Abstract
HIV-derived lentiviral vectors have been widely employed to transduce non-dividing cells, such hematopoietic stem cells (HSC), in the setting of gene therapy. Here, we screened lentiviral vectors for their ability to drive expression of the murine MHC class II (MHCII) chaperone, invariant chain (Ii) and a GFP reporter. The vectors included T2A vector with T2A-separated Ii and GFP under the same MSCV promoter, dual-promoter vectors with separate promoters for Ii and GFP (called MSCV or EF1a according to the promoter driving Ii expression), and a vector with EF1a driving a fusion of Ii/GFP (called Fusion vector). T2A and MSCV induced highest levels of Ii and GFP expression, respectively, after direct transfection of 293T cells. All vectors except the Fusion vector drove expression of functional Ii, based on the enhancement of MHC II level, which is a known consequence of Ii expression. Comparing the vectors after they were packaged into lentiviruses and used to transduce 293T, we found MSCV and EF1a vectors mediated higher Ii and GFP expression. In ckit+ bone marrow (BM) cells, MSCV still induced the highest Ii and GFP expression, whereas EF1a only induced robust Ii expression. Regardless of the vector, both Ii and GFP levels were significantly reduced in BM cells compared to 293T cells. When in vivo expression was assessed in cells derived from MSCV-transduced BM-HSC, up to 80% of myeloid cells were GFP+, but no Ii expression was observed. In contrast, transplantation of EF1a-transduced BM-HSC led to much higher in vivo Ii expression. Thus, among those compared, dual-promoter vector-based lentivirus with the EF1a promoter driving the gene of interest is optimal for murine BM-HSC transduction.
HIV-based lentiviral vectors with large coding capability, relative high transduction efficiency and low immunogenicity have been widely used as gene delivery vectors[1]. The stable expression of transgenes after their integration into host genome in both dividing and non-dividing mammalian cells makes these vectors suitable for transduction of quiescent hematopoietic stem cells (HSC). The unique self-renewal and differentiation capabilities of HSC guarantee that the transgenes expressed in a small number of HSC result in long term gene correction of much greater numbers of mature progeny, making HSC promising target cells for leukemia, genetic anemia and immunodeficiency[2-4]. However, the low titer of lentiviral vectors carrying large transgene cassettes and thus lower than desired multiplicity of infection (MOI) leading to low transduction efficiency of HSC even after cytokine activation, as well as the low engraftment of HSC, remain key challenges for the applications of lentiviral vectors in preclinical and clinical settings[5, 6].
Two transgenes can be expressed by a single lentiviral vector under discrete promoters (i.e. dual-promoter) or under the same promoter, in the presence or absence of a separating sequence, like an internal ribosomal entry site (IRES) or a T2A site[7]. IRES is a nucleotide sequence mediating new translation initiation for the 2nd gene; however its expression is usually lower than that of the 1st gene[8]. The Thosea asigna virus-derived T2A sequence mediates co-translational self-cleavage to split introduced proteins, resulting in expression at comparable levels[9]. A previous study showed that a dual-promoter vector carrying two transcriptional units is better than a vector with an IRES sequence for transducing human HSC[8], although promoter interference may occur in some cell types[10]. However, there are differences between human and murine HSC[11], suggesting that direct assessment of transduction of murine HSC is necessary to choose an optimal vector for these cells.
To screen for a lentivirus co-expressing a gene of interest and a reporter gene with improved transduction efficiency of murine HSC, we used several types of GFP-containing lentiviral pCDH vectors to express the murine MHC class II chaperone, invariant chain (Ii, wild type (WT) or a mutant (M98A) Ii cDNA, both engineered to with a Flag epitope tag. M98A can improve the stability and abundance of a particular murine class II I-Ag7[12]). Two ubiquitous promoters-Murine Stem Cell Virus (MSCV) and the housekeeping Elongation Factor 1a (EF1a) were chosen, because they drive high transgene expression in human HSC[13, 14]. Vectors including Fusion (GFP expressed immediately downstream of Ii), dual-promoter (MSCV or EF1a for Ii or GFP respectively) and T2A (the MSCV promoter driving Ii and GFP separated by T2A sequence) were constructed. The transfection efficiency of the constructs as DNA plasmids and the transduction efficiency of lentiviruses packaged from corresponding vectors were tested in 293T and BM-derived ckit+ cells. The long-term expression of Ii and GFP in various cell compartments was assessed in NOD mouse recipients reconstituted with lentivirus-transduced (cKit+Sca-1hiLin−) HSC. We also evaluated the integration into the host genome and the transcriptional expression of introduced GFP and Ii transgenes. Our results allowed for selection of a promising lentiviral vector for murine HSC gene therapy.
Methods
Lentiviral vectors and virus packaging
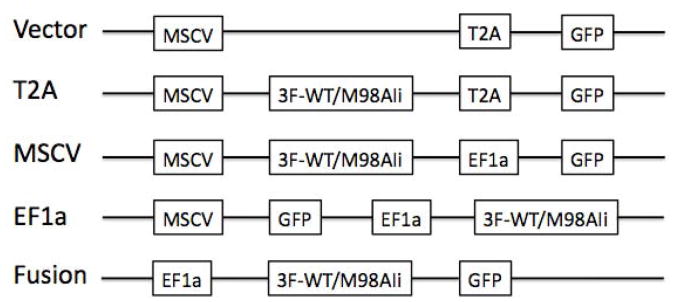
cDNAs of 3xflag-tagged WT and mutant (M98A) Ii[12] were cloned into MCS of pCDH vectors (T2A vector and dual promoter vector MSCV) purchased from System Bioscience (Mountain view, CA). Dual promoter vector EF1a was derived from MSCV vector by switching the positions of GFP and Ii. Fusion vector was constructed by addition of Ii cDNA in front of GFP under EF1a promoter in the MSCV vector backbone (Fig. 1).
Fig. 1.
Schemes of different pCDH lentiviral vectors. Vectors are named according to the presence or absence of linker (T2A or Fusion) or promoter for Ii in dual-promoter vectors (MSCV or EF1a). Only promoters, transgene (3×Flag tagged Ii) and reporter gene (GFP) are shown. Other elements can be found in the vector maps on System Biosciences website: http://www.systembio.com/lentiviral-technology/expression-vectors/cdna/vector-maps
Lentiviruses were produced in 293T cells by transfection (calcium phosphate precipitation) with the vectors, envelope plasmid VSV and packaging plasmid PAX2. Lentivirus containing supernatants were harvested at 24h. Supernatants were filtered, precipitated and concentrated with PEG-it Virus Precipitation Solution (System Bioscience). Lentiviral titer was determined by assessing GFP+% of 293T cells after transduction with time diluted viruses and confirmed by qPCR to determine the number of vector copies integrated into host genomic DNA extracted from lentivirus-transduced 293T cells[15].
Transient transfection and transfected Ii functional analysis
293T cells were transfected by various lentiviral vectors using calcium phosphate precipitation. Cells were harvested the next day and Ii and GFP expression were measured by flow cytometry. To assess transfected Ii function, 2A12 cells (293T cells engineered to express I-Ag7) were transfected by vectors using calcium phosphate precipitation. I-Ag7 levels were compared between the GFP+ (representing transfected cells) and GFP− (representing non-transfected cells) populations in WT and mutant Ii transfected 2A12 cells.
In vitro transduction
293T cells were transduced with different lentiviral vector-derived viruses at 1:300 dilution in the presence of 5ug/ml polybrene. GFP and Ii levels were monitored by FACS 48h after transduction. BM cells of femora and tibiae harvested from 3-5m NOD mice were enriched for c-kit+ cells with CD117 microbeads (Miltenyi Biotec, Auburn, CA) and then transduced with various lentiviruses at MOI 40 in the presence of 8ug/ml polybrene. GFP and Ii levels were measured by FACS 3d afterward.
HSC transplantation
ckit+ BM cells from 3-5m NOD mice (CD45.1+, 50% of which had high blood glucose, i.e. >250mg/dl) enriched by CD117 microbeads were stained with monoclonal antibodies for linage (Lin) markers (CD3, CD4, CD8, B220, Gr1, Mac1, Ter119) and stem/progenitor cell markers (ckit and Sca-l) then sorted for HSC (cKit+Sca-1hiLin−) using FACS-Aria (BD Bioscience, San Jose, CA)[16]. HSC were transduced with dual-promoter vector-derived lentiviruses at MOI 80 for 8h in the presence of 8ug/ml polybrene[17] after pre-activation by 100ng/ml SCF and 100ng/ml TPO overnight. The toxicity of MOI 80 lentivirus together with 8ug/ml polybrene was negligible, based on trypan blue staining of cultured cells (i.e. viability > 90%); in addition, the survival and reconstitution rate of recipients (i.e. >90% of each APC type is donor-derived, data not shown) also indicated viability of the transduced cells. 10,000 transduced HSC/mouse were transplanted by tail vein injection into 8-12w NOD recipients (CD45.2+, with normal blood glucose) that had been lethally irradiated at 980 cGy. Chimerism (%CD45.1 vs. %CD45.2) as well as levels of GFP and Ii were detected by FACS up to 8m post-transplantation. Abs and cytokines were purchased from eBioscience (San Diego, CA) and Peptrotech (Rocky Hill, NJ) respectively. NOD mice were bred and housed in the Stanford Veterinary Service Center under the approval of Administrative Panel for Laboratory Animal Care.
Flow cytometry analyses
293T or BM cells transfected with lentiviral vectors or transduced with corresponding lentiviruses were harvested and divided into two parts. Half of the cells were tested for GFP expression directly, whereas the other half were treated with a fixation/permeabilization reagent (eBioscience), followed by sequential staining with biotin-labeled anti-Flag Ab (Sigma-Aldrich, St. Louis, MO) and APC-conjugated Streptavidin (BD Bioscience) for Flag expression, which reflects Ii level. For in vivo GFP and Ii expression, blood, bone marrow (BM), lymph nodes (LNs) and spleens were harvested from lentivirus-transduced HSC recipients. After lysis with RBC lysis buffer, 1E6 cells were stained with Abs recognizing different compartment markers, i.e. B220+ for B cells, CD11b+ for myeloid cells, CD11b+F4/80+ for macrophages, CD11b+F4/80− for monocytes, CD11c+ for dendritic cells (DC), CD4+CD25− for T effector cells (Teff), CD4+CD25+ for regulatory T cells (Treg), CD8+ for cytotoxic T-cell (CTL) and CD3+CD4−CD8−NK1.1+ for natural killer T cells (NKT). GFP expression was measured in different compartments. 2E6 cells stained first with compartment markers then were fixed, permeabilized and stained with Abs against Flag to measure Ii level. Stained cells were collected on a Caliber or LSR II flow cytometer (BD Bioscience) and analyzed with Flowjo software (Tree Star Inc., Ashland, OR). Abs were purchased from Biolegend (San Diago, CA), unless otherwise mentioned.
Quantitative real time PCR (qPCR)
Genomic DNA (gDNA) and cellular total RNA from 293T cells transduced with equal volumes of lentiviruses were extracted with a DNeasy tissue kit and an RNeasy Mini kit (Qiagen, Valencia, CA) respectively, per manufacturer's instruction. cDNA was generated from RNA using an iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad, Hercules, CA). Primers for PCR amplification were designed using Custom Primers-OligoPerfect Designer software (Invitrogen, Grand Island, NY). The primers used were: WPRE, 5′-GGACGTCCTTCTGCTACGTC-3′ (sense) and 5′-GAGATCCGACTCGTCTGAGG-3′ (antisense); GFP, 5′-TGATGGGCTACGGCTTCTAC-3′ (sense) and 5′-GTACTTCTCGATGCGGGTGT-3′ (antisense); Flag, 5′-ATGGACTACAAAGACCATGACGG-3′ (sense) and 5′-GAGCTTGTCATCGTCATCCTTG-3′ (antisense); human RRP30, 5′-GAGGCCTGGCTTTTGAACTT- 3′ (sense) and 5′-GCATCAAATTGAGGGCACTG-3′ (antisense). Sequences of human PPP1CC primers were obtained from NIH (http://primerdepot.nci.nih.gov/). Amplification reactions were performed using SsoAdvanced™ SYBR® Green Supermix (Bio-Rad) with different cycle conditions for gDNA and cDNA in a C1000 thermal cycler, according to the manufacturer's instructions (Bio-Rad). RRP30 and PPP1CC were used as the endogenous references for gDNA and cDNA respectively. Transgenes expressed by T2A-3FWT lentivirus-transduced 293T cells were used as the calibrators and with expression levels set to 1.
Results
Design of lentiviral vectors for co-expression of wild type (WT) or mutant (M98A) invariant chain (Ii) and GFP marker
To screen for an efficient lentiviral vector expressing both Ii and GFP, we constructed T2A vector encoding both proteins under the same MSCV promoter but separated by the T2A peptide, dual-promoter vectors with separate promoters (i.e. MSCV or EF1a) for Ii and GFP respectively, and a Fusion vector that expresses GFP immediately following Ii. Dual-promoter vectors were named according to the promoter for Ii, i.e. MSCV or EF1a. To distinguish the introduced Ii from endogenous Ii, three Flag sequences in tandem were added to N-terminal of Ii. The design of the vectors is shown schematically in Figure 1.
T2A and MSCV vectors drove the highest levels of Ii and GFP expression in 293T cells, respectively
To compare gene expression driven by each of the PCDH vectors in the panel, replicate transient transfections of 293T cells (n=3) were performed using calcium phosphate. By FACS, MSCV induced the highest GFP expression, comparable to vector alone. EF1a and T2A expressed intermediate levels of GFP, whereas Fusion expressed the lowest level of GFP (Fig. 2A). For Ii, T2A induced the highest expression, followed by EF1a and Fusion. MSCV expressed the lowest level of Ii (Fig. 2B). Flag expression correlated highly with Ii expression, as detected by anti-Ii antibody (not shown). As there is no endogenous Ii in 293T cells, we used the Flag level to indicate Ii expression in the subsequent experiments. Notably, GFP and Ii levels encoded by vectors containing either wild type (WT) or M98A mutant Ii were always comparable, indicating that mutant Ii did not affect either expression of Ii itself or GFP.
Fig. 2.
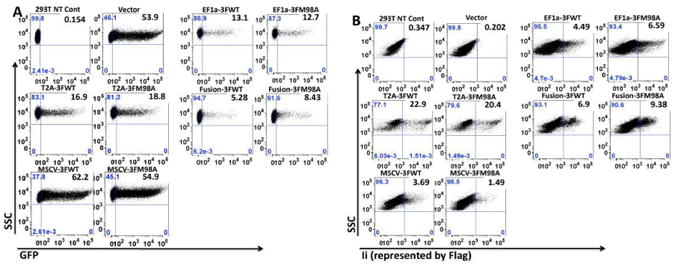
Highest proportion of GFP- and Ii-expressing cells are in MSCV and T2A vector-transfected 293T cells, respectively. A. GFP expression. GFP levels in 293T cells, directly transfected with different vectors, were assessed by FACS. B. Ii (indicated by Flag) expression. Flag levels in the same transfectants were detected by FACS, after intracellular staining with biotin-labeled Flag-specific Ab, followed by allophycocyanin (APC)-conjugated streptavidin. Data shown are representatives of three independent experiments.
Lentiviruses carrying dual-promoter vectors MSCV and EF1a expressed the highest levels of both GFP and Ii in 293T cells
The ultimate goal of this study was to transduce HSC using the optimal PCDH construct, packaged into lentiviruses. To optimize this step, we tested 293T cells transduced with the different lentiviruses, generating by packaging of the vectors with the VSV (vesicular stomatitis virus)-PAX2 system. Both dual-promoter vector-derived viruses (MSCV and EF1a) induced the highest GFP and the highest Ii expression. Lower levels of GFP and Ii expression were detected in 293T cells transduced with T2A-derived and Fusion-derived viruses (Fig. 3A & B). The change in expression pattern of both Ii and GFP after packaging into viruses suggested that the packaging procedure may affect transgene expression.
Fig. 3.
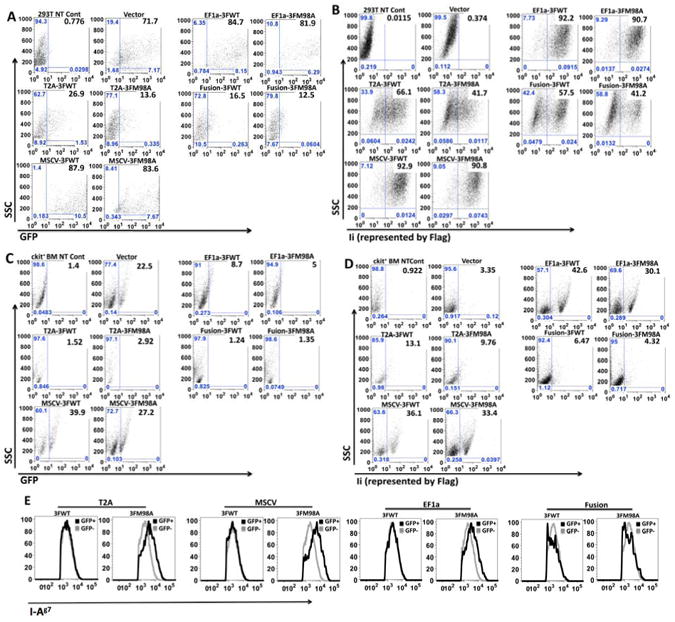
Highest GFP and Ii levels are induced by dual-promoter vector-derived lentiviruses; Ii expressed by Fusion vector is dysfunctional. FACS analysis of A. GFP expression in 293T cells; B. Ii expression (represented by Flag expression) in 293T cells; C. GFP levels in murine ckit+ BM cells; and D. Ii (Flag) expression in murine ckit+ BM cells transduced with various lentiviruses. Flag levels were detected by intracellular staining prior to FACS analysis, as described in Fig. 2. E. Ii expressed by Fusion vector is not functional. 293T cells expressing I-Ag7 transfected with various vectors were stained with phycoerythrin (PE)-conjugated I-Ag7 specific Ab and then analyzed by FACS. I-Ag7 levels in GFP negative (grey line) and positive (black line) populations were compared. Untransfected cells were used to set the GFP negative threshold (not shown). Data shown are representatives of three independent experiments.
MSCV-derived lentiviruses induced the highest GFP and Ii expression in ckit+ BM cells whereas EF1a-carrying lentiviruses induced only high levels of Ii
To test the efficiency of different vector-based lentiviruses for transduction of HSC, each kind of virus was used to transduce cytokine-pre-activated bone marrow (BM)-derived ckit+ cells (which include both stem and progenitor cells) at MOI 40. As in 293T cells, MSCV-based lentiviruses induced significant levels of both GFP and Ii, whereas EF1a carrying viruses induced only Ii, but not GFP, expression (Fig. 3C & D). Notably, both GFP and Ii levels were significantly reduced in ckit+ cells compared to their levels in 293T cells, suggesting non-dividing HSC are harder to transduce than other cells, even with lentiviruses (which target mitotically inactive cells) and cytokine pre-activation pushing HSC from G0 to G1 (Fig. 3 A & B vs. C & D).
Ii expressed by all vectors except Fusion was functional
To test if the introduced Ii protein is functional, class II levels were compared in I-Ag7 expressing 293T cells transfected with WT vs. M98A mutant Ii carrying vectors. I-Ag7 molecules are expressed by NOD mice, a faithful model for type 1 diabetes (T1D). I-Ag7, like its human counterpart, DQ8, is closely associated with T1D[18]. I-Ag7 has low affinity for a region of Ii known as the CLIP fragment [19]. Previously, we showed that mutation of Ii at a key residue in the CLIP region (M98A) increases affinity for I-Ag7 and leads to increased I-Ag7 abundance in transfected cells[12]. In contrast, transfection of WT Ii did not affect I-Ag7 stability or function[12]. Consistent with those published results, we observed that mutant Ii, but not WT Ii, increased I-Ag7 abundance, except in the case of M98A expression by the Fusion vector (Fig. 3E). The failure of this GFP-fused mutant Ii to enhance I-Ag7 level suggested possible structural interference with the Ii/MHC interaction because no linker separated GFP and Ii.
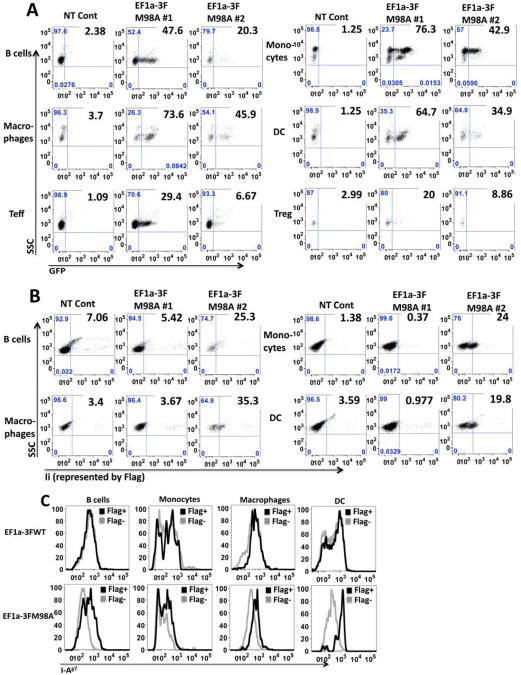
In vivo expression of GFP and Ii derived from HSC transduced with dual-promoter-based lentiviruses
To study long-term in vivo expression of GFP and Ii, the lentivirus with the best performance in vitro, MSCV, was selected to transduce HSC (ckit+Scal1hiLin−). MSCV-transduced HSC were then transplanted into lethally irradiated NOD mice. GFP and Ii levels in different blood compartments were assessed by FACS. As shown in Figures 4A and B, at 4w post-transplantation, about 70% of CD11b+ myeloid cells, 60% of CD11c+ DC and 40% of B220+ B cells were GFP+. Lower percentage (about 20%) of T cells (Teff, Treg, CTL and NKT cells) expressed GFP. Similar GFP levels were detected in other organs like spleen, lymph nodes and BM (including both ckit+ and more mature ckit− cells) up to 8m post-transplantation (data not shown). In contrast, no significant Ii expression was observed in any assayed compartment after transplantation with MSCV-transduced HSC (Fig. 4C and D). Because EF1a induced the highest Ii expression in ckit+ BM cells in vitro (Fig. 3D), GFP and Ii expression levels by EF1a-transduced HSC (ckit+Scal1hiLin−) in vivo were also tested. In contrast with MSCV, EF1a induced lower percentage of GFP+ cells at 4w post-transplantation. Specifically, only 20% of myeloid cells, 15% of DC, 10% of B cells and <10% of various kinds of T cells were GFP+ (Fig. 4E & F). However, almost 20% of macrophage cells were Ii+ after transplantation with EF1a-transduced HSC. EF1a also induced a little bit higher percentage of Ii+ cells in other antigen presenting cell (APC) compartments than MSCV transduction, although the total positive levels were still low (Fig. 4G & H). Both GFP and Ii expression levels increased in EF1a transplanted mice over time. At 4-5m post-transplantation, we detected significant enhancement of GFP and Ii expression from blood, spleen, pancreatic LNs and ckit+ and ckit- BM (Fig. 5A & B and data not shown). GFP and Ii expression did not correlate, i.e. mice did not express highest levels of both GFP and Ii (for example, compare GFP and Ii levels of EF1a-3FM98A #1 with those of EF1a-3FM98A #2 in Fig. 5A & B). Ii with the M98A mutation introduced by the EF1a vector was functional, as shown by the increased level of I-Ag7 in the Ii+ population compared to the Ii− population in M98A-transduced HSC recipients, but not the WT recipients (Fig. 5 C).
Fig. 4.
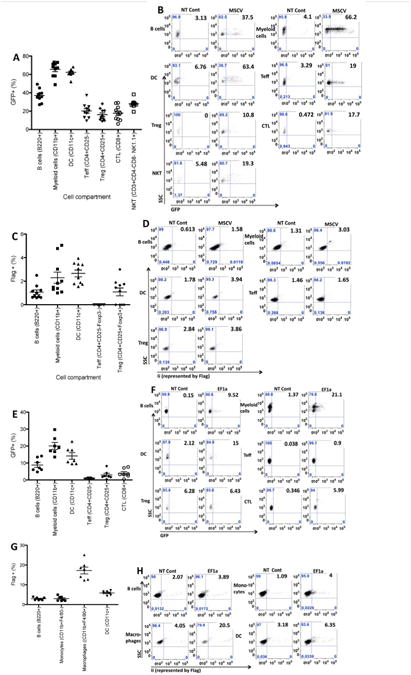
In vivo expression of GFP and Ii derived from MSCV or EF1a-transduced HSC. GFP levels (A & B from MSCV; E & F from EF1a) and Ii levels (represented by Flag, C & D from MSCV; G & H from EF1a) were assessed in various cell compartments in blood from NOD mice 4w post-transplantation of HSC. MSCV (A to D) or EF1a (E to H) viruses at MOI=80 were used to transduce donor HSC, which were then transplanted into lethally irradiated recipients. A & C (n=10) and E & G (n=7) summarize data from multiple mice. B & D are representative FACS graphs from one non-transplanted (NT) control and one MSCV lentivirus-transduced mouse. F & H are FACS graphs from another NT control and one EF1a-transduced mouse. Cell markers for each lineage are indicated in parentheses. Note the maximal number on y axis of panel C is 6%.
Fig. 5.
Increased GFP and functional Ii expression 4-5m post-transplantation of EF1a-transduced HSC. GFP (A) and Ii (represented by Flag, B) levels were assessed in various cell compartments in blood from one non-transplanted (NT) control and two EF1a-3FM98A transduced NOD mice. Note the levels of GFP and Flag were not parallel, i.e. EF1a-3FM98A #1 had higher GFP but no Flag expression, whereas EF1a-3FM98A #2 had lower GFP but higher Flag expression. C. I-Ag7 levels in exogenous Ii-expressing (represented by Flag expression, black line) vs. non-expressing populations (grey line) were compared in splenocytes from an EF1a-3FWT transduced mouse (top row of panel C) and an EF1a-3FM98A transduced mouse (bottom row of panel C) 5m post-transplantation. Data shown are representative from two independent experiments.
Transgene integration to host genome and expression at mRNA level
To investigate the step when transgene expression is lost, we assessed GFP and Flag integration into the host genome (detected using genomic DNA or gDNA) and the expression of these genes, as measured by mRNA levels (detected using complementary DNA or cDNA). We used quantitative real time PCR (qPCR) to analyze 293T cells transduced with various lentiviruses at equivalent volumes. We determined integration and expression of a vector backbone component-Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), which enhances the stability of the viral transcripts, as evidence for viral presence. As shown in figure 6A, the highest amount of integration was observed in MSCV-transduced 293T cells, followed by those transduced with EF1a and T2A. MSCV also induced the highest level of GFP transcript, but only drove a level of Flag that was comparable to that of EF1a transduced-cells (Fig. 6B). EF1a induced higher transgene expression (mRNA) than T2A, although these 2 viruses integrated into the host genome to a similar degree. Fusion lentivirus was integrated and expressed at the lowest level among the vectors tested (Fig. 6A & B). The transcript levels of the transgenes correlated better with their protein levels than did their integration levels (Fig. 3A, B & Fig. 6), suggesting that the expression of the transgenes in the settings tested was regulated at the transcriptional level.
Fig. 6.
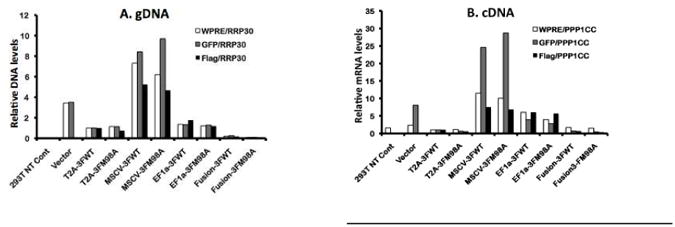
GFP and Ii expression at integration and transcriptional levels. Genomic DNA (gDNA) (A) and complementary DNA (cDNA) from RNA (B) levels for a vector backbone component-WPRE, GFP and Flag were assessed by real time PCR after extraction from 293T cells transduced with various lentiviruses at equivalent volumes using corresponding primers. RRP30 and PPP1CC were used as endogenous controls for gDNA and cDNA respectively. Relative expression levels of corresponding genes were normalized to those derived from T2A-3FWT transduced 293T cells (relative value=1).
Discussion
To select a lentiviral vector for transducing murine HSC, we performed a series of in vitro and in vivo transfection and transduction experiments. Among all the lentiviral vectors we screened, dual-promoter vectors performed best to express both the gene of interest and the reporter gene. These results are similar to those in previous work showing that a dual-promoter vector is superior to an IRES-containing vector in transducing human HSC, driving comparable expression levels of two proteins[8]. However, we observed some imbalance between the expression of the gene of interest and the marker gene. For example, in ckit+ BM cells transduced in vitro by EF1a-based lentiviruses, we could not detect expression of GFP (Fig. 3C). We also could not detect in vivo Ii expression after reconstitution of mice with MSCV-transduced (ckit+Scal1hiLin−) HSC (Fig. 4C & D). The failure to express one of the two transgenes driven by two separate promoters may be due to interference and/or silencing of promoters, vector rearrangements and deletions[20]. Notably, the activity of the MSCV promoter is suppressed both in vivo and in vitro (Fig. 3 & 4). These findings suggest that the MSCV promoter may be specifically inhibited in the murine HSC environment (discussed below), although it has been found to function in human HSC[21] and with additional CPG mutations at LTR region, it works well in murine embryonic stem cells[22]. The low transgene expression driven by MSCV in murine HSC may contribute to lower Ii and GFP expression with the T2A vector (Fig. 3). However, levels of Ii and of GFP driven by separate promoters including MSCV are higher than levels of proteins when the genes are driven together by MSCV in the T2A vector (Fig. 3). The level of GFP as the 2nd transgene in the T2A vector is always lower than the level of Ii in the context of lentiviral transduction (Fig. 3), whereas GFP and Ii expression are comparable in 293T cells, directly transfected with T2A vector (Fig. 2). Thus, the T2A vector with the MSCV promoter is not as efficient as expected, at least in a lentiviral-transduced murine BM-HSC system.
Our result that lentiviruses carrying a dual-promoter vector are better than those based on vectors with a separating sequence for expression of two transgenes corroborates a previous study of IRES vector-based lentivirus use in human HSC[8]. However, our report extends their study by examining transgene expression is several organs (versus BM only) and by determining integrated copy number of the different vectors tested. In addition, they did not see significant differences in transgene expression level among dual-promoter vectors with various promoters, including EF1a, CMV, PGK or DRa in human HSC. This result suggests that human HSC may be a more favorable environment for a variety of promoters than murine HSC.
The differential levels of GFP and Ii in EF1a-transduced mice also demonstrated imbalance in expression of genes driven by EF1a and MSCV. As has been observed with CMV and EF1a promoters[20], the interference between promoters MSCV and EF1a may be epigenetic and be dependent on the strength of individual promoter. Competition between promoters for transcription factors, for their binding proteins and for enhancers as well as for ribosomal association at the post-transcriptional level may also contribute to the interference[20].
Low transduction efficiency of lentivirus and loss of long term transgene expression remain critical issues for HSC gene therapy. Indeed, in our study, the maximal Ii+ percentage is 35.3% in EF1a-3FM98A-transduced HSC-derived macrophage cells (Fig. 5B). The mutant Ii was sufficient to enhance I-Ag7 level (Fig. 5C) in Ii+ cells. However, most antigen presenting cells were still Ii−, and the Ii+ percentage was even lower at the beginning of reconstitution (Fig. 4G & H). While low percentages of cells expressing the gene of interest may be sufficient to address some clinical needs[23], other settings appear to call for larger proportions of “corrected” cells and require improved strategies or reagents.
Lentivirus is one genus of retrovirus that can transduce post-mitotic and non-dividing cells by active transport through the nucleopore[24-26]; these properties have made them attractive for gene therapy use. However, gene silencing remains a problem. Gene silencing mainly refers to transcriptional silencing observed immediately after retroviral transduction. It also includes variegation (when genetically identical sister cells inheriting the same provirus express different levels of the transgene) and extinction or progressive loss of an initially expressed provirus during differentiation[27]. Silencing is thought to be initiated by a stem cell specific “somno-complex” composed of negative cis-elements of the virus acted on additively by several trans-acting factors of the host cells. Chromatin over the bound provirus is then remodeled by ATP-dependent chromatin remodeling helicases and modified by histone deacetylases or histone H3-specific methyltransferases. The resulting repressive histone codes are recognized by chromatin-associated proteins, such as members of the polycomb-group. De novo methylases (DNMT3A and DNMT3B) are then recruited to these sites to methylate the cytosine of CpG sites of proviruses. Confirming this model, improved transgene expression has been achieved via cis-modifications of retroviral vector sequences, including mutations/deletions of silencer elements and/or addition of positive regulatory elements. Transgene reactivation by HDAC and DNMT inhibitors also has been reported[28, 29]. Using limiting dilution reconstitution of lethally irradiated mice with FACS-sorted GFP+ HSC after retroviral transduction, it was observed that transcriptional silencing of GFP occurred in most integration events at the HSC level. Extinction under this condition is restricted primarily to lymphoid cells, possibly due to chromatin remodeling accompanying B cell commitment, the absence of positively acting transcription factors or the presence of silencing factors in T cells, and/or higher levels of DNMT3 activity in lymphoid cells than in myeloid cells[30]. These factors may lead to higher expression of GFP and Ii in myeloid compared to lymphoid cells in our study.
Lentiviruses, which are capable of stabilizing their RNA genomes during replication, suffer the same silencing effects as retroviruses[27]. Even the currently used self-inactivating (SIN) lentiviruses are subject to silencing at half the integration sites due to position effects, although the multiple provirus integrations achieved with high multiplicities of infection may enhance transgene expression levels[27]. In our study, GFP expression driven by the EF1a promoter in the MSCV lentivirus was higher (∼7×) than GFP expression driven by MSCV promoter in EF1a lentivirus, due apparently to both improved integration and transcription. However, despite increased integration of the MSCV compared to the EF1a lentivirus, both lentiviruses expressed similar levels of Ii RNA (Fig. 6), suggesting transcriptional silencing of MSCV promoter-driven Ii expression. With the T2A virus, where GFP and Ii genes are driven together by the MSCV promoter, the relative levels of both were reduced at transcriptional level compared to the integration level (Fig. 6), confirming the transcriptional silencing of MSCV promoter. In vitro manipulation of human CD34+ cells by cytokine activation and lentiviral transduction induces genome-wide epigenetic modifications including enhanced expression of DNMT1[31], which may also contribute to gene silencing. The same strategies for modification of retroviral vectors might apply to lentiviruses. However, putatively positive additions, such as locus control regions and insulators, may damage retroviral replication and DNMT is indispensible for development. Thus, better interventions are still needed.
In conclusion, we found that a dual-promoter vector is the best for murine BM-derived HSC transduction among the lentiviruses we tested. EF1a is a better promoter than MSCV for gene of interest expression in this setting. In the case of invariant chain, a component of the MHC class II antigen presentation pathway, expression may be improved by using an APC-specific class II promoter[32] and by using codon optimization[33]. In addition, transient treatment of HSC by a proteasome inhibitor may enhance lentiviral transduction efficiency[5]. Future studies may reveal other approaches to modify vectors or transduction conditions for murine and human HSC.
Acknowledgments
We thank Drs. Rong Lu, Kai Chen, Claudia Macaubas, Mark Kay, and Jiamiao Lu (Stanford University) for technical support, review and comments.
Support: This work was supported by grants from Juvenile Diabetes Research Foundation, Stanford Medical School Child Health Research Institute funded by Stanford NIH/NCRR CTSA award number UL1 RR025744 and by the Lucile Packard Foundation for Children's Health (to NW), Arthritis Foundation (to NR), NIH/NIAID F32AI089080 for postdoctoral fellows (to TH), the Berry Fellowship Foundation (to LL), American College of Rheumatology Research and Education Foundation, the Juvenile Diabetes Research Foundation and NIH AI075253 and DK079163 (to EDM).
Footnotes
Financial Disclosure Declaration: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hu B, Tai A, Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol Rev. 2011;239:45–61. doi: 10.1111/j.1600-065X.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrette S, Douglas JL, Seidel NE, Bodine DM. Lentivirus-based vectors transduce mouse hematopoietic stem cells with similar efficiency to moloney murine leukemia virus-based vectors. Blood. 2000;96:3385–3391. [PubMed] [Google Scholar]
- 3.Bank A. Hematopoietic stem cell gene therapy: selecting only the best. J Clin Invest. 2003;112:1478–1480. doi: 10.1172/JCI20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanisch K, Yanez-Munoz RJ. Integration-deficient lentiviral vectors: a slow coming of age. Mol Ther. 2009;17:1316–1332. doi: 10.1038/mt.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leuci V, Mesiano G, Gammaitoni L, et al. Transient proteasome inhibition as a strategy to enhance lentiviral transduction of hematopoietic CD34(+) cells and T lymphocytes: implications for the use of low viral doses and large-size vectors. J Biotechnol. 2011;156:218–226. doi: 10.1016/j.jbiotec.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Hangoc G, Campbell TB, et al. Identification of parameters required for efficient lentiviral vector transduction and engraftment of human cord blood CD34(+) NOD/SCID-repopulating cells. Exp Hematol. 2008;36:947–956. doi: 10.1016/j.exphem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Felipe P. Polycistronic viral vectors. Curr Gene Ther. 2002;2:355–378. doi: 10.2174/1566523023347742. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Zhan X, D'Costa J, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 9.Szymczak-Workman AL, Vignali KM, Vignali DA. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb Protoc. 2012;2012:199–204. doi: 10.1101/pdb.ip067876. [DOI] [PubMed] [Google Scholar]
- 10.Tian J, Andreadis ST. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009;16:874–884. doi: 10.1038/gt.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitnicka E, Buza-Vidas N, Larsson S, Nygren JM, Liuba K, Jacobsen SE. Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c-kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood. 2003;102:881–886. doi: 10.1182/blood-2002-06-1694. [DOI] [PubMed] [Google Scholar]
- 12.Rinderknecht CH, Lu N, Crespo O, et al. I-Ag7 is subject to post-translational chaperoning by CLIP. Int Immunol. 2010;22:705–716. doi: 10.1093/intimm/dxq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varma NR, Janic B, Ali MM, Iskander A, Arbab AS. Lentiviral Based Gene Transduction and Promoter Studies in Human Hematopoietic Stem Cells (hHSCs) J Stem Cells Regen Med. 2011;7:41–53. doi: 10.46582/jsrm.0701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramezani A, Hawley TS, Hawley RG. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- 15.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran N, Wang N, Truong P, et al. Host-derived CD4+ T cells attenuate stem cell-mediated transfer of autoimmune arthritis in lethally irradiated C57BL/6.g7 mice. Arthritis Rheum. 2013;65:681–692. doi: 10.1002/art.37800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29:928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausmann DH, Yu B, Hausmann S, Wucherpfennig KW. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J Exp Med. 1999;189:1723–1734. doi: 10.1084/jem.189.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JA, Dane AP, Swanson A, Alexander IE, Ginn SL. Bidirectional promoter interference between two widely used internal heterologous promoters in a late-generation lentiviral construct. Gene Ther. 2008;15:384–390. doi: 10.1038/sj.gt.3303105. [DOI] [PubMed] [Google Scholar]
- 21.Choi JK, Hoang N, Vilardi AM, Conrad P, Emerson SG, Gewirtz AM. Hybrid HIV/MSCV LTR enhances transgene expression of lentiviral vectors in human CD34(+) hematopoietic cells. Stem Cells. 2001;19:236–246. doi: 10.1634/stemcells.19-3-236. [DOI] [PubMed] [Google Scholar]
- 22.Swindle CS, Kim HG, Klug CA. Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells. J Biol Chem. 2004;279:34–41. doi: 10.1074/jbc.M309128200. [DOI] [PubMed] [Google Scholar]
- 23.Steptoe RJ, Ritchie JM, Harrison LC. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. J Clin Invest. 2003;111:1357–1363. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchschacher GL, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95:2499–2504. [PubMed] [Google Scholar]
- 25.Frimpong K, Spector SA. Cotransduction of nondividing cells using lentiviral vectors. Gene Ther. 2000;7:1562–1569. doi: 10.1038/sj.gt.3301283. [DOI] [PubMed] [Google Scholar]
- 26.Chicurel M. Virology. Probing HIV's elusive activities within the host cell. Science. 2000;290:1876–1879. doi: 10.1126/science.290.5498.1876. [DOI] [PubMed] [Google Scholar]
- 27.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 28.Pannell D, Ellis J. Silencing of gene expression: implications for design of retrovirus vectors. Rev Med Virol. 2001;11:205–217. doi: 10.1002/rmv.316. [DOI] [PubMed] [Google Scholar]
- 29.Schlesinger S, Goff SP. Silencing of proviruses in embryonic cells: efficiency, stability and chromatin modifications. EMBO Rep. 2013;14:73–79. doi: 10.1038/embor.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klug CA, Cheshier S, Weissman IL. Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- 31.Yamagata Y, Parietti V, Stockholm D, et al. Lentiviral Transduction of CD34(+) Cells Induces Genome-Wide Epigenetic Modifications. PLoS One. 2012;7:e48943. doi: 10.1371/journal.pone.0048943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, Golob J, Kelleher E, Ye Z, Pardoll D, Cheng L. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- 33.Moreno-Carranza B, Gentsch M, Stein S, et al. Transgene optimization significantly improves SIN vector titers, gp91phox expression and reconstitution of superoxide production in X-CGD cells. Gene Ther. 2009;16:111–118. doi: 10.1038/gt.2008.143. [DOI] [PubMed] [Google Scholar]