Abstract
Background
Clonorchiasis is an important zoonotic parasitic disease worldwide. Past estimates showed the prevalence increased based on studies undertaken ten years or more ago. However, control strategies, changing ecology and migration may have resulted to changes in the prevalence of clonorchiasis. The purpose of the present study was to analysis the prevalence and epidemiological characterisation of clonorchiasis in Heilongjiang Province, Northeast China.
Methodology/Principal Findings
A total of 4951 clinically suspected outpatients were examined from January 2009 to December 2012. Overall prevalence of clonorchiasis was 25.93% (1284/4951) by the combination strategy of the Kato-Katz technique (KK) and enzyme-linked immunosorbent assay (ELISA), with a significant increase from 22.53% in 2009 to 34.25% in 2012. Apart from Daxinganling city, clonorchiasis was reported throughout Heilongjiang Province and mainly along the Songhua River and Nen River basin, with an increased annual prevalence. The annual prevalence in men increased significantly in 2012 and was higher than that in women over 4 years. A similar pattern was seen for the annual infection rate in rural and urban areas. Farm labourers accounted for the majority of cases (65.93%), with a higher prevalence than in other occupations. Consumption of freshwater fish was considered the strongest risk factor of clonorchiasis. The infection rates in the 40–49 and 50–59 years age groups showed a significant increasing trend in 2012. Cases of re-infection were common.
Conclusions/Significance
The present study revealed that clonorchiasis remained widespread and prevalent in Heilongjiang Province. An integrated control programme is urgently needed to reduce the public health impact of clonorchiasis in this endemic area.
Introduction
Clonorchiasis, caused by Clonorchis sinensis (C. sinensis), is an important foodborne zoonotic disease, with an estimated 35 million people infected worldwide [1,2]. Humans and mammals can be infected by consuming raw or inadequately cooked freshwater fish containing the infective metacercariae. Infection by C. sinensis induces several pathological changes including chronic inflammation, periductal fibrosis, and epithelial desquamation in the bile duct. Hepatobiliary diseases such as cholangitis, hepatic fibrosis, and even cholangiocarcinoma and liver cancer can be associated with C. sinensis infection [3–5]. Recently, C. sinensis has been classified as a type 1 carcinogen to humans and among the neglected tropical diseases [6,7].
Clonorchiasis has been an important public health problem in China, especially in Heilongjiang Province [8]. According to national sampling surveys in China, the prevalence of clonorchiasis increased by 75% from 1990 to 2003, and Heilongjiang Province had been confirmed as an endemic focus [4,9]. However, this latest investigation had been published for ten years. Furthermore, a few recent large-scale studies have focused on prevalence of C. sinensis in this endemic region. It is imperative to evaluate further the epidemiological factors and prevalence due to changes in socioeconomic conditions, human behaviour and environmental factors.
The present cross-sectional investigation from January 2009 to December 2012 was performed on outpatients using a combination of the Kato–Katz (KK) method and ELISA. The aim of this study was to assess the change in prevalence and epidemiological characterisation of clonorchiasis in Heilongjiang Province, Northeast China over time, which will provide a basis for effective disease control planning and monitoring.
Materials and Methods
Ethics statement
The procedures of sample collection and use were approval by the Ethics Committees of Harbin Medical University. The objectives, procedures and potential risks were explained to all participants. Written informed consents were obtained from all adult participants and the parents or legal guardians of children. Individuals with positive fecal examination results were treated with a single 40 mg/kg dose of praziquantel three times a day.
Study area and participants
This study was conducted from January 2009 to December 2012 in Heilongjiang Province. The province is situated in the northeast of China, stretching from 121° 11' to 135° 05 'E longitude and 43° 25' to 53° 33' N latitude. The winters are cold and snowy, and the summers are hot and rainy. The province has an area of approximately half a million square kilometers, with about 38 million population. It owns 13 administrative regions, with the city of Harbin as its capital. In terms of ecology, 37% of the province is characterized by plains.
Based on the national standardized diagnostic criteria for suspended patients published by the Ministry of Health of China (WS309-2009), a total of 4951 outpatients (male 3635 and female 1316) raging in age from 5 to 86 years old were collected who referred to the Department of Parasitology, Harbin Medical University for parasite examination.
Questionnaire
An individual questionnaire was used to obtain information on socio-demographic variables, including name, age, sex, occupation, residence, history of eating raw freshwater fish and/or shellfish, as well as any previous history of the disease treatment recorded etc.
Immunological tests
Blood samples from all participants were collected and tested by well trained technicians using an indirect ELISA diagnostic kit from Shenzhen Combined Biotech Co. Ltd., China. The technicians were unaware of the subject’s medical status. The incubation procedure, washing steps and detection steps were carried out according to instructions supplied by the manufacturer. Absorbance was read at 450 nm zeroed by the reagent blank wells. For each run, positive and negative control sera were measured simultaneously. A positive result was defined as an optical density (OD) value greater than 2.1 times the OD value of the negative control serum provided by the kit.
Stool examination
The fecal samples were collected from each patient (1–3 specimens per patient) and detected C. sinensis eggs by the modified kato thick smear method (Kato-Katz technique) [10]. Briefly, the procedure of the method was as follows: each fecal sample was sieved through a fine screen and filled into a hole of a plastic template (41.7mg); the calibrated stool in slide was covered with cellophane soaked in glycerol and malachite green, then pressed against a hard surface so that the stool can spread evenly; after clarification overnight, triple slides were made from each stool specimen. Slides were read 1-12h after their initial preparation by two experienced technicians in a blinded manner. To maximize the diagnostic accuracy, the seropositive and egg-negative cases were re-examined by repeated egg counts and/or the number of KK slides. Patients were considered positive when at least one stool sample was found eggs of C. sinensis, which was considered to be the standard. The prevalence of C. sinesis infection was referred to as egg-positive rate.
Statistical analysis
SPSS (version 10.0 software for windows; Chicago, IL, USA ) was used for analyzing the date. "Number positive" in Table 1 through 3 was referred to the number of egg-positive individuals. The chi-square test was used to evaluate the assessment between qualitative variable to check for statistical differences. Unconditional multivariate analysis was used to calculate odds ratios (Ors) and corresponding 95% confidence intervals (CIs) of being C. sinensis egg positive according to various characteristics. P<0.05 was regarded as statistically significant.
Table 1. Prevalence of clonorchiasis among Patients in Heilongjiang Province, China.
| Year | No. examined | No. positive | Prevalence (%) |
|---|---|---|---|
| 2009 | 870 | 196 | 22.53 |
| 2010 | 1489 | 317 | 21.29 |
| 2011 | 1316 | 334 | 25.38 |
| 2012 | 1276 | 437 | 34.25* |
| Total | 4951 | 1284 | 25.93 |
P<0.001, significantly different from 2009 vs 2012, 2010 vs 2012, 2011 vs 2012.
Results
Annual trends of clonorchiasis prevalence
Within the last 4 years (2009–2012) a total of 4951 were requested for C. sinensis diagnosis and 1284 (25.93%) microscopically confirmed clonorchiasis cases. There was a fluctuating trend of clonorchiasis within the last 4 years with lower prevalence (21.29%; 317/1489) in 2010 and higher (34.25%; 437/1276) in 2012. In addition, the prevalence of clonorchiasis in 2012 increased significantly (P<0.05) (Table 1).
Geographical distribution of clonorchiasis
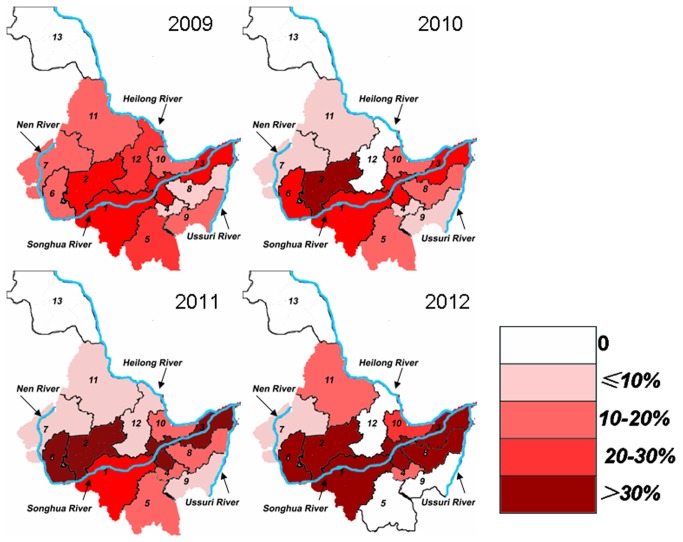
Clonorchiasis geographical distribution in various sites was shown in Table 2 and Figure 1. In the Songhua River and Nen River basin, the prevalence rate (35.67%; 412/1155) in 2012 was considerably higher than that in the past 3 years (P<0.05). The prevalence in Harbin, Daqing and Jiamusi cities remained relatively stable in 2009–2011, and increased significantly in 2012 (P<0.05). Although not significant, the prevalence rates in Suihua showed an increasing trend, Qiqihar showed a declining trend, and Qitaihe and Mudanjiang fluctuated.
Table 2. Geographical distribution of clonorchiasis among Patients in Heilongjiang Province.
| Locality a | 2009 |
2010 |
2011 |
2012 |
||||
|---|---|---|---|---|---|---|---|---|
| % | No./N | % | No./N | % | No./N | % | No./N | |
| Songhua River and Nen River | 23.60 | 181/767 | 22.41 | 298/1330 | 26.60‡ | 315/1184 | 35.67* | 412/1155 |
| (1) Harbin | 24.58 | 117/476 | 21.93 | 191/871 | 25.83 | 203/786 | 34.53* | 280/811 |
| (2) Suihua | 22.43 | 24/107 | 30.77 | 44/143 | 30.43 | 42/138 | 36.21 | 42/116 |
| (3) Jiamusi | 26.51 | 22/83 | 25.78 | 33/128 | 30.16 | 38/126 | 44.34† | 47/106 |
| (4) Qitaihe | 10.00 | 1/10 | 8.33 | 1/12 | 16.67 | 4/24 | 20.00 | 1/5 |
| (5) Mudanjiang | 30.00 | 6/20 | 11.11 | 4/36 | 16.67 | 2/12 | 0.00 | 0/6 |
| (6) Daqing | 14.81 | 8/54 | 22.12 | 23/104 | 31.25 | 25/80 | 45.05† | 41/91 |
| (7) Qiqihar | 17.65 | 3/17 | 5.56 | 2/36 | 5.56 | 1/18 | 5.00 | 1/20 |
| Ussuri River | 11.11 | 4/36 | 14.06 | 9/64 | 17.86 | 10/56 | 22.22 | 10/45 |
| (8) Shuangyashan | 8.33 | 2/24 | 16.67 | 7/42 | 20.00 | 9/45 | 32.26 | 10/31 |
| (9) Jixi | 16.67 | 2/12 | 9.09 | 2/22 | 9.09 | 1/11 | 0.00 | 0/14 |
| Heilong River | 16.42 | 11/67 | 10.53 | 10/95 | 11.84 | 9/76 | 19.74 | 15/76 |
| (10) Hegang | 18.52 | 5/27 | 15.09 | 8/53 | 25.93 | 7/27 | 28.89 | 13/45 |
| (11) Heihe | 16.00 | 4/25 | 7.69 | 2/26 | 5.00 | 1/20 | 13.33 | 2/15 |
| (12) Yichun | 25.00 | 2/8 | 0.00 | 0/4 | 4.17 | 1/24 | 0.00 | 0/9 |
| (13)Daxinganling area | 0.00 | 0/7 | 0.00 | 0/12 | 0.00 | 0/5 | 0.00 | 0/7 |
a locality from the province in Figure 1. No.: Number positive; N: Number examined; * p<0.05 2012 vs 2009, 2012 vs 2010, 2012 vs 2011; † p<0.05 2012 vs 2009, 2012 vs 2010; ‡p<0.05, 2010 vs 2011
Figure 1. Geographical distribution of clonorchiasis among Patients in Heilongjiang Province.
In the Ussuri River basins, the prevalence rate increased year by year from 2009 (11.11%; 4/36) to 2012 (22.22%; 10/45). The prevalence rates in Shuangyashan showed an upward tendency, and Jixi showed a downward trend within 4 years.
In the Heilong River basins, there was also a fluctuating trend of clonorchiasis within the last 4 years with lower prevalence (10.53%; 10/95) in 2010 and higher (19.74%; 15/76) in 2012. The prevalence of Hegang, Heihe and Yichun also showed fluctuated. However, no C. sinensis cases were detected in Daxinganling region.
The overall prevalence of the Songhua River and Nen River basin during the past 4 years was 27.19% (1206/4436), which was higher than in Heilong River (14.33%; 45/314) and Ussuri River (16.42%; 33/201) (P<0.05).
Age distribution of clonorchiasis in Heilongjiang Province
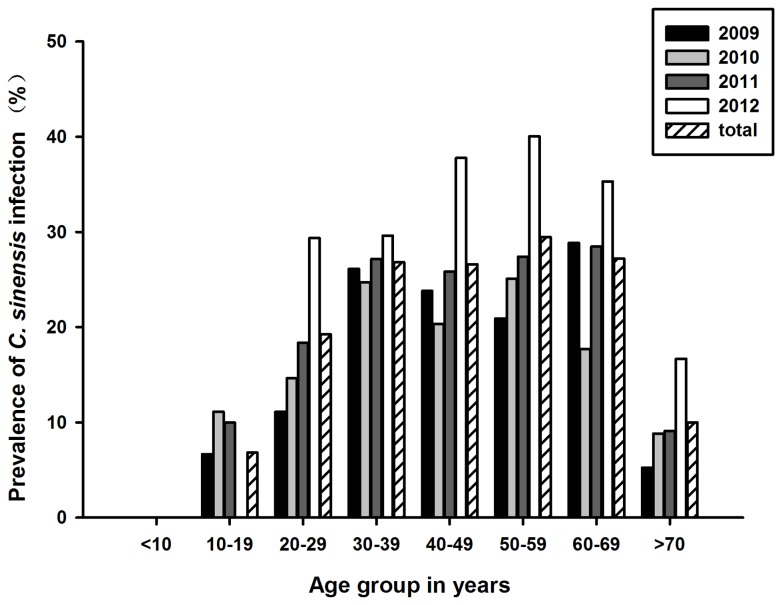
Clonorchiasis was reported in all age groups in the area except for <10 years group and the prevalence increased with age. The youngest person infected was 10 years old and the oldest was 82 years old. In 2009 and 2011 years, the prevalence rate reached a peak at 60–69 years with 28.85% and 28.46%, respectively. While in 2010 and 2012 years, it was higher in the 50–59 age group with a prevalence rate of 25.08% and 40.06%, respectively (Figure 2).
Figure 2. Prevalence of clonorchiasis in different age groups of Patients.
Epidemiological characteristics of clonorchiasis
In the last four years, males showed a significantly higher (P<0.001) egg-positive rate (28.67%) than females (18.39%). The rate of infection in rural areas (30.48%) was significantly higher than that found in the urban (14.11%) (P<0.001). The majority of human cases (82.00%; 4060/4951) had eaten raw freshwater fish and/or shellfish. In 4060 patients with a past history of ingestion, the infection rate was 31.13%. Raw freshwater fish consumption was strongly associated with clonorchiasis (OR=16.30; 95% CI=10.38–25.58). With regard to occupations, the farm labourers accounted for the majority 65.93% (3264/4951), with a higher prevalence (30.88%), followed by civil servants with the prevalence rate (18.29%), significantly greater (P<0.001) than the proportion of any other occupation (8.31%). There was no difference of prevalence in the history of treatment and the different each season, respectively. (Table 3).
Table 3. Univariate analysis of factors associated with clonorchiasis among Patients in Heilongjiang Province.
| Variable | Subcategory | Positive n (%) | Negative n (%) | β | SX | Wald χ2 | P |
|---|---|---|---|---|---|---|---|
| Total | 1284 (25.93) | 3667 (74.07) | |||||
| Gender | Male | 1042 (28.67) | 2593 (71.33) | ref. | |||
| Female | 242 (18.39) | 1074 (81.61) | -0.5785 | 0.0801 | 52.2263 | <0.001 | |
| Environment | Rural | 1090 (30.48) | 2486 (69.52) | ref. | |||
| Urban | 194 (14.11) | 1181 (85.89) | -0.9817 | 0.0856 | 131.6520 | <0.001 | |
| History of treatment | No | 1112 (25.96) | 3172 (74.04) | ref. | |||
| Yes | 172 (25.79) | 495 (74.21) | -0.0089 | 0.0951 | 0.0087 | 0.9259 | |
| Habit of eating raw fish | no | 20 (2.24) | 871 (97.76) | ref. | |||
| Yes | 1264 (31.13) | 2796 (68.87) | 2.9799 | 0.2287 | 169.8107 | <0.001 | |
| Season | Spring | 277 (26.26) | 778 (73.74) | ref. | |||
| Summer | 396 (26.67) | 1089 (73.33) | 0.0211 | 0.0913 | 0.0534 | 0.8172 | |
| Autumn | 322 (25.64) | 934 (74.36) | -0.0322 | 0.0952 | 0.1144 | 0.7352 | |
| Winter | 289 (25.02) | 866 (74.98) | -0.0647 | 0.0975 | 0.4408 | 0.5067 | |
| Occupation | Other | 27 (8.31) | 298 (91.69) | ref. | |||
| Farmer | 1008 (30.88) | 2256 (69.12) | 1.5956 | 0.2045 | 60.8691 | <0.001 | |
| Civil servants | 248 (18.29) | 1113 (81.71) | 0.9039 | 0.2129 | 18.0330 | <0.001 |
Multivariate logistic regression analysis using forward stepwise confirmed that sex, eating raw fish, location, and occupation were closely related to clonorchiasis (Table 4).
Table 4. Multivariate logistic regression analysis of factors associated with clonorchiasis among Patients in Heilongjiang Province.
| Variable | β | SX | Wald χ2 | P | OR (95%CI) |
|---|---|---|---|---|---|
| Intercept | -4.6803 | 0.3082 | 230.6364 | <0.001 | — |
| Gender | -0.3494 | 0.0848 | 16.9748 | <0.001 | 0.71 (0.60-0.83) |
| Habit of eating raw fish | 2.7910 | 0.2300 | 147.2322 | <0.001 | 16.30 (10.38-25.58) |
| Environment | -0.6111 | 0.1057 | 33.4441 | <0.001 | 0.54 (0.44-0.67) |
| Occupation | |||||
| Farmer / Other | 1.4100 | 0.2097 | 45.2066 | <0.001 | 4.10 (2.72-6.18) |
| Civil servants / Other | 1.1933 | 0.2186 | 29.7997 | <0.001 | 3.30 (2.15-5.06) |
Change of epidemiological characteristics in different patient populations
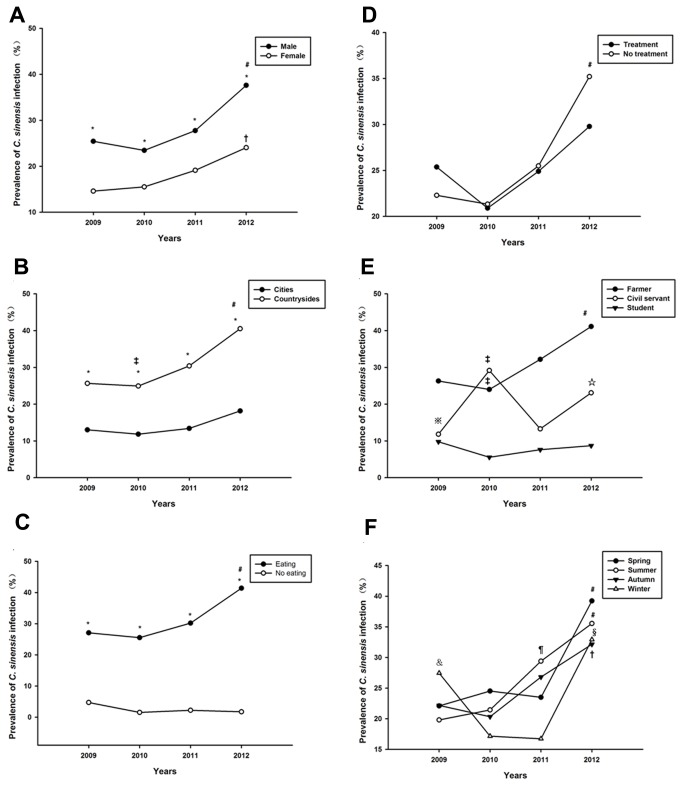
Figure 3 showed the changes in epidemiological characteristics of clonorchiasis in different patient populations. For the period 2009–2012, the annual prevalence in men increased significantly, and was higher than that in women every year (P<0.05) (Figure 3 A). A similar pattern was seen for the annual infection rate in surrounding and habit of eating raw fish, respectively. The annual prevalence in rural increased significantly, and was higher than that in urban areas every year (P<0.05) (Figure 3 B). Figure 3 C also showed that the infection rates of human cases had eaten raw freshwater fish owed a higher prevalence than that without habit.
Figure 3. Prevalence of clonorchiasis in different patient populations.
A. Gender. B. History of treatment. C. Environment. D. Occupation. E. Habit of eating raw fish. F. Season. *P<0.05, compared at the same time point of prevalence of Clonorchis sinensis infection in different patient populations. ﹟P<0.05, 2009 vs 2012, 2010 vs 2012, 2011 vs 2012; †p<0.05, 2009 vs 2012, 2010 vs 2012; § p<0.05, 2010 vs 2012, 2011 vs 2012; ☆p<0.05, 2011 vs 2012; ¶ p<0.05, 2009vs 2011, 2010 vs 2011; ‡p<0.05, 2010 vs 2011; &p<0.05, 2010vs 2009, 2011 vs 2009; ※p<0.05, 2010vs 2009, 2012vs 2009.
With regard to other factors including history of treatment, occupation and season, the annual infection rates revealed fluctuated irregularly (Figure 3 D, E, F).
Discussion
The findings from this study proved that the prevalence of clonorchiasis, as a representative food-borne parasitic disease, was still a serious public health problem in Heilongjiang Province. The prevalence of C. sinensis was 1.2% in 1988 [1,11]. However, the mean prevalence was 9.5% in studies conducted from 1988 to 2002, with 67 666 people from 21 different sites being subjected to fecal examination [1]. In the present study, 1284/4951 (25.93%) individuals were infected with C. sinensis over 4 years. The infection rate was lower than that of 31.6% in Hunan Province [12], but higher compared to that in other studies on the general population in Korea (16%) and Vietnam (17.2%) [13,14]. Different infection rates are related to many factors, including the sensitivity and specificity of diagnostic methods, the size and structure of specimens, the study subjects, and study areas. In addition, socioeconomic, demographic, biological and environmental factors are related to the transmission dynamics of C. sinensis [15].
From 2009 to 2012, the prevalence of clonorchiasis increased from 22.53% to 34.25%. Although other diseases such as schistosomiasis and soil-transmitted helminthiasis have declined significantly in China as a result of long-term treatment and control efforts [16,17], the prevalence of clonorchiasis has been consistently high. The change in epidemiological and socioeconomic factors, including more migration, more opportunities for eating, rapid growth of aquaculture, and lack of self-protection awareness has contributed to the high infection rates [18]. This present study suggests that our future parasite prevention and control work should focus on C. sinensis in this province.
In terms of geographical distribution, our data showed that clonorchiasis was widely prevalent in Heilongjiang Province, and mainly along the mainstream and tributaries of the Songhua River and its northern source Nen River. The reason might be that Songhua River and Nen River are important sites for freshwater fish in the northeast, such as carp, grass carp, and catfish.
Furthermore, there was a significant upward trend in cities located along the middle and lower reaches of the Songhua River. Besides, the infection rate in Suihua has been more than 30% since 2010. This may be attribute to rich fish resources in the basin and local residents like eating raw fish. Thus, further prevention and control should been strengthened in these cities, especially promoting public health education among local residents [19]. No infection was found in the Daxinganling area. It may be that this area is located in the North of Heilongjiang Province, around the Greater Higgnan Mountains, with a long winter and short summer, and the average temperature is low.
In this present study, men had a significantly higher egg-positive rate than women, which was consistent with other studies conducted in Vietnam, Korea and China [20–22]. Furthermore, the annual prevalence in men increased significantly. It is commonly assumed that this is due to the different way of life between men and women [23]. Men prefer to eat more fish and have more frequent social activities and eating opportunities at restaurants compared with women [14].
People of all ages are at risk and can be infected with C. sinensis. The prevalence rate increased gradually with age, reached a plateau among adolescents and young adults, and then decreased in elderly people. C. sinensis can survive for many years in the human body [1], therefore, this increase with age is considered to be the consequence of parasite accumulation. The decrease beyond the 70s is attributed to a higher death rate among infected people. Worryingly, the annual infection rate showed a significant increase in the 40–49 and 50–59 years groups. Therefore, they have become the main age groups that need prevention and treatment.
The increased annual infection rate in rural areas was probably due to poor sanitary conditions, poor self-protection awareness, and lack of large-scale prevention education. This leads to a greater economic burden in rural populations. Furthermore, the infection rate among farm labourers was significantly higher than in the previous 3 years. Therefore, there seems to be a greater need for treatment, prevention and control of C. sinensis in rural areas. The infection rate of the civil servants in 4 years was increased, which might have been related to the rapid development of freshwater industries, the relatively slow detection and quarantine, development of fish food, and increase in staff eating out [24].
The strongest risk factor is thought to be the consumption of raw or uncooked freshwater fish and/or shrimps [2]. This habit has been prevalent in this endemic area for a long time. It is difficult to change this habit in the short-term for prevention and control, so the infection rate remains high. Otherwise, some people without a history of raw fish consumption were also found to be infected. It is probable that these people are infected by accidental ingestion of C. sinensis metacercariae from kitchen knives, towels and hands contaminated after catching and handling freshwater fish [25]. If food hygiene supervision is reinforced, infection of these populations could be reduced to some extent.
With regard to seasonality, individuals infected with C. sinensis were found all year. There is more opportunity to eat fish and shrimps in the summer. Although the rivers froze in the winter, many cases of infection were also found at that time. That is because it might take a period time to find the C. sinensis eggs or adults from initial infection to pathogenesis.
It is interesting to note that was some re-infection after treatment, as a result of further consumption of raw fish. Re-infection is still common in endemic areas and may be the main cause of consistent transmission [26]. The majority of people believe that clonorchiasis can be easily treated with oral praziquantel and will not result in serious hepatobiliary disease. So, re-infection is a major obstacle to control of clonorchiasis in endemic areas. In addition, the practice of home animal slaughter and poor sanitary conditions also increase the risk of re-infection [27]. Therefore, long-term follow-up and health education should be undertaken instead of a short period of patient evaluation in this group of patients [19].
Currently, praziquantel-based symptomatic chemotherapy is still the main treatment strategy [28]. However, it has not reduced the infection rate and prevented the increase in endemic areas. A multi-component integrated control programme like the integrated prevention and control strategies for schistosomiasis is required, such as avoiding eating raw fish, enhancement of health education programmes, and elimination of intermediate host snails [18,19].
This study had some limitations that need to be considered. First, the subjects were outpatients, which might have contributed to selection bias. This should be taken into account when extrapolating these findings to the general population in Heilongjiang Province. Second, this was a laboratory-based study, so exhaustive information related to patients’ clinical variables was unknown. Finally, we did not investigate the intermediate and reservoir hosts.
In conclusion, the present study highlights that clonorchiasis remains a serious public health problem in Heilongjiang Province. Several risk factors have been shown to affect the prevalence, including eating raw freshwater fish and/or shellfish, sex, environment, and occupation. An integrated control programme including early diagnosis, medical intervention and promoting health awareness are imperative to reduce the public health impact of human clonorchiasis in this area of China.
Funding Statement
This work was supported by China, Heilongjiang postdoctoral Fund (Grant LRB08-304), Heilongjiang Province Education Bureau (Grant 12531347), Heilongjiang postdoctoral scientific research fund (Grant LBH-Q11056), Heilongjiang Province Ministry Bureau 2012-753, and Heilongjiang Province ordinary colleges and universities Medical Etiology (BSL3) Key Laboratory Open Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ et al. (2005) Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis 5: 31-41. doi: 10.1016/S1473-3099(04)01252-6. PubMed: 15620559. [DOI] [PubMed] [Google Scholar]
- 2. Keiser J, Utzinger J (2005) Emerging foodborne trematodiasis. Emerg Infect Dis 11: 1507-1514. doi: 10.3201/eid1110.050614. PubMed: 16318688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ et al. (2006) Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg 75: 93-96. PubMed: 16837714. [PubMed] [Google Scholar]
- 4. Rim HJ (2005) Clonorchiasis: an update. J Helminthol 79: 269-281. doi: 10.1079/JOH2005300. PubMed: 16153321. [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Jin Z, Da R, Dong Y, Song W et al. (2008) Fas/FasL-dependent apoptosis of hepatocytes induced in rat and patients with Clonorchis sinensis infection. Parasitol Res 103: 393-399. doi: 10.1007/s00436-008-0985-5. PubMed: 18427836. [DOI] [PubMed] [Google Scholar]
- 6. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B et al. (2009) A review of human carcinogens--Part B: biological agents. Lancet Oncol 10: 321-322. doi: 10.1016/S1470-2045(09)70096-8. PubMed: 19350698. [DOI] [PubMed] [Google Scholar]
- 7. Sripa B (2008) Concerted action is needed to tackle liver fluke infections in Asia. PLOS Negl Trop Dis 2: e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han S, Zhang X, Wen J, Li Y, Shu J et al. (2012) A combination of the kato-katz methods and ELISA to improve the diagnosis of clonorchiasis in an endemic area, china. PLOS ONE 7: e46977. doi: 10.1371/journal.pone.0046977. PubMed: 23056547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coordinating Office of the National Survey on the Important Human Parasitic Diseases (2005) A national survey on current status of the important parasitic diseases in human population. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 23: 332-340. PubMed: 16562464. [PubMed] [Google Scholar]
- 10. Hong ST, Choi MH, Kim CH, Chung BS, Ji Z (2003) The Kato-Katz method is reliable for diagnosis of Clonorchis sinensis infection. Diagn Microbiol Infect Dis 47: 345-347. doi: 10.1016/S0732-8893(03)00113-5. PubMed: 12967748. [DOI] [PubMed] [Google Scholar]
- 11. Niu H, Liu Y, Ren L (2001) Investigation on clonorchiasis in the Korean villages in Haerbing. Chin J Prevent Med 35: 308. [Google Scholar]
- 12. Yu SH, Kawanaka M, Li XM, Xu LQ, Lan CG et al. (2003) Epidemiological investigation on Clonorchis sinensis in human population in an area of South China. Jpn J Infect Dis 56: 168-171. [PubMed] [Google Scholar]
- 13. Kim BJ, Ock MS, Kim IS, Yeo UB (2002) Infection status of Clonorchis sinensis in residents of Hamyang-gun, Gyeongsangnam-do, Korea. Korean J Parasitol 40: 191-193. doi: 10.3347/kjp.2002.40.4.191. PubMed: 12509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nontasut P, Thong TV, Waikagul J, Anantaphruti MT, Fungladda W et al. (2003) Social and behavioral factors associated with Clonorchis infection in one commune located in the Red River Delta of Vietnam. Southeast Asian J Trop Med Public Health 34: 269-273. PubMed: 12971548. [PubMed] [Google Scholar]
- 15. Qian MB, Chen YD, Yan F (2013) Time to tackle clonorchiasis in China. Infect Dis Poverty 2: 4. doi: 10.1186/2049-9957-2-4. PubMed: 23849773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YD, Tang LH, Xu LQ (2008) Current status of soil-transmitted nematode infection in China Biomed Environ Sci 21: 173-179. PubMed: 18548859. [DOI] [PubMed] [Google Scholar]
- 17. Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW et al. (2005) The public health significance and control of schistosomiasis in China--then and now. Acta Trop 96: 97-105. doi: 10.1016/j.actatropica.2005.07.005. PubMed: 16125655. [DOI] [PubMed] [Google Scholar]
- 18. Wu W, Qian X, Huang Y, Hong Q (2012) A review of the control of clonorchiasis sinensis and Taenia solium taeniasis/cysticercosis in China. Parasitol Res 111: 1879-1884. doi: 10.1007/s00436-012-3152-y. PubMed: 23052782. [DOI] [PubMed] [Google Scholar]
- 19. Guoqing L, Xiaozhu H, Kanu S (2001) Epidemiology and control of Clonorchiasis sinensis in China. Southeast Asian J Trop Med Public Health 32 (Suppl 2): 8-11. PubMed: 12041609. [PubMed] [Google Scholar]
- 20. Dang TC, Yajima A, Nguyen VK, Montresor A (2008) Prevalence, intensity and risk factors for clonorchiasis and possible use of questionnaires to detect individuals at risk in northern Vietnam. Trans R Soc Trop Med Hyg 102: 1263-1268. doi: 10.1016/j.trstmh.2008.06.002. PubMed: 18632126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ju YH, Oh JK, Kong HJ, Sohn WM, Kim JI et al. (2005) Epidemiologic study of Clonorchis sinensis infestation in a rural area of Kyongsangnam-do, South Korea. J Prev Med Public Health 38: 425-430. [PubMed] [Google Scholar]
- 22. Peng ZQ, Geng YJ, Gao ST, Huang DN, Yu L et al. (2007) Epidemiological studies on Clonorchis sinensis infection along the Zhujiang River in Lou village of Shenzhen. Zhonghua Liu Xing Bing Xue Za Zhi 28: 544-546. PubMed: 17939380. [PubMed] [Google Scholar]
- 23. Qian MB, Chen YD, Fang YY, Xu LQ, Zhu TJ et al. (2011) Disability weight of Clonorchis sinensis infection: captured from community study and model simulation. PLOS Negl Trop Dis 5: e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ (2008) Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol 24: 190-196. doi: 10.1016/j.pt.2008.01.001. PubMed: 18314393. [DOI] [PubMed] [Google Scholar]
- 25. Kim HG, Han J, Kim MH, Cho KH, Shin IH et al. (2009) Prevalence of clonorchiasis in patients with gastrointestinal disease: a Korean nationwide multicenter survey. World J Gastroenterol 15: 86-94. doi: 10.3748/wjg.15.86. PubMed: 19115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi MH, Park SK, Li Z, Ji Z, Yu G et al. (2010) Effect of control strategies on prevalence, incidence and re-infection of clonorchiasis in endemic areas of China. PLOS Negl Trop Dis 4: e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Upatham ES, Viyanant V, Brockelman WY, Kurathong S, Lee P et al. (1988) Rate of re-infection by Opisthorchis viverrini in an endemic northeast Thai community after chemotherapy. Int J Parasitol 18: 643-649. doi: 10.1016/0020-7519(88)90099-9. PubMed: 3170073. [DOI] [PubMed] [Google Scholar]
- 28. Prichard RK, Basanez MG, Boatin BA, McCarthy JS, Garcia HH et al. (2013) A research agenda for helminth diseases of humans: intervention for control and elimination. PLOS Negl Trop Dis 6: e1549. [DOI] [PMC free article] [PubMed] [Google Scholar]