Abstract
Currently, the evaluation of thyroid cancer relies on the use of fine needle aspiration biopsy as non-invasive imaging methods do not provide sufficient levels of accuracy for the diagnosis of this disease. In this study, the potential of quantitative ultrasound methods for characterizing thyroid tissues was studied using a rodent model ex vivo. A high-frequency ultrasonic scanning system (40 MHz) was used to scan thyroids extracted from mice that had spontaneously developed thyroid lesions (cancerous or benign). Three sets of mice were acquired having different predispositions to developing thyroid anomalies (a C-cell adenoma, a papillary thyroid carcinoma (PTC), and a follicular variant papillary thyroid carcinoma (FV-PTC)). A fourth set of mice did not develop thyroid anomalies (normal mice) and were used as controls. The backscatter coefficient was estimated from excised thyroid lobes for the different mice. From the backscatter coefficient versus frequency (25 to 45 MHz), the effective scatterer diameter (ESD) and effective acoustic concentration (EAC) were estimated. From the envelope of the backscattered signal, the homodyned K distribution was used to estimate the k parameter (ratio of coherent to incoherent signal energy) and the μ parameter (number of scatterers per resolution cell). Statistically significant differences were observed between the malignant thyroids and the normal thyroids based on the ESD, EAC and μ parameters. The mean values of the ESDs were 18.0 ± 0.92, 15.9 ± 0.81, and 21.5 ± 1.80 µm for the PTC, FV-PTC and the normal thyroids, respectively. The mean values of the EACs were 59.4 ± 1.74, 62.7 ± 1.61, and 52.9 ± 3.42 dB (mm−3) for the PTC, FV-PTC and the normal thyroids, respectively. The mean values of the μ parameters were 2.55 ± 0.37, 2.59 ± 0.43, and 1.56 ± 0.99 for the PTC, FV-PTC and the normal thyroids, respectively. Statistically significant differences were observed between the malignant thyroids and the C-cell adenomas based on the ESD and EAC parameters with estimated values for the ESD of 21.3 ± 1.50 µm and EAC of 54.7 ± 2.24 dB (mm−3) for the C-cell adenomas. These results suggest that high frequency quantitative ultrasound may enhance the ability to detect and classify diseased thyroid tissues.
Keywords: Quantitative Ultrasound, Tissue Characterization, Cancer Diagnosis, Thyroid
Introduction
Thyroid nodules are a very common occurrence and present challenges to clinical diagnosis. In the United States between 4 and 7% of the general population have clinically palpable nodules [Lewis et al., 2002]. The American Cancer Society estimates in 2012 there will be 56,460 new cases of thyroid cancer [2012]. While the prognosis of thyroid cancer is relatively good, with 20-year survival rates are around 90% [Cotran et al., 1999], clearly differentiating between benign and malignant nodules is problematic. Using ultrasonic imaging, certain studies predicted up to 70% of the adult population have detectable thyroid nodules [Tan and Gharib, 1997; Marqusee et al., 2000; Ross, 2002]. With the increased use of ultrasound to image the head and neck, the management problem has skyrocketed over the past few decades to an epidemic proportion. The number of incidentally found nodules from ultrasound examinations of asymptomatic patients has complicated the debate on proper management of thyroid nodules [Chidiac and Aron, 1997; Mirilas and Skandalakis, 2002].
The problem of thyroid cancer management is usually one of diagnosis rather than detection. Clinicians will take a conservative approach if there is a modest level of suspicion of malignancy in detected thyroid nodules. This results in many fine needle aspiration (FNA) biopsies with benign diagnosis, and many with undetermined diagnosis or just a level of suspicion. As a result, there are many unnecessary surgical procedures in hindsight and testing and treatments that carry with them their own set of risks [Silver and Parangi, 2004].
While the advent of ultrasonography of the thyroid has contributed to the management crisis concerning the prevalence of nodules in asymptomatic adults, it has been used to alleviate some of the costs and burden associated with proper management. Abundant research has been conducted to determine the ability of ultrasonic imaging to predict the malignancy of thyroid nodules with some success [Brander et al., 2000; Ahuja and Metreweli, 2000; Ahuja and Ying, 2002; Koike et al., 2002; Papini et al., 2002; Kim et al., 2002; Frates et al., 2003; Chan et al., 2003; Liebeskind et al., 2005]. The diagnostic approach of ultrasound currently falls under two branches: ultrasonographic features (i.e., echogenicity of the nodule, the type of border around the nodule, whether calcifications are present, composition and size) and color Doppler characteristics of the nodules [Ahuja and Metreweli, 2000].
Several studies using these ultrasonic features have yielded a variety of results. The majority of the results in the literature, however, suggest that current ultrasonic imaging methods may only provide a level of suspicion for a particular nodule [Papini et al., 2002, Frates et al., 2003, Iannuccilli et al., 2004, Moon et al., 2008]. This is reflected in the current guidelines provided by the American Thyroid Association, which state that no single sonographic feature or combination of features has the sufficient sensitivity and specificity to allow for non-invasively diagnosing all thyroid nodules and therefore recommend FNA as the procedure of choice in the evaluation of thyroid nodules [Cooper et al., 2009].
Another difficulty with using sonographic features and color Doppler to diagnose thyroid nodules is the lack of system independence. The ability to map out the feature set is highly dependent not only on the equipment used but also on the operator and the training of the operator. As a result, the statistics of sonographic feature detection vary from one study to the next. In the end, accurate diagnosis comes from a cellular level (optical microscopy) and the feature sets proposed using conventional ultrasound diagnosis have little basis in structure at the cellular level.
Recently, elastography techniques involving ultrasound have been examined for their ability to improve thyroid cancer diagnostics. In one study shear wave elastography was used to diagnose 146 nodules from 93 patients [Sebag et al., 2010]. Using shear wave elastography and ultrasound features of the thyroids resulted in a sensitivity of 81.5% and specificity of 97.0%. Similar experiments using strain imaging were observed to increase the sensitivity and specificity of ultrasound B-mode imaging alone [Rago et al., 2010]. Ultrasound elastography has been used to correlate the stiffness of thyroid nodules to malignancy where increases stiffness of thyroid nodules was associated with cancer and decreased stiffness was associated with benign nodules [Vorlander et al., 2010]. These techniques show promise but still need to be evaluated in larger clinical studies.
Another ultrasonic imaging mode that can potentially improve thyroid cancer diagnosis and help alleviate the management crisis resulting from the detection of thyroid nodules is quantitative ultrasound (QUS) techniques based on ultrasound backscatter. QUS techniques (spectral-based parameters and parameters based on envelope statistics) have been used to characterize different disease states such as prostate cancer, ocular tumors, mammary cancer in rodent models, detection of micrometastases in lymph nodes, and fatty liver disease [Feleppa et al., 1997; Lizzi et al., 1997; Silverman et al., 2003; Oelze and Zachary, 2006; Mamou et al., 2011; Ghoshal et al, 2012].
Based on these earlier successes, where changes in tissue microstructure led to new sources of image contrast using QUS parameters, it is possible that high frequency QUS could also provide sources of image contrast to detect and classify thyroid cancer. Studies on QUS applied to thyroid imaging are however sparse. Scatterer size imaging was used to examine a couple of cases of thyroid cancer in [Wilson et al., 2006], and preliminary data suggested that scatterer size imaging could increase contrast between thyroid abnormalities and healthy thyroid tissue. However, no subsequent correlation with pathology provided a comparison between actual underlying microstructure and the values of the lone QUS parameter analyzed in that study. The few studies on quantitative envelope characterization are based on texture analysis [Catherine et al., 2006, Rajendra et al., 2012] instead of envelope statistics parameters that have a closer connection with tissue microstructure [Destrempes et al., 2010].
In this study, multiple QUS parameters based on the frequency-dependent backscatter coefficient and the envelope statistics of the backscattered ultrasound were estimated from mouse models of thyroid cancer. These QUS estimates were then compared to histological slides of the analyzed thyroid tissues.
Methods
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign and satisfied all university and National Institutes of Health rules for the humane use of laboratory animals.
A high-frequency ultrasonic scanning system was used to scan thyroids extracted from mice that had spontaneously developed thyroid lesions (cancerous or benign). One set of mice was used as controls and three sets of mice were acquired having different predispositions to developing thyroid anomalies. The first set of mice (n = 8) did not develop thyroid lesions, were judged to be normal thyroids by a pathologist and were used as controls. The second set of mice (n = 6) were from the Rb 1+/− mouse strain and acquired from the mouse cancer model repository at the National Cancer Institute (courtesy of the Jacks Lab at the Koch Institute for Integrative Cancer Research at MIT) [Jacks et al., 1992]. Approximately 50% of these mice will develop C-cell adenomas or C-cell hyperplasia in the thyroid. These growths would typically be benign in nature. The third set of mice (n = 6) was from the TG-BRAF mouse line and acquired from the Fagin lab (Sloan-Kettering Institute for Cancer Research) [Knauf et al., 2005]. These mice would develop papillary thyroid carcinomas (PTCs). The fourth set of mice (n = 5) were acquired from Dr. Cheng’s lab (Center for Cancer Research, NIH) [Suzuki et al., 2002] and consisted of mutant mice that had introduced a dominant negative mutant thyroid nuclear receptor gene, TRβPV, into the TRβ gene locus. As a result of this mutation, as the TRβPV/PV mice aged they developed metastatic thyroid tumors consistent with follicular variant papillary thyroid carcinomas (FV-PTC).
All mice were examined weekly and scanned with a VisualSonics Vevo 2100 (VisualSonics, Toronto, CA) to determine the size of the thyroid of a particular mouse or if a mouse had developed a detectable lesion in the thyroid. When the size of a thyroid was detected to be larger than normal or if the thyroid appeared to have lesions, a mouse was taken for experimental examination using the QUS scanning system and analysis.
When a mouse was selected for scanning, the mouse was euthanized and both thyroid lobes were extracted along with a portion of the trachea. The thyroid lobes were placed in a tank of degassed 0.9% saline maintained at 37 °C for ultrasonic scanning. After scanning, both thyroid lobes were excised, fixed in 10% neutral buffered formalin, processed and embedded in paraffin, sectioned and stained for routine histologic evaluation by light microscopy. A diagnosis was obtained for all animals following histopathologic evaluation.
The ultrasonic scanning system consisted of a weakly-focused (f/3) single-element transducer (USC, Ultrasonic Transducer Resource Center, Los Angeles, CA) with nominal center frequency of 40 MHz (the actual -6-dB bandwidth of the transducer was 25 to 45 MHz) and active element diameter of 3 mm. The transducer was operated using a Panametrics 5900 pulser/receiver (Olympus NDT, Waltham, MA). Backscattered waveforms were acquired with a PC via a 14-bit UF3-4121 A/D card with 250 MHz sampling (Strategic Test Corporation, Woburn, MA) and were saved to a computer for post-processing. For most thyroids, more than a dozen slices of the thyroids were acquired by translating the transducer using a micropositioning system (Daedal, Inc, Harrisburg, PA) controlled with custom LabView (National Instruments, Austin, TX) software. Slices were taken at 0.2 to 0.4 mm apart (larger than a beamwidth) across the thyroid and perpendicular to the axial direction of the trachea. For each slice a number of scan lines were acquired depending on the size of the thyroid and each scan line was separated by 0.05 mm, i.e., approximately half a beamwidth at the transducer center frequency.
From the scan lines, the envelope was detected and a B-mode image was constructed of the thyroid lobes. For processing, custom Matlab (MathWorks, Natick, MA) software was used to draw regions of interest in each slice corresponding to the actual thyroid lobes. Within the regions of interest, data blocks were automatically selected for QUS analysis. Each data block was 0.5 mm by 0.5 mm with a 75% overlap in the axial and lateral directions. Two QUS parameters were examined based on the backscatter coefficient versus frequency (spectral-based parameters: effective scatterer diameter (ESD) and effective acoustic concentration (EAC)) and two QUS parameters were estimated from the envelope statistics (the k parameter and the μ parameter).
From each data block the backscatter coefficient versus frequency was estimated by the method of Chen et al [1997] and based on studies by Lavarello et al [2011]. For calculation of the backscatter coefficient, a reference spectrum for each depth location was acquired by measuring the signal reflected from a smooth planar surface of known reflectivity (Plexiglas). To correct for attenuation, estimates from different thyroids were estimated using insertion loss techniques. The mean attenuation slope value from the estimates from all the thyroids in the frequency range between 25 and 45 MHz was 1.19 ± 0.256 dB/MHz/cm and this value was used for attenuation compensation when calculating the backscatter coefficient. Similar values of attenuation in human thyroids have been estimated, i.e., 0.91 to 1.5 dB/MHz/cm when operating at 10 MHz [Fuji et al., 2003].
The spectral-based parameters were estimated by applying a spherical Gaussian model to the data and using an estimator that has been previously reported [Oelze et al., 2002]. Under plane wave incidence and no multiple scattering assumptions, the backscatter coefficient can be modeled as
where f is the frequency in MHz, L is the gate length in mm, and q is the ratio of the aperture radius to the data block depth. From a physical point of view, the ESD is indicative of the size of the scatterers giving rise to the measured ultrasonic echoes and the EAC is proportional both to the number density of scatterers and the square of the impedance mismatch between the scatterers and background. In this work, the analysis bandwidth used for deriving the spectral-based parameters was 25 to 45 MHz.
The envelope statistics were estimated through a routine to parameterize the homodyned K distribution [Hruska and Oelze, 2009]. The envelope of the backscattered signal was detected and the values of the envelope corresponding to a particular data block were stored in a vector. The SNR, skewness and kurtosis were calculated from the envelope amplitude values in the data block vector corresponding to two fractional-order moments (i.e., 0.72 and 0.88). Level curves previously stored for values of SNR, skewness and kurtosis were generated for k and μ parameters for each fractional-order moment. The intersection of the curves in the k-μ space represented the values obtained for the particular data block. In our estimator, μ parameter estimates greater than 10 were excluded because these values were found to be unreliable estimates [Hruska, 2009]. The k parameter quantifies the ratio of the coherent scattering signal to the incoherent scattered signal. If scatterers are regularly spaced or large single scatterers are present, the k parameter will increase. The μ parameter provides an estimate of the number of scatterers per resolution cell. If the resolution cell of the imaging system can be estimated, then an estimate of the number density of scatterers can be obtained. Because these parameters are related to the organization of underlying scatterers, it may be possible to correlate these parameters to underlying structure.
Results
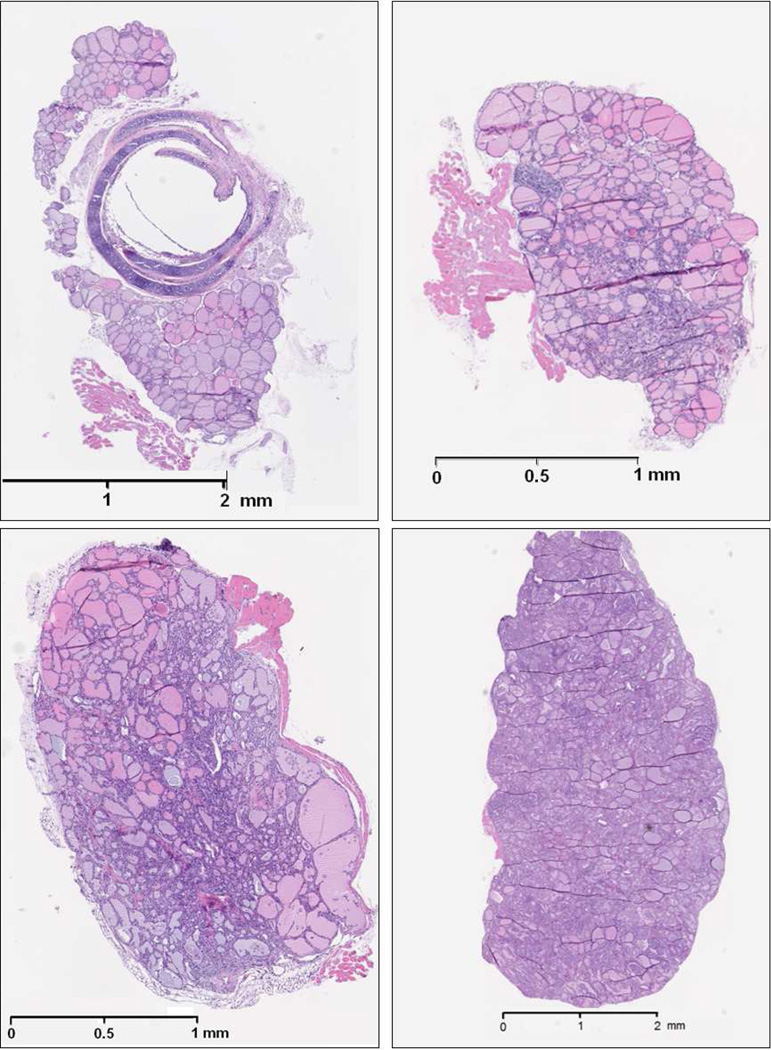
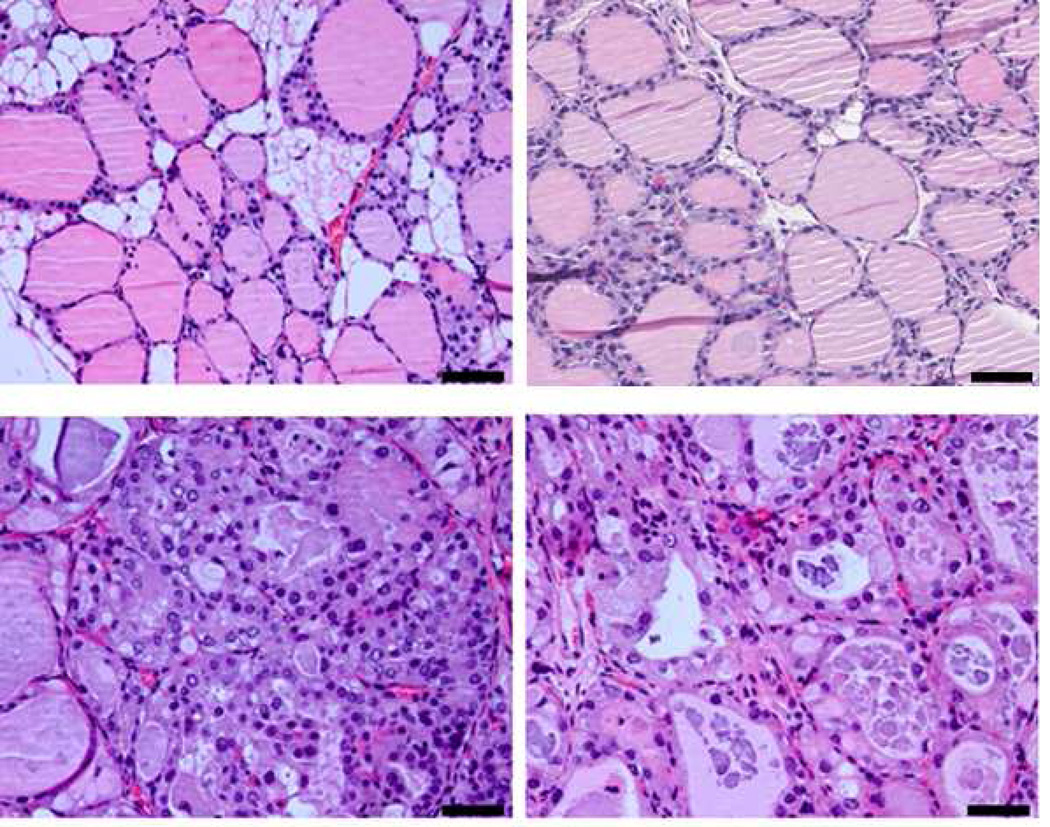
Histological slides of tissues stained with H&E are shown in Figs. 1 (no magnification) and 2 (40× magnification). The normal thyroid gland consisted of round to oval thyroid follicles lined by a single layer of epithelial cells. The nucleoli were small and the chromatin was dense. The follicles contained eosinophilic colloid material, and were separated by a small amount of benign adipose tissue.
Figure 1.
Histology images of (Top left) Normal thyroid, (top right) C-cell adenoma, (bottom left) papillary thyroid carcinoma, and (bottom right) papillary thyroid carcinoma showing follicular pattern.
The C-cell tumors were characterized by solid sheets of monotonous cells. The glands had much more cellular proliferation compared to the normal thyroid and some areas of the thyroid were completely taken over by sheets of cells. The nuclear features were very characteristic of neuroendocrine cells. The chromatin was granular dispersed usually described as “salt and pepper” chromatin. Nucleoli were inconspicuous and cytoplasm was barely visible. Large tumor masses had areas of necrosis and rather brisk mitotic activity.
The papillary thyroid carcinomas had two main patterns. The complex papillary structures in some areas formed nodular masses (PTC) and in other areas had a follicular pattern (FV-PTC). In both patterns the follicles were lined by enlarged follicular cells. The nuclei were large and the chromatin was granular. There were optically clear nuclei and well defined intra-nuclear pseudo inclusions. Colloid material was either scant or absent. In other areas the follicular lumen was obliterated by malignant cells. The cancer was spread diffuse throughout the thyroids and was not contained to small nodules, as can be the case for human thyroid cancer.
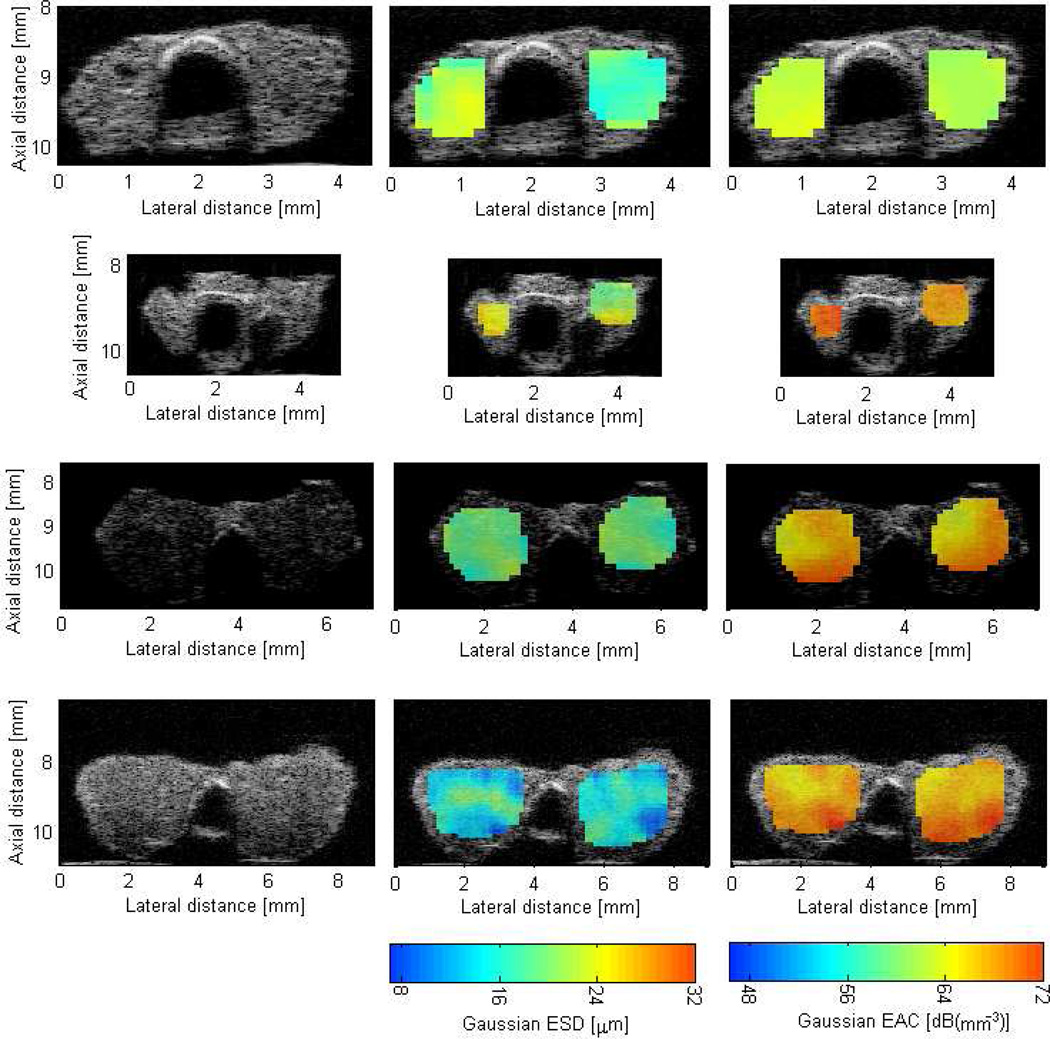
Figure 3 shows B-mode images of mice thyroids (normal and cancerous) along with QUS images enhanced by either the ESD or EAC. From B-mode images it would be difficult to differentiate between different thyroids based on their visible appearance. However, using the QUS images, differentiating between the malignant and cancerous cases is possible. Nevertheless, the benign C-cell adenoma could not be differentiated from the normal thyroids based on the appearance of the QUS images.
Figure 3.
B-mode (left column) and QUS images of thyroids enhanced by ESD (middle column) and EAC (right column). The top row is a normal thyroid (no tumor observed), the second row is a C-cell adenoma, the third row is PTC, and the last row is a FV-PTC.
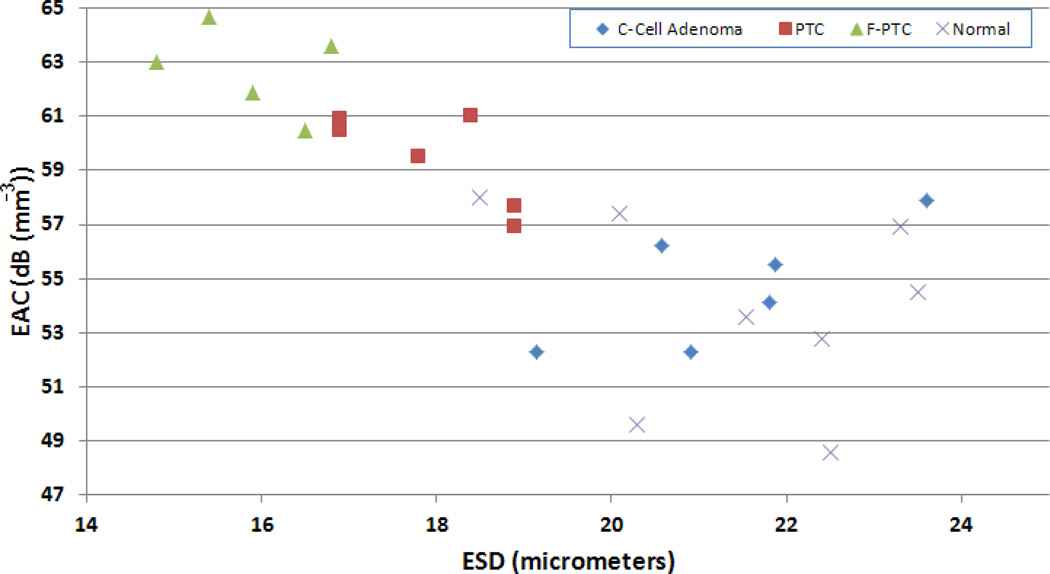
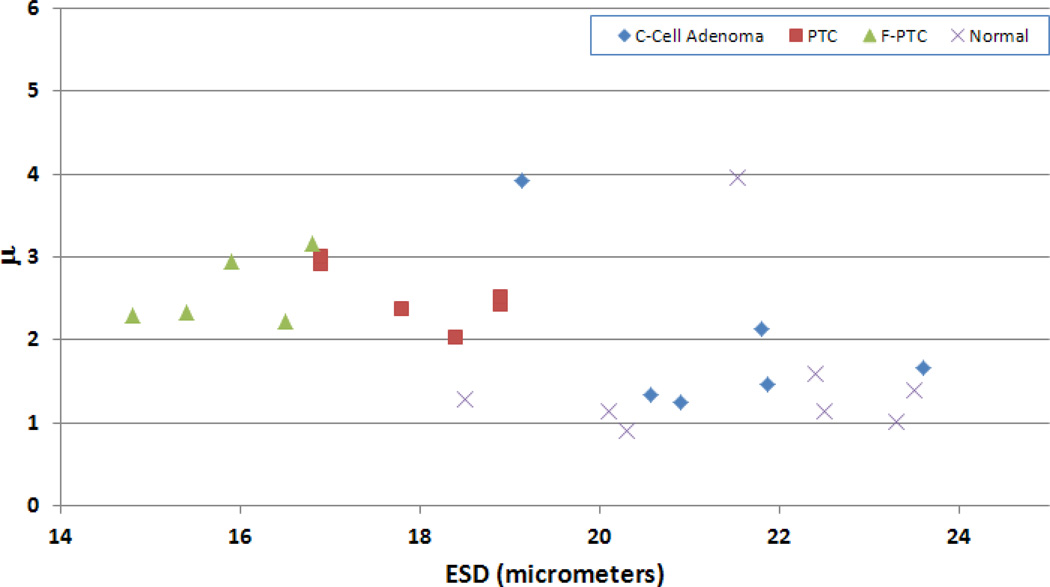
Table 1 provides a tabulation of the average results from the different thyroid conditions examined. Scatter plots of ESD versus EAC and of the ESD versus μ parameter were also generated to illustrate the ability of QUS to differentiate the different thyroids (Figs. 4 and 5, respectively). The scatter plots indicate that different groups were clustered together based on the values of the estimated parameters for each thyroid condition.
Table 1.
Tabulation of the mean and standard deviation of the parameters estimated for each thyroid condition examined.
| ESD (µm) | EAC (dB (mm−3)) | k parameter | μ parameter | |
|---|---|---|---|---|
| Normal | 21.5 ± 1.80 | 52.9 ± 3.42 | 0.518 ± 0.07 | 1.56 ± 0.99 |
| C-Cell Adenoma | 21.3 ± 1.50 | 54.7 ± 2.24 | 0.537 ± 0.12 | 1.96 ± 1.01 |
| PTC | 18.0 ± 0.92 | 59.4 ± 1.74 | 0.508 ± 0.05 | 2.55 ± 0.37 |
| FV-PTC | 15.9 ± 0.81 | 62.7 ± 1.61 | 0.558 ± 0.04 | 2.59 ± 0.43 |
Figure 4.
Scatter plot of ESD versus EAC for the different kinds of thyroids examined.
Figure 5.
Scatter plot of ESD versus μ parameter for the different kinds of thyroids examined.
Given the relatively small population used in this study (i.e., 25 animals in total), statistically significant differences between the different groups were estimated using a non-parametric Kruskal-Wallis test. Table 2 lists the p-values associated with the different sets of tumors and if statistically significant differences (p < 0.05) were observed between the different kinds of thyroids scanned. Statistically significant differences were observed between the cancerous thyroids (PTC and FV-PTC) and the normal thyroids using the ESD, EAC, and μ parameters. The k parameter did not yield statistically significant differences between groups. No parameter was able to differentiate the C-cell adenomas from the normal thyroids. Only the ESD and EAC could differentiate the cancerous thyroids from the C-cell adenomas. However, if the outlier for the C-cell adenoma in terms of the μ parameter was taken out (see Fig. 5), then statistically significant differences were also observed between the cancerous cases and the C-cell adenomas. Finally, the ESD and EAC provided the ability to distinguish the PTC from the FV-PTC. The μ parameter did not provide statistically significant differences between the PTC and FV-PTC thyroids.
Table 2.
P-values from tests of statistically significant differences between groups for the ESD (first column in each block), the EAC (second column in each block) and the μ parameter (third column in each block).
| C-Cell | PTC | FV-PTC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESD | EAC | μ | k | ESD | EAC | μ | k | ESD | EAC | μ | k | |
| Normal | 0.90 | 0.90 | 0.12 | 0.44 | < 0.05 | < 0.05 | < 0.05 | 0.27 | < 0.05 | < 0.05 | < 0.05 | 0.07 |
| C-Cell | < 0.05 | < 0.05 | 0.08 | 0.63 | < 0.05 | < 0.05 | 0.07 | 0.52 | ||||
| PTC | < 0.05 | < 0.05 | 0.86 | 0.12 | ||||||||
Discussion and Conclusions
High-frequency QUS provided a new source of image contrast with the ability to differentiate cancerous thyroids in mice from both normal mouse thyroids and mouse thyroids that had developed a benign lesion (C-cell adenoma). Further, spectral based QUS techniques provided the ability to differentiate two types of thyroid cancer from each other. These results suggest that the additional information provided by QUS can improve the diagnostic potential of ultrasound for thyroid classification. Improving the ability of ultrasound to detect and classify thyroid cancer would greatly improve the management of thyroid cancer and could potentially alleviate the need for many biopsies.
Based on comparing the histological slides of the different thyroid conditions examined and the QUS parameters, some correlations exist. The feature analysis plots of Figs. 4 and 5 indicate that the ESD estimates are smallest for the FV-PTC thyroids, next smallest for the PTC thyroids, and largest for the normal and C-Cell adenomas. From the histological slides, for the normal thyroids the structure is dominated by follicles. The follicles are lined by follicular cells and the lumen contains colloid. The benign follicles varied in size from 40 µm to 100 µm in diameter. The benign cells measured approximately 10 µm in diameter. The malignant follicles varied from 60 µm to 200 µm and the malignant cells varied from 15 µm to 24 µm in diameter. As the cancer invaded the thyroid, the thyroid space was filled up more and more by the malignant cells and less and less by the follicles. If the scattering of ultrasound occurred from a combination of the follicles and the follicular cells, then as the thyroid tissue was taken over by the cancer the scattering may have been more dominated by the malignant cells, which have a much smaller size than the follicles.
Furthermore, both the EAC and the μ parameter were observed to increase between the normal thyroid and the cancerous cases. The increases in the EAC mean that the product of the number density and the impedance mismatch between the scatterer and background increased. The increases in the μ parameter correspond to an increase in the number density of scatterers. The cancerous thyroids are characterized by cellular hyperplasia compared to the normal thyroids. Assuming that the cells contribute significantly to the backscattered signal, this increase in the number of cells may result in an increase in the number of scatterers per unit volume. Therefore, an increase in the estimated number density (as reflected in both EAC and μ parameter estimates) is consistent with an increased proliferation of cells observed in histology for the cancerous cases.
High-frequency QUS was required because the size of the mouse thyroids were small (i.e., only a few millimeters in size) and sufficient signal samples were needed to provide estimates of QUS parameters. The QUS parameters estimated from ultrasound signals over the frequency range from 25 to 45 MHz produced contrast that allowed the differentiation of malignant thyroids from normal thyroids or thyroids with benign growths. The high frequency ultrasound used in these studies may have enabled a greater sensitivity to the microstructural changes occurring in the thyroid cancer compared to the normal thyroids. These microstructural changes were evident in the histological slides of the different thyroids examined. Whether or not this contrast exists at more clinical ultrasound frequency ranges (1 to 20 MHz) has yet to be determined. However, because of the location of the thyroid gland in humans, it may be possible to use ultrasound signals at the higher end of the clinical ultrasound frequencies (~ 20 MHz). Furthermore, the contrast in QUS estimates detected in the mouse models of thyroid cancer may not translate into contrast in humans. Therefore, additional studies to quantify the QUS contrast in thyroid nodules of humans are warranted.
Another difference between scanning in humans and the mouse thyroid samples used in this study is the use of excised tissue samples. The main objective of this study was to determine if significant differences between QUS parameters of normal and diseased thyroid tissues existed. Therefore, it was decided to scan the thyroid glands ex vivo in order to eliminate effects caused by intervening tissues (i.e., heterogeneous attenuation profiles, aberration, clutter, blood flow). In humans the thyroid nodules would be examined in vivo and therefore the impact of the aforementioned effects on the accuracy and precision of the QUS estimates would need to be assessed.
Essentially, the RF backscattered signals from a whole thyroid lobe were used to produce an average QUS estimate for that particular thyroid sample. The justification for doing this was that histologically the cancer in the thyroids appeared to be diffuse throughout most of the gland, as observed in Fig. 1. The diffuse nature of the cancer in the diseased mouse thyroid may be partially attributable to the procedures used to monitor the progression of the disease. Mice were taken for QUS scanning and histology after the thyroids were enlarged based on their sonographic appearance using the Vevo 2100 scanner. If thyroids were excised at earlier time points, then it may be possible that the cancer would have been less diffuse throughout the glands. On the other hand, it is still highly unlikely that for the cancerous cases the whole thyroid lobes were affected by the malignancy. This could potentially increase the variance of estimates coming from the malignant thyroids depending on how diffuse the disease was at the time of scanning. In humans with much larger thyroids and the potential to observe thyroid nodules, it may be possible to select signals only from suspicious thyroid lesions thereby reducing the variance of QUS estimates and improving diagnostics.
This study has provided the first ever QUS results demonstrating significant contrast in thyroids having cancer and normal thyroids. However, as a preliminary study, many questions remain to be answered. Specifically, future work will look at determining the QUS contrast in human thyroids at more clinical frequency ranges, more detailed modeling of thyroid nodules to correlate structural changes to QUS parameter estimates, and providing QUS estimates from in vivo samples as opposed to excised samples. In addition, future studies should also include the estimation of attenuation from thyroid nodules to determine if attenuation can also be used as a discriminating property.
Figure 2.
High power view (40×) of: (Top left) Normal thyroid, where the round to oval follicles contain colloid material and benign fatty tissue separates groups of follicles; (top right) C-cell neoplasm; (bottom left) papillary thyroid carcinoma; and (bottom right) papillary thyroid carcinoma showing follicular pattern. The scale bar is 50 µm.
Acknowledgements
The authors would like to thank Rita Miller and Emily Hartman for their assistance in animal handling and scanning. This work was supported by a grant from NIH (R21 CA139095) and grants from Pontificia Universidad Católica del Perú (DGI2010-0105 and DGI2012-0149).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja AT, Metreweli C. Ultrasound of thyroid nodules. Ultrasound Quart. 2000;16:111–121. [Google Scholar]
- Ahuja A, Ying M. An overview of neck node sonography. Invest. Radiol. 2002;37:333–342. doi: 10.1097/00004424-200206000-00005. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2012. Atlanta, Ga: American Cancer Society; 2012. [Google Scholar]
- Brander AEE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000;215:801–806. doi: 10.1148/radiology.215.3.r00jn07801. [DOI] [PubMed] [Google Scholar]
- Catherine S, Maria L, Aristides A, Lambros V. Quantitative image analysis in sonograms of the thyroid gland. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2006 Dec;vol. 569(no. 2):606–609. [Google Scholar]
- Chan BY, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB., Jr Common and uncommon sonographic features of papillary thyroid carcinoma. J. Ultrasound Med. 2003;22:1083–1090. doi: 10.7863/jum.2003.22.10.1083. [DOI] [PubMed] [Google Scholar]
- Chen X, Phillips D, Schwarz KQ, Mottley JG, Parker KJ. The measurement of backscatter coefficient from a broadband pulse-echo system: a new formulation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1997;44:515–525. doi: 10.1109/58.585136. [DOI] [PubMed] [Google Scholar]
- Chidiac RM, Aron DC. Epidemiology and clinical decision making. Endocrin. Metab. Clin. 1997;26:233–254. doi: 10.1016/s0889-8529(05)70242-5. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver, Pacini F, Schlumberger M, Sherman SI, Steward DL, Turtle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;vol. 19(no. 11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Pathologic Basis of Disease. 6th Ed. Philadelphia, PA: W.B. Saunders Company; 1999. [Google Scholar]
- Destrempes F, Cloutier G. A critical review and uniformized representation of statistical distributions modeling the ultrasound echo envelope. Ultrasound in Medicine and Biology. 2010;vol. 36(no. 7):1037–1051. doi: 10.1016/j.ultrasmedbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Feleppa EJ, Liu T, Kalisz A, Shao MC, Fleshner N, Reuter V, Fair WR. Ultrasonic spectral-parameter imaging of the prostate. Int J Imaging Syst Technol. 1997;8(1):11–25. [Google Scholar]
- Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules. J. Ultrasound Med. 2003;22:127–131. doi: 10.7863/jum.2003.22.2.127. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Taniguchi N, Itoh K, Omoto K. Attenuation coefficient measurement in the thyroid. J. Ultrasound Med. 2003;22:1067–1073. doi: 10.7863/jum.2003.22.10.1067. [DOI] [PubMed] [Google Scholar]
- Ghoshal G, Lavarello RJ, Kemmerer JP, Miller RJ, Oelze ML. Ex vivo study of quantitative ultrasound parameters in fatty rabbit livers. Ultrasound Med. Biol. 2012 doi: 10.1016/j.ultrasmedbio.2012.08.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska DP, Oelze ML. Improved Parameter Estimates Based on the Homodyned K Distribution. IEEE Trans Ultrason, Ferroelec, Freq Contr. 2009;56:2471–2481. doi: 10.1109/TUFFC.2009.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska DP. Master’s Thesis. Vol. 29 University of Illinois at Urbana-Champaign; 2009. Improved techniques for statistical analysis of the envelope of backscattered ultrasound using the Homodyned K Distribution. [Google Scholar]
- Insana MF, Wagner RF, Brown DG, Hall TJ. Describing small-scale structure in random media using pulse-echo ultrasound. J Acoust Soc Am. 1990;87:179–192. doi: 10.1121/1.399283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Nikiforov YE, Fagin JA. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, Yamashita H. Ultrasonographic characteristics of thyroid nodules. Arch. Surg. 2002;136:334–337. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- Lannuccilli JD, Cronana JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria. J. Ultrasound Med. 2004;23:1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- Lavarello R, Ghoshal G, Oelze ML. On the Estimation of Backscatter Coefficients Using Single-Element Focused Transducers. J. Acoust. Soc. Amer. 2011;129:2903–2911. doi: 10.1121/1.3557036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinung MC, Gianoukakis A, Lee DW. Letter to the editor: Ultrasonography in management of nodular thyroid disease. Ann. Int. Med. 2001;135:383. doi: 10.7326/0003-4819-135-5-200109040-00023. [DOI] [PubMed] [Google Scholar]
- Lewis BD, Charboneau JW, Reading CC. Ultrasound-guided biopsy and ablation in the neck. Ultra. Quart. 2002;18:3–12. doi: 10.1097/00013644-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Liebeskind A, Sikora AG, Komisar A, Slavit D, Fried K. Rates of malignancy in incidentally discovered thyroid nodules evaluated with sonography and fine-needle aspiration. J. Ultrasound Med. 2005;24:629–634. doi: 10.7863/jum.2005.24.5.629. [DOI] [PubMed] [Google Scholar]
- Lizzi FL, Astor M, Liu T, Deng C, Coleman DJ, Silverman RH. Ultrasonic spectrum analysis for tissue assays and therapy evaluation. International Journal of Imaging Systems and Technology. 1997;8(1):3–10. [Google Scholar]
- Mamou J, Coron A, Oelze ML, Saegusa-Beecroft E, Hata M, Lee P, Machi J, Yanagihara E, Laugier P, Feleppa EJ. Three-Dimensional High-Frequency Backscatter and Envelope Quantification of Cancerous Human Lymph Nodes. Ultrasound Med Biol. 2011;37:345–357. doi: 10.1016/j.ultrasmedbio.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, Mandel SJ. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann. Inter. Med. 2000;133:696–700. doi: 10.7326/0003-4819-133-9-200011070-00011. [DOI] [PubMed] [Google Scholar]
- McFarlin BL, Bigelow TA, Labyed Y, O'Brien WD, Jr, Oelze ML. Ultrasonic Attenuation Estimation of the Pregnant Cervix: A Preliminary Report. Ultrasound Obstet Gyn. 2010;36:218–225. doi: 10.1002/uog.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirilas P, Skandalakis JE. Benign anatomical mistakes: incidentaloma. Am Surg. 2002;68:1026–1028. [PubMed] [Google Scholar]
- Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Benign and malignant thyroid nodules: US differentiation – multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- Oelze ML, Zachary JF, O'Brien WD., Jr Characterization of Tissue Microstructure using Ultrasonic Backscatter: Theory and Technique Optimization using a Gaussian Form Factor. J Acoust Soc Amer. 2002;112:1202–1211. doi: 10.1121/1.1501278. [DOI] [PubMed] [Google Scholar]
- Oelze ML, O’Brien WD, Jr, Blue JP, Zachary JF. Differentiation and characterization of rat mammary fibroadenomas and 4T1 mouse carcinomas using quantitative ultrasound imaging. IEEE Trans Med Imag. 2004;23:764–771. doi: 10.1109/tmi.2004.826953. [DOI] [PubMed] [Google Scholar]
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J. Clin. Endo. & ZMetab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- Rajendra Acharya U, Vinitha Sree S, Muthu Rama Krishnan M, Molinari F, Garberoglio R, Suri JS. Non-invasive automated 3D thyroid lesion classification in ultrasound: A class of ThyroScan systems. Ultrasonics. 2012 Apr;vol. 52(no. 4):508–520. doi: 10.1016/j.ultras.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Rago T, Scutari M, Santini F, Loiacono V, Piaggi D, Di Coscio G, Basolo F, Berti P, Pinchera A, Vitti P. Real-time elastosonography: Useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J. Clin. Endocrin. Metab. 2010;95:5274–5280. doi: 10.1210/jc.2010-0901. [DOI] [PubMed] [Google Scholar]
- Ross DS. Editorial: nonpalpable thyroid nodules – managing an epidemic. J. Clin. Endocrin. &. Metab. 2002;87:1938–1940. doi: 10.1210/jcem.87.5.8552. [DOI] [PubMed] [Google Scholar]
- Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, Oliver C. Shear wave elastography: a new ultrasound imaging mode for differential diagnosis of benign and malignant thyroid nodules. J Clin. Endocrin. Metab. 2010;95:5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- Silver RJ, Parangi S. Management of thyroid incidentalomas. Surg. Clin. N. Am. 2004;84:907–919. doi: 10.1016/j.suc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Silverman RH, Folberg R, Rondeau MJ, Boldt HC, Lloyd HO, Chen X, Lizzi FL, Weingeist TA, Coleman DJ. Spectral parameter imaging for detection of prognostically significant histologic features in uveal melanoma. Ultrasound Med Biol. 2003;29(7):951–959. doi: 10.1016/s0301-5629(03)00907-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng S. Mice with a mutation in thyroid hormone receptor β gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann. Intern. Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- Vorlander C, Wolff J, Saalabian S, Lienenluke RH, Wahl RA. Real-time ultrasound elastography – a noninvasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbecks Arch. Surg. 2010;395:865–871. doi: 10.1007/s00423-010-0685-3. [DOI] [PubMed] [Google Scholar]
- Wilson T, Chen Q, Zagzebski JA, Varghese T, VanMiddlesworth L. Initial Clinical Experience Imaging Scatterer Size and Strain in Thyroid Nodules. J Ultrasound Med. 2006;25:1021–1029. doi: 10.7863/jum.2006.25.8.1021. [DOI] [PubMed] [Google Scholar]