Abstract
We investigated whether rimonabant, a type 1 cannabinoid receptor antagonist, reduces visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) in dogs maintained on a hypercaloric high-fat diet (HHFD). To determine whether energy expenditure contributed to body weight changes, we also calculated resting metabolic rate. Twenty male dogs received either rimonabant (1.25 mg·kg−1·day−1, orally; n = 11) or placebo (n = 9) for 16 wk, concomitant with a HHFD. VAT, SAT, and nonfat tissue were measured by magnetic resonance imaging. Resting metabolic rate was assessed by indirect calorimetry. By week 16 of treatment, rimonabant dogs lost 2.5% of their body weight (P = 0.029), whereas in placebo dogs body weight increased by 6.2% (P < 0.001). Rimonabant reduced food intake (P = 0.027), concomitant with a reduction of SAT by 19.5% (P < 0.001). In contrast with the VAT increase with placebo (P < 0.01), VAT did not change with rimonabant. Nonfat tissue remained unchanged in both groups. Body weight loss was not associated with either resting metabolic rate (r2 = 0.24; P = 0.154) or food intake (r2 = 0.24; P = 0.166). In conclusion, rimonabant reduced body weight together with a reduction in abdominal fat, mainly because of SAT loss. Body weight changes were not associated with either resting metabolic rate or food intake. The findings provide evidence of a peripheral effect of rimonabant to reduce adiposity and body weight, possibly through a direct effect on adipose tissue.
Keywords: adipose tissue, body weight, energy expenditure, magnetic resonance, resting metabolic rate
the association among body fat accumulation, insulin resistance, and other metabolic disorders, including type 2 diabetes, is well documented (3, 13, 19, 26). Obesity is a known risk factor for cardiovascular diseases, cancer, insulin resistance, and the development of type 2 diabetes, this latter being the fifth leading cause of death in the United States (American Diabetes Association) and a growing problem worldwide (46). Of recent interest in this regard is the cannabinoid system and its role in the regulation of metabolism, body weight, and energy expenditure (8, 33). One of the compounds that can affect obesity is rimonabant, a selective type 1 cannabinoid receptor (CB1R) antagonist (7, 34, 39). Rimonabant has been reported to decrease appetite and body weight, although the former effect seems to be transient and might not explain the sustained body weight loss (9, 11, 35, 36).
Studies conducted in rodents have shown that rimonabant decreases adiposity in different regions of the body. Obese mice treated with rimonabant decreased epididymal, perirenal, and lumbar fat depots (36) after 40 days of treatment. Similarly, rats treated with rimonabant for 10–14 days showed a decrease in total body fat (9) and subcutaneous, retroperitoneal, mesenteric, and epididymal fat (11). A recent report has demonstrated a significant reduction in visceral fat in mice treated with rimonabant for 6 wk concomitant with a candy diet (16). However, what constitutes true visceral fat in the mouse model remains to be clearly identified.
To date, human studies testing the effect of rimonabant on obesity have demonstrated a significant reduction in waist circumference (7, 34, 43), which serves as a surrogate of visceral adiposity. Increased fat accumulation in the abdomen, particularly visceral adiposity, has been associated with a variety of metabolic abnormalities, including insulin resistance and cardiovascular diseases (4, 14, 30, 42, 44). Interestingly, dogs, unlike rodents, have the typical abdominal fat compartments seen in humans, i.e., clearly defined as visceral and subcutaneous fat depots. These compartments are enhanced in dogs after a prolonged high-fat diet (24, 25) and are associated with the canine metabolic syndrome. Thus, it is of interest to study the effects of a cannabinoid antagonist in an animal model with fat compartments similar to humans to gain insight into possible therapies for fat deposition.
In this study, we monitored the effect of rimonabant treatment on fat distribution in the abdominal trunk of dogs maintained on a high-fat diet. In addition, because obesity results from an imbalance between caloric intake and energy expenditure, and resting metabolic rate is the major component of energy expenditure, we examined whether resting metabolic rate contributes to the body weight loss induced by rimonabant.
MATERIALS AND METHODS
Animals.
A total of 20 male adult mongrel dogs were included in this study. Animals were housed in kennels at the vivarium of the Keck School of Medicine at the University of Southern California. This study was approved by the ethics committee of the University of Southern California.
Induction of fat accumulation.
Dogs were initially maintained on a standard diet for a 3-wk acclimation period (3,885 kcal/day, 39.4% carbohydrates, 33.2% fat, 27.4% proteins). Food was presented from 0900 to 1200 daily. To induce fat accumulation, we switched from the standard diet to a hypercaloric high-fat diet (HHFD) that consisted of 5,527 kcal/day (27.7% carbohydrates, 53.0% fat, 19.3% proteins), also presented from 0900 to 1200 daily. We have previously reported that 6 wk is an adequate period to observe significant increases in adiposity (24, 25) and body weight (23). Thus the HHFD was maintained for a minimum of 6 wk (9 ± 2 wk) before any drug treatment.
Rimonabant treatment.
After the initial fat feeding period of rimonabant (n = 11) or placebo (n = 9), animals were randomly segregated into two groups. Animals were matched for body weight (31.7 ± 1.3 and 31.8 ± 1.5 kg; rimonabant vs. placebo group, respectively). Rimonabant (Sanofi-aventis, Paris, France) was encapsulated (AMC Pharmacy, Burbank, CA) and administered orally at 1.25 mg·kg−1·day−1, whereas placebo-treated animals received inert capsules. The dose was chosen based on tolerability to the drug. We used three criteria to assess tolerability in five dogs that were not included in the current study. We measured food intake, blood pressure, and gross motor movements (muscle fasciculations evaluated by visual observations). The first dose selected was 5 mg/kg. At this dose, animals did not eat, exhibited blood pressure >160/100 mmHg, and showed severe muscle fasciculations. After a washout period in which all parameters returned to normal, the dose was titrated down to 2.5 mg/kg. At 2.5 mg/kg, similar observations were made to those at the 5 mg/kg dose; therefore, it was decided to decrease the dose to 1.25 mg/kg. At this dose, we observed a transient decrease in food intake and blood pressure. In addition, we did not observe evidence of muscle fasiculations. The final dose selected is 2–2.5 times higher than the efficacious 20-mg dose administered to humans. During the 16 wk of treatment, all animals were continued on the HHFD.
Food intake and body weight.
We performed daily recordings of food intake throughout the study as well as weekly recordings of body weight. Food intake data for each specific week are presented in the text as means of the 7-day recordings for that week.
Abdominal trunk composition.
The amount of fat tissue and nonfat tissue in the abdominal trunk was quantified by magnetic resonance imaging (MRI) using a 1.5 T Gemsow Scanner (General Electric). The MRI scanning included the following six sessions: before the beginning of the HHFD (week −6), after induction of increased body weight and adiposity and before initiation of drug (week 0), and thereafter at 2, 6, 12, and 16 wk of treatment. For scans, dogs were preanesthetized with 1.35 mg of atropine sulfate plus 5 mg of acepromazine (sc) and then anesthetized with a single intravenous dose of a mixed solution of ketamine (10 mg/kg) and diazepam (0.5 mg/kg), slowly administered. Dogs were placed on the scanner table in supine position with the front legs flexed and the hind legs extended. The scanning consisted of 30 contiguous slices 1-cm thick (longitudinal relaxation time T1-weighted, repetition time = 500 ms, echo time = 14.0 ms), placing the first slice at the level of the junction between the inferior limb and the trunk. In an attempt to get comparable repeated measurements along the study, the left renal hilum was chosen as a landmark for all sessions. The analysis of the data was based on 11 consecutive slices, 5 upper and 5 below the landmark and the slice at the level of the landmark. Body composition in the abdominal trunk was divided in the following five categories: visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), total fat (VAT + SAT), nonfat tissue, and total tissue (total fat + nonfat tissue). Images were retrieved to a personal computer with eFILM Lite software (versions 2.0.1. and 2.1.2.; Merge Healthcare, Milwaukee, WI). Every image was analyzed using the software Scion Image for Windows (Alpha 4.0.3.2.; Scion, Frederick, MD) to differentiate fat from nonfat tissue based on pixel intensity as previously described (25). VAT was defined as the fat located in the intra- and retroperitoneal region, and this region was separated from the SAT by manual trace. For each slice, the area was calculated in square centimeters (area of 226 × 256 pixels equivalent to 34.9 × 34.9 cm2) and multiplied by the thickness of the slice to obtain the volume. The total volume of each component of the trunk was obtained by summing the partial volumes from each slice.
Resting metabolic rate.
In a subset of dogs (rimonabant, n = 7; placebo, n = 5), we assessed resting metabolic rate by indirect calorimetry. The calorimetric chamber used consisted of a standard dog kennel adapted for the experiments. All dogs were acclimated to the chamber 3 wk before the initiation of the protocol. Dogs were fasted for at least 12 h before each experiment. We assessed resting metabolic rate at weeks 0, 2, 6, 12, and 16. For each weekly session, the recordings were obtained between 0630 and 1030 over a period of 2 days, randomly assigning six dogs to each day. The gas analyzer module VacuMed Vista MX (VacuMed, Venture, CA) was calibrated before each experiment, which consisted of 25 min, with recordings every 30 s. Data were analyzed using TurboFit software (VacuMed), and the resting metabolic rate was calculated using the modified Weir's equation: resting metabolic rate = 1.44(3.9 × V̇o2 + 1.1 × V̇co2), where V̇o2 is the oxygen consumed and V̇co2 the CO2 exhaled (45). For a given animal, we considered as an accurate value for resting metabolic rate when six consecutive measurements of the levels of CO2 in the chamber and the resting metabolic rate were within 3% difference of each other.
Statistical analysis.
All data were expressed as means ± SE. Comparison of means within groups were performed using the repeated-measures one-way ANOVA test, with Tukey's test as a post hoc analysis. Nonpaired t-test was used to compare means between groups. General linear model was used to determine multiple correlation (r) between variables measured repeatedly throughout the study. All of the analyses were done using Statistica (StatSoft, Tulsa, OK). Differences were assumed to be significant if the P value was <0.05.
RESULTS
Initial induction of fat accumulation (weeks −6 to 0).
The initial 6 wk of HHFD resulted in a 7.1% increase in body weight (n = 20) from 29.6 ± 0.8 to 31.8 ± 1.0 kg (P < 0.001). These changes were accompanied by a 35.8% increase in total fat in the abdominal trunk (733.3 ± 58.5 to 995.5 ± 90.8 cm3, P < 0.001) with no changes in nonfat tissue (P = 0.166). There were increases in both VAT (28.1%, P < 0.001) and SAT (66.8%, P < 0.001), with a predominance of SAT accumulation in absolute numbers (145.7 ± 32.0 vs. 116.6 ± 21.0 cm3, SAT and VAT, respectively) (Fig. 1). The fat percentage of the total tissue increased from 24.9 to 31.5% (P < 0.001).
Fig. 1.
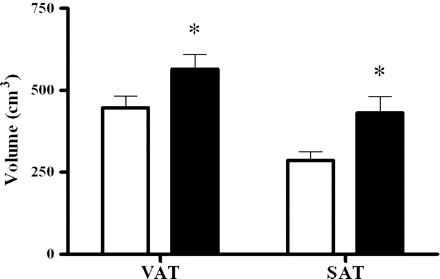
Bars represent changes in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), measured by magnetic resonance imaging, in the abdominal trunk from dogs (n = 20) maintained exclusively on a hypercaloric high-fat diet (HHFD) for 6 wk. Open bars, week −6 (pre-HHFD); filled bars, week 0 (post-HHFD). Data are means ± SE. *P < 0.001, compared with week −6.
Effect of rimonabant on body weight and food intake (weeks 0 to 16).
By week 16 of treatment and continued HHFD, rimonabant treated dogs lost 2.8% of their body weight, whereas placebo dogs increased their body weight by 6.5%. However, body weight was not different between groups (P = 0.156). In the rimonabant group, we observed transient decreases in food intake, at week 2 (P < 0.001) and at week 16 (P = 0.027). Placebo group showed no changes in food intake throughout the study. When we normalized food intake to body weight, we found a significant difference between groups only at week 2 (65.1 ± 4.1 vs. 81.2 ± 3.0 kcal·kg−1·day−1, rimonabant vs. placebo, respectively; P = 0.007) (Table 1).
Table 1.
Food intake, body weight, and abdominal trunk composition in dogs maintained on a HHFD
| Variable | Week 0 | Week 2 | Week 6 | Week 12 | Week 16 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rimonabant group (n = 11) | |||||||||
| FI, kcal·kg−1·day−1 | 80.2±3.7 | 65.1±4.1a,d | 80.6±5.0 | 73.7±3.2 | 70.0±3.1 | ||||
| Body wt, kg | 31.7±1.3 | 29.9±1.1c | 30.1±1.1c | 30.6±1.2a | 30.8±1.1b | ||||
| Abdominal trunk | |||||||||
| TT, cm3 | 3,105.6±145.5 | 2,953.7±115.9 | 2,898.9±125.5b | 3,056.3±123.4 | 3,062.6±134.4 | ||||
| NFT, cm3 | 2,016.0±88.0 | 2,004.2±75.3e | 2,010.0±71.1 | 2,103.0±82.1 | 2,126.9±85.5 | ||||
| TF, cm3 | 1,089.5±118.3 | 949.5±95.2b | 888.9±109.3c | 953.3±93.1b | 935.6±108.2b | ||||
| VAT, cm3 | 611.3±57.9 | 523.6±41.6 | 519.7±60.0 | 574.0±48.7 | 555.9±63.9 | ||||
| SAT, cm3 | 478.3±63.5 | 425.8±59.1 | 369.2±54.7c | 379.3±46.9c | 379.7±48.9c | ||||
| VAT/SAT | 1.4±0.1 | 1.2±0.1 | 1.5±0.1 | 1.6±0.1b | 1.5±0.1 | ||||
| TF (%) of the TT | 34.5±2.4 | 31.7±2.3 | 29.9±2.7c | 30.8±2.2a | 29.9±2.6c | ||||
| VAT (%) of the TF | 57.1±1.4 | 56.3±1.9 | 59.2±1.9 | 61.0±1.3b | 59.7±1.4 | ||||
| SAT (%) of the TF | 42.9±1.4 | 43.7±1.9 | 40.8±1.9 | 39.0±1.3b | 40.3±1.4 | ||||
| Placebo group (n = 9) | |||||||||
| FI, kcal·kg−1·day−1 | 84.8±4.1 | 81.2±3.0 | 83.4±3.6 | 80.2±5.7 | 76.3±4.3 | ||||
| Body wt, kg | 31.8±1.5 | 31.8±1.5 | 32.4±1.5 | 33.5±1.7c | 33.9±1.9c | ||||
| Abdominal trunk: | |||||||||
| TT, cm3 | 3,113.7±143.4 | 3,173.4±146.0 | 3,220.9±161.6 | 3,371.8±218.7b | 3,404.5±207.4b | ||||
| NFT, cm3 | 2,233.1±106.8 | 2,229.2±63.9 | 2,241.1±91.7 | 2,283.7±113.2 | 2282.7±99.2 | ||||
| TF, cm3 | 880.6±138.2 | 944.1±138.6 | 979.8±165.9 | 1,088.1±202.9a | 1,121.8±194.7c | ||||
| VAT, cm3 | 506.0±70.3 | 551.2±76.6 | 555.9±83.9 | 624.9±112.4b | 641.0±103.8a | ||||
| SAT, cm3 | 374.6±74.8 | 393.0±70.5 | 423.9±88.3 | 463.2±95.9a | 480.8±97.5c | ||||
| VAT/SAT | 1.5±0.2 | 1.3±0.2 | 1.4±0.2 | 1.4±0.1 | 1.4±0.1 | ||||
| TF (%) of the TT | 27.7±3.2 | 28.9±2.8 | 29.5±3.3 | 30.9±3.6a | 31.7±3.4c | ||||
| VAT (%) of the TF | 58.6±2.8 | 58.9±2.8 | 57.8±2.6 | 57.8±2.3 | 57.8±2.6 | ||||
| SAT (%) of the TF | 41.4±2.8 | 41.1±2.8 | 42.2±2.6 | 42.2±2.3 | 42.2±2.6 | ||||
Values are means ± SE. FI, food intake; HHFD, hypercaloric high-fat diet; NFT, nonfat tissue; SAT, subcutaneous adipose tissue; TF, total fat; TT, total tissue; VAT, visceral adipose tissue.
P < 0.01,
P < 0.05, and
P < 0.001 compared with week 0.
P < 0.01 and
P < 0.05 compared with placebo.
Effect of rimonabant on the abdominal fat composition.
Fat changes in the abdominal trunk from representative dogs in each group are shown in Fig. 2. The body weight loss seen in the rimonabant group was consistent throughout the study, and occurred concomitant with a loss of total fat in the abdominal trunk. Interestingly, VAT remained unchanged in the rimonabant group, whereas SAT decreased consistently from week 6 to the end of the study. Conversely, the placebo group showed a progressive increase in total fat, including VAT and SAT, reaching significant changes in the last 4 wk of the study (Table 1). Trends of total fat, VAT, and SAT changes in both groups throughout the study are shown in Fig. 3.
Fig. 2.
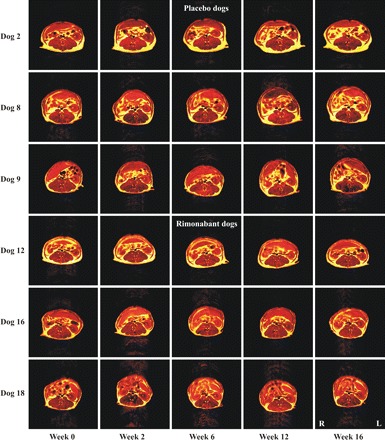
Magnetic resonance imaging (T1-weighted) of the abdominal trunk in 3 representative dogs from each group, placebo and rimonabant, maintained on a HHFD. Images correspond to scans at the level of the left renal hilum. Placebo dogs progressively gained more fat (represented in yellow) during the high-fat-diet period, whereas, in rimonabant dogs, fat accumulation was prevented. Nonfat tissue is shown in red/brown. R, right; L, left.
Fig. 3.
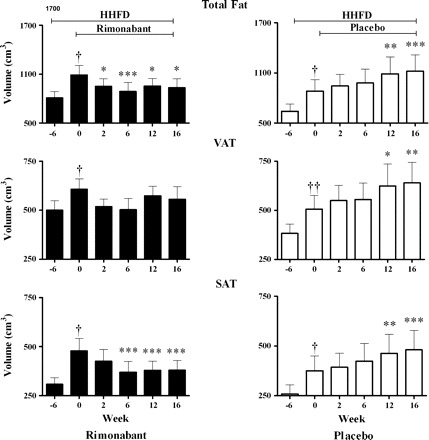
Changes in total fat, VAT, and SAT in the abdominal trunk from dogs receiving rimonabant (n = 11) or placebo (n = 9) concomitant with a HHFD during 16 wk. Data are means ± SE. †P < 0.05 and ††P < 0.01 compared with week −6. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with week 0.
At the end of the study, total fat, VAT, and SAT, but not nonfat tissue, expressed as percent changes of the baseline (Δ), were significantly different between groups (rimonabant vs. placebo, Δtotal fat: −14.0 vs. 26.2%, P < 0.001; ΔVAT: −9.8 vs. 26.7%, P = 0.004; ΔSAT: −19.5 vs. 28.8%, P < 0.001; Δnonfat tissue: 5.9 vs. 2.6%, P = 0.303). Although absolute values of VAT and SAT seemed to be higher at week −6 in the rimonabant group, compared with placebo (Fig. 3), they were not statistically different (P = 0.101 and 0.381, for VAT and SAT, respectively). Importantly, body weight changes were associated with fat tissue and nonfat tissue changes in the rimonabant group (vs. total fat: r2 = 0.43, P = 0.001; vs. VAT: r2 = 0.37, P = 0.001; vs. SAT: r2 = 0.39, P < 0.001; vs. nonfat tissue: r2 = 0.22, P = 0.041) and in the placebo group (vs. total fat: r2 = 0.8, P < 0.001; vs. VAT: r2 = 0.70, P < 0.001; vs. SAT: r2 = 0.72, P < 0.001; vs. nonfat tissue: r2 = 0.47, P < 0.001). Hence, total fat and nonfat tissue were predictors of body weight changes in the rimonabant group.
Body weight changes are not associated with either resting metabolic rate or food intake changes.
In a subset of dogs (n = 12), we evaluated the contribution of food intake and resting metabolic rate to body weight changes induced by rimonabant. The dogs treated with rimonabant (n = 7) lost 4.3% of their body weight (P < 0.001), whereas the placebo dogs (n = 5) increased their body weight by 5.9% (P = 0.003). However, the difference in body weight between the groups was not significant (Fig. 4A). Rimonabant reduced food intake only during the first 2 wk of the study (baseline: 2,570.9 ± 185.5 kcal/day; week 2: 1,808.4 ± 152.3 kcal/day; P < 0.001). The placebo group showed no changes on food intake at any week. When normalized to body weight, food intake was significantly less in the rimonabant group compared with the placebo group only at week 2 (60.8 ± 5.6 vs. 81.4 ± 4.4 kcal·kg−1·day−1; P = 0.022) (Fig. 4B).
Fig. 4.
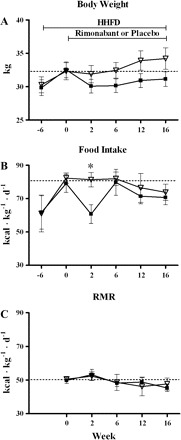
Effect of rimonabant on body weight, food intake, and resting metabolic rate in dogs fed a HHFD. ▪, Rimonabant group (n = 7); ▵, placebo group (n = 5). Dogs received either rimonabant or placebo from week 0 through week 16, as indicated in A, for all panels. Rimonabant dogs significantly reduced their body weight from week 2 until the end of the study (A); however, food intake was reduced only at week 2 (B). There were no changes in resting metabolic rate (C). Dashed lines indicate the baseline reference values immediately before the beginning of treatment. Data are means ± SE. *P < 0.05 between groups.
At the end of the study, rimonabant treatment did not result in changes in resting metabolic rate (week 0: 1,618.8 ± 75.9; week 16: 1,414.1 ± 85.5 kcal/day; P = 0.264; repeated-measures ANOVA). Likewise, resting metabolic rate remained unchanged in the placebo group (week 0: 1,632.9 ± 63.7; week 16: 1,628.5 ± 139.9 kcal/day; P = 0.999; repeated-measures ANOVA). Moreover, there was no difference in resting metabolic rate between groups at any week (Fig. 4C). We found that body weight changes in the rimonabant group were not associated with either resting metabolic rate (r2 = 0.24; P = 0.154) or food intake (r2 = 0.24; P = 0.166) changes. Furthermore, in the rimonabant group, total fat, VAT, and SAT changes were not associated with resting metabolic rate (vs. total fat: r2 = 0.10, P = 0.660; vs. VAT: r2 = 0.08, P = 0.756; vs. SAT: r2 = 0.15, P = 0.420), but were associated with food intake changes (vs. total fat: r2 = 0.41, P = 0.013; vs. VAT: r2 = 0.34, P = 0.044; vs. SAT: r2 = 0.38, P = 0.024).
DISCUSSION
Excessive abdominal fat is a high risk factor for the development of insulin resistance and type 2 diabetes. Clinical trials have shown a reduction in waist circumference in subjects treated with rimonabant. However, to date, direct evidence showing the effects of rimonabant on visceral and SAT in the abdominal region has not been reported. In the present study, we induced abdominal fat accumulation in lean dogs (an animal model similar to human obesity) using a high-fat hypercaloric diet. We examined the effect of rimonabant treatment on visceral and subcutaneous fat in the abdomen. We also examined the contribution of food intake and energy expenditure to body weight changes induced by rimonabant. The present study confirms previous findings, demonstrating an effect of rimonabant to reduce adiposity and body weight with only a transient reduction in food intake. In addition, we clearly demonstrate that rimonabant prevents additional fat accumulation in the VAT depot while significantly reducing SAT content during the maintenance of a HHFD. These changes in fat depots occurred in the absence of any changes in nonfat tissue. Thus CB1R antagonism has a profound effect to reduce the amount of stored fat in an animal model reminiscent of human obesity.
The decrease in body weight induced by rimonabant was significant after 2 wk of treatment until the end of the study (Fig. 4A). However, the dogs exhibited only a transient decrease in food intake at week 2 (Fig. 4B). This transience in the effect of rimonabant on food intake has been consistently reported in rodent studies (9, 11, 35, 36). Importantly, we did not find a correlation between food intake and body weight changes. Together, these results suggest that food intake alone cannot explain the profound effect of rimonabant to reduce weight.
The observation that rimonabant induced a preferential loss of SAT, rather than VAT, does not appear to be in concordance with the notion that adipocytes from VAT have a higher basal lipolytic rate than adipocytes from SAT. However, it must be noted that, in the present study, fat feeding was maintained throughout the treatment period. We have recently hypothesized that fat accumulates initially in the VAT depot and then spills over into the SAT depot, known as the overflow hypothesis (2). Hence, in the presence of continued fat feeding, rimonabant may act to prevent further fat accumulation in the VAT while reducing SAT. These actions of rimonabant on adipose tissue are indicative of direct peripheral effects of rimonabant. Several in vitro studies support a direct effect of rimonabant on adipose tissue. The expression of CB1R has been found in both rodent (1, 6) and human (10, 32, 38) adipocytes. Interestingly, rimonabant has been shown to block the rate of lipogenesis induced by the CB1R agonist WIN-55,212 in primary adipocytes (6) and stop the accumulation of lipid droplets in cultured clonal preadipocytes (12). Whether CB1R expression is found in canine fat tissue is largely unknown. However, we have recently shown that rimonabant treatment prevents the accumulation of large adipocytes in subcutaneous and omental fat in dogs fed a HHFD (17).
It has been reported that increased fat accumulation in adipocytes leads to an increase in lipolysis (47). Although we found an increase in the number of large adipocytes (>75 μm) with fat feeding, we did not observe any differences in basal lipolysis measured in vitro (data not shown). These observations lead us to speculate that there is a threshold in which lipid accumulation in adipocytes must reach to observe changes in lipolytic activity. Consistent with this notion is a recent report in rats that demonstrate no change in basal lipolysis in high-fat-fed rodents at a time when mesenteric and retroperitoneal fat pads are significantly increased (5). However, after prolonged periods of fat feeding and consequently increased fat accumulation, differences in lipolytic activity were noted. Moreover, some studies suggest that the antiobesity effects of rimonabant are due to increased lipolysis, yet we observed no differences between the two groups. The molecular mechanism as to how rimonabant prevents visceral fat accumulation and reduces subcutaneous fat content remains to be elucidated. Clearly, the roles of lipogenesis, lipoprotein lipase activity, and reesterification rates must be taken into account. A potential mechanism may be gleaned from mouse studies that demonstrate inhibition of lipid accumulation in adipocytes by blocking preadipocyte proliferation (12).
An alternative mechanism for the reduction in body adiposity and weight loss may be attributed to changes in energy expenditure. Energy expenditure has been shown to be enhanced with rimonabant treatment. Because resting metabolic rate represents the largest component of total energy expenditure (29, 37), we examined whether body weight loss induced by rimonabant was dependent on resting metabolic rate changes. In a subset of dogs, we conducted a study to determine whether increases in energy expenditure could explain the body weight changes. Surprisingly, we found no significant changes on resting metabolic rate during the study (Fig. 4C). Moreover, there was no association between resting metabolic rate and body weight changes. These results suggest that other components of total energy expenditure might explain the body weight loss (29) (i.e., physical activity and adaptive thermogenesis). It has been reported that rimonabant increases grooming (18) and scratching activity (18, 28) in rodents. In addition, it has been shown that acute (28) and chronic (15) administration of rimonabant increases total energy expenditure in rats. Physical activity was not monitored in this study. However, weekly core body temperatures (data not shown) in the dogs remained invariable throughout the study, indirectly suggesting that the body heat production was not altered. Thus the reduction of body weight may be due to changes in spontaneous physical activity.
Clinical studies aimed to determine the effect of other anti-obesity drugs in combination with a low-calorie diet on abdominal fat distribution, either by computed tomography or MRI, demonstrate a decrease in both VAT and SAT compartments. Orlistat, an inhibitor of pancreatic lipase (40), and sibutramine, a blocker of serotonin and norepinephrine reuptake, (20, 21, 48) were able to significantly reduce abdominal fat content in obese subjects. However, unlike our study, the aforementioned studies are treatment therapies in combination with a low-calorie diet. In the face of continued fat feeding, it may be difficult to see a true reduction in visceral fat, particularly if fat is preferentially deposited in this depot.
It has been reported that overexpression of adiponectin in mice resulted in expansion of SAT and was associated with an improvement of the metabolic profile (22). In addition, it has recently been reported that transplantation of SAT in the VAT depot improved insulin sensitivity in mice (41). Apparently, these results might be in conflict with the fact that the effect of rimonabant was more pronounced in SAT. Although the findings from these studies are interesting, the extrapolation of those results to human obesity is unknown, given the differences in fat distribution and composition between humans and rodents. In fact, if SAT were beneficial, liposuction would significantly impair the metabolic profile in humans that undergo this procedure. For example, some studies have not shown changes in metabolic parameters after 10–12 wk of follow-up (27) or within 1–4 yr of a significant sustained reduction of SAT (31). Together, these human studies suggest that SAT does not have a beneficial effect on metabolic disease.
In conclusion, this study provides the first direct evidence of the effect of rimonabant on fat distribution in the abdominal region in a canine model which parallels fat distribution in humans. Chronic treatment with rimonabant resulted in a reduction of body weight and fat content in the abdominal trunk with no changes in nonfat tissue, suggesting a direct effect of rimonabant on adipocytes. In contrast, with the significant increases of both SAT and VAT in placebo, rimonabant prevented additional VAT accumulation and decreased SAT. The reduction in overall body weight was not associated with either resting metabolic rate or food intake changes. However, an increase in spontaneous physical activity cannot be excluded as a possible mechanism to induce weight loss. These findings provide evidence of a peripheral effect of rimonabant to reduce adiposity and body weight, possibly through a direct effect on adipose tissue.
GRANTS
This work was supported by Sanofi-aventis.
Acknowledgments
We are very grateful to radiologist Linda Needham for help with the MRI scans and to Rita Thomas for excellent assistance with the MRI images. We also thank our lead laboratory animal technician Edward Zuñiga and assistant Edgardo Paredes. We give special recognition to Sophia Yae and Justin Dittmann for assistance with experiments. Additionally, we express our extreme gratitude to Dr. Erlinda Kirkman for excellent veterinarian expertise in facilitating these studies.
Parts of this study have previously been presented at the 43rd and 44th Annual Meetings of the European Association for the Study of Diabetes (Amsterdam, 2007 and Rome, 2008; respectively) and at the 67th and 68th Scientific Sessions of the American Diabetes Association (Chicago, 2007 and San Francisco, 2008; respectively).
REFERENCES
- 1.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63: 908–914, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: Mechanisms of the metabolic syndrome. Obesity 14: 16S–S19, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 120: S3–S8, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45: 633–638, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Collin P, Chapados N, Dufresne E, Corriveau P, Imbeault P, Lavoie JM. Time course of changes in in vitro lipolysis of intra-abdominal fat depots in relation to high-fat diet-induced hepatic steatosis in rats. Br J Nutr 96: 268–275, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353: 2121–2134, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7: 438–455, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Doyon C, Denis RG, Baraboi ED, Samson P, Lalonde J, Deshaies Y, Richard D. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes 55: 3403–3410, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Lorente R, Cabot C, Fernandez-Lopez JA, Alemany M. Effects of combined oleoyl-estrone and rimonabant on overweight rats. J Pharmacol Sci 104: 176–182, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gary-Bobo M, Elachouri G, Scatton B, Le Fur G, Oury-Donat F, Bensaid M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol 69: 471–478, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med 120: S10–S16, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 57: 1269–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed wistar rats. Endocrinology 149: 2557–2566, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Herling AW, Kilp S, Juretschke HP, Neumann-Haefelin C, Gerl M, Kramer W. Reversal of visceral adiposity in candy-diet fed female Wistar rats by the CB1 receptor antagonist rimonabant. Int J Obes (Lond) 32: 1363–1372, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hsu IR, Kabir M, Stefanovski D, Woolcott O, Zheng D, Catalano KJ, Chiu JD, Kim SP, Harrison LN, Lottati M, Ionut V, Bergman RN, Richey JM. Accumulation of large adipocytes in omental and subcutaneous fat depots during development of obesity in dogs prevented by rimonabant (Abstract). Diabetologia 50, Suppl 1: P0664, 2007 [Google Scholar]
- 18.Jarbe TU, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Delta 9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacol Biochem Behav 73: 911–919, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord 24: 607–613, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kim DM, Yoon SJ, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC, Huh KB. Sibutramine improves fat distribution and insulin resistance, and increases serum adiponectin levels in Korean obese nondiabetic premenopausal women. Diabetes Res Clin Pract 66, Suppl 1: S139–S144, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. β-Cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 292: E1581–E1589, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kim SP, Ellmerer M, Van Citters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 52: 2453–2460, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kissebah AH. Intra-abdominal fat: is it a major factor in developing diabetes and coronary artery disease? Diabetes Res Clin Pract Suppl 30: 25–30, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350: 2549–2557, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W. Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 32: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 283: E1135–E1143, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mohammed BS, Cohen S, Reeds D, Young VL, Klein S. Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring) 16: 2648–2651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, Bianchi K, Rizzuto R, Bernante P, Federspil G, Vettor R. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 92: 4810–4819, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27: 73–100, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, and for the RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295: 761–775, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 7: 65–72, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 35: 566–573, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, Haffaf Y, Cesari M, Festy F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol 126: 177–187, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368: 1660–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tiikkainen M, Bergholm R, Rissanen A, Aro A, Salminen I, Tamminen M, Teramo K, Yki-Jarvinen H. Effects of equal weight loss with orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. Am J Clin Nutr 79: 22–30, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tulloch-Reid MK, Hanson RL, Sebring NG, Reynolds JC, Premkumar A, Genovese DJ, Sumner AE. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in African Americans. Obesity Res 12: 1352–1359, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365: 1389–1397, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 52: 2490–2496, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Weir JBdV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 52: 541–546, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Yip I, Go VL, Hershman JM, Wang HJ, Elashoff R, DeShields S, Liu Y, Heber D. Insulin-leptin-visceral fat relation during weight loss. Pancreas 23: 197–203, 2001 [DOI] [PubMed] [Google Scholar]


