Abstract
Ebola is a highly virulent pathogen causing severe hemorrhagic fever with a high case fatality rate in humans and non-human primates (NHPs). Although safe and effective vaccines or other medicinal agents to block Ebola infection are currently unavailable, a significant effort has been put forth to identify several promising candidates for the treatment and prevention of Ebola hemorrhagic fever. Among these, recombinant-virus based vectors have been identified as potent vaccine candidates with some affording both pre- and post-exposure protection from the virus. Recently, Investigational New Drug (IND) applications have been approved by the United States (U.S.) Food and Drug Administration (FDA) and Phase I clinical trials initiated for two small molecule therapeutics, 1) anti-sense phosphorodiamidate morphino oligomers (PMOs: AVI-6002, AVI-6003), and 2) lipid-nanoparticle/small interfering RNA (LNP/siRNA: TKM-Ebola). These potential alternatives to vector-based vaccines require multiple doses to achieve therapeutic efficacy which is not ideal with regard to patient compliance and outbreak scenarios. These concerns have fueled a quest for even better vaccination and treatment strategies. Here, we summarize recent advances in vaccines or post-exposure therapeutics for prevention of Ebola hemorrhagic fever. The utility of novel pharmaceutical approaches to refine and overcome barriers associated with the most promising therapeutic platforms will also be discussed.
Keywords: ebolavirus, vaccines, therapeutics, formulations
1. Introduction: Ebola Biology and Pathogenesis
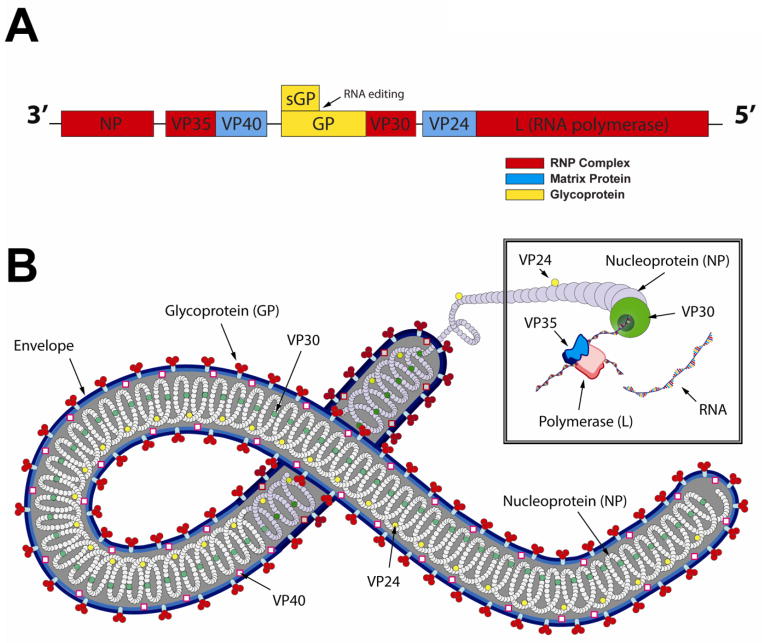
Ebola virus is a filamentous, negative-stranded RNA virus of the Filoviridae family that causes a severe, often fatal viral hemorrhagic fever in humans and non-human primates (NHPs)1. The single-stranded, negative sense 18.9 kb RNA genome encodes seven structural proteins and two non-structural proteins as shown in Figure 1A. The nucleoprotein (NP) is an essential component of the nucleocapsid that intimately binds to the virus genome. It, along with virion proteins (VPs) 30 and 35 and the RNA-dependent RNA polymerase (L) form the ribonucleoprotein (RNP) complex responsible for transcription and virus replication (Figure 1B) 2–4. Matrix proteins VP40 and VP24, linked to the RNP complex and the inner surface of the viral envelope respectively, are also involved in nucleocapsid formation. They also play a role in viral budding, assembly, and host range determination 5–10. The virus particle is enclosed in a lipid bilayer envelope derived from the host cell membrane during the budding process (Figure 1B).
Figure 1. The Ebola Virus.
A. Schematic Representation of the Zaire Ebola (EBOV) Genome. The non-segmented negative-stranded RNA genome contains seven structural proteins (NP, VP24, VP30, VP35, VP40, L, GP) and two non-structural proteins (secreted GP (sGP) and small soluble sGP (ssGP) not shown). B. Configuration of the Ebola Virus Particle. During replication, NP, VP30, VP35, VP24 and L protein form the ribonucleoprotein (RNP) complex with the viral genomic RNA. The rod-shaped virus is 80 nm in diameter. The length of the virion, ranging from 1,028 to 1,978 nm is dictated by the number and length of the genomes that are incorporated into a single virus capsid during replication and assembly.
Ebola glycoprotein (GP), dispersed throughout the viral envelope as trimeric spikes, consists of two fragments; an extracellular protein (GP1) and a membrane-anchored protein (GP2). These are held together by disulfide bonds 11–14. Preferential binding of the Ebola virus to endothelial and monocytic cells is mediated by a 17 amino acid sequence within the GP1 domain that resembles an immunosuppressive motif in several human and animal retrovirus envelope proteins 15–21. Interaction of this peptide sequence with target cells is thought to play a key role in apoptosis and the immunopathology of Ebola infection 22. Proteolysis of a precursor protein (pre-sGP) by furin generates a non-structural secretory glycoprotein (sGP) homodimer and a smaller Δ-peptide. sGP shares neutralizing epitopes with the envelope GP1,2 trimer spike and is released from cells in large quantity early in infection 23–25. This would suggest that it may be a decoy produced by the virus to bind circulating neutralizing antibodies (NABs). Additional studies evaluating the function of the Δ-peptide have produced evidence that it plays a role in viral entry and prevents superinfection of cellular targets. It also prevents trapping of mature virions in the endoplasmic reticulum 26. A third GP gene product, a smaller, soluble secreted glycoprotein (ssGP) has recently been discovered. Although its role in Ebola infection is currently unclear, it has very distinct properties from the sGP and Δ-peptide27.
Ebola virus infection in humans generally occurs through direct contact with mucosal surfaces, skin abrasions or contaminated needles28. Antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs) located at the site of infection, are primary targets of Ebola replication. Despite the fact that the virus enters immature DCs through typical C-type lectin (DC-SIGN) or other pattern recognition receptors, the cells become functionally deregulated and are unable to express co-stimulatory molecules or stimulate lymphocytes, namely naïve T cells 29, 30. VP24 and 35 most likely play a pivotal role in preventing DCs from responding to infection as they block the type 1 interferon (IFN) anti-viral response in infected cellular targets by preventing nuclear accumulation of signal transducer and activator 1 (STAT1) and impeding the activity of interferon regulatory factor (IRF)-3 and 731, 32. This effect is further propagated by VP24 as it also blocks the p38 MAP kinase pathway in a JAK-STAT independent manner and by VP35 as it prevents activation of a dsRNA-dependent protein kinase required for production of IFN 33–35. Unresponsiveness of DCs to Ebola infection most likely contributes to the massive lymphocyte apoptosis routinely observed during clinical cases of infection in humans 36.
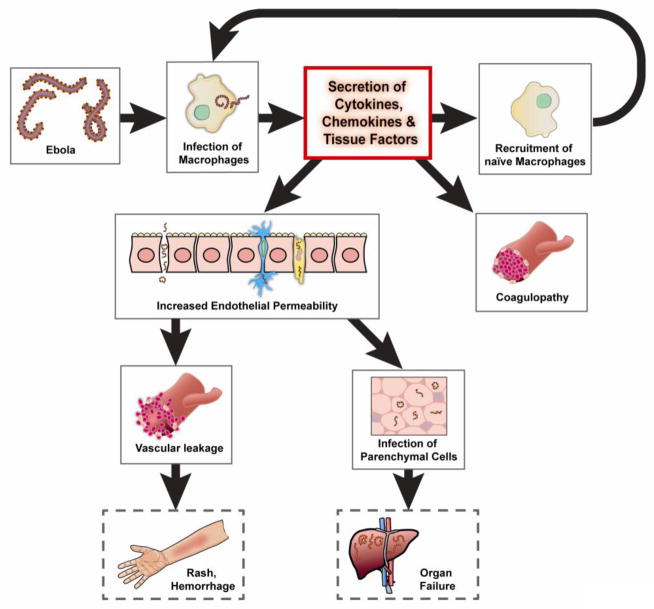
Ebola infection of monocytes and macrophages elicits the release of massive amounts of pro-inflammatory cytokines and chemokines including interleukins (IL)-1β, 2, 6, 8 and 10; tumor necrosis factor (TNF)-α monocyte chemo-attractant protein (MCP)-1; regulated on activation normal T cell expressed and secreted (RANTES) and reactive and nitrogen oxygen species (ROS and NO, respectively) 37–39. This “cytokine storm” recruits additional APCs to the site of infection, increasing the number of hosts to support virus replication. It also contributes to the pathogenesis at the late stage of disease by increasing endothelial permeability, and vascular leakage which, in turn, foster rapid dissemination of infected APCs throughout the systemic circulation to release Ebola in secondary lymphoid organs, lungs, liver and other ancillary sites of virus replication (Figure 2) 40–43.
Figure 2. Ebola Pathogenesis.
Ebola infects a variety of cellular targets (endothelial cells, fibroblasts, hepatocytes and adrenal cortical cells) as well as macrophages, monocytes and dendritic cells. While infected dendritic cells fail to activate naïve T cells to combat infection, infected macrophages and monocytes release a large number of cytokines and chemokines in a “cytokine storm”. This cytokine storm supports virus replication and dissemination as it recruits new hosts (naïve antigen presenting cells) to the site of infection. Excess cytokines tissue factor released from macrophages also interfere with the coagulation cascade and increase endothelial permeability that leads to vascular leakage, hemorrhage and a notable maculopapular rash. Repeated cycles of rapid virus replication in parenchymal cells eventually overcome a dysregulated immune response and lead to severe tissue damage, necrosis, septic shock, multi-organ failure and eventually death.
Over the last 35 years, numerous Ebola outbreaks have been recorded44. The Ebola virus was first identified during two near-simultaneous outbreaks in Central Africa in 1976 of two different species with case fatality rates of up to 90%: Zaire ebolavirus (EBOV) and Sudan ebolavirus (SUDV). Since then, additional species have been identified: Reston (RESTV), Tai Forest (TAFV) and Bundigbugyo (BDBV) 45. RESTV, isolated in 1989 from cynomolgus macaques exported from the Philippines to the United States, is the only species that has not been associated with human disease 46–48. Although cases of Ebola virus infection have been primarily limited to Africa, the number of outbreaks and associated fatalities has slowly increased over time. This, coupled with documented reports that Ebola can be transmitted across species through aerosolization of virus particles 49, 50, has raised significant concerns over its possible use as a biological weapon, makes the virus a NIAID Category A Priority Pathogen and restricts experiments using all Ebola species to biosafety level (BSL)-4 containment laboratories 50–52.
There is generally a 2~21 day incubation period before symptoms of Ebola virus-induced hemorrhagic fever are noted. They initially manifest as non-specific flu-like symptoms (malaise, chills, fever) and rapidly progress to severe nausea, diarrhea, shortness of breath, hypotension, bleeding and coma 53. Vascular injury due to endothelial cell damage, hepatocyte necrosis caused by virus replication, coagulation disorders and uncontrolled cytokine/chemokine secretion by infected monocytes and macrophages contribute to EBOV-induced hemorrhagic shock and eventual death of the patient (Figure 2) 36, 51, 54. Although Ebola is the focus of many cutting-edge, coordinated, interdisciplinary research programs around the world, effective vaccines or medicinal agents to combat this deadly pathogen are currently unavailable for human use. These efforts, however, have accelerated identification of many new molecular targets and promising therapeutic candidates currently in pre-clinical testing.
2. Vaccine Targets: Ebola Proteins
The first Ebola vaccine consisted of whole virions inactivated by heat, formalin and gamma-irradiation 55, 56 and was largely ineffective in rodents and non-human primates. Since then, overexpression of genes that encode Ebola virus proteins has been the primary approach to vaccine development. The rationale behind this strategy was to induce cellular targets to produce enough virus protein to elicit potent T and B cell-mediated immune responses that would confer protection against Ebola (Figure 3). Because Ebola GP was known to play a key role in virus entry and facilitate cell death and vascular permeability in the latter stages of infection, most of the early recombinant vaccine platforms centered around overexpression of GP alone or in combination with NP and other VPs (Figure 3). A variety of viral and non-viral vectors have been used to deliver genes for these antigens and encourage strong B and T cell-mediated responses (Table 1).
Figure 3. Generalized Approach to Ebola Vaccine Development.
The most promising platforms under development for clinical testing involve overexpression of Ebola glycoprotein and/or nucleoprotein. This is accomplished through administration of replication deficient recombinant adenoviruses or plasmid vectors (Panel A) that transduce various cellular targets (Panel B) to make large amounts of the Ebola antigens that enter the general circulation. Other platforms use attenuated recombinant viruses, such as VSV or HPIV3, that bear Ebola glycoprotein on their surface. These viruses can replicate in cellular targets (Panel B). These particles, like the glycoprotein and nucleoprotein made from cells transduced with adenovirus or plasmid, are taken up and processed by macrophages and dendritic cells (Panel C) to generate strong B and T cell-mediated immune responses against Ebola (Panel D).
Table 1.
Ebola Vaccine Platforms Currently Tested In Non-Human Primates
| Platform | Targets in vaccine | Prophylactic efficacy | Therapeutic efficacy | Concerns | References |
|---|---|---|---|---|---|
| Recombinant adenovirus serotype 5 (rAd5) | GP, GP+NP | Yes, 1 ×1010 ~ 2 × 1012 VP | Not tested | Pre-existing immunity | 58, 189 |
| Rare adenovirus serotypes (rAd26 prime /rAd35 boost) | GP | Yes, 1 ×1011 VP (Prime boost) | Not tested | Boost immunization required | 66 |
| DNA/rAd5 (Prime/Boost) | GP+NP | Yes DNA: 4 mg (3 doses)/rAd5: 1 × 1010 PFU, 1 × 1011 PU | Not tested | Boost immunization required, pre-existing immunity | 57, 58, 190 |
| Vesicular stomatitis virus (VSV) | GP | Yes 1 × 107 PFU | Yes 2 × 107 PFU | Safety (replication competent) | 74–76, 79–81 |
| Human parainfluenza virus type 3 (HPIV-3) | GP, GP+NP | Yes 4 × 106 TCID50 (single dose) 2 × 106 TCID50 (Prime/boost) |
Not tested | Boost immunization required, Safety (Replication competent) | 86, 191 |
| Venezuelan Equine Encephalitis virus (VEE) replicon | GP | Yes 1 × 107 FFU | Not tested | Boost immunization required | 192 |
| Virus-like particles (VLPs) | GP+NP+VP40 | Yes 250 μg (3 doses) | Not tested | Boost immunization required | 193 |
Abbreviations: VP: total number of virus particles (infectious and non-infectious); PFU: plaque forming units; PU: particle units; TCID50: median tissue culture infective dose; FFU: focus forming units.
2.1 Recombinant Adenovirus and Plasmid DNA-Based Ebola Vaccines
The first vaccine platform that successfully protected NHPs from Ebola virus infection was a recombinant adenovirus serotype 5 (rAd5) vector expressing EBOV GP 57. A single intramuscular (IM) dose of adenovirus after three consecutive priming doses of plasmid DNA encoding EBOV GP and NP, SUDV GP and TAFV GP fully protected primates against lethal challenge. This combinatorial approach, DNA prime/rAd5 boost, greatly improved circulating anti-GP antibody levels and generated notable antigen-specific CD4+ and CD8+ T cell proliferative responses in cynomolgus macaques. The high level of transgene expression and the inherent adjuvant properties of the adenovirus capsid were fully appreciated in subsequent studies in which a single IM injection of the virus alone could protect animals from lethal challenge 58. Further refinements of the rAd5-based vaccine platform by Richardson et al. involved optimizing the GP expression cassette so that more antigen was produced 59. As a result, the dose of this vaccine could be reduced 100 fold without compromising antigen-specific immune responses. This approach was so successful that a single IM injection of the re-engineered vaccine fully protected mice when it was given 30 minutes after exposure to a lethal dose of EBOV, suggesting that this platform might be useful for both prophylaxis and post-exposure applications.
Despite these promising results, the concern remains that rAd5-based vaccines may have limited clinical utility due to the fact that a significant portion of the global population have considerable amounts of anti-Ad5 neutralizing antibodies (NABs) in their circulation 60, 61. In the United States, approximately 30–60% of the population have measurable levels anti-adenovirus NABs in their circulation, while 40–80% of those in Europe and Asia contain similar levels of NABs 62, 63. The highest levels recorded to date are found in sub-Saharan Africa (80–100% positive) 64. Increasing the vaccination dose can override pre-existing immunity (PEI) and achieve notable antigen expression. This approach, however, is not desirable since high doses of adenovirus particles can precipitate severe, toxic inflammatory responses in humans 65. Another strategy to circumvent PEI to Ad5 involves immunization with rare adenovirus serotypes since anti-Ad5 NABs do not completely cross-react with and neutralize these viruses 66–69. Vaccine platforms using these viruses have partially protected rodents and NHPs with PEI to Ad5 from lethal challenge (Table 1) 66. Mucosal administration of rAd5 has also been shown to avoid neutralization of the virus by anti-Ad5 NABs in the circulation. Although this route of immunization generally induces lower systemic antigen-specific T cell responses, it induces strong local T and B cell responses not impaired by PEI that afford full protection in rodent and NHP models of disease 60, 61. Recently, a Phase I clinical trial conducted with 31 healthy adults demonstrated that a rAd5-based Ebola vaccine is capable of inducing antigen-specific T cell and antibody responses without notable side effects, however, prior exposure to adenovirus did compromise immunogenicity of the vaccine when it was given by IM injection 70.
2.2 Live Attenuated Virus-Based Ebola Vaccines
Another promising vaccine platform involves the use of live attenuated recombinant viruses bearing the Ebola GP (Figure 3). One particular candidate, a recombinant vesicular stomatitis virus (VSV) in which the wild-type VSV surface glycoprotein was replaced with EBOV GP demonstrated attenuated growth kinetics and tropism of EBOV in vitro 71. A single dose of the virus given by the IM, intranasal (IN) or oral (PO) route completely protected mice, guinea pigs and NHPs from lethal challenge in the absence of any clinical symptoms or measurable viremia (Table 1) 72–78. In contrast, administration of a gamma-irradiated, inactive form of the virus did not protect animals, suggesting that replication is a critical component of the potency of this vaccine 79. This vector is a promising therapeutic option for post-exposure therapy since a single intraperitoneal dose 24 hours after lethal Ebola infection fully protected mice 75, 76, 80. Fifty percent of guinea pigs also survived lethal challenge when given the vector in a similar manner 24 hours after exposure 80. Fifty percent survival was also noted when rhesus macaques were given the vector 20~30 min after lethal challenge. These animals did develop notable clinical signs of disease (fever, lymphocytopenia) on day 6, but had low-level serum viremia that resolved 10 days later. High levels of GP-specific IgG NAB and relatively low IgM responses were also found in the serum of survivors.
Although more than 80 NHPs have been given this vaccine platform without notable toxicity 73, progress of the VSV-based construct to the clinic has been limited by concerns about its safety. To resolve this issue, the vector was first evaluated in immune-compromised mice lacking functional B and T cell 77 and NHPs infected with simian/human immunodeficiency virus (SHIV) 81. Administration of the vector to Non-Obese Diabetic/Severe Combined Immunodeficiency (NOD-SCID) mice at a dose that was 10 times that previously given to healthy mice was well tolerated 77. Four of six vaccinated SHIV infected NHPs survived Ebola challenge without vaccine-induced toxicity despite the fact that a relatively high dose of vaccine (1×107 PFU) was given to each animal 81. In an effort to address concerns associated with neurotoxicity of the VSV vector in healthy subjects, 21 NHPs were given either wild type VSV or recombinant VSVs containing either EBOV or Marburg GP on the surface by intrathalamic injection 82. Results from this study clearly indicate that recombinant VSV vectors lack neurovirulence properties associated with the wild type virus. An important observation made during this study was that, even though animals given the recombinant VSV vector did not elicit notable neurovirulence throughout the course of the study, the recombinant virus was detected in mucosal swabs, indicating that it could leave the central nervous system by an unknown mechanism. This vaccine was first used in humans when a laboratory scientist working with Ebola in a BSL-4 laboratory was exposed through an accidental needle-stick 83. The vaccine was given 48 hours after exposure. The patient developed a mild fever and myalgia 12 hours after injection. Other laboratory parameters (blood chemistry, coagulation and hematology) remained normal. Although the protective efficacy of the vaccine could not be determined in this case since Ebola infection could not be confirmed through serological testing, the scientist remains healthy to date.
Recombinant human parainfluenza virus 3 (HPIV3) expressing EBOV GP alone (HPIV3/EboGP) or together with nucleoprotein (HPIV3/EboGP-NP) has also been developed as a live-attenuated vaccine platform. Each of these constructs has conferred complete protection in guinea pigs and NHPs after EBOV challenge (Table 1) 84–86. Much like adenovirus, HPIV-3 is a common respiratory virus, making PEI to the vector in humans a major limitation of this platform. To address this issue, Bukreyev and colleagues developed a chimeric HPIV-3 vector expressing EBOV GP as the sole surface protein to circumvent impact of PEI on vaccine potency 87. This vector, HPIV3/Δ F-HN/EboGP, is resistant to HPIV-3-specific NABs in vitro and a single intranasal dose (4×106 PFU) protected guinea pigs from EBOV infection. Additional studies in animals given the vaccine in the presence of PEI to HPIV-3 and evaluation of toxicity of the vaccine in NHPs are needed to evaluate the clinical utility of this platform more precisely.
2.3 Future Perspectives: Vaccine Development
Although many of the vaccine platforms under development have fully protected NHP against lethal Ebola infection and some have entered clinical testing 70, each contain antigen sequences for one species of Ebola. Because each species of Ebola is antigenically distinct 88, 89, development of multivalent vaccines capable of conferring protection against several different species of Ebola would be practical since outbreaks are sporadic and difficult to predict. While a rAd5-based vector expressing both SUDV and EBOV GP and several different VSV-based vectors expressing GPs from SUDV, EBOV and Marburg virus (MARV) have been constructed, they have successfully protected a limited number of animals after lethal challenge with several different Ebola species 90–92. This multivalent approach is currently extremely labor intensive and limited by the cloning capacity of recombinant viral vectors. Development of mosaic antigens that contain protein fragments of potential T-cell epitopes from multiple Ebola species might be a good alternative strategy to overcome these issues. This concept has recently been illustrated with a recombinant viral vector expressing multivalent mosaic proteins that could elicit strong antigen-specific CD8+ T cell responses against several model antigens 93–95. Even though the protective efficacy of this strategy has not yet been demonstrated in infectious disease models, recent studies that highlight the importance of the CD8+ T cell response in conferring immune protection against EBOV infection in NHPs support this approach for development of multivalent Ebola vaccines 96, 97. As technology for production of protein-based vaccines progresses, large scale production of these novel antigens in combination with immunization scaffolds to ease purification and augment the immune response will be possible as recently demonstrated with the production of a potent Ebola GP immune complex vaccine in N. benthamiana 98.
3. Development of Novel Anti-Viral Molecules as Treatments for Ebola Infection
Even though Ebola has been identified as the cause of many lethal outbreaks of hemorrhagic fever for more than three decades, current treatment options for infected individuals are limited. Crude approaches like administration of convalescent serum from infected survivors 99 or equine anti-Ebola immunoglobulin with interferon 100 have been successfully used to reduce the severity of infection in laboratory settings (Figure 4A). Although each person that received these preparations survived, the mechanism for protection is not clear since the composition of each product was complex and one specific component could not reproducibly be linked to survival. For example, it was initially thought that anti-Ebola IgG was responsible for the protection of 8 patients with Ebola hemorrhagic fever who survived after they were given whole blood known to contain anti-EBOV IgG antibodies101. While this association seemed logical and straightforward, it has not been successfully reproduced in a controlled laboratory setting. In a study conducted by Jahrling et al., naïve rhesus macaques given convalescent-phase whole blood from 3 macaques that survived EBOV infection developed notable serum anti-EBOV IgG antibody titers 102. This, however, was not sufficient to control virus replication and disease in these animals progressed in a manner similar to that seen in untreated macaques. The efficacy of a highly characterized human anti-EBOV monoclonal antibody (KZ52) has been evaluated in guinea pigs and NHPs (Tables 2 and 3) 103. Even though this antibody had a reported IC50 of 0.5~2 μg/ml in vitro and in vivo, administration of a dose of 25 mg/kg, one hour after EBOV challenge conferred only partial protection (9 of 15) in guinea pigs. This preparation also failed to control virus replication and protect NHPs at a dose of 50 mg/kg 103. Additional detailed molecular studies revealed that monoclonal neutralizing antibodies interact with Ebola GP at different sites and combinations of monoclonal isolates are most effective for pre- and post- challenge applications 104–107. Once it was clear that direct transfer of immunity from infected survivors was an unreliable approach to combat Ebola infection, large multi-center collaborative research projects were initiated to screen libraries of well characterized and novel compounds to identify cellular and molecular targets associated with virus entry and replication. Compounds that have currently been evaluated in rodents and NHPs are summarized in Tables 2 and 3.
Figure 4. Common Targets of Small Molecules for Treatment of Ebola Infection.
A. Cellular Entry. Early attempts to mitigate the progress of Ebola infection involved administration of serum collected from convalescent survivors. Moderate success with this approach led to the development of monoclonal antibodies specific for Ebola glycoprotein that should, in theory, prevent virus entry into cellular targets. Additional work demonstrated that polyclonal antibodies or combinations of monoclonal isolates capable of interacting with Ebola GP at multiple sites are most effective for pre- and post-challenge treatments. High throughput screens identified one molecule, Compound 7, which could prevent virus entry in vitro. Computational analysis revealed that it fit in a pocket in the GP1,2 trimer. B. Endosomal Escape. High throughput analyses have identified several compounds that are effective in preventing endosomal escape of the virus particle. Other screens to identify host proteins that interact with Ebola throughout infection have led to the development of silencing technologies that make these proteins inaccessible to prevent release of virus particles from the endosome. C. Virus Replication, Budding and Release. Compounds that have demonstrated efficacy in stopping Ebola replication target various components of the RNP complex. Compounds that target VP 40 and 24 affect cellular transport of the virus particle and budding and release from the cellular host.
Table 2.
Anti-Viral Compounds Currently Tested in Rodent Models of Ebola Infection
| Therapeutic targets | Prophylactic efficacy | Therapeutic efficacy | Concerns | References | |
|---|---|---|---|---|---|
| FGI-103, 104 and 106 | Unknown | Yes FGI-106: 2 ~ 5 mg/kg BW * (single dose) | Yes FGI-103: 10 mg/kg BW*, (single dose) FGI-104: 10 mg/kg BW*/day (11 doses), FGI-106: 5 mg/kg BW* (3 doses) | Multiple doses required | 131–133 |
| NSC62914 | Reactive oxygen species (ROS) | Partial protection 2 mg/kg BW * (3 times) | Partial protection 2 ~ 5 mg/kg BW* (3 doses) | Multiple doses required | 144 |
| Small interfering RNAs (siRNA) | L polymerase + VP24 + VP35 | Not tested | Yes 0.75 ~ 1 mg/kg BW* (7 doses) | Multiple doses required | 122 |
| Phosphorodiamid ate morphino oligo nucleotides (PMOs) | VP24+VP35; L polymerase + VP24 + VP35 | Yes 5–50 μg (twice) | Yes 12.5 ~ 100 mg (11 doses) | Multiple doses required | 127, 128 |
| Monoclonal neutralizing Antibodies (NABs) | Ebola Virion (KZ52) | Not tested | Yes 50 mg/kg BW* (single dose) | Multiple doses required | 194 |
| Triple monoclonal antibody cocktail | Ebola GP | Yes 100 μg (single dose) | Yes 100 μg (single dose) | Must be used early before/after exposure. | 107 |
| S-adenosyl-L-homocysteine hydrolase inhibitors | S-adenosyl-L-homocysteine hydrolase | Yes Ca-C3Ado: 80 mg/kg BW*, (single dose) C-NpcA: 1 mg/kg BW* (single dose) | Yes Ca-C3Ado: 80 mg/kg BW*, (3 doses) C-NpcA: 1 mg/kg BW* (3 doses) | Manipulation of host immune system | 139, 140 |
BW: body weight
Table 3.
Anti-Viral Compounds Currently Tested in NHP Models of Ebola Infection
| Platform | Therapeutic targets | Prophylactic efficacy | Therapeutic efficacy | Concerns | References |
|---|---|---|---|---|---|
| Recombinant human activated protein C (rhAPC) | Abnormal coagulation | Not tested | Partial protection 2 mg/m2/h (i.v. infusion) until day 7 post-exposure | Low efficacy, manipulation of coagulant pathway, withdrawn from global market (2011) | 147 |
| Recombinant nematode anticoagulant protein C2 (rNAPC2) | Factor VIIa: tissue factor complex | Not tested | Partial protection 30 μg/kg BW*/day until day 14 post-exposure | Low efficacy, manipulation of coagulant pathway | 146 |
| Small interfering RNAs (siRNA) | L polymerase + VP24 + VP35 | Not tested | Yes 2 mg/kg BW* (7 doses) | Multiple doses required | 123 |
| Phosphorodiamidate morphino oligo nucleotides (PMOs) (LNP/siRNA: TKM-Ebola) | VP24+VP35; L polymerase + VP24 + VP35 | Not tested | Yes 12.5 ~ 200 mg (11 doses) | Multiple doses required | 128 |
| Monoclonal neutralizing Antibodies (NABs) | Ebola Virion (KZ52) | No | No | No efficacy | 103, 194 |
| Triple monoclonal antibody cocktail | Ebola GP | Not tested | Full protection 24H, Partial Protection 48H, 25 mg/kg BW* (3 doses) | Multiple doses required, must be used early after exposure. | 106 |
BW: body weight
3.1 Small Molecule Inhibitors of Virus Entry and Endosomal Escape
Virus entry is an essential step in the virus life cycle and is often an attractive target for therapy since inhibition of this process blocks replication at an early stage, significantly reducing the chance for the virus to evolve and develop drug resistance. High-throughput cell-based screening (HTS) assays performed in tandem with recombinant EBOV expressing green fluorescent protein or recombinant pseudotyped viruses coated with EBOV GP that do not require high-level containment have been used to identify proteins that mediate filovirus entry 108. This approach, used in combination with structural analysis of Ebola GP, has led to the discovery of several small molecules that may be useful therapeutics for EBOV hemorrhagic fever. A benzodiazepine derivative (compound 7) was identified from HTS of libraries of novel molecules as a compound that could prevent infection of a recombinant human immunodeficiency virus (HIV) pseudotyped with EBOV GP (HIV/EBOV-GP) 109. Computational analysis of the crystal structure of EBOV GP was subsequently used to determine how compound 7 interacted with the virus. Results from this study indicated that compound 7 could fit within the hydrophobic pocket between the GP1 and GP2 subunits in a manner that could interfere with virus entry (Figure 4A).
Ebola must also closely interact with multiple host proteins for endosomal escape, replication, assembly, budding and release processes. HTS also identified a compound derived from benzylpiperazine adamantane diamide(2-((3r,5r,7r)-adamantan-1-yl)-N-(2-(4-benzylpiperazin-1-yl)-2-oxoethyl)acetamide), known as compound 3.47, that inhibited EBOV infection in vivo 110. Biochemical studies revealed that this molecule binds to the Niemann-Pick C1 (NPC1) protein, a cholesterol transporter protein responsible for removal of cholesterol from late-endosomes/lysosomes and intracellular cholesterol homeostasis 111. Further analysis with this and other chemically similar compounds revealed that this protein, which mediates a devastating neurodegenerative condition, Niemann-Pick Type C disease, also plays a role in EBOV entry 112, 113. In a similar manner, efforts to characterize EBOV-host protein interactions identified two novel molecules: EBOV Δ-peptide conjugated to the Fc region of a human IgG1 antibody and the endosome targeting C-peptide derived from native C-terminal heptad repeat regions of EBOV GP2 conjugated to the arginine rich sequence of HIV-1 Tat as promising candidates to prevent virus release from the endosome (Figure 4B) 114. In a somewhat opposing manner, Spargers and colleagues identified several host proteins (HSPA-5, RPL18) by liquid chromatography-linked tandem mass spectrometry that were key in Ebola replication and confirmed their findings by using small interfering RNA sequences (siRNA) to target host protein expression 115. Various protease inhibitors have also been evaluated as potential anti-viral compounds for EBOV. This strategy is based upon the fact that EBOV GP is processed by endosomal cysteine proteases; cathepsin, CatB and CatL 116–118. Addition of cysteine protease inhibitors E64 and CA074 in culture media effectively blocked cellular cathepsins and suppressed EBOV replication and virus-induced cytopathic effects in vitro 117. These compounds also demonstrated anti-viral activity against many other viruses (Marburg, Rift Valley fever, Lassa) in vitro and protected mice from challenge with mouse-adapted EBOV when given as prophylaxis (80%) or post-exposure (50%) therapy (Figure 4B).
3.2 Compounds that Block Virus Replication
For many years, ribavirin, a common anti-viral drug that interferes with the replication of many RNA viruses like influenza and polio by fostering mutations in the viral genome with increasing incidence (“error catastrophe”) and through other contributing mechanisms, has been used in hemorrhagic fever 119. Despite its success in mitigating hemorrhagic fevers arising from arenaviruses and bunyaviruses 120, 121, ribavirin did not control EBOV replication and failed to protect animals from lethal challenge. An alternative to this approach is to block virus transcription and/or replication with antisense oligonucleotides complementary to sequences in the EBOV genome or within the RNA polymerase complex (Figure 4C) 122, 123. Geisbert and colleagues have identified siRNAs that specifically bind to sequences within the EBOV polymerase L (EK-1), VP24 (VP-24-1160) and VP35 (VP-35-855) regions 123. When these compounds were combined and formulated as stable nucleic acid-lipid particles (SNALPs; LNP/siRNA: TKM-Ebola) and given to NHPs in 4 separate doses of 2 mg/kg each intravenously, 66% of the population survived lethal challenge with EBOV (Table 3). A seven-dose regimen effectively halted virus replication with a moderate increase in serum aspartate aminotransferase levels noted. All animals given this regimen survived challenge.
In a similar approach, c-Abl1 and related tyrosine kinases, known to affect replication of certain DNA viruses and bacteria, were evaluated for their role in EBOV replication. A series of silencing studies revealed that phosphorylation of the VP40 protein by c-Abl1 is necessary for transport of the nucleocapsid complex to the cell membrane and release of complete virions from the cell 124. Blocking this process with compounds that have been approved for the treatment of leukemia in humans such as imatinib (Gleevec®) and nilotinib (Tasigna®) which target this enzyme significantly limited the amount of infectious Ebola virions released in culture medium (Figure 4C) 124. Use of compounds that target host gene products rather than the virus itself in larger models of Ebola infection may effectively prevent the development of drug-resistant escape mutants over time.
Another therapeutic platform which holds promise in preventing EBOV replication involves the use of third generation synthetic antisense oligonucleotides, phosphorodiamidate morphino oligomers (PMOs), which are RNase H incompetent and arrest translation and mRNA processing through steric hindrance 125. These molecules, in which the ribose rings are replaced with 6 membered morpholine rings and traditional phosphodiester bonds replaced with phosphorodiamidate linkages, demonstrate improved solubility and are more chemically stable in biological fluids and during storage with respect to their first generation counterparts 126. Several recent reports have demonstrated that these molecules could be valuable therapeutics for filovirus infection 115, 127–129. Administration of positively charged EBOV-specific PMOs (AVI-6002) targeting mRNA sequences within the VP24 and VP35 regions 30–60 minutes after challenge suppressed virus replication and subsequent inflammatory responses and fully protected 5 of 8 macaques (Figure 4C, Table 3) 130. Post-exposure administration of Marburg virus-specific PMOs (AVI-6003) targeting MARV VP24, 35 and L protein also fully protected NHPs from lethal challenge 130. Although New Investigational Drug applications are on file with the United States Food and Drug Administration and Phase I clinical trials are in progress to evaluate the safety of these compounds 126, additional studies must be performed to accurately define the timeframes within which they offer post-exposure protection before they can be used in post-exposure therapeutic regimens.
High throughput screening systems have also aided in identifying three small molecule inhibitors of EBOV infection: FGI-103 131, FGI-104 132 and FGI-106 133. These compounds have provided 80~100% protection in mice challenged with EBOV (Table 2). Although the mechanisms of anti-viral activity of FGI-103 and FGI-104 are not completely understood, the broad activity of FGI-106 against Ebola, Marburg, Rift Valley fever, Dengue, HIV and hepatitis C viruses suggests that this compound targets a host cellular pathway involved in and common to the replication of many different viruses.
3.3 Compounds for the Symptoms of Ebola Infection: Inflammatory Modulators
To date, non-adapted strains of EBOV have been found to only induce hemorrhagic fever in humans and non-human primates 41, 134–136. Although this species-specific restriction has been problematic since adapted strains are required for modeling disease in rodents 137, studies conducted in rodents have been pivotal in identifying ways to augment the immune response and block virus replication. For example, NOD-SCID, IFN-α/β receptor knockout and immune-competent mice treated with anti-mouse IFN-α/β antibodies succumb to non-adapted (wild-type) EBOV infection 138. In a separate series of studies, treatment with s-adenosyl-homocysteine (SAH) hydrolase inhibitors, 3-deazaadenosine (c3-NpcA) 139 and carbocyclic 3-dezaadenosine (C-c3Ado) 140, which inhibit replication of a variety DNA and RNA viruses, fully protected immune-competent mice during lethal infection with mouse-adapted-EBOV (Table 2). Further investigation revealed that the protective effect could be completely eliminated by co-administration of SAH hydrolase inhibitors and anti-mouse IFN-α/β antibodies 138. Although these results strongly suggest that resistance and/or susceptibility to wild type ZEBOV infection is mediated by the type I interferon response, re-invigoration of the anti-viral response in this manner has not been therapeutically effective in rhesus macaques 141, baboons 142 and African green monkeys 143. This might be improved by using species-specific interferon. Further evaluation of this approach has not yet been performed.
In a recent effort to identify novel therapeutics to treat and mitigate the pathological symptoms associated with filovirus hemorrhagic fevers, compound NSC62914, an antioxidant that acts as a scavenger of reactive oxygen species (ROS), was found to inhibit replication of EBOV, Marburg, Lassa and Rift Valley Fever viruses in vitro 144. Although the role of ROS in the pathogenesis of filovirus infection is not currently understood, this compound protected mice from lethal challenge with EBOV (Table 2). This was significant, given that other known antioxidants have had no impact on EBOV infection. It also suggests that NSC62914 and other compounds with antioxidant properties may maintain other cell signaling pathways common to many viruses that are arrested during EBOV infection.
3.4 Compounds for the Symptoms of Ebola Infection: Coagulation Modulators
Overproduction of pro-coagulant tissue factors during Ebola infection facilitate clotting disorders that progress to multi-organ failure, often indicated by a reduction in circulating serum protein C 145. Thus, stimulating coagulation through the tissue factor pathway by administering a factor VIIa/tissue factor inhibitor (recombinant nematode anticoagulant protein c2: rNAPc2) 146 or by activating the natural anticoagulant protein C pathway with recombinant human activated protein C (rhAPC) 147 were logical choices in the design of supportive post-exposure therapeutic regimens. Each of these compounds significantly decreased production of pro-inflammatory cytokines and extended the mean time to death with respect to non-treated controls in animal models of infection (Table 3). Despite these promising results, routine use of these agents in humans has been hindered by conflicting reports from Phase II trials designed to evaluate their clinical efficacy for treatment of septic shock 148, 149 and subsequent withdrawal of one product from the U.S. market 150.
4. Future Directions: Development of Small Molecule Therapeutics for Ebola Infection
Although there is a clear need for effective regimens for prevention and protection against Ebola-mediated hemorrhagic fevers, many significant hurdles have limited the ability of promising candidates to reach those in need of them. One of the primary reasons for the modest progress made in this area over the last two decades is the fact that meaningful “proof-of-principle” studies can only be handled in maximum-containment biosafety level 4 (BSL-4) laboratories. Significant progress in the development of high throughput systems that allow rapid screening of potential anti-viral compounds at a much lower containment level (BSL-2) and the use of RNA interference technology to confirm these findings has led to the discovery of several novel compounds that have demonstrated protective efficacy in animal models of infection (Tables 2 and 3) 123, 128, 130, 131. Since this approach will significantly accelerate the discovery of new therapeutics to combat Ebola, it will also heighten the need for studies in larger animal models, as data obtained from rodents may not accurately reflect the pharmacological and toxicological responses of humans.
Most of the recently discovered small molecule therapeutics to treat filovirus hemorrhagic fevers have limited serum half-lives and poor bioavailability in target tissues, making administration of excessively large amounts of compound in multiple dosing regimens necessary to achieve an optimal therapeutic effect 115, 127, 128, 130, 131, 144. It is also well established that the potency of any therapeutic or vaccine is heavily influenced by the physical stability of the active compound and the chosen delivery system. Half-life and bioavailability can be greatly extended through formulations that maintain the structural integrity of a medicinal agent and protect it from nucleases and other degradative enzymes in vivo and during long-term storage 151, 152. Thus, focused efforts in development of novel formulations that can stabilize or alter the physical conformation of these promising compounds will be vital to improve their efficacy and reduce their toxicity as they progress to the clinic. Incorporating these formulations in the proper delivery platform will then move them toward single dose regimens that are easy to administer in outbreak and post-exposure scenarios. Some of the most relevant formulation/delivery strategies for novel Ebola therapeutics are discussed in detail below and have application to both small molecule and vaccine platforms.
4.1. Formulation Development: Mucoadhesive/Absorption Enhancers
Mucoadhesive agents bring therapeutic molecules in close contact with the mucosal cell surface and prolong their residence time along the surface of the airways, oral cavity, digestive and genitourinary tracts and the skin 153. They also suppress mucociliary clearance (MCC) processes that rapidly remove foreign particulates from these areas 154. Mucoadhesive compounds can be divided into three categories 155. The first group includes hydrophilic polymers like sodium alginate, sodium carboxymethylcellulose, hydroxypropyl methylcellulose, and Carbopol that can form covalent hydrogen bonds with the mucus layer 156. The second group consists of cationic polymers like chitosan and synthetic polymethacrylates that interact with the negatively charged mucin through the formation of ionic or hydrogen bonds. The third group is made up of thiolated polymers, or thiomers, that form covalent bonds with free sulfhydryl groups in mucin 157. The thiomers are currently the strongest mucoadhesives available for delivery of drugs to mucosal surfaces 158. While mucoadhesives clearly facilitate direct contact with the mucosal surface, most but not all are relatively poor at getting therapeutic molecules across the underlying epithelial cell monolayer, which is impermeable to most compounds in the absence of specific transporters. Thus, mucoadhesives are often paired with an absorption enhancer, a compound that gently weakens cellular membranes or loosens tight junctions to allow the medicinal agent to be absorbed through transcellular or paracellular pathways 159. Examples of absorption enhancers commonly used in therapeutic formulations approved for human use include carbohydrates, surfactants, bile salts and their derivatives, phospholipids, cyclodextrins and poly(ethylene) glycols 160. Cell penetrating peptides, derived from the human immunodeficiency virus (HIV-1) transactivator of transcription (Tat) protein 161 and the Drosophilia melanogaster Antennapedia homeodomain (penetratin) 162 have also been used to increase cellular uptake of large molecules. Although the exact mechanism by which they exert their effect is not clearly understood, several recent in vivo studies demonstrate that these novel molecules can improve delivery of siRNA molecules with minimal toxicity 163.
4.2. Formulation Development: Lipid-based Carriers
Liposomes are vesicles consisting of lipid or phospholipid bilayers with an aqueous core. Depending upon the manufacturing process, these particles are composed of a single (uni-lamellar) or several concentric (multi-lamellar) lipid bilayers and range in size from 50 to 2,000 nm 164. Lipid micelles are monolayer structures composed of poly(ethylene) glycol (PEG)-conjugated phospholipids that self-assemble spontaneously at concentrations above their critical micelle concentrations (CMCs) in aqueous solution 165. In these relatively small particles (7–35 nm), the hydrophobic acyl chains of the lipids form the micelle core while the polar head groups make up the outer hydrophilic corona. Solid lipid nanoparticles consist of sub-micron sized lipid emulsions where the liquid lipid (oil) has been replaced by a solid lipid dispersed in an aqueous surfactant solution 166. Each of these systems employ phospholipids, triglycerides and cholesterol derivatized or extracted from natural sources that are biocompatible and biodegradable in vivo 167. Of these systems, liposomes have been the most extensively studied with over 40 years of documented research describing their suitability as carriers for hydrophilic and hydrophobic small molecules and antigens for vaccines.
Early studies to evaluate the ability of liposome formulations to improve drug absorption revealed that these particles were efficiently taken up by the reticuloendothelial system (RES), thus making them suitable candidates for vaccine development 168, 169. Since then, liposome-antigen preparations have been shown to induce Th1 and Th2 type responses with respect to lipid composition 170, 171. Liposomes have been found to be quite versatile for vaccine development in that lipid compositions can easily be tailored to the type of immune response desired, they are compatible with most adjuvants and can accommodate antigens of varying size 172, 173. They also serve as the platform for virosomes which can be used for immunization or drug targeting 174, 175. While the inherent uptake of liposomes in the RES was highly regarded within the vaccine field, it was not acceptable for most small molecule therapeutics as they were rapidly cleared from the circulation before they could exert their therapeutic effect. Development of “stealth” liposomes in which PEG and other biocompatible polymers have been placed on the liposome surface to prevent recognition by opsonins have greatly reduced uptake by the RES and resulted in the first liposomal formulation to be approved for clinical use in the United States and Europe 164, 176. Incorporation of these polymers in liposome preparations has also been of additional benefit in that they allow for chemical attachment of a wide array of ligands to redirect the liposome from the RES to specific organs and cell types 164, 167, 177.
4.3. Formulation Development: Biocompatible Polymers
Liposome preparations are limited by poor physical and chemical stability in biological fluids and as formulated products at ambient temperatures. Large-scale manufacturing of these products is also difficult with batch-to batch reproducibility a significant concern 178. To address these issues, “entrapment and encapsulation” methods to embed small molecules, proteins and peptides within nanoparticles made from biodegradable polymers were developed and have been widely used for several decades in the field of pharmaceutical science 179. These preparations allow continuous release of compound over extended periods of time to improve the half-life of drugs with poor bioavailability profiles 180, 181. They also minimize the overall surface charge of a therapeutic compound and foster interaction with target tissues and organs to improve bioavailability profiles 182. Many naturally occurring polymers such as alginate, chitosan, gelatin, albumin, pullulan, gliadin and dextran have been the focus of many pioneering studies evaluating this carrier system for vaccine and drug delivery 179. Synthetic polymers like poly(caprolactone), poly(methyl acrylate) and poly(lactic-co-glycolic acid) are less immunogenic than those listed above. Nanoparticles consisting of these polymers in various combinations and molecular weights can be prepared in a highly reproducible manner. Of these polymers, poly(lactic-co-glycolic acid) (PLGA) has been studied and characterized extensively and is approved by the U.S. FDA and European Medicine Agency (EMA) in various drug delivery systems in humans 183. As with liposome carriers, proteins, antibodies and other known ligands can be placed on the surface of these particles to direct them to specific physiological targets. Recent studies have elucidated methods where targeting molecules can be imprinted directly in the polymer matrix to minimize the need for additional complex modifications of the surface of these particles once the therapeutic compound is embedded in them 184, 185. Advances in polymer chemistry have also facilitated the development of “smart” particles capable of delivering their therapeutic payload in response to changes in temperature, oxygen content, pH, and light which may be useful in the future development of therapeutics to treat Ebola hemorrhagic fevers 186–188.
5. Final Thoughts
From what we have summarized here, it is clear that the development of an effective vaccine against Ebola has progressed further than efforts to identify small molecule therapeutics to treat infection. Although several vaccine platforms have entered the early stages of clinical testing, the longevity of the immune response elicited by each is not fully characterized. This is of some concern since recurrent immunization programs for Ebola hemorrhagic fever seem unrealistic and costly since the disease burden is currently limited to a specific region of the world. Although the exact correlates of protection for Ebola in humans continue to be an issue of debate, a systems vaccinology approach employing microarrays, mass spectrometry-based proteomics, metabolomics and computational modeling would define the immune responses necessary for protection. Data obtained from these types of studies will also allow for further refinement of current vaccine platforms with formulations that foster specific types of immune responses that are long lasting. It is also important to note that most of the potential post-exposure treatments described above have shown protective efficacy in animals treated very soon (30~60 minutes) after exposure to Ebola. These are impractical with respect to natural outbreaks where infected individuals will not seek treatment for days. Studies evaluating gene expression profiles during active Ebola infection would be a valuable tool in developing post-exposure treatments for those that have progressed to symptomatic illness, however, facilities, funding and reagents to support them are very limited. Although discovery of small molecule therapeutics to combat Ebola hemorrhagic fever has been lagging behind that of vaccine development, recent efforts to develop high-throughput screening systems which do not require use of maximum containment facilities will foster an exponential increase in the discovery and development new therapeutics for Ebola-mediated and other hemorrhagic fevers in the next five years. This trend may reinvigorate interest and funding to support clinical testing of highly promising therapeutic compounds and solidify plans for how a given vaccine or therapeutic would be used once it is licensed as well as for the infrastructure necessary to deliver it to regions where it is needed the most.
Acknowledgments
This work was funded by the National Institutes of Health NIAID Grant U01AI078045 (MAC). The content and the views expressed in this manuscript do not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases. The authors would like to thank Mr. Stephen C. Schafer for excellent technical assistance in designing the figures presented in this manuscript.
Footnotes
The authors do not have any conflicts nor financial interests to declare.
References
- 1.Bray M, Murphy FA. Filovirus Research: Knowledge Expands to Meet a Growing Threat. J Infect Dis. 2007;196(Suppl 2):S438–S443. doi: 10.1086/520552. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Xu L, Sun Y, Nabel GJ. The Assembly of Ebola Virus Nucleocapsid Requires Virion-Associated Proteins 35 and 24 and Posttranslational Modification of Nucleoprotein. Mol Cell. 2002;10(2):307–316. doi: 10.1016/s1097-2765(02)00588-9. [DOI] [PubMed] [Google Scholar]
- 3.Muhlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the Transcription and Replication Strategies of Marburg Virus and Ebola Virus by Using Artificial Replication Systems. J Virol. 1999;73(3):2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volchkov VE, Volchkova VA, Muhlberger E, Kolesnikova LV, Weik M, Dolnik O, Klenk HD. Recovery of Infectious Ebola Virus from Complementary DNA: RNA Editing of the GP Gene and Viral Cytotoxicity. Science. 2001;291(5510):1965–1969. doi: 10.1126/science.1057269. [DOI] [PubMed] [Google Scholar]
- 5.Bamberg S, Kolesnikova L, Moller P, Klenk HD, Becker S. VP24 of Marburg Virus Influences Formation of Infectious Particles. J Virol. 2005;79(21):13421–13433. doi: 10.1128/JVI.79.21.13421-13433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Z, Boshra H, Sunyer JO, Zwiers SH, Paragas J, Harty RN. Biochemical and Functional Characterization of the Ebola Virus VP24 Protein: Implications for a Role in Virus Assembly and Budding. J Virol. 2003;77(3):1793–1800. doi: 10.1128/JVI.77.3.1793-1800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noda T, Halfmann P, Sagara H, Kawaoka Y. Regions in Ebola Virus VP24 That Are Important for Nucleocapsid Formation. J Infect Dis. 2007;196 (Suppl 2):S247–S250. doi: 10.1086/520596. [DOI] [PubMed] [Google Scholar]
- 8.Noda T, Watanabe S, Sagara H, Kawaoka Y. Mapping of the VP40-Binding Regions of the Nucleoprotein of Ebola Virus. J Virol. 2007;81(7):3554–3562. doi: 10.1128/JVI.02183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. Ebola Virus VP40 Drives the Formation of Virus-Like Filamentous Particles Along with GP. J Virol. 2002;76(10):4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe S, Watanabe T, Noda T, Takada A, Feldmann H, Jasenosky LD, Kawaoka Y. Production of Novel Ebola Virus-Like Particles from cDNAs: An Alternative to Ebola Virus Generation by Reverse Genetics. J Virol. 2004;78(2):999–1005. doi: 10.1128/JVI.78.2.999-1005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. The Virion Glycoproteins of Ebola Viruses Are Encoded in Two Reading Frames and Are Expressed Through Transcriptional Editing. Proc Natl Acad Sci US A. 1996;93(8):3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. Processing of the Ebola Virus Glycoprotein by the Proprotein Convertase Furin. Proc Natl Acad Sci US A. 1998;95(10):5762–5767. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volchkov VE, Volchkova VA, Muhlberger E, Kolesnikova LV, Weik M, Dolnik O, Klenk HD. Recovery of Infectious Ebola Virus from Complementary DNA: RNA Editing of the GP Gene and Viral Cytotoxicity. Science. 2001;291(5510):1965–1969. doi: 10.1126/science.1057269. [DOI] [PubMed] [Google Scholar]
- 14.Volchkov VE, Volchkova VA, Slenczka W, Klenk HD, Feldmann H. Release of Viral Glycoproteins During Ebola Virus Infection. Virology. 1998;245(1):110–119. doi: 10.1006/viro.1998.9143. [DOI] [PubMed] [Google Scholar]
- 15.Chan SY, Empig CJ, Welte FJ, Speck RF, Schmaljohn A, Kreisberg JF, Goldsmith MA. Folate Receptor-Alpha Is a Cofactor for Cellular Entry by Marburg and Ebola Viruses. Cell. 2001;106(1):117–126. doi: 10.1016/s0092-8674(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 16.Chan SY, Speck RF, Ma MC, Goldsmith MA. Distinct Mechanisms of Entry by Envelope Glycoproteins of Marburg and Ebola (Zaire) Viruses. J Virol. 2000;74 (10):4933–4937. doi: 10.1128/jvi.74.10.4933-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chepurnov AA, Tuzova MN, Ternovoy VA, Chernukhin IV. Suppressive Effect of Ebola Virus on T Cell Proliferation in Vitro Is Provided by a 125-Kda GP Viral Protein. Immunol Lett. 1999;68(2–3):257–261. doi: 10.1016/s0165-2478(99)00058-9. [DOI] [PubMed] [Google Scholar]
- 18.Volchkov VE, Blinov VM, Netesov SV. The Envelope Glycoprotein of Ebola Virus Contains an Immunosuppressive-Like Domain Similar to Oncogenic Retroviruses. FEBS Lett. 1992;305(3):181–184. doi: 10.1016/0014-5793(92)80662-z. [DOI] [PubMed] [Google Scholar]
- 19.Wool-Lewis RJ, Bates P. Characterization of Ebola Virus Entry by Using Pseudotyped Viruses: Identification of Receptor-Deficient Cell Lines. J Virol. 1998;72 (4):3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Distinct Cellular Interactions of Secreted and Transmembrane Ebola Virus Glycoproteins. Science. 1998;279(5353):1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola Virus Glycoprotein as the Main Viral Determinant of Vascular Cell Cytotoxicity and Injury. Nat Med. 2000;6(8):886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 22.Yaddanapudi K, Palacios G, Towner JS, Chen I, Sariol CA, Nichol ST, Lipkin WI. Implication of a Retrovirus-Like Glycoprotein Peptide in the Immunopathogenesis of Ebola and Marburg Viruses. FASEB J. 2006;20(14):2519–2530. doi: 10.1096/fj.06-6151com. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. Biochemical Analysis of the Secreted and Virion Glycoproteins of Ebola Virus. J Virol. 1998;72(8):6442–6447. doi: 10.1128/jvi.72.8.6442-6447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volchkova VA, Feldmann H, Klenk HD, Volchkov VE. The Nonstructural Small Glycoprotein sGP of Ebola Virus Is Secreted as an Antiparallel-Orientated Homodimer. Virology. 1998;250(2):408–414. doi: 10.1006/viro.1998.9389. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Watanabe S, Takada A, Kawaoka Y. Ebola Virus Glycoprotein: Proteolytic Processing, Acylation, Cell Tropism, and Detection of Neutralizing Antibodies. J Virol. 2001;75(3):1576–1580. doi: 10.1128/JVI.75.3.1576-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radoshitzky SR, Warfield KL, Chi X, Dong L, Kota K, Bradfute SB, Gearhart JD, Retterer C, Kranzusch PJ, Misasi JN, Hogenbirk MA, Wahl-Jensen V, Volchkov VE, Cunningham JM, Jahrling PB, Aman MJ, Bavari S, Farzan M, Kuhn JH. Ebola virus Delta-Peptide Immunoadhesins Inhibit Marburgvirus and Ebola virus Cell Entry. J Virol. 2011;85(17):8502–8513. doi: 10.1128/JVI.02600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehedi M, Falzarano D, Seebach J, Hu X, Carpenter MS, Schnittler HJ, Feldmann H. A New Ebola Virus Nonstructural Glycoprotein Expressed through RNA Editing. J Virol. 2011;85(11):5406–5414. doi: 10.1128/JVI.02190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaki SR, Goldsmith CS. Pathologic Features of Filovirus Infections in Humans. Curr Top Microbiol Immunol. 1999;235:97–116. doi: 10.1007/978-3-642-59949-1_7. [DOI] [PubMed] [Google Scholar]
- 29.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. Ebola and Marburg Viruses Replicate in Monocyte-Derived Dendritic Cells without Inducing the Production of Cytokines and Full Maturation. J Infect Dis. 2003;188(11):1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 30.Mahanty S, Hutchinson K, Agarwal S, Mcrae M, Rollin PE, Pulendran B. Cutting Edge: Impairment of Dendritic Cells and Adaptive Immunity by Ebola and Lassa Viruses. J Immunol. 2003;170(6):2797–27801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 31.Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Muhlberger E, Bray M, Klenk HD, Palese P, Garcia-Sastre A. The Ebola Virus VP35 Protein Inhibits Activation of Interferon Regulatory Factor 3. J Virol. 2003;77(14):7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola Virus VP24 Binds Karyopherin Alpha1 and Blocks STAT1 Nuclear Accumulation. J Virol. 2006;80(11):5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Z, Cerveny M, Yan Z, He B. The VP35 Protein of Ebola Virus Inhibits the Antiviral Effect Mediated by Double-Stranded RNA-Dependent Protein Kinase PKR. J Virol. 2007;81(1):182–192. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halfmann P, Neumann G, Kawaoka Y. The Ebola virus VP24 Protein Blocks Phosphorylation of P38 Mitogen-Activated Protein Kinase. J Infect Dis. 2011;204 (Suppl 3):S953–S956. doi: 10.1093/infdis/jir325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann M, Gantke T, Muhlberger E. Ebola Virus VP35 Antagonizes Pkr Activity through Its C-Terminal Interferon Inhibitory Domain. J Virol. 2009;83(17):8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez O, Leung LW, Basler CF. The Role of Antigen-Presenting Cells in Filoviral Hemorrhagic Fever: Gaps in Current Knowledge. Antiviral Res. 2012;93(3):416–428. doi: 10.1016/j.antiviral.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, Lansoud-Soukate J, Mavoungou E. Inflammatory Responses in Ebola Virus-Infected Patients. Clin Exp Immunol. 2002;128(1):163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, Mccormick JB, Georges AJ. Defective Humoral Responses and Extensive Intravascular Apoptosis Are Associated with Fatal Outcome in Ebola Virus-Infected Patients. Nat Med. 1999;5(4):423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 39.Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. Markedly Elevated Levels of Interferon (IFN)-Gamma, IFN-Alpha, Interleukin (IL)-2, IL-10, and Tumor Necrosis Factor-Alpha Associated with Fatal Ebola Virus Infection. J Infect Dis. 1999;179 (Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 40.Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, Jahrling PB. Pathogenesis of Experimental Ebola Virus Infection in Guinea Pigs. J Infect Dis. 1999;179 (Suppl 1):S203–S217. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 41.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. Pathogenesis of Ebola Hemorrhagic Fever in Cynomolgus Macaques: Evidence That Dendritic Cells Are Early and Sustained Targets of Infection. Am J Pathol. 2003;163(6):2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory Response During Ebola Virus Infection of Primate Models: Possible Involvement of the Tumor Necrosis Factor Receptor Superfamily. Immunol Lett. 2002;80(3):169–179. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 43.Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of Highly Virulent Pathogens: Insights from Ebola Virus. Nat Immunol. 2007;8(11):1159–1164. doi: 10.1038/ni1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leroy EM, Gonzalez JP, Baize S. Ebola and Marburg Haemorrhagic Fever Viruses: Major Scientific Advances, but a Relatively Minor Public Health Threat for Africa. Clin Microbiol Infect. 2011;17(7):964–976. doi: 10.1111/j.1469-0691.2011.03535.x. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brister JR, Bukreyev AA, Caì Y, Chandran K, Davey RA, Dolnik O, Dye JM, Enterlein S, Gonzalez JP, et al. Virus Nomenclature Below the Species Level: A Standardized Nomenclature for Laboratory Animal-Adapted Strains and Variants of Viruses Assigned to the Family Filoviridae. Arch Virol. 2013;158(1):301–311. doi: 10.1007/s00705-012-1454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayes CG, Burans JP, Ksiazek TG, Del Rosario RA, Miranda ME, Manaloto CR, Barrientos AB, Robles CG, Dayrit MM, Peters CJ. Outbreak of Fatal Illness among Captive Macaques in the Philippines Caused by an Ebola-Related Filovirus. Am J Trop Med Hyg. 1992;46(6):664–671. doi: 10.4269/ajtmh.1992.46.664. [DOI] [PubMed] [Google Scholar]
- 47.Jahrling PB, Geisbert TW, Dalgard DW, Johnson ED, Ksiazek TG, Hall WC, Peters CJ. Preliminary Report: Isolation of Ebola Virus from Monkeys Imported to USA. Lancet. 1990;335(8688):502–505. doi: 10.1016/0140-6736(90)90737-p. [DOI] [PubMed] [Google Scholar]
- 48.Rollin PE, Williams RJ, Bressler DS, Pearson S, Cottingham M, Pucak G, Sanchez A, Trappier SG, Peters RL, Greer PW, Zaki S, Demarcus T, et al. Ebola (Subtype Reston) Virus among Quarantined Nonhuman Primates Recently Imported from the Philippines to the United States. J Infect Dis. 1999;179 (Suppl 1):S108–S114. doi: 10.1086/514303. [DOI] [PubMed] [Google Scholar]
- 49.Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G. Transmission of Ebola Virus from Pigs to Non-Human Primates. Sci Rep. 2012;2:811. doi: 10.1038/srep00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed DS, Lackemeyer MG, Garza NL, Sullivan LJ, Nichols DK. Aerosol Exposure to Zaire Ebola virus in Three Nonhuman Primate Species: Differences in Disease Course and Clinical Pathology. Microbes Infect. 2011;13(11):930–936. doi: 10.1016/j.micinf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Feldmann H, Geisbert TW. Ebola Haemorrhagic Fever. Lancet. 2011;377(9768):849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhn JH, Dodd LE, Wahl-Jensen V, Radoshitzky SR, Bavari S, Jahrling PB. Evaluation of Perceived Threat Differences Posed by Filovirus Variants. Biosecur Bioterror. 2011;9(4):361–371. doi: 10.1089/bsp.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kortepeter MG, Bausch DG, Bray M. Basic Clinical and Laboratory Features of Filoviral Hemorrhagic Fever. J Infect Dis. 2011;204 (Suppl 3):S810–S816. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 54.Paessler S, Walker DH. Pathogenesis of the Viral Hemorrhagic Fevers. Annu Rev Pathol Mech Dis. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- 55.Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated Vaccine for Ebola Virus Efficacious in Guineapig Model. Lancet. 1980;2 (8207):1294–1295. doi: 10.1016/s0140-6736(80)92352-1. [DOI] [PubMed] [Google Scholar]
- 56.Chupurnov AA, Chernukhin IV, Ternovoi VA, Kudoiarova NM, Makhova NM, Azaev Ms, Smolina MP. Attempts to Develop a Vaccine against Ebola Fever. Voprosy Virusologii. 1995;40(6):257–260. [PubMed] [Google Scholar]
- 57.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a Preventive Vaccine for Ebola Virus Infection in Primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Accelerated Vaccination for Ebola Virus Haemorrhagic Fever in Non-Human Primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson JS, Yao MK, Tran KN, Croyle MA, Strong JE, Feldmann H, Kobinger GP. Enhanced Protection against Ebola Virus Mediated by an Improved Adenovirus-Based Vaccine. PloS One. 2009;4(4):e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi JH, Schafer SC, Zhang L, Kobinger GP, Juelich T, Freiberg AN, Croyle MA. A Single Sublingual Dose of an Adenovirus-Based Vaccine Protects Against Lethal Ebola Challenge in Mice and Guinea Pigs. Mol Pharm. 2012;9(1):156–167. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal Delivery of an Adenovirus-Based Vaccine Bypasses Pre-Existing Immunity to the Vaccine Carrier and Improves the Immune Response in Mice. PLoS One. 2008;3(10):e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, et al. International Epidemiology of Human Pre-Existing Adenovirus (Ad) Type-5, Type-6, Type-26 and Type-36 Neutralizing Antibodies: Correlates of High Ad5 Titers and Implications for Potential Hiv Vaccine Trials. Vaccine. 2010;28(4):950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 63.Pilankatta R, Chawla T, Khanna N, Swaminathan S. The Prevalence of Antibodies to Adenovirus Serotype 5 in an Adult Indian Population and Implications for Adenovirus Vector Vaccines. J Med Virol. 2010;82(3):407–414. doi: 10.1002/jmv.21721. [DOI] [PubMed] [Google Scholar]
- 64.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. Prevalence of Neutralizing Antibodies to Adenoviral Serotypes 5 and 35 in the Adult Populations of the Gambia, South Africa, and the United States. Clin Diagn Lab Immunol. 2004;11(2):351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aldhamen YA, Seregin SS, Amalfitano A. Immune Recognition of Gene Transfer Vectors: Focus on Adenovirus as a Paradigm. Front Immunol. 2011;2:40. doi: 10.3389/fimmu.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Pau MG, Custers J, Vellinga J, Hendriks J, Jahrling P, et al. Recombinant Adenovirus Serotype 26 (Ad26) and Ad35 Vaccine Vectors Bypass Immunity to Ad5 and Protect Nonhuman Primates against Ebola virus Challenge. J Virol. 2011;85(9):4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel A, Tikoo S, Kobinger G. A Porcine Adenovirus with Low Human Seroprevalence Is a Promising Alternative Vaccine Vector to Human Adenovirus 5 in an H5N1 Virus Disease Model. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine Adenoviral Vector-Based H5N1 Influenza Vaccine Overcomes Exceptionally High Levels of Pre-Existing Immunity against Human Adenovirus. Mol Ther. 2008;16(5):965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, Jones S, Wilson JM. Chimpanzee Adenovirus Vaccine Protects Against Zaire Ebola Virus. Virology. 2006;346(2):394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 70.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, Koup RA, Roederer M, Bailer R, et al. Immunogenic in Healthy Adults. Vaccine. 2010;29(2):304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 71.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. Properties of Replication-Competent Vesicular Stomatitis Virus Vectors Expressing Glycoproteins of Filoviruses and Arenaviruses. J Virol. 2004;78(10):5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuda Y, Safronetz D, Brown K, Lacasse R, Marzi A, Ebihara H, Feldmann H. Protective Efficacy of a Bivalent Recombinant Vvesicular Stomatitis Virus Vaccine in the Syrian Hamster Model of Lethal Ebola Virus Infection. J Infect Dis. 2011;204 (Suppl 3):S1090–S1097. doi: 10.1093/infdis/jir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisbert TW, Feldmann H. Recombinant Vesicular Stomatitis Virus-Based Vaccines against Ebola and Marburg Virus Infections. J Infect Dis. 2011;204(Suppl 3):S1075–S1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Stroher U, Feldmann H, Jones SM. Mucosal Immunization of Cynomolgus Macaques with the VSVdeltaG/ZEBOVGP Vaccine Stimulates Strong Ebola GP-Specific Immune Responses. PLoS One. 2009;4(5):e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Stroher U, Fritz EA, Hensley LE, Jones SM, Feldmann H. Vesicular Stomatitis Virus-Based Vaccines Protect Nonhuman Primates against Aerosol Challenge with Ebola and Marburg Viruses. Vaccine. 2008;26(52):6894–900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geisbert TW, Daddario-Dicaprio KM, Williams KJ, Geisbert JB, Leung A, Feldmann F, Hensley LE, Feldmann H, Jones SM. Recombinant Vesicular Stomatitis Virus Vector Mediates Postexposure Protection against Sudan Ebola Hemorrhagic Fever in Nonhuman Primates. J Virol. 2008;82(11):5664–5668. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones SM, Stroher U, Fernando L, Qiu X, Alimonti J, Melito P, Bray M, Klenk HD, Feldmann H. Assessment of a Vesicular Stomatitis Virus-Based Vaccine by Use of the Mouse Model of Ebola Virus Hemorrhagic Fever. J Infect Dis. 2007;196 (Suppl 2):S404–S412. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 78.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live Attenuated Recombinant Vaccine Protects Nonhuman Primates Against Ebola and Marburg Viruses. Nat Med. 2005;11(7):786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 79.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live Attenuated Recombinant Vaccine Protects Nonhuman Primates against Ebola and Marburg Viruses. Nat Med. 2005;11(7):786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 80.Feldmann H, Jones SM, Daddario-Dicaprio KM, Geisbert JB, Ströher U, Grolla A, Bray M, Fritz EA, Fernando L, Feldmann F, Hensley LE, Geisbert TW. Effective Post-Exposure Treatment of Ebola Infection. PLoS Pathog. 2007;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, Paragas J, Matthias L, Smith MA, Jones SM, Hensley LE, Feldmann H, Jahrling PB. Vesicular Stomatitis Virus-Based Ebola Vaccine Is Well-Tolerated and Protects Immunocompromised Nonhuman Primates. PLoS Pathog. 2008;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, Feldmann H, Hensley LE, Geisbert TW. Recombinant Vesicular Stomatitis Virus Vaccine Vectors Expressing Filovirus Glycoproteins Lack Neurovirulence in Nonhuman Primates. PLoS Negl Trop Dis. 2012;6(3):e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gunther S, Feldmann H, Geisbert TW, Hensley LE, Rollin PE, Nichol ST, Stroher U, Artsob H, Peters CJ, Ksiazek TG, Becker S, Ter Meulen J, Olschlager S, Schmidt-Chanasit J, Sudeck H, Burchard GD, Schmiedel S. Management of Accidental Exposure to Ebola Virus in the Biosafety Level 4 Laboratory, Hamburg, Germany. J Infect Dis. 2011;204 (Suppl 3):S785–S790. doi: 10.1093/infdis/jir298. [DOI] [PubMed] [Google Scholar]
- 84.Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. Nonsegmented Negative-Strand Viruses as Vaccine Vectors. J Virol. 2006;80(21):10293–10306. doi: 10.1128/JVI.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, Murphy BR, Collins PL, Sanchez A. A Single Intranasal Inoculation with a Paramyxovirus-Vectored Vaccine Protects Guinea Pigs against a Lethal-Dose Ebola Virus Challenge. J Virol. 2006;80(5):2267–2279. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. Mucosal Parainfluenza Virus-Vectored Vaccine against Ebola Virus Replicates in the Respiratory Tract of Vector-Immune Monkeys and Is Immunogenic. Virology. 2010;399(2):290–298. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, Dorward DW, Pickles RJ, Murphy BR, Feldmann H, Collins PL. Chimeric Human Parainfluenza Virus Bearing the Ebola Virus Glycoprotein as the Sole Surface Protein Is Immunogenic and Highly Protective against Ebola Virus Cchallenge. Virology. 2009;383(2):348–361. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carroll SA, Towner JS, Sealy TK, Mcmullan LK, Khristova ML, Burt FJ, Swanepoel R, Rollin PE, Nichol ST. Molecular Evolution of Viruses of the Family Filoviridae Based on 97 Whole-Genome Sequences. J Virol. 2013;5(5):2608–2616. doi: 10.1128/JVI.03118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hensley L, Mulangu S, Asiedu C, Johnson J, Honko AN, Stanley D, Fabozzi G, Nichol ST, Ksiazek TG, Rollin PE, Wahl-Jensen V, Bailey M, Jahrling PB, Roederer M, Koup RA, Sullivan NJ. Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebola virus Species. PLoS Pathog. 2010;6(5):e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, Feldmann H. Vesicular Stomatitis Virus-Based Ebola Vaccines with Improved Cross-Protective Efficacy. J Infect Dis. 2011;204 (Suppl 3):1066–1074. doi: 10.1093/infdis/jir348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, Holman DH, Dong JY. Protection of Nonhuman Primates against Two Species of Ebola Virus Infection with a Single Complex Adenovirus Vector. Clin Vaccine Immunol. 2010;17(4):572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geisbert TW, Geisbert JB, Leung A, Daddario-Dicaprio KM, Hensley LE, Grolla A, Feldmann H. Single-Injection Vaccine Protects Nonhuman Primates against Infection with Marburg Virus and Three Species of Ebola Virus. J Virol. 2009;83 (14):7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ndhlovu ZM, Piechocka-Trocha A, Vine S, Mcmullen A, Koofhethile KC, Goulder PJ, Ndung’u T, Barouch DH, Walker BD. Mosaic HIV-1 Gag Antigens Can Be Processed and Presented to Human Hiv-Specific CD8+ T Cells. J Immunol. 2011;186(12):6914–6924. doi: 10.4049/jimmunol.1004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, Quinn D, Parks RJ, Tsao CY, Carville, et al. Mosaic Vaccines Elicit CD8+ T Lymphocyte Responses That Confer Enhanced Immune Coverage of Diverse HIV Strains in Monkeys. Nat Med. 2010;16(3):324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barouch DH, O’brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, et al. Mosaic HIV-1 Vvaccines Expand the Breadth and Depth of Cellular Immune Responses in Rhesus Monkeys. Nat Med. 2010;16(3):319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zahn R, Gillisen G, Roos A, Koning M, Van Der Helm E, Spek D, Weijtens M, Grazia Pau M, Rado evi K, Weverling GJ, Custers J, Vellinga J, et al. Ad35 and Ad26 Vaccine Vectors Induce Potent and Cross-Rreactive Antibody and T-Cell Responses to Multiple Filovirus Species. PLoS One. 2012;7(12):e44115. doi: 10.1371/journal.pone.0044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fenimore PW, Muhammad MA, Fischer WM, Foley BT, Bakken RR, Thurmond JR, Yusim K, Yoon H, Parker M, Hart MK, Dye JM, Korber B, Kuiken C. Designing and Testing Broadly-Protective Filoviral Vaccines Optimized for Cytotoxic T-Lymphocyte Epitope Coverage. PLoS One. 2012;7(10):e4469. doi: 10.1371/journal.pone.0044769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phoolcharoen W, Dye JM, Kilbourne J, Piensook K, Pratt WD, Arntzen CJ, Chen Q, Mason HS, Herbst-Kralovetz MM. A Nonreplicating Subunit Vaccine Protects Mice against Lethal Ebola Virus Challenge. Proc Natl Acad Sci US A. 2011;108(51):20695–20700. doi: 10.1073/pnas.1117715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emond RT, Evans B, Bowen ET, Lloyd G. A Case of Ebola Virus Infection. BMJ. 1977;2(6086):541–544. doi: 10.1136/bmj.2.6086.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudoyarova-Zubavichene NM, Sergeyev NN, Chepurnov AA, Netesov SV. Preparation and Use of Hyperimmune Serum for Prophylaxis and Therapy of Ebola Virus Infections. J Infect Dis. 1999;179 (Suppl 1):S218–S223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 101.Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, Colebunders R, Muyembe-Tamfum JJ. Treatment of Ebola Hemorrhagic Fever with Blood Transfusions from Convalescent Patients. International Scientific and Technical Committee. J Infect Dis. 1999;179 (Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 102.Jahrling PB, Geisbert JB, Swearengen JR, Larsen T, Geisbert TW. Ebola Hemorrhagic Fever: Evaluation of Passive Immunotherapy in Nonhuman Primates. J Infect Dis. 2007;196 (Suppl 2):S400–S403. doi: 10.1086/520587. [DOI] [PubMed] [Google Scholar]
- 103.Oswald WB, Geisbert TW, Davis KJ, Geisbert JB, Sullivan NJ, Jahrling PB, Parren PW, Burton DR. Neutralizing Antibody Fails to Impact the Course of Ebola Virus Infection in Monkeys. PLoS Pathog. 2007;3(1):e9. doi: 10.1371/journal.ppat.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shedlock DJ, Bailey MA, Popernack PM, Cunningham JM, Burton DR, Sullivan NJ. Antibody-Mediated Neutralization of Ebola Virus Can Occur by Two Distinct Mechanisms. Virology. 2010;401(2):228–235. doi: 10.1016/j.virol.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu X, Alimonti JB, Melito PL, Fernando L, Stroher U, Jones SM. Characterization of Zaire Ebola virus Glycoprotein-Specific Monoclonal Antibodies. Clin Immunol. 2011;141(2):218–227. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. Successful Treatment of Ebola Virus-Infected Cynomolgus Macaques with Monoclonal Antibodies. Sci Transl Med. 2012;4(138):138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 107.Qiu X, Fernando L, Melito PL, Audet J, Feldmann H, Kobinger G, Alimonti JB, Jones SM. Ebola Gp-Specific Monoclonal Antibodies Protect Mice and Guinea Pigs from Lethal Ebola Virus Infection. PLoS Negl Trop Dis. 2012;6(3):e1575. doi: 10.1371/journal.pntd.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, Mccray PB, Chiorini J, Maury W. T-Cell Immunoglobulin and Mucin Domain 1 (TIM-1) Is a Receptor for Zaire Ebola virus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011;108(20):8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Basu A, Li B, Mills DM, Panchal RG, Cardinale SC, Butler MM, Peet NP, Majgier-Baranowska H, Williams JD, Patel I, Moir DT, Bavari S, Ray R, Farzan MR, Rong L, Bowlin TL. Identification of a Small-Molecule Entry Inhibitor for Filoviruses. J Virol. 2011;85(7):3106–3119. doi: 10.1128/JVI.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. Small Molecule Inhibitors Reveal Niemann-Pick C1 Is Essential for Ebola Virus Infection. Nature. 2011;477(7364):344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.King G, Sharom FJ. Proteins that Bind and Move Lipids: MsbA and NPC1. Crit Rev Biochem Mol Biol. 2012;47(1):75–95. doi: 10.3109/10409238.2011.636505. [DOI] [PubMed] [Google Scholar]
- 112.Lee K, Ren T, Côté M, Gholamreza B, Misasi J, Bruchez A, Cunningham J. Inhibition of Ebola Virus Infection: Identification of Niemann-Pick C1 as the Target by Optimization of a Chemical Probe. ACS Med Chem Lett. 2013;4(2):239–243. doi: 10.1021/ml300370k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shoemaker CJ, Schornberg KL, Delos SE, Scully C, Pajouhesh H, Olinger GG, Johansen LM, White JM. Multiple Cationic Amphiphiles Induce a Niemann-Pick C Phenotype and Inhibit Ebola Virus Entry and Infection. PLoS One. 2013;8(2):e56265. doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller EH, Harrison JS, Radoshitzky SR, Higgins CD, Chi X, Dong L, Kuhn JH, Bavari S, Lai JR, Chandran K. Inhibition of Ebola Virus Entry by a C-Peptide Targeted to Endosomes. J Biol Chem. 2011;286(18):15854–15861. doi: 10.1074/jbc.M110.207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spurgers KB, Alefantis T, Peyser BD, Ruthel GT, Bergeron AA, Costantino JA, Enterlein S, Kota KP, Boltz RC, Aman MJ, Delvecchio VG, Bavari S. Identification of Essential Filovirion-Associated Host Factors by Serial Proteomic Analysis and RNAi Screen. Mol Cell Proteomics. 2010;9(12):2690–2703. doi: 10.1074/mcp.M110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barrientos LG, Rollin PE. Release of Cellular Proteases into the Acidic Extracellular Milieu Exacerbates Ebola Virus-Induced Cell Damage. Virology. 2007;358(1):1–9. doi: 10.1016/j.virol.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 117.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal Proteolysis of the Ebola Virus Glycoprotein Is Necessary for Infection. Science. 2005;308(5728):1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. Role of Endosomal Cathepsins in Entry Mediated by the Ebola Virus Glycoprotein. J Virol. 2006;80(8):4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crotty S, Cameron CE, Andino R. RNA Virus Error Catastrophe: Direct Molecular Test by Using Ribavirin. Proc Natl Acad Sci US A. 2001;98(12):6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huggins JW. Prospects for Treatment of Viral Hemorrhagic Fevers with Ribavirin, a Broad-Spectrum Antiviral Drug. Rev Infect Dis. 1989;11 (Suppl 4):S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 121.Ignat’ev GM, Strel’tsova MA, Agafonov AP, Kashentseva EA, Prozorovskii NS. Experimental Study of Possible Treatment of Marburg Hemorrhagic Fever with Desferal, Ribavirin, and Homologous Interferon. Voprosy virusologii. 1996;41(5):206–209. [PubMed] [Google Scholar]
- 122.Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-Dicaprio K, Fritz EA, Jahrling PB, Mcclintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, Maclachlan I. Postexposure Protection of Guinea Pigs against a Lethal Ebola Virus Challenge Is Conferred by RNA Interference. J Infect Dis. 2006;193(12):1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, De Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I. Postexposure Protection of Non-Human Primates against a Lethal Ebola Virus Challenge with RNA Interference: A Proof-of-Concept Study. Lancet. 2010;375(9729):1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia M, Cooper A, Shi W, Bornmann W, Carrion R, Kalman D, Nabel GJ. Productive Replication of Ebola Virus Is Regulated by the C-Abl1 Tyrosine Kinase. Sci Transl Med. 2012;4(123):123ra24. doi: 10.1126/scitranslmed.3003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Warren TK, Shurtleff AC, Bavari S. Advanced Morpholino Oligomers: A Novel Approach to Antiviral Therapy. Antiviral Res. 2012;94(1):80–88. doi: 10.1016/j.antiviral.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dirin M, Winkler J. Influence of Diverse Chemical Modifications on the Adme Characteristics and Toxicology of Antisense Oligonucleotides. Expert Opin Biol Ther. 2013 doi: 10.1517/14712598.2013.774366. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 127.Swenson DL, Warfield KL, Warren TK, Lovejoy C, Hassinger JN, Ruthel G, Blouch RE, Moulton HM, Weller DD, Iversen PL, Bavari S. Chemical Modifications of Antisense Morpholino Oligomers Enhance Their Efficacy against Ebola Virus Infection. Antimicrob Agents Chemother. 2009;53(5):2089–2099. doi: 10.1128/AAC.00936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Warfield KL, Swenson DL, Olinger GG, Nichols DK, Pratt WD, Blouch R, Stein DA, Aman MJ, Iversen PL, Bavari S. Gene-Specific Countermeasures against Ebola Virus Based on Antisense Phosphorodiamidate Morpholino Oligomers. PLoS Pathog. 2006;2(1):e1. doi: 10.1371/journal.ppat.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, Panchal RG, Bavari S. Discovery and Early Development of AVI-7537 and AVI-7288 for the Treatment of Ebola Virus and Marburg Virus Infections. Viruses. 2012;4(11):2806–2830. doi: 10.3390/v4112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. Advanced Antisense Therapies for Postexposure Protection against Lethal Filovirus Infections. Nat Med. 2010;16(9):991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 131.Warren TK, Warfield KL, Wells J, Enterlein S, Smith M, Ruthel G, Yunus AS, Kinch MS, Goldblatt M, Aman MJ, Bavari S. Antiviral Activity of a Small-Molecule Inhibitor of Filovirus Infection. Antimicrob Agents Chemother. 2010;54 (5):2152–2159. doi: 10.1128/AAC.01315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kinch MS, Yunus AS, Lear C, Mao H, Chen H, Fesseha Z, Luo G, Nelson EA, Li L, Huang Z, Murray M, Ellis WY, Hensley L, Christopher-Hennings J, Olinger GG, Goldblatt M. FGI-104: A Broad-Spectrum Small Molecule Inhibitor of Viral Infection. Am J Transl Res. 2009;1(1):87–98. [PMC free article] [PubMed] [Google Scholar]
- 133.Aman MJ, Kinch MS, Warfield K, Warren T, Yunus A, Enterlein S, Stavale E, Wang P, Chang S, Tang Q, Porter K, Goldblatt M, Bavari S. Development of a Broad-Spectrum Antiviral with Activity against Ebola Virus. Antiviral Res. 2009;83(3):245–251. doi: 10.1016/j.antiviral.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 134.Bradfute SB, Warfield KL, Bray M. Mouse Models for Filovirus Infections. Viruses. 2012;4(9):1477–1508. doi: 10.3390/v4091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Subbotina E, Dadaeva A, Kachko A, Chepurnov A. Genetic Factors of Ebola Virus Virulence in Guinea Pigs. Virus Res. 2010;153(1):121–133. doi: 10.1016/j.virusres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 136.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. Pathogenesis of Ebola Hemorrhagic Fever in Primate Models: Evidence That Hemorrhage Is Not a Direct Effect of Virus-Induced Cytolysis of Endothelial Cells. Am J Pathol. 2003;163(6):2371–282. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A Mouse Model for Evaluation of Prophylaxis and Therapy of Ebola Hemorrhagic Fever. J Infect Dis. 1998;178(3):651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 138.Bray M. The Role of the Type I Interferon Response in the Resistance of Mice to Filovirus Infection. J Gen Virol. 2001;82(Pt 6):1365–1373. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 139.Bray M, Raymond JL, Geisbert T, Baker RO. 3-Deazaneplanocin a Induces Massively Increased Interferon-Alpha Production in Ebola Virus-Infected Mice. Antiviral Res. 2002;55(1):151–159. doi: 10.1016/s0166-3542(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 140.Bray M, Driscoll J, Huggins JW. Treatment of Lethal Ebola Virus Infection in Mice with a Single Dose of an S-Adenosyl-L-Homocysteine Hydrolase Inhibitor. Antiviral Res. 2000;45(2):135–147. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 141.Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, Huggins JW, Leduc JW, Peters CJ. Evaluation of Immune Globulin and Recombinant Interferon-Alpha2b for Treatment of Experimental Ebola Virus Infections. J Infect Dis. 1999;179 (Suppl 1):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 142.Markin VA, Mikhailov VV, Krasnianskii VP, Borisevich IV, Firsova IV. Developing Principles for Emergency Prevention and Treatment of Ebola Fever. Voprosy virusologii. 1997;42(1):31–34. [PubMed] [Google Scholar]
- 143.Kolokoltsov AA, Davidovich IA, Streltsova MA, Nesterov AE, Agafonova OA, Agafonov AP. The Use of Interferon for Emergency Prophylaxis of Marburg Hemorrhagic Fever in Monkeys. Bull Exp Biol Med. 2001;132(1):686–688. doi: 10.1023/a:1012540614713. [DOI] [PubMed] [Google Scholar]
- 144.Panchal RG, Reid SP, Tran JP, Bergeron AA, Wells J, Kota KP, Aman J, Bavari S. Identification of an Antioxidant Small-Molecule with Broad-Spectrum Antiviral Activity. Antiviral Res. 2012;93(1):23–29. doi: 10.1016/j.antiviral.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms Underlying Coagulation Abnormalities in Ebola Hemorrhagic Fever: Overexpression of Tissue Factor in Primate Monocytes/Macrophages Is a Key Event. J Infect Dis. 2003;188(11):1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 146.Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, Young HA, Fredeking TM, Rote WE, Vlasuk GP. Treatment of Ebola Virus Infection with a Recombinant Inhibitor of Factor Viia/Tissue Factor: A Study in Rhesus Monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 147.Hensley LE, Stevens EL, Yan SB, Geisbert JB, Macias WL, Larsen T, Daddario-Dicaprio KM, Cassell GH, Jahrling PB, Geisbert TW. Recombinant Human Activated Protein C for the Postexposure Treatment of Ebola Hemorrhagic Fever. J Infect Dis. 2007;196 (Suppl 2):S390–S399. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 148.Mungall D. rNAPc2. Nuvelo. Curr Opin Invet Drugs. 2004;5(3):327–333. [PubMed] [Google Scholar]
- 149.Ranieri V, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, et al. Drotrecogin Alfa (Activated) in Adults with Septic Shock. N Engl J Med. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 150.Food and Drug Administraiton. Voluntary Withdrawal of Xigris (Drotrecogini Alfa (Activated)) Due to Failure to Show a Survival Benefit. 2011 [cited 2013 May 1]; http://www.fda.gov/drugs/drugsafety/ucm277114.htm.
- 151.Foged C. siRNA Delivery with Lipid-Based Systems: Promises and Pitfalls. Curr Top Med Chem. 2012;12(2):97–107. doi: 10.2174/156802612798919141. [DOI] [PubMed] [Google Scholar]
- 152.Jiskoot W, Randolph TW, Volkin DB, Middaugh CR, Schoneich C, Winter G, Friess W, Crommelin DJ, Carpenter JF. Protein Instability and Immunogenicity: Roadblocks to Clinical Application of Injectable Protein Delivery Systems for Sustained Release. J Pharm Sci. 2012;101(3):946–954. doi: 10.1002/jps.23018. [DOI] [PubMed] [Google Scholar]
- 153.Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive Drug Delivery Systems. J Pharm Bioallied Sci. 2011;3(1):89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leucuta SE. Systemic and Biophase Bioavailability and Pharmacokinetics of Nanoparticulate Drug Delivery Systems. Curr Drug Deliv. 2013;10(2):208–240. doi: 10.2174/1567201811310020007. [DOI] [PubMed] [Google Scholar]
- 155.Khutoryanskiy VV. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol Biosci. 2011;11(6):748–764. doi: 10.1002/mabi.201000388. [DOI] [PubMed] [Google Scholar]
- 156.Das Neves J, Bahia MF, Amiji MM, Sarmento B. Mucoadhesive Nanomedicines: Characterization and Modulation of Mucoadhesion at the Nanoscale. Expert Opin Drug Deliv. 2011;8(8):1085–1104. doi: 10.1517/17425247.2011.586334. [DOI] [PubMed] [Google Scholar]
- 157.Laffleur F, Bernkop-Schnürch A. Thiomers: Promising Platform for Macromolecular Drug Delivery. Future Med Chem. 2013;5(5):511–522. doi: 10.4155/fmc.12.165. [DOI] [PubMed] [Google Scholar]
- 158.Hauptstein S, Bernkop-Schnürch A. Thiomers and Thiomer-Based Nanoparticles in Protein and DNA Drug Delivery. Expert Opin Drug Deliv. 2012;9(9):1069–1081. doi: 10.1517/17425247.2012.697893. [DOI] [PubMed] [Google Scholar]
- 159.Aungst BJ. Absorption Enhancers: Applications and Advances. AAPS J. 2012;14(1):10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hamman J, Steenekamp J. Excipients with Specialized Functions for Effective Drug Delivery. Expert Opin Drug Deliv. 2012;9(2):219–230. doi: 10.1517/17425247.2012.647907. [DOI] [PubMed] [Google Scholar]
- 161.Frankel AD, Pabo CO. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 162.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The Third Helix of the Antennapedia Homeodomain Translocates through Biological Membranes. J Biol Chem. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- 163.Gooding M, Browne LP, Quinteiro FM, Selwood DL. siRNA Delivery: From Lipids to Cell-Penetrating Peptides and Their Mmimics. Chem Biol Drug Des. 2012;80 (6):787–809. doi: 10.1111/cbdd.12052. [DOI] [PubMed] [Google Scholar]
- 164.Sen K, Mandal M. Second Generation Liposomal Cancer Therapeutics: Transition from Laboratory to Clinic. Int J Pharm. 2013;448(1):28–43. doi: 10.1016/j.ijpharm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 165.Musacchio T, Torchilin VP. Recent Developments in Lipid-Based Pharmaceutical Nanocarriers. Front Biosci. 2011;16:1388–1412. doi: 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- 166.Pardeshi C, Rajput P, Belgamwar V, Tekade A, Patil G, Chaudhary K, Sonje A. Solid Lipid Based Nanocarriers: An Overview. Acta Pharm. 2012;62(4):433–472. doi: 10.2478/v10007-012-0040-z. [DOI] [PubMed] [Google Scholar]
- 167.Lim SB, Banerjee A, Önyüksel H. Improvement of Drug Safety by the Use of Lipid-Based Nanocarriers. J Control Release. 2012;163(1):34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 168.Van Rooijen N, Van Nieuwmegen R. Liposomes in Immunology: The Immune Response against Antigen-Containing Liposomes. Immunol Commun. 1977;6(5):489–498. doi: 10.3109/08820137709094148. [DOI] [PubMed] [Google Scholar]
- 169.Hsu MJ, Juliano RL. Interactions of Liposomes with the Reticuloendothelial Ssystem. Ii: Nonspecific and Receptor-Mediated Uptake of Liposomes by Mouse Peritoneal Macrophages. Biochim Biophys Acta. 1982;720(4):411–419. doi: 10.1016/0167-4889(82)90120-3. [DOI] [PubMed] [Google Scholar]
- 170.Shahum E, Thérien HM. Liposomal Adjuvanticity: Effect of Encapsulation and Surface-Linkage on Antibody Production and Proliferative Response. Int J Immunopharmacol. 1995;17(1):9–20. doi: 10.1016/0192-0561(94)00082-y. [DOI] [PubMed] [Google Scholar]
- 171.Agrewala JN, Owais M, Gupta CM, Mishra GC. Antigen Incorporation into Liposomes Results in the Enhancement of Il-4 and IgG1 Secretion: Evidence for Preferential Expansion of Th-2 Cells. Cytokines Mol Ther. 1996;2(1):59–65. [PubMed] [Google Scholar]
- 172.Watson DS, Endsley AN, Huang L. Design Considerations for Liposomal Vaccines: Influence of Formulation Parameters on Antibody and Cell-Mediated Immune Responses to Liposome Associated Antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Christensen D, Korsholm KS, Andersen P, Agger EM. Cationic Liposomes as Vaccine Adjuvants. Expert Rev Vaccines. 2011;10(4):513–521. doi: 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- 174.Chang HI, Yeh MK. Clinical Development of Liposome-Based Drugs: Formulation, Characterization, and Therapeutic Efficacy. Int J Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-Inspired, Bio-Engineered and Biomimetic Drug Delivery Carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 176.Salmaso S, Caliceti P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J Drug Deliv. 2013 doi: 10.1155/2013/374252. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Webster DM, Sundaram P, Byrne ME. Injectable Nanomaterials for Drug Delivery: Carriers, Targeting Moieties, and Therapeutics. Eur J Pharm Biopharm. 2013;84(1):1–20. doi: 10.1016/j.ejpb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 178.Mandal B, Bhattacharjee H, Mittal N, Sah H, Balabathula P, Thoma LA, Wood GC. Core-Shell-Type Lipid-Polymer Hybrid Nanoparticles as a Drug Delivery Platform. Nanomedicine. 2013;9(4):474–491. doi: 10.1016/j.nano.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 179.Hudson D, Margaritis A. Biopolymer Nanoparticle Production for Controlled Release of Biopharmaceuticals. Crit Rev Biotechnol. 2013 doi: 10.3109/07388551.2012.743503. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 180.Balmayor ER, Azevedo HS, Reis RL. Controlled Delivery Systems: From Pharmaceuticals to Cells and Genes. Pharm Res. 2011;28(6):1241–1258. doi: 10.1007/s11095-011-0392-y. [DOI] [PubMed] [Google Scholar]
- 181.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The Mechanisms of Drug Rrelease in Poly(Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems--A Review. Int J Pharm. 2011;415(1–2):34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 182.Naeye B, Raemdonck K, Remaut K, Demeester J, De Smedt SC. Matrix Systems for siRNA Delivery. Curr Top Med Chem. 2012;12(2):89–96. doi: 10.2174/156802612798919150. [DOI] [PubMed] [Google Scholar]
- 183.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 184.Lv Y, Tan T, Svec F. Molecular Imprinting of Proteins in Polymers Attached to the Surface of Nanomaterials for Selective Recognition of Biomacromolecules. Biotechnol Adv. 2013 doi: 10.1016/j.biotechadv.2013.02.005. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 185.Cheong WJ, Yang SH, Ali F. Molecular Imprinted Polymers for Separation Science: A Review of Reviews. J Sep Sci. 2013;36(3):609–628. doi: 10.1002/jssc.201200784. [DOI] [PubMed] [Google Scholar]
- 186.Wrenn SP, Dicker SM, Small EF, Dan NR, Mleczko M, Schmitz G, Lewin PA. Bursting Bubbles and Bilayers. Theranostics. 2012;2(12):1140–1159. doi: 10.7150/thno.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials. 2013;34(14):3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 188.Gibson MI, O’reilly RK. To Aggregate, or Not to Aggregate? Considerations in the Design and Application of Polymeric Thermally-Responsive Nanoparticles. Chem Soc Rev. 2013 doi: 10.1039/c3cs60035a. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 189.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, Reimann KA, Bao S, Rao S, Roederer M, Jahrling PB, Koup RA, Nabel GJ. CD8+ Cellular Immunity Mediates rAd5 Vaccine Protection against Ebola Virus Infection of Nonhuman Primates. Nat Med. 2011;17(9):1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 190.Geisbert TW, Bailey M, Geisbert JB, Asiedu C, Roederer M, Grazia-Pau M, Custers J, Jahrling P, Goudsmit J, Koup R, Sullivan NJ. Vector Choice Determines Immunogenicity and Potency of Genetic Vaccines against Angola Marburg Virus in Nonhuman Primates. J Virol. 2010;84(19):10386–10394. doi: 10.1128/JVI.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. Successful Topical Respiratory Tract Immunization of Primates against Ebola Virus. J Virol. 2007;81(12):6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg Virus Vaccines Based Upon Alphavirus Replicons Protect Guinea Pigs and Nonhuman Primates. Virology. 1998;251(1):28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 193.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola Virus-Like Particle-Based Vaccine Protects Nonhuman Primates against Lethal Ebola Virus Challenge. J Infect Dis. 2007;196 (Suppl 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 194.Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and Postexposure Prophylaxis of Ebola Virus Infection in an Animal Model by Passive Transfer of a Neutralizing Human Antibody. J Virol. 2002;76(12):6408–6412. doi: 10.1128/JVI.76.12.6408-6412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]