Abstract
This prospective case-controlled clinical study was undertaken to investigate to what extent the manually assisted treadmill stepping Locomotor Training with body weight support (LT) can change respiratory function in individuals with chronic Spinal Cord Injury (SCI). Pulmonary function outcomes (Forced Vital Capacity /FVC/, Forced Expiratory Volume one second /FEV1/, Maximum Inspiratory Pressure /PImax/, Maximum Expiratory Pressure /PEmax/) and surface electromyographic (sEMG) measures of respiratory muscles activity during respiratory taskswere obtained from eight individuals with chronic C3-T12 SCI before and after 62±10 (Mean ± SD) sessions of the LT. FVC, FEV1, PImax, PEmax, amount of overall sEMG activity and rate of motor unit recruitment were significantly increased after LT (p<0.05) These results suggest that these improvements induced by the LT are likely the result of neuroplastic changes in spinal neural circuitry responsible for the activation of respiratory muscles preserved after injury.
Keywords: Spinal Cord Injury, Respiratory Function, Motor Control, Locomotor Training
1. Introduction
Spinal cord injury (SCI) alters the control of respiratory muscles innervated below level of injury so as to cause weakness, paralysis and spasticity (Laffont et al., 2003; Zimmer et al., 2008), leading to, among other problems, respiratory insufficiency (Schilero et al., 2009) which significantly impedes recovery (Linn et al., 2000) and diminishes quality of life (Walter et al., 2002). In fact, respiratory deficiency and pneumonia are common secondary problems that are the leading causes of death in people with chronic SCI (Garshick et al., 2005).
Exercise programs involving limb muscles are known to increase fitness and improve ventilatory function in individuals with SCI (Hoffman, 1986; Le Foll-de Moro et al., 2005). It has been shown that robot-assisted locomotion in individuals with chronic SCI elicits a metabolic response characterized by increased oxygen consumption, minute-ventilation, and heart rate (Nash et al., 2004). Locomotor Training (LT) with stepping assisted by trained therapists (Harkema et al., 2011) and LT combined with functional electrical stimulation (Carvalho et al., 2005) in people with SCI were found to improve not only balance and gait function, but it has also been shown to increase oxygen consumption, heart rate and pulmonary ventilation. However, the mechanisms of this effect are not fully understood. The results of LT studies from our center indicate that spinal interneuronal networks are highly dependent on afferent feedback specific to the motor tasks providing intensive proprioceptive feedback to the spinal network and generating significant electromyographic activity in extremities and trunk muscles (Harkema, 2008; Harkema et al., 2003). Therefore, this study was designed to examine the effects of the LT, specifically the neuroplastic changes that might be induced, as physiologically measured by a surface electromyographic (sEMG) method to evaluate spinal motor output to the muscles of respiration.
2. Methods
This study was conducted in the Neuroscience Collaborative Center at the Frazier Rehab Institute after informed consent was obtained as approved by the Institutional Review Board for Human Research of the University of Louisville. Eight persons, one female and seven males, with chronic SCI, 25 ± 12 (mean ± SD) months post injury participated in this study (Table 1). Data from fourteen physically matched non-injured (NI) subjects, five females and nine males, with no history of respiratory dysfunction or smoking were used to calculate the normative sEMG-based values for volitional respiratory tasks (Ovechkin et al., 2010).
Table 1.
Characteristics of Spinal Cord Injured (SCI) participants
| Subjects (n = 8) | Age (years) | Sex | Height (cm) | Weight (Kg) | Level of SCI | AIS category | Time after SCI (mo) | Number of LT sessions |
|---|---|---|---|---|---|---|---|---|
| A33 (C3A) | 51 | M | 188 | 90 | C3 | AIS-A | 24 | 70 |
| A41 (C4A) | 19 | M | 180 | 82 | C4 | AIS-A | 17 | 56 |
| B07 (T2B) | 23 | M | 185 | 79 | T2 | AIS-B | 12 | 58 |
| C19 (C4C) | 59 | F | 155 | 59 | C4 | AIS-C | 36 | 62 |
| C09 (C7C) | 26 | M | 183 | 99 | C7 | AIS-C | 24 | 51 |
| C11 (T12C) | 31 | M | 170 | 77 | T12 | AIS-C | 24 | 58 |
| D20 (C5D) | 63 | M | 183 | 73 | C5 | AIS-D | 12 | 82 |
| D18(T5D) | 20 | M | 183 | 80 | T5 | AIS-D | 48 | 60 |
| Mean ± SD | 37 ±18 | N/A | 178 ±11 | 80 ±12 | N/A | N/A | 25 ±12 | 62 ±10 |
ID codes include the AIS level and category of each SCI subject.
2.1. Clinical assessment
The International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) (Kirshblum et al., 2011b) was used to determine the neurological level and clinical severity of the spinal cord lesion according to the American Spinal Cord Injury Association Impairment Scale (AIS) (Kirshblum et al., 2011a). The ISNCSCI categorizes based on the subjective estimation of voluntary contraction strengths from five upper-limb (C5 to T1) and five lower-limb (L2 to S1) muscles on each side. It also determines a sensory level for light touch and pin prick for C2 through S5 dermatomes. For this study, three SCI participants were classified as clinically motor-complete (AIS-A or AIS-B) and five were motor-incomplete (AIS-C or AIS-D), with neurological levels of SCI ranging from C3 to T12 (Tab. 1).
2.2. Pulmonary function test (PFT)
Standard spirometry testing was performed in the seated position before and after LT. Forced Vital Capacity (FVC) and Forced Expiratory Volume one second (FEV1) were obtained and expressed as the percent of a predicted value for each subject (Hart et al., 2005). Three acceptable spirograms were obtained and the result of the best attempt was used (American Thoracic Society/European Respiratory Society, 2002). A Differential Pressure Transducer (MP45-36-871-350) with UPC 2100 PC card and software from Validyne Engineering (Northridge, CA) was used to measure Maximum Inspiratory Pressure (PImax) and Maximum Expiratory Pressure (PEmax). The PImax was measured during maximal inspiratory effort beginning at near residual volume and PEmax was measured during maximal expiratory effort starting from near total lung capacity (American Thoracic Society/European Respiratory Society, 2002). Subjects were asked to use a three-way valve system with rubber tube as mouthpiece (Airlife 001504, Allegiance Healthcare Corp., McGaw Park, IL). The pressure meter incorporated a 1.5 mm diameter leak to prevent glottic closure and to reduce buccal muscle contribution during measurements (Griffiths and McConnell, 2007; Smyth et al., 1984). The assessment required that a sharp, forceful effort be maintained for a minimum of 2 seconds. The maximum pressure was taken as the highest value that is sustained for one second (Smyth et al., 1984). The maximum value was the average of three trials that varied by less than 20%.
2.3 Respiratory Motor Control Assessment (RMCA)
Surface electromyography (sEMG) and airway pressure were recorded simultaneously from selected respiratory muscles to measure the motor activity produced during selected voluntary motor tasks attempted in the supine position as previously published (Ovechkin et al., 2010). The RMCA protocol consisted of 5 minutes of quiet breathing followed by three repetitions each of Maximum Inspiratory Pressure Task (MIPT), Maximum Expiratory Pressure Task (MEPT), and cough. During MIPT/MEPT, subjects produced maximum inspiratory or expiratory efforts for 5 seconds, cued by an audible tone (Fig. 1 and 2). Pairs of recessed, silver-silver chloride cup surface electrodes (FE9, Grass Instruments, W Warwick, RI) were placed centered over the muscle belly and placed 3 cm apart for the right (R) and left (L): clavicular portion of Pectoralis (PEC) on the midclavicular line;6th Intercostals on the anterior axillary line (IC6); Rectus Abdominus at the umbilical level (RA); Oblique Abdominus on the midaxillary line at the umbilical level (OBL); and Paraspinal (PSP) at the iliac intercrestal line (American Thoracic Society/European Respiratory Society, 2002). Skin was prepared to reduce intra-electrode impedance. The ground electrode was placed over the acromion process. The incoming sEMG signals were amplified with a gain of 500, filtered at 30–1000 Hz and sampled at 2000 Hz using an Eclipse Neurological Workstation (Axon Systems Inc., Hauppauge, NY).
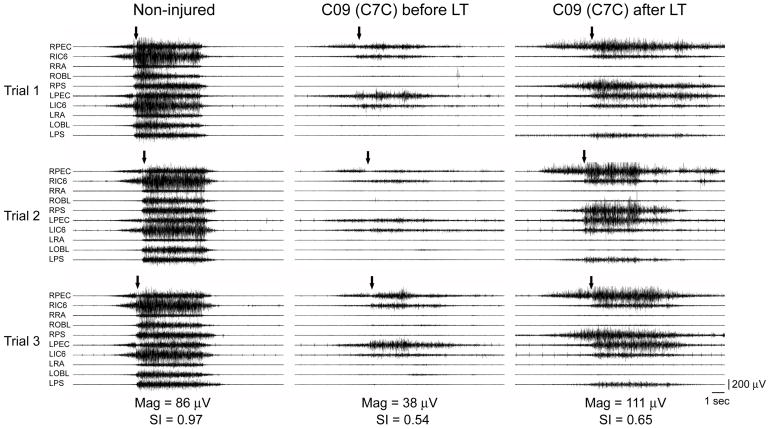
Fig. 1.
Surface electromyography (sEMG) recorded during a Maximum Expiratory Pressure Task (MEPT) in a Non-injured individual and a person (C09) with C7, AIS-C SCI. Muscles shown are right and left pectoralis (RPEC/LPEC), 6th intercostals (RIC6/LIC6), rectus abdominus (RRA/LRA), oblique abdominus (ROBL/LOBL), and paraspinals (RPS/LPS). Arrows indicate volitional initiation of the task. Note the considerably reduced amount of sEMG activity (Mag) before LT and its increase with more rapid recruitment of motor units and improvement in similarity index (SI) value after LT.
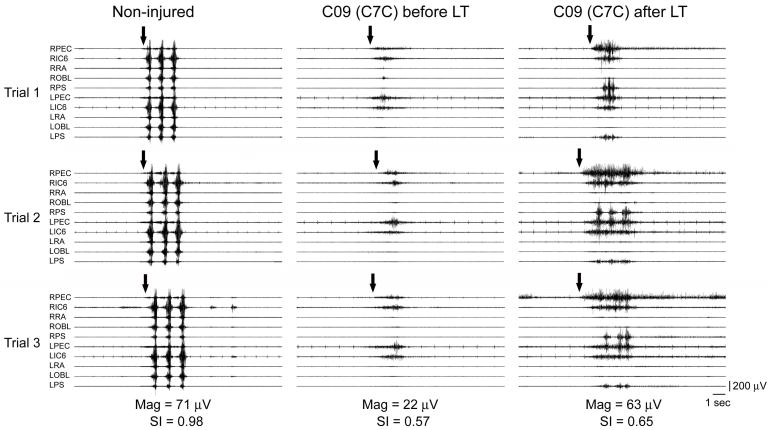
Fig. 2.
Surface electromyography (sEMG) recorded during a Coughing Task in a Non-injured individual and a person (C09) with C7, AIS-C (C7C) SCI. Muscles shown are right and left pectoralis (RPEC/LPEC), 6th intercostals (RIC6/LIC6), rectus abdominus (RRA/LRA), oblique abdominus (ROBL/LOBL), and paraspinals (RPS/LPS). Arrows indicate volitional initiation of the task. Note the improved ability to produce repeated coughs with more rapid recruitment of motor units after LT in the SCI subject.
2.4. sEMG data analysis
Multi-muscle activity distributions were analyzed for 5-second window provided by the event mark denoting the cuing tone for each of the MIPT and MEPT attempts and for the actual burst duration of each of the three cough attempts. The amount of sEMG activity for each muscle was calculated using a root mean square (RMS) algorithm (American Thoracic Society/European Respiratory Society, 2002; Sherwood et al., 2000), averaged for three repeated trials for each task (McKay et al., 2004) and used to calculate a vector-based voluntary response index (VRI) (Lee et al., 2004). The VRI calculation produces two values: Magnitude (Mag) and the Similarity Index (SI) for each maneuver. Mag represents the length of the Response Vector (RV) which is the resultant vector calculated from the RMS values for all muscles, that expresses the total amount of sEMG activity recorded during the time window analyzed. The SI quantifies how closely the multi-muscle activity distribution in a test subject pattern matches the Prototype Response Vector (PRV) developed from non-injured subjects for the same volitional task (Ovechkin et al., 2010). PRVs and RVs for all tasks examined in this study were calculated from the right and left PEC, IC6, RA, OBL and PSP muscles.
Motor unit recruitment rate was measured as the raising slope of the sEMG from the onset of the muscle activation to the peak amplitude using standard curve fitting analysis (MATLAB, The MatWorks, Natick, MA) for expiratory muscles: IC6, RA, and OBL. The onset of the muscle activation was defined as the moment when EMG reached levels higher than the mean background activity plus three-times its standard deviation. Values for three trials of each expiratory task (MEPT and cough) were averaged for each muscle to generate pre- and post-training values that were then compared with non-injured group (13), and to determine individual and group training effects.
2.5. Locomotor Training (LT)
Subjects underwent 62 ± 10 sessions (60 min/session; 5 days/week) of LT with manual stepping assistance. During training session subject was suspended by a harness (Robertson Harness, Henderson, NV) and body weight support system (Innoventor, St. Louis, MO) over a treadmill (Biodex Medical Systems, Shirley, NY) to provide the body weight support. The level of weight support was adjusted to maximize bilateral limb loading without the knee buckling during stance. A trainer positioned behind the subject aided in pelvis and trunk stabilization, as well as appropriate weight shifting and hip rotation during the step cycle. Trainers positioned at each limb provide manual assistance that facilitated knee extension during stance and knee flexion and toe clearance during swing. The treadmill speed was adjusted to promote the best stepping pattern at the given body weight load. Speeds were maintained within a normal walking speed range (0.89–1.34 m/s). Body weight support was continuously reduced over the course of the training sessions as subjects increased their ability to bear weight on the lower limbs (Beres-Jones and Harkema, 2004; Harkema et al., 2003).
2.6. Statistical analysis
A paired t-test to detect the difference for each independent parametric variable (FVC, FEV1, SI and raising slope speed) and Wilcoxon Signed-Rank Test for each non-parametric variable (PImax, PEmax and Mag) were used. Significance was reached at p = ≤.05. Statistical analysis was conducted using NCSS/PASS Software (v. 2002, Kaysville, UT).
3. Results
3.1. Pulmonary function test
Spirometrical measures of both inspiratory and expiratory function (FVC, PImax, and PEmax) were significantly increased post the LT as compared to the pre-LT values (Tab. 2). The increase in FEV1 was modest but still significant (p = 0.021).
Table 2.
Summary of Pulmonary Function Testing (PFT) values obtained before and after the Locomotor Training (LT)
| Subjects (n=8) | PFT | |||||||
|---|---|---|---|---|---|---|---|---|
| FVC (% predicted) | FEV1 (% predicted) | PImax (cm H2O) | PEmax (cm H2O) | |||||
| Before LT | After LT | Before LT | After LT | Before LT | After LT | Before LT | After LT | |
| A33 (C3A) | 31 | 61 | 36 | 41 | −66 | −71 | 28 | 48 |
| A41 (C4A) | 60 | 61 | 51 | 55 | −22 | −31 | 23 | 26 |
| B07 (T2B) | 85 | 95 | 85 | 86 | −70 | −91 | 69 | 89 |
| C19 (C4C) | 94 | 92 | 76 | 77 | −30 | −55 | 30 | 40 |
| C09 (C7C) | 70 | 73 | 61 | 61 | −78 | −80 | 70 | 85 |
| C11 (T12C) | 119 | 124 | 112 | 113 | −83 | −89 | 80 | 103 |
| D20 (C5D) | 99 | 97 | 100 | 101 | −93 | −81 | 75 | 73 |
| D18 (T5D) | 82 | 98 | 79 | 79 | −85 | −98 | 97 | 103 |
| Mean ± SD | 80 ±27 | 88±21* | 75 ±25 | 77 ±24* | −66 ± 26 | −75 ± 22* | 59 ±28 | 71 ± 29* |
Note that all PFT values (FVC. FEV1, PImax. and Pemax) were significantly increased after the LT (*p<0.05).
3.2. Respiratory muscle activation
During MEPT, baseline muscle activation patterns in SCI individuals were altered showing decreased or absent activity in expiratory muscles, bringing a reduction in calculated Mag and SI values (Fig. 1 and Tab. 3). Similarly altered activation patterns and associated VRI values were seen during the volitional coughing task (Fig. 2 and Tab 3). The overall amount of sEMG activity represented as Mag values was significantly increased after LT for all tasks (Tab. 3). At the same time, the SI values, which quantitatively describe the distribution of activity across recorded muscles, were not significantly changed after LT (Tab. 3).
Table 3.
Magnitude (Mag) and Similarity Index (SI) values obtained during Maximum Inspiratory & Expiratory Tasks (MIPT. MEPT) and Cough before and after the Locomotor training (LT)
| Subjects (n = 8) | Mag (μV) | SI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIPT | MEPT | Cough | MIPT | MEPT | Cough | |||||||
| Before LT | After LT | Before LT | After LT | Before LT | After LT | Before LT | After LT | Before LT | Alter LT | Before LT | After LT | |
| A33 (C3A) | 52 | 64 | 6 | 30 | 19 | 25 | 0.73 | 0.76 | 0.36 | 0.25 | 0.36 | 0.61 |
| A41 (C4A) | 62 | 65 | 69 | 75 | 30 | 36 | 0.79 | 0.78 | 0.59 | 0.41 | 0.51 | 0.52 |
| B07 (T2B) | 161 | 187 | 153 | 266 | 172 | 217 | 0.90 | 0.85 | 0.88 | 0.86 | 0.81 | 0.82 |
| C19 (C4C) | 50 | 56 | 24 | 29 | 28 | 71 | 0.80 | 0.77 | 0.61 | 0.61 | 0.47 | 0.36 |
| C09 (C7C) | 26 | 47 | 38 | 111 | 22 | 63 | 0.96 | 0.83 | 0.54 | 0.65 | 0.57 | 0.65 |
| C11 (T12C) | 8 | 15 | 52 | 94 | 80 | 97 | 0.82 | 0.83 | 0.66 | 0.79 | 0.90 | 0.95 |
| D20 (C5D) | 105 | 100 | 119 | 86 | 126 | 122 | 0.75 | 0.69 | 0.82 | 0.71 | 0.91 | 0.77 |
| D18 (T5D) | 29 | 57 | 50 | 98 | 45 | 119 | 0.73 | 0.68 | 0.91 | 0.91 | 0.92 | 0.94 |
| Mean ± SD | 62±49 | 74±51* | 64±49 | 99±74* | 65±57 | 94±61* | 0.81±0.08 | 0.78±0.06 | 0.68±0.18 | 0.65±0.22 | 0.70±0.22 | 0.70±0.21 |
Note the significant increase in Mag after LT for all tasks (*p<0.05, Wilcoxon Signed-Rank Test).
All three clinically motor complete subjects with AIS-A (A33; A41) or B (B07) categorization were able to volitionally activate trunk muscles caudal to their diagnosed motor level during the forced expiratory tasks. Conversely, one subject whose injury was diagnosed as being in the lumbar region, caudal to trunk innervation, showed quite poor pre-training activation of trunk muscles which increased after locomotor training but remained different from the NI subjects, lacking PEC activation during the MEPT (C11). Two other subjects (C09; C19) produced distribution patterns that were the same whether the task was inspiratory or expiratory. Common to all but one subject (D20) was an increase in sEMG amplitudes for all respiratory tasks following LT. This individual did not show an increase and had considerable long-lasting ongoing sEMG activity in many muscles, a pattern that was decreased following LT. Finally, after LT, only one subject developed an ability to activate, albeit at very low amplitudes, muscles not activated during pre-LT testing (A33).
The rate of motor unit recruitment, measured as the raising slope of the sEMG envelope during expiratory tasks, was lower and more variable within the SCI group than among NI control subjects (Fig. 3). Further, muscle activation was sometimes absent or too small to allow peak recognition in the SCI group. Finally, after LT, motor unit recruitment rate was faster as indicated by higher speed of raising slope (Fig. 3).
Fig. 3.
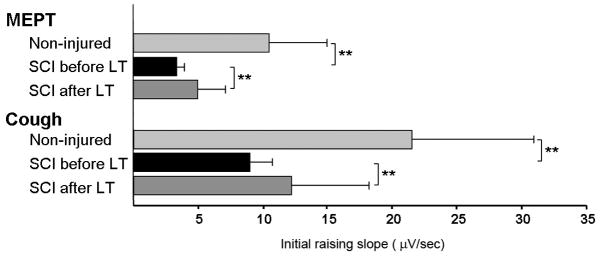
Group mean values for the speed of the sEMG raising slope from the onset to the peak of the muscle activity during MEPT and Cough. Note that prior to LT, the raising slope was significantly lower in the SCI group as compared to the control group (** p<.01). However, the slope was significantly increased post-LT as compared to the pre-LT values (** p<.01).
4. Discussion
It has been previously shown that expiratory related spirometrical values (FVC, FEV1, and PEmax) obtained during this study, correlate well with the clinically determined injury level (Linn et al., 2001; Mateus et al., 2007; Ovechkin et al., 2010). However, this finding is somewhat suspect if not irrelevant since the AIS does not truly examine trunk or respiratory muscles, but rather assumes that, within the thoracic region, motor function is at the same level as sensory function. Further, those with high cervical, clinically motor-complete lesions were able to activate trunk respiratory muscles caudal to their lesion, which would be indicative of clinically unrecognized incompleteness. Volitional inspiration was less impaired than expiration due to the fact that all SCI subjects in this study had lesions that spared phrenic innervation of the diaphragm. However, all participants showed significantly altered multi-muscle activation patterns for both inspiration and expiration tasks. Indeed, even in this relatively small number of SCI subjects, there was substantial diversity of lesion levels, severity and motor control. Regardless, the results showed that LT improved forced respiratory function primarily by increasing the rate and amount of muscle activation.
Although LT brought improved group average forced respiratory performance, one individual (D20) with considerable post-traumatic dystonia (McKay et al., 2011) of the trunk muscles did not reveal such increased respiratory function. This could be due to unorganized activity likely occurring within the spinal interneuronal circuitry obscuring the afferent input provided during training. Persons with such neurophysiological characteristics may not be ideal candidates for this training, but additional investigations in participants with similar characteristics may be needed to confirm this suggestion. Another subject (A33) acquired the ability to activate an additional muscle after LT but no pre-training parameters predicted such improvement. Otherwise, increases in spinal motor output to respiratory muscles occurred only in muscles that could be volitionally activated prior to training. This could be related to the fact that LT is not a task that is specific to respiratory control.
Improvements in pulmonary function in people with SCI have been known to result from general exercise training. For example, six weeks of wheelchair ergometer training by a group of subjects with SCI brought an increase in peak ventilation and tidal volumes (Le Foll-de Moro et al., 2005). Also, LT coupled with functional electrical stimulation improved metabolic and cardiorespiratory function in tetraplegic subjects (Carvalho et al., 2005). However, in another study of 10 subjects with incomplete SCI who remained passive during robot-assisted stepping, no such effect was found and they concluded that patients must actively contribute to the exercise in order to achieve the training intensity needed to increase cardiopulmonary fitness (Jack et al., 2011). Thus, the study suggests that LT, as administered here, contains the active component and the level of the training intensity that is prerequisite for respiratory improvement.
The current study examined volitional control of respiratory muscles during forced breathing tasks as opposed to automatic, bulbo-spinally managed quiet breathing (Butler, 2007) in which expiration is thought to be largely passive (De Troyer et al., 1987). Although the regulation of respiratory rate is largely thought to occur as a result of changes in blood-gas concentrations (Saupe et al., 1992), there is evidence from cat models that vestibular nuclei of the brainstem also modulate respiratory motor output (Yates et al., 1993) and that postural changes altered activation of diaphragm and abdominal muscles (Cotter et al., 2001). Further, peripheral nerve input from sensory end organs in the trachea and lungs (Coleridge and Coleridge, 1986) and muscles (Aminoff and Sears, 1971; De Troyer et al., 1986; De Troyer et al., 2005; Decima et al., 1969; Murray et al., 2008) have been shown to contribute to respiratory regulation. In addition, volitional effort likely plays an active role in respiratory control and the effects of LT on respiratory function. From studies carried out in humans, increased metabolic activation was shown to occur in regions of the motor cortex associated with respiratory muscle control and brainstem respiratory centers during voluntary breathing (McKay et al., 2003). Also, leg exercise increased activation of the cerebral cortex and limbic and reticular activating systems (Bell, 2006). Finally, corticospinal tract fibers from the motor cortex have been shown to functionally connect to the spinal motor circuitry for the diaphragm (Davey et al., 1996; Gandevia and Rothwell, 1987), intercostal (Ellaway et al., 2007; Misawa et al., 2001), pectoralis (Bawa et al., 2004), rectus abdominus (Tsao et al., 2008) oblique (Strutton et al., 2004) and paraspinal (Nowicky et al., 2001) muscles to provide voluntary control during inspiration and expiration. Thus, all of these mechanisms would likely be utilized during LT to increase respiratory muscle activity that could lead to neuroplastic changes in their control. Further, all SCI subjects in this study had some ability to volitionally activate, to varying degrees, some of the recorded trunk muscles demonstrating that at least some surviving long-tract fibers reached the spinal motor nuclei for their muscles of respiration.
In non-injured individuals, positron emission tomography has been able to show that there is increased regional cerebral blood flow in the primary motor cortex of the legs and regions associated with volitional breathing immediately after leg exercising and bicycling (Fink et al., 1995). Thus, the corticospinal output to the spinal motor nuclei of respiratory muscles can be increased by exercise involving the legs as in the current study. Further, for leg muscles, locomotor training has been shown to improve corticospinal connectivity to spinal motor circuitry for leg muscles in people with chronic incomplete SCI (Thomas and Gorassini, 2005). The increased responsiveness to transcranial motor cortex stimulation that they observed was not likely due to any increase in the number of long tract fibers conduction across there chronic lesions but rather from increased synaptic strength in surviving connections (Brus-Ramer et al., 2007; Warraich and Kleim, 2010) or new synaptic connections resulting from increased arborization of the surviving long-tract fibers on the spinal level (Fouad and Tse, 2008).
It has been suggested that sEMG signal acquired during a voluntary muscle contraction is a summation of all the active motor units contributes (Basmajian et al., 1985), and it is largely dependent on the properties of motor units and their firing patterns as well as muscle innervation zones (Saitou et al., 2000). In addition, muscle strength is affected by peripheral factors associated with electrophysiological and anatomical properties of the muscles itself (Reyes et al., 2013). It is reasonable to expect that these same mechanisms were available to the circuitry driving respiratory muscle activation and peripheral changes resulting in in morphological and functional changes. These mechanisms may include muscle hypertrophy (Ruther et al., 1995; Schoenfeld, 2010), change in muscle fiber types and size (Andersen and Aagaard, 2010), altered activation of fast- and slow-twitch fibers (Orozco-Levi, 2003) and changes at the cellular level (Baar, 2006). However, this study does not provide a direct means of separating the effects of alterations in muscular and neural factors in response to the LT.
Therefore, these results suggest that even though LT does not directly drive contractions of respiratory muscles, the metabolic changes, cortical and peripheral inputs to the neural control of respiration can induce neuroplastic change within the neural circuitry that sub-serves respiration. However, the changes were confined largely to the rate and amount of muscle activation rather than the pattern of multi-muscle distribution. It remains to be seen if more specific exercise such as strength training of respiration by loading inspiration and expiration might bring changes in multi-muscle distribution as well. Further study is needed to determine the degree to which motor control of respiration and respiratory muscle function can be improved through physical training and to elucidate the mechanisms of such improvements.
5. Conclusion
LT significantly improves pulmonary function in individuals with chronic SCI. These improvements are likely the result of neuroplastic changes in spinal neural circuitry responsible for the activation of respiratory muscles preserved after injury.
Acknowledgments
This work was supported by grants from University of Louisville (UofL/IRI 50814); Paralyzed Veterans of America Research Foundation (PVA RFG 2613); Christopher and Dana Reeves Foundation (CDRF OA2-0802-2); and National Institutes of Health (R01NS049209, P01NS16333, and 1R01HL103750).
References
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Aminoff MJ, Sears TA. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971;215:557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Aagaard P. Effects of strength training on muscle fiber types and size; consequences for athletes training for high-intensity sport. Scand J Med Sci Sports. 2010;20(Suppl 2):32–38. doi: 10.1111/j.1600-0838.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- Baar K. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc. 2006;38:1939–1944. doi: 10.1249/01.mss.0000233799.62153.19. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Gopal DN, Ghista DN. Electrodiagnostic model for motor unit action potential (MUAP) generation. Am J Phys Med. 1985;64:279–294. [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res. 2004;158:385–390. doi: 10.1007/s00221-004-2031-x. [DOI] [PubMed] [Google Scholar]
- Bell HJ. Respiratory control at exercise onset: an integrated systems perspective. Respir Physiol Neurobiol. 2006 May;152(1):1–15. doi: 10.1016/j.resp.2006.02.005. Epub 2006 Mar 13 1521–15. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159:115–126. doi: 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Carvalho DC, de Cassia ZM, Sereni JM, Cliquet A. Metabolic and cardiorespiratory responses of tetraplegic subjects during treadmill walking using neuromuscular electrical stimulation and partial body weight support. Spinal Cord. 2005;43:400–405. doi: 10.1038/sj.sc.3101730. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Reflexes evoked from the tracheobronchial tree and lungs. American Physiological Society; Bethesda, USA: 1986. [Google Scholar]
- Cotter LA, Arendt HE, Jasko JG, Sprando C, Cass SP, Yates BJ. Effects of postural changes and vestibular lesions on diaphragm and rectus abdominis activity in awake cats. J Appl Physiol. 2001;91:137–144. doi: 10.1152/jappl.2001.91.1.137. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Murphy K, Maskill DW, Guz A, Ellaway PH. Site of facilitation of diaphragm EMG to corticospinal stimulation during inspiration. Respir Physiol. 1996;106:127–135. doi: 10.1016/s0034-5687(96)00069-2. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M, Heilporn A. Mechanism of active expiration in tetraplegic subjects. N Engl J Med. 1986;314:740–744. doi: 10.1056/NEJM198603203141203. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiological Reviews. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Ninane V, Gilmartin JJ, Lemerre C, Estenne M. Triangularis sterni muscle use in supine humans. J Appl Physiol. 1987;62:919–925. doi: 10.1152/jappl.1987.62.3.919. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand. 1969;75:568–579. [PubMed] [Google Scholar]
- Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, Jamous A, Savic G. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev. 2007;44:69–76. doi: 10.1682/jrrd.2005.08.0140. [DOI] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS, et al. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol. 1995;489 ( Pt 3):663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30:17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol. 1987;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths LA, McConnell AK. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur J Appl Physiol. 2007;99:457–466. doi: 10.1007/s00421-006-0367-6. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57:255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Beres-Jones JA, Ferreira C. Neural adaptation with locomotor training in the functionally isolated human spinal cord. Society For Neuroscience. 2003:493.10. [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz D, Edgerton VR, Behrman AL. Balance and Ambulation Improvements in Individuals With Chronic Incomplete Spinal Cord Injury Using Locomotor Training-Based Rehabilitation. Arch Phys Med Rehabil. 2011 doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Hart N, Laffont I, de la Sota AP, Lejaille M, Macadou G, Polkey MI, Denys P, Lofaso F. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Arch Phys Med Rehabil. 2005;86:1447–1451. doi: 10.1016/j.apmr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Hoffman MD. Cardiorespiratory fitness and training in quadriplegics and paraplegics. Sports Med. 1986;3:312–330. doi: 10.2165/00007256-198603050-00002. [DOI] [PubMed] [Google Scholar]
- Jack LP, Purcell M, Allan DB, Hunt KJ. The metabolic cost of passive walking during robotics-assisted treadmill exercise. Technol Health Care. 2011;19:21–27. doi: 10.3233/THC-2011-0608. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011a;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011b;34:547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont I, Durand MC, Rech C, De La Sotta AP, Hart N, Dizien O, Lofaso F. Breathlessness associated with abdominal spastic contraction in a patient with C4 tetraplegia: a case report. Arch Phys Med Rehabil. 2003;84:906–908. doi: 10.1016/s0003-9993(02)04898-0. [DOI] [PubMed] [Google Scholar]
- Le Foll-de Moro D, Tordi N, Lonsdorfer E, Lonsdorfer J. Ventilation efficiency and pulmonary function after a wheelchair interval-training program in subjects with recent spinal cord injury. Arch Phys Med Rehabil. 2005;86:1582–1586. doi: 10.1016/j.apmr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Lee DC, Lim HK, McKay WB, Priebe MM, Holmes SA, Sherwood AM. Toward an objective interpretation of surface EMG patterns: a voluntary response index (VRI) J Electromyogr Kinesiol. 2004;14:379–388. doi: 10.1016/j.jelekin.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Linn WS, Spungen AM, Gong H, Jr, Adkins RH, Bauman WA, Waters RL. Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord. 2001;39:263–268. doi: 10.1038/sj.sc.3101155. [DOI] [PubMed] [Google Scholar]
- Mateus SR, Beraldo PS, Horan TA. Maximal static mouth respiratory pressure in spinal cord injured patients: correlation with motor level. Spinal Cord. 2007;45:569–575. doi: 10.1038/sj.sc.3101998. [DOI] [PubMed] [Google Scholar]
- McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil Neural Repair. 2004;18:144–153. doi: 10.1177/0888439004267674. [DOI] [PubMed] [Google Scholar]
- McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Long-lasting involuntary motor activity after spinal cord injury. Spinal Cord. 2011;49:87–93. doi: 10.1038/sc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa T, Ebara S, Kamimura M, Tateiwa Y, Kinoshita T, Takaoka K. Evaluation of thoracic myelopathy by transcranial magnetic stimulation. J Spinal Disord. 2001;14:439–444. doi: 10.1097/00002517-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Murray NP, McKenzie DK, Gorman RB, Gandevia SC, Butler JE. Reproducibility of the short-latency reflex inhibition to loading of human inspiratory muscles. Respir Physiol Neurobiol. 2008;162:216–222. doi: 10.1016/j.resp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Nash MS, Jacobs PL, Johnson BM, Field-Fote E. Metabolic and cardiac responses to robotic-assisted locomotion in motor-complete tetraplegia: a case report. J Spinal Cord Med. 2004;27:78–82. doi: 10.1080/10790268.2004.11753734. [DOI] [PubMed] [Google Scholar]
- Nowicky AV, McGregor AH, Davey NJ. Corticospinal control of human erector spinae muscles. Motor Control. 2001;5:270–280. doi: 10.1123/mcj.5.3.270. [DOI] [PubMed] [Google Scholar]
- Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J Suppl. 2003;46:41s–51s. doi: 10.1183/09031936.03.00004607. [DOI] [PubMed] [Google Scholar]
- Ovechkin A, Vitaz T, Terson de Paleville D, Aslan S, McKay W. Evaluation of respiratory muscle activation in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2010;173:171–178. doi: 10.1016/j.resp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Reyes A, Ziman M, Nosaka K. Respiratory muscle training for respiratory deficits in neurodegenerative disorders: a systematic review. Chest. 2013;143:1386–1394. doi: 10.1378/chest.12-1442. [DOI] [PubMed] [Google Scholar]
- Ruther CL, Golden CL, Harris RT, Dudley GA. Hypertrophy, resistance training and the nature of skeletal muslce activation. J Strength Cond Res. 1995;9:155–159. [Google Scholar]
- Saitou K, Masuda T, Michikami D, Kojima R, Okada M. Innervation zones of the upper and lower limb muscles estimated by using multichannel surface EMG. Journal of human ergology. 2000;29:35–52. [PubMed] [Google Scholar]
- Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory muscle recruitment during selective central and peripheral chemoreceptor stimulation in awake dogs. J Physiol. 1992;448:613–631. doi: 10.1113/jphysiol.1992.sp019061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129–141. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- Sherwood AM, Graves DE, Priebe MM. Altered motor control and spasticity after spinal cord injury: subjective and objective assessment. J Rehabil Res Dev. 2000;37:41–52. [PubMed] [Google Scholar]
- Smyth RJ, Chapman KR, Rebuck AS. Maximal inspiratory and expiratory pressures in adolescents. Normal values Chest. 1984;86:568–572. doi: 10.1378/chest.86.4.568. [DOI] [PubMed] [Google Scholar]
- Strutton PH, Beith ID, Theodorou S, Catley M, McGregor AH, Davey NJ. Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. Exp Brain Res. 2004;158:474–479. doi: 10.1007/s00221-004-1939-5. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- Tsao H, Galea MP, Hodges PW. Concurrent excitation of the opposite motor cortex during transcranial magnetic stimulation to activate the abdominal muscles. J Neurosci Methods. 2008;171:132–139. doi: 10.1016/j.jneumeth.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, Wheeler JS. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev. 2002;39:53–61. [PubMed] [Google Scholar]
- Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PM R. 2010;2:S208–219. doi: 10.1016/j.pmrj.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res. 1993;629:209–217. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol. 2008;209:399–406. doi: 10.1016/j.expneurol.2007.05.015. [DOI] [PubMed] [Google Scholar]