Scholar]
- 11.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) Otolaryngol Head Neck Surg. 2002;126:41–47. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo J, Edwards D, Haiduk A. Psychometric and clinimetric validity of the 31 item rhinosinusitis outcome measure (RSOM-31) Am J Rhinol. 1995;9:297–306. [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–164. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Neu D, Mairesse O, Hoffmann G, et al. Do ‘sleepy’ and ‘tired’ go together? Rasch analysis of the relationships between sleepiness, fatigue and nonrestorative sleep complaints in a nonclinical population sample. Neuroepidemiol. 2010;35:1–11. doi: 10.1159/000301714. [DOI] [PubMed] [Google Scholar]
- 18.Patarca R. Cytokines and chronic fatigue syndrome. Ann N Y Acad Sci. 2001;933:185–200. doi: 10.1111/j.1749-6632.2001.tb05824.x. [DOI] [PubMed] [Google Scholar]
- 19.Soler ZM, Smith TL. Quality of life outcomes after functional endoscopic sinus surgery. Otolaryngol Clin North Am. 2010;43:605–612. doi: 10.1016/j.otc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chester AC, Sindwani R, Smith TL, Bhattacharyya N. Fatigue improvement following endoscopic sinus surgery: a systematic review and meta-analysis. Laryngoscope. 2008;118:730–739. doi: 10.1097/MLG.0b013e318161e57b. [DOI] [PubMed] [Google Scholar]
- 21.Sautter NB, Mace J, Chester AC, Smith TL. The effects of endoscopic sinus surgery on level of fatigue in patients with chronic rhinosinusitis. Am J Rhinol. 2008;22:420–426. doi: 10.2500/ajr.2008.22.3196. [DOI] [PubMed] [Google Scholar]
- 22.Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882–1889. doi: 10.1002/ibd.21580. [DOI] [PubMed] [Google Scholar]
- 23.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 25.Neu D, Linkowski P, le Bon O. Clinical complaints of daytime sleepiness and fatigue: how to distinguish and treat them, especially when they become ‘excessive’ or ‘chronic’? Acta Neurol Belg. 2010;110:15–25. [PubMed] [Google Scholar]
- 26.Zwillich CW, Pickett C, Hanson FN, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis. 1981;124:158–160. doi: 10.1164/arrd.1981.124.2.158. [DOI] [PubMed] [Google Scholar]
- 27.Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest. 1986;90:324–329. doi: 10.1378/chest.90.3.324. [DOI] [PubMed] [Google Scholar]
- 28.Olsen KD, Kern EB. Nasal influences on snoring and obstructive sleep apnea. Mayo Clin Proc. 1990;65:1095–1105. doi: 10.1016/s0025-6196(12)62722-0. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99:S757–762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 30.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosun F, Kemikli K, Yetkin S, Ozgen F, Durmaz A, Gerek M. Impact of endoscopic sinus surgery on sleep quality in patients with chronic nasal obstruction due to nasal polyposis. J Craniofac Surg. 2009;20:446–449. doi: 10.1097/SCS.0b013e31819b97ef. [DOI] [PubMed] [Google Scholar]
- 32.Fokkens W, Lund V, Bachert C, et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy. 2005;60:583–601. doi: 10.1111/j.1398-9995.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 33.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22:297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 34.Lavie P, Gertner R, Zomer J, Podoshin L. Breathing disorders in sleep associated with “microarousals’ in patients with allergic rhinitis. Acta Otolaryngol. 1981;92:529–533. doi: 10.3109/00016488109133292. [DOI] [PubMed] [Google Scholar]
- 35.McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126:625–628. doi: 10.1164/arrd.1982.126.4.625. [DOI] [PubMed] [Google Scholar]
- 36.Nakata S, Noda A, Yasuma F, et al. Effects of nasal surgery on sleep quality in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol. 2008;22:59–63. doi: 10.2500/ajr.2008.22.3120. [DOI] [PubMed] [Google Scholar]
- 37.Li HY, Lee LA, Wang PC, Chen NH, Lin Y, Fang TJ. Nasal surgery for snoring in patients with obstructive sleep apnea. Laryngoscope. 2008;118:354–359. doi: 10.1097/MLG.0b013e318158f73f. [DOI] [PubMed] [Google Scholar]
- 38.Lindemann J, Keck T, Leiacker R, Dzida R, Wiesmiller K. Early influence of bilateral turbinoplasty combined with septoplasty on intranasal air conditioning. Am J Rhinol. 2008;22:542–545. doi: 10.2500/ajr.2008.22.3224. [DOI] [PubMed] [Google Scholar]
- 39.Li HY, Wang PC, Chen YP, Lee LA, Fang TJ, Lin HC. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25:45–49. doi: 10.2500/ajra.2011.25.3558. [DOI] [PubMed] [Google Scholar]
- 40.Li HY, Lin Y, Chen NH, Lee LA, Fang TJ, Wang PC. Improvement in quality of life after nasal surgery alone for patients with obstructive sleep apnea and nasal obstruction. Arch Otolaryngol Head Neck Surg. 2008;134:429–433. doi: 10.1001/archotol.134.4.429. [DOI] [PubMed] [Google Scholar]
- 41.Craig TJ, Mende C, Hughes K, Kakumanu S, Lehman EB, Chinchilli V. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc. 2003;24:53–58. [PubMed] [Google Scholar]
- 42.Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol. 1998;101:633–637. doi: 10.1016/s0091-6749(98)70171-x. [DOI] [PubMed] [Google Scholar]
- 43.Gurevich F, Glass C, Davies M, et al. The effect of intranasal steroid budesonide on the congestion-related sleep disturbance and daytime somnolence in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2005;26:268–274. [PubMed] [Google Scholar]
- 44.Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy. 2003;58:380–385. doi: 10.1034/j.1398-9995.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 45.Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004;59:50–55. [PMC free article] [PubMed] [Google Scholar]
- 46.Mintz M, Garcia J, Diener P, Liao Y, Dupclay L, Georges G. Triamcinolone acetonide aqueous nasal spray improves nocturnal rhinitis-related quality of life in patients treated in a primary care setting: the Quality of Sleep in Allergic Rhinitis study. Ann Allergy Asthma Immunol. 2004;92:255–261. doi: 10.1016/S1081-1206(10)61557-8. [DOI] [PubMed] [Google Scholar]
- 47.Kakumanu SS, Mende CN, Lehman EB, Hughes K, Craig TJ. Effect of topical nasal corticosteroids on patients with chronic fatigue syndrome and rhinitis. J Am Osteopath Assoc. 2003;103:423–427. [PubMed] [Google Scholar]
- 48.Colas C, Galera H, Anibarro B, et al. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAAR study) Clin Exp Allergy. 2012;42:1080–1087. doi: 10.1111/j.1365-2222.2011.03935.x. [DOI] [PubMed] [Google Scholar]
- 49.Ishimori K. True cause of sleep - a hypnogenic substance as evidenced in the brain sleep -deprived animals. Tokyo Igakkai Zasshi. 1909;23:429–459. [Google Scholar]
- 50.Krueger JM, Obal F, Jr, Fang J. Why we sleep: a theoretical view of sleep function. Sleep Med Rev. 1999;3:119–129. doi: 10.1016/s1087-0792(99)90019-9. [DOI] [PubMed] [Google Scholar]
- 51.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Bolger WE, Leonard D, Dick EJ, Jr, Stierna P. Gram negative sinusitis: a bacteriologic and histologic study in rabbits. Am J Rhinol. 1997;11:15–25. doi: 10.2500/105065897781446766. [DOI] [PubMed] [Google Scholar]
- 54.Ophir D, Hahn T, Schattner A, Wallach D, Aviel A. Tumor necrosis factor in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1988;114:1256–1258. doi: 10.1001/archotol.1988.01860230050021. [DOI] [PubMed] [Google Scholar]
- 55.Higgins GC, Foster JL, Postlethwaite AE. Interleukin 1 beta propeptide is detected intracellularly and extracellularly when human monocytes are stimulated with LPS in vitro. J Exp Med. 1994;180:607–614. doi: 10.1084/jem.180.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim DH, Jeon EJ, Park SN, Park KH, Park YS, Yeo SW. Effects of a tumor necrosis factor-alpha antagonist on experimentally induced rhinosinusitis. J Biomed Biotechnol. 2011;2011:360457. doi: 10.1155/2011/360457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erbek SS, Yurtcu E, Erbek S, Atac FB, Sahin FI, Cakmak O. Proinflammatory cytokine single nucleotide polymorphisms in nasal polyposis. Arch Otolaryngol Head Neck Surg. 2007;133:705–709. doi: 10.1001/archotol.133.7.705. [DOI] [PubMed] [Google Scholar]
- 58.Mfuna Endam L, Cormier C, Bosse Y, Filali-Mouhim A, Desrosiers M. Association of IL1A, IL1B, and TNF gene polymorphisms with chronic rhinosinusitis with and without nasal polyposis: A replication study. Arch Otolaryngol Head Neck Surg. 2010;136:187–192. doi: 10.1001/archoto.2009.219. [DOI] [PubMed] [Google Scholar]
- 59.Cormier C, Bosse Y, Mfuna L, Hudson TJ, Desrosiers M. Polymorphisms in the tumour necrosis factor alpha-induced protein 3 (TNFAIP3) gene are associated with chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2009;38:133–141. [PubMed] [Google Scholar]
- 60.Karosi T, Csomor P, Sziklai I. Tumor necrosis factor-alpha receptor expression correlates with mucosal changes and biofilm presence in chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2012;122:504–510. doi: 10.1002/lary.23190. [DOI] [PubMed] [Google Scholar]
- 61.Lin SK, Kok SH, Shun CT, et al. Tumor necrosis factor-alpha stimulates the expression of C-C chemokine ligand 2 gene in fibroblasts from the human nasal polyp through the pathways of mitogen-activated protein kinase. Am J Rhinol. 2007;21:251–255. doi: 10.2500/ajr.2007.21.2958. [DOI] [PubMed] [Google Scholar]
- 62.Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000;14:367–373. doi: 10.2500/105065800779954329. [DOI] [PubMed] [Google Scholar]
- 63.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 64.Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun. 2010;25:53–58. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis--a pilot study. Rheumatol Int. 2005;25:481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- 66.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 68.Baune BT, Ponath G, Rothermundt M, Riess O, Funke H, Berger K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Palin K, Bluthe RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1beta mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus Calmette-Guerin in the rat hippocampus. Brain Behav Immun. 2004;18:223–230. doi: 10.1016/j.bbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Opp MR, Krueger JM. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994;639:57–65. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- 71.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 72.Susic V, Totic S. “Recovery” function of sleep: effects of purified human interleukin-1 on the sleep and febrile response of cats. Metab Brain Dis. 1989;4:73–80. doi: 10.1007/BF00999497. [DOI] [PubMed] [Google Scholar]
- 73.Lue FA, Bail M, Jephthah-Ochola J, Carayanniotis K, Gorczynski R, Moldofsky H. Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. Int J Neurosci. 1988;42:179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- 74.Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- 75.Darko DF, Miller JC, Gallen C, et al. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- 77.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szymusiak R, Steininger T, Alam N, McGinty D. Preoptic area sleep-regulating mechanisms. Arch Ital Biol. 2001;139:77–92. [PubMed] [Google Scholar]
- 79.Alam MN, McGinty D, Bashir T, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 80.Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- 81.Terao A, Matsumura H, Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J Neurosci. 1998;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tashiro M, Mochizuki H, Iwabuchi K, et al. Roles of histamine in regulation of arousal and cognition: functional neuroimaging of histamine H1 receptors in human brain. Life Sci. 2002;72:409–414. doi: 10.1016/s0024-3205(02)02276-2. [DOI] [PubMed] [Google Scholar]
- 83.Okuda M, Watase T, Mezawa A, Liu CM. The role of leukotriene D4 in allergic rhinitis. Ann Allergy. 1988;60:537–540. [PubMed] [Google Scholar]
- 84.Miadonna A, Tedeschi A, Leggieri E, et al. Behavior and clinical relevance of histamine and leukotrienes C4 and B4 in grass pollen-induced rhinitis. Am Rev Respir Dis. 1987;136:357–362. doi: 10.1164/ajrccm/136.2.357. [DOI] [PubMed] [Google Scholar]
- 85.Sri Kantha S, Matsumura H, Kubo E, et al. Effects of prostaglandin D2, lipoxins and leukotrienes on sleep and brain temperature of rats. Prostaglandins Leukot Essent Fatty Acids. 1994;51:87–93. doi: 10.1016/0952-3278(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 86.Bellia V, Bonanno A, Cibella F, et al. Urinary leukotriene E4 in the assessment of nocturnal asthma. J Allergy Clin Immunol. 1996;97:735–741. doi: 10.1016/s0091-6749(96)80149-7. [DOI] [PubMed] [Google Scholar]
- 87.Alt JA, Sautter N, Mace JC, Smith TL. Anti-Somnogenic Cytokines, Quality of Life and Chronic Rhinosinusitis: a Pilot Study. Laryngoscope. 2013 doi: 10.1002/lary.24412. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krouse HJ, Davis JE, Krouse JH. Immune mediators in allergic rhinitis and sleep. Otolaryngol Head Neck Surg. 2002;126:607–613. doi: 10.1067/mhn.2002.125300. [DOI] [PubMed] [Google Scholar]
- 89.Kushikata T, Fang J, Krueger JM. Interleukin-10 inhibits spontaneous sleep in rabbits. J Interferon Cytokine Res. 1999;19:1025–1030. doi: 10.1089/107999099313244. [DOI] [PubMed] [Google Scholar]
- 90.Kushikata T, Fang J, Wang Y, Krueger JM. Interleukin-4 inhibits spontaneous sleep in rabbits. Am J Physiol. 1998;275:R1185–1191. doi: 10.1152/ajpregu.1998.275.4.R1185. [DOI] [PubMed] [Google Scholar]
- 91.Kubota T, Fang J, Kushikata T, Krueger JM. Interleukin-13 and transforming growth factor-beta1 inhibit spontaneous sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2000;279:R786–792. doi: 10.1152/ajpregu.2000.279.3.R786. [DOI] [PubMed] [Google Scholar]
- 92.von Kanel R, Dimsdale JE, Ancoli-Israel S, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 93.Mullington JM, Hinze-Selch D, Pollmacher T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Ann NY Acad Sci. 2001;933:201–210. doi: 10.1111/j.1749-6632.2001.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 94.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 95.Mullol J, Xaubet A, Lopez E, Roca-Ferrer J, Picado C. Comparative study of the effects of different glucocorticosteroids on eosinophil survival primed by cultured epithelial cell supernatants obtained from nasal mucosa and nasal polyps. Thorax. 1995;50:270–274. doi: 10.1136/thx.50.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Interleukin 1 receptor antagonist blocks somnogenic and pyrogenic responses to an interleukin 1 fragment. Proc Natl Acad Sci USA. 1992;89:3726–3730. doi: 10.1073/pnas.89.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1004–1012. doi: 10.1152/ajpregu.2001.281.3.R1004. [DOI] [PubMed] [Google Scholar]
- 98.Selezn’ov KH, Barynov EF, Iel’s’kyi KV, Ziablitsev SV. Interleukin-2 and interleukin-4 blood levels in sinusitis. Fiziol Zh. 2001;47:69–73. [PubMed] [Google Scholar]
- 99.Garcia-Garcia F, Yoshida H, Krueger JM. Interleukin-8 promotes non-rapid eye movement sleep in rabbits and rats. J Sleep Res. 2004;13:55–61. doi: 10.1111/j.1365-2869.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 100.Bauer J, Hohagen F, Ebert T, et al. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin Investig. 1994;72:315. doi: 10.1007/BF00180048. [DOI] [PubMed] [Google Scholar]
- 101.Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. J Neuroimmunol. 2003;137:59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 102.Ghaffar O, Lavigne F, Kamil A, Renzi P, Hamid Q. Interleukin-6 expression in chronic sinusitis: colocalization of gene transcripts to eosinophils, macrophages, T lymphocytes, and mast cells. Otolaryngol Head Neck Surg. 1998;118:504–511. doi: 10.1016/s0194-5998(98)70209-8. [DOI] [PubMed] [Google Scholar]
- 103.Opp MR, Smith EM, Hughes TK., Jr Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J Neuroimmunol. 1995;60:165–168. doi: 10.1016/0165-5728(95)00066-b. [DOI] [PubMed] [Google Scholar]
- 104.Toth LA, Opp MR. Cytokine- and microbially induced sleep responses of interleukin-10 deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1806–1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- 105.Jyonouchi H, Sun S, Le H, Rimell FL. Evidence of dysregulated cytokine production by sinus lavage and peripheral blood mononuclear cells in patients with treatment-resistant chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2001;127:1488–1494. doi: 10.1001/archotol.127.12.1488. [DOI] [PubMed] [Google Scholar]
- 106.al Ghamdi K, Ghaffar O, Small P, Frenkiel S, Hamid Q. IL-4 and IL-13 expression in chronic sinusitis: relationship with cellular infiltrate and effect of topical corticosteroid treatment. J Otolaryngol. 1997;26:160–166. [PubMed] [Google Scholar]
- 107.Reh DD, Ramanathan M, Jr, Sultan B, Wang Y, May L, Lane AP. The role of hepatocyte growth factor/c-Met in chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2010;24:266–270. doi: 10.2500/ajra.2010.24.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R404–413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 109.Xu R, Xu G, Shi J, Wen W. A correlative study of NF-kappaB activity and cytokines expression in human chronic nasal sinusitis. J Laryngol Otol. 2007;121:644–649. doi: 10.1017/S0022215106001824. [DOI] [PubMed] [Google Scholar]
- 110.Kubota T, Fang J, Brown RA, Krueger JM. Interleukin-18 promotes sleep in rabbits and rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R828–838. doi: 10.1152/ajpregu.2001.281.3.R828. [DOI] [PubMed] [Google Scholar]
- 111.Okano MFT, Makihara S, Fujiwara R, Higaki T, Kariya S, Noda Y, Haruna T, Nishizaki K. Characterization of IL-18 Expression and Release in the Pathogenesis of Chronic Rhinosinusitis. Int Arch Allergy Immunol. 2012;160:275–286. doi: 10.1159/000341668. [DOI] [PubMed] [Google Scholar]
- 112.Bohnet SG, Traynor TR, Majde JA, Kacsoh B, Krueger JM. Mice deficient in the interferon type I receptor have reduced REM sleep and altered hypothalamic hypocretin, prolactin and 2′,5′-oligoadenylate synthetase expression. Brain Res. 2004;1027:117–125. doi: 10.1016/j.brainres.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 113.Kubota T, Majde JA, Brown RA, Krueger JM. Tumor necrosis factor receptor fragment attenuates interferon-gamma-induced non-REM sleep in rabbits. J Neuroimmunol. 2001;119:192–198. doi: 10.1016/s0165-5728(01)00382-4. [DOI] [PubMed] [Google Scholar]
- 114.Shin SH, Ponikau JU, Sherris DA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Kou W, Hu GH, Yao HB, et al. Regulation of transforming growth factor-beta1 activation and expression in the tissue remodeling involved in chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2012;74:172–178. doi: 10.1159/000338799. [DOI] [PubMed] [Google Scholar]
- 116.Perez-Novo CA, Claeys C, Van Cauwenberge P, Bachert C. Expression of eicosanoid receptors subtypes and eosinophilic inflammation: implication on chronic rhinosinusitis. Respir Res. 2006;7:75. doi: 10.1186/1465-9921-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wisor JP, Clegern WC, Schmidt MA. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep. 2011;34:1335–1345. doi: 10.5665/SLEEP.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sartorius T, Lutz SZ, Hoene M, et al. Toll-like receptors 2 and 4 impair insulin-mediated brain activity by interleukin-6 and osteopontin and alter sleep architecture. FASEB J. 2012;26:1799–1809. doi: 10.1096/fj.11-191023. [DOI] [PubMed] [Google Scholar]
- 119.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006;20:117–123. [PMC free article] [PubMed] [Google Scholar]
- 120.Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- 121.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Krueger JM, Kapas L, Opp MR, Obal F., Jr Prostaglandins E2 and D2 have little effect on rabbit sleep. Physiol Behav. 1992;51:481–485. doi: 10.1016/0031-9384(92)90168-2. [DOI] [PubMed] [Google Scholar]
- 123.Zhang SQ, Inoue S, Kimura M. Sleep-promoting activity of prolactin-releasing peptide (PrRP) in the rat. Neuroreport. 2001;12:3173–3176. doi: 10.1097/00001756-200110290-00006. [DOI] [PubMed] [Google Scholar]
- 124.Obal F, Jr, Garcia-Garcia F, Kacsoh B, et al. Rapid eye movement sleep is reduced in prolactin-deficient mice. J Neurosci. 2005;25:10282–10289. doi: 10.1523/JNEUROSCI.2572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Platt MP, Soler ZM, Kao SY, Metson R, Stankovic KM. Topographic gene expression in the sinonasal cavity of patients with chronic sinusitis with polyps. Otolaryngol Head Neck Surg. 2011;145:171–175. doi: 10.1177/0194599811402030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- 127.Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigne S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 2012;16:265–279. doi: 10.1016/j.smrv.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Endam LM, Filali-Mouhim A, Bosse Y, Castano R, Desrosiers M. Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:e49–54. doi: 10.2500/ajra.2011.25.3588. [DOI] [PubMed] [Google Scholar]


