Abstract
The Syrian Cardiomyopathic Hamster (BIO-14.6/53.58 strains) model of cardiac failure, resulting from naturally occurring deletion at the SGCD (delta-sarcoglycan) locus, displays widespread disturbances in catecholamine metabolism. Rare Mendelian myopathy disorders of human SGCD occur, though common naturally occurring SGCD genetic variation has not been evaluated for effects on human norepinephrine (NE) secretion. This study investigated the effect of SGCD genetic variation on control of NE secretion in healthy twin pairs. Genetic associations profiled SNPs across the SGCD locus. Trait heritability (h2) and genetic covariance (pleiotropy; shared h2) were evaluated. Sympathochromaffin exocytosis in vivo was probed in plasma by both catecholamines and CHGB. Plasma NE is substantially heritable (P=3.19E-16, at 65.2±5.0% of trait variance), sharing significant (P<0.05) genetic determination with circulating and urinary catecholamines, CHGB, eGFR and several cardio-metabolic traits. Participants with higher pNE showed significant (P<0.05) differences in several traits, including increased BP and hypertension risk factors. Peak SGCD variant rs1835919 predicted elevated systemic vascular compliance, without changes in specifically myocardial traits. We used a chimeric regulated secretory pathway photoprotein (CHGA-EAP) to evaluate the effect of SGCD on the exocytotic pathway in transfected PC12 cells; in transfected cells, expression of SGCD augmented CHGA trafficking into the exocytotic regulated secretory pathway. Thus our investigation determined human NE secretion to be a highly heritable trait, influenced by common genetic variation within the SGCD locus. Circulating NE aggregates with BP and hypertension risk factors. Additionally, coordinate NE and CHGB elevation by rs1835919 implicates exocytosis as the mechanism of release.
Keywords: Norepinephrine, catecholamine, delta-sarcoglycan, secretion, cytoskeleton
INTRODUCTION
The sympathetic neurotransmitter norepinephrine (NE, noradrenaline) plays an essential role in circulatory homeostasis, with actions on both the heart and vessels (Parati & Esler 2012). Using plasma concentration as a rough index of sympathetic activity, investigators have shown NE secretion governs diverse metabolic and hemodynamic pathways.
In congestive heart failure, sympathetic indexes are systematically elevated as a function of severity, including steady-state plasma NE (pNE) concentration, with increased NE spillover from sympathetic terminals, especially cardiac and renal spillover (Hasking et al. 1986), as well as epinephrine release from the adrenal medulla, and finally decreased plasma clearance of the amines.
The Syrian Cardiomyopathic Hamster (SCH; BIO 14.6 and 53.58 strains) is a widely studied experimental model of hereditary hypertrophic cardiomyopathy progressing to heart failure and its associated autonomic dysfunction. These animals are characterized by substantially increased catecholamine secretion, turnover, and elevated plasma concentrations (Yamada et al. 1997) in the face of congestive heart failure. Such phenotypic traits of the SCH begin relatively early in life, from 40 days of age onward, then increasing until death or sacrifice. Literature on the SCH model is substantial.
Italian (Nigro et al. 1997) and Japanese (Okazaki et al. 1996) investigators demonstrated that the cardiomyopathic traits of the SCH emerge from a single deletion mutation across the promoter and exon-1 in the delta-sarcoglycan (SGCD) locus, effectively deleting expression of SGCD mRNA and protein from myocytes (Escobales & Crespo 2008, Nigro et al. 1997). Thus, the SCH constitutes a Mendelian model for heart failure and its associated disorders of catecholamine storage and release (Escobales & Crespo 2008, Nigro et al. 1997, Sole et al. 1975, Vainzof et al. 2008, Yamada et al. 1997). Targeted ablation of the Sgcd gene in the mouse results in cardiomyopathy (Coral-Vazquez et al. 1999), while inactivating mutations in human SGCD, on chromosome 5q33, have been associated with autosomal recessive limb-girdle muscular dystrophy or dilated cardiomyopathy <http://omim.org/entry/601411>.
Thus profound inactivating mutations of SGCD eventuate in cardiomyopathy and disordered sympathetic activity, in both animal models and humans. However, subtle naturally occurring alterations (i.e., common allelic variants) of SGCD have not been evaluated for effects upon NE secretion or cardiac function. Here we coupled autonomic phenotyping with extensive genotyping at the SGCD locus, to probe potential involvement of SGCD in control of NE secretion in a series of healthy twin pairs and siblings. We found that NE secretion is a heritable trait influenced by common genetic variation within SGCD.
MATERIALS AND METHODS
Twin and sibling subjects
The UCSD twin/sibling study has previously been described (Davis et al. 2012). Twin and sibling participants were recruited from southern California by access to a population birth record-based twin registry (Cockburn et al. 2002), as well as by newspaper advertisement, as described previously (Zhang et al. 2004). The protocol was approved by the UCSD Human Research Protection Program, and each subject gave written informed consent prior to participation. Subjects included dizygotic (DZ) and monozygotic (MZ) twin pairs. Zygosity of twins was confirmed genetically by microsatellite and single nucleotide polymorphisms (SNP) markers (Zhang et al. 2004). Initially ethnicity was established by self-identification, including information on geographic origin of both parents and all four grandparents, and only individuals of Caucasian or Hispanic (Mexican-American) ancestry/ethnicity were included here. The age of the subjects ranged from 14 to 78 years. Phenotyping (biochemical and physiological) was conducted as previously described (Zhang et al. 2004). Blood pressure (BP) status (high vs. normal) was defined by history (medical record or self-report), presence of antihypertensive medications, and measurement of seated BP by arm cuff (hypertension: either/or ≥140/≥90 mmHg systolic BP (SBP)/diastolic BP (DBP), or both). None of the subjects had a history of cardiovascular disease or renal failure, and serum creatinine concentrations were ≤1.5 mg/dl.
Twin/sibling physiological phenotyping
Brachial cuff BPs with SBP/DBP measured as K1/K4, heart rate, cardiac output, stroke volume, systemic vascular resistance (SVR), and systemic vascular compliance (SVC) were obtained noninvasively in seated subjects in triplicate using an oscillometric device (DynaPulse; PulseMetric Inc., San Diego, CA) as described (Davis et al. 2012). Triplicate values (within ±10% of individual's mean) were averaged. We and others previously validated DynaPulse measurements against more invasive devices (Brinton et al. 1996, Davis et al. 2012).
Biochemical phenotyping
Subjects were instructed to fast during the 6 h preceding the evaluation. Fasting blood and urine samples were collected from each subject. Plasma and urine samples were quickly frozen to −70°C, in preparation for catecholamine concentration assessment. A sensitive radioenzymatic assay was used to measure plasma and urine catecholamines (dopamine (DA), NE, and epinephrine (EPI)) through the catechol-O-methylation process (Ziegler et al. 1988). The radioenzymatic assay for catecholamines involved transfer of a 3H label to catecholamines from S-adenosylmethionine during O-methylation, mediated by the enzyme catechol-O-methyltransferase (COMT). Prior to O-methylation, plasma catecholamines were extracted into dilute acetic acid to remove COMT inhibitors in plasma. Assay sensitivities (lower limits of detection) were 10 pg for NE and 6 pg for EPI. Intra-assay coefficients of variation were 4% for NE and 13% for EPI, while inter-assay coefficients of variation were 10% for NE and 13% for EPI. Urine catecholamine values were normalized to the level of creatinine excretion of the subject's same sample. Plasma lipids were measured as previously described (Wessel et al. 2007). Plasma insulin, leptin, and renin were measured by immunoassay. Plasma glucose and creatinine were measured by autoanalyzer (Beckman-Coulter; Brea, CA).
Heritability(h2)
Estimates of heritability (h2 = VG/VP, where VG is additive genetic variance and VP is total phenotypic variance) were obtained with variance-component methods implemented in Sequential Oligogenic Linkage Analysis Routines (SOLAR) (Almasy & Blangero 1998), available at <http://solar.txbiomedgenetics.org>. This method maximizes the likelihood assuming a normal distribution of phenotypes in twin pairs (MZ versus DZ). The null hypothesis (H0 of h2=0) is tested by comparing the full model, which assumes non-zero genetic variation (VG), and a reduced model, which assumes no genetic variation, by a likelihood ratio test. SOLAR was also used to evaluate whether allelic variation at the locus contributed to a significant fraction of the trait heritability (i.e., locus-specific VG) by comparing models including or excluding the genotype as a covariate. Heritability estimates were adjusted for age and sex, since these covariates affected several traits.
Pleiotropy
Pleiotropy (genetic covariance for two correlated, heritable traits) was estimated as the parameter RhoG (ρG) in SOLAR, as described (Wessel et al. 2007). SOLAR was also used to estimate the environmental covariance, as parameter RhoE (ρE).
Genotyping
Genomic DNA was isolated from blood leukocytes on Qiagen columns, after proteinase K digestion of protein, as previously described (Zhang et al. 2004). SNP genotyping across the SGCD locus was accomplished using the Illumina 610-Quad genotyping array. Results across the locus, centered on the peak association at rs1835919, were displayed by local SNAP (SNP Annotation and Proxy Search) plot as described at <http://www.broadinstitute.org/mpg/snap/ldplot.php>. Marker-on-trait significance was estimated using the variance components software MERLIN <http://www.sph.umich.edu/csg/abecasis/Merlin/>, which accounts for intra-twin-pair correlations. Analyses were adjusted by age and sex. Marker-on-trait significance was also estimated by GEE (generalized estimating equations).
Calculations
Body surface area (BSA, in m2), as a measure of overall body size, was estimated by the Mosteller formula <http://www.halls.md/body-surface-area/refs.htm>. Glomerular filtration rate (GFR) was estimated from ethnicity, sex, and plasma creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) algorithm (Levey et al. 2009). Endogenous insulin sensitivity (or resistance) was estimated from fasting plasma glucose and insulin values by QUICKI (QUantitative Insulin sensitivity ChecK Index; an index of insulin sensitivity), calculated as previously described (Lee et al. 2004).
Statistical analyses
Descriptive and inferential statistics (mean ± SEM) were computed by GEE using IBM SPSS predictive analytics software, to account for intra-twin-pair correlations. GEE also tested for allele and diploid genotype effects on traits. The SNP was defined as a 3-level variable representing the 3 possible genotypes (homozygous variant, heterozygous, and homozygous wild-type), initially assuming an additive effect in the model. Analyses were adjusted for age and sex. False Discovery Rate (FDR): When testing the effects of a gene on multiple correlated traits, we estimated the FDR, in order to minimize false negative results while maximizing true positive results, using the Excel calculator of FDR from a distribution of p-values, at <http://www.rowett.ac.uk/~gwh/fdr.html>.
Bioinformatics
We viewed the likely exon/intron structure for human SGCD, as well as putative promoter domains, at <http://genome.ucsc.edu>, focusing on long (~887 kbp) genomic clone uc0031wa.1 (in which the peak trait-associated region is in intron-4), or shorter RefSeq clone NM_000337 (~447 kbp), in which the peak trait-associated region is in the distal promoter, ~200 kbp upstream of exon-1. Transcription factor binding or epigenetic marks, documented by Chip-Seq in a variety of cell lines, were explored on the EnCode displays at <http://genome.ucsc.edu/ENCODE>. Transcription factor motif matches in the region were evaluated further on CONSITE (Sandelin et al. 2004) at <http://asp.ii.uib.no:8090/cgi-bin/CONSITE/consite>. Chromatin Conformation Capture (“Hi-C”) <http://chromosome.sdsc.edu/mouse/hi-c/index.html>, evaluated the boundaries of regions subject to local trans-interactions, based on interactions in human embryonic stem cells and human IMR90 fetal lung fibroblasts. Evidence for cis-QTLs (sometimes known as allelic expression imbalance) was generated on the eQTL browser at the Pritchard lab from the University of Chicago, linking transcriptome and GWAS human studies <http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/>.
Construction of expression plasmids
The pCMV (pcDNA6 vector) eukaryotic expression plasmid for a chromogranin A (CHGA) / secretory embryonic alkaline phosphatase (CHGA-EAP) chimera was prepared as previously described (Taupenot 2007, Taupenot et al. 2002). Plasmid constructs were verified by restriction digestion and nucleotide sequence analysis. The full-length human SGCD cDNA (SC309561) was obtained in a pCMV (pCMV6-XL vector) eukaryotic expression plasmid, from Origene <www.origene.com>.
Cell culture and transient transfections
PC12 cells were cultured in Ham's F12K medium supplemented with 15% heat-inactivated horse serum and 2.5% heat-inactivated fetal bovine serum (Gemini Bioproducts), streptomycin (100 g/ml), and penicillin (100 units/ml) (Invitrogen). Supercoiled plasmid DNA for transfection was grown in Escherichia coli strain DH5α (Invitrogen) and purified on columns (Qiagen). Two days before transfection, PC12 cells were split onto either poly-L-lysine (Sigma) plus collagen (Upstate)-coated 18-mm round glass #1.5 coverslips (Fisher) in 12-well Costar plates or onto poly-L-lysine-coated 6- or 12-well Costar plates. Cells were co-transfected with pCMV-CHGA-EAP and pCMV-SGCD at 1.25 μg (12-well plate) or 2 μg (6-well plate) of supercoiled plasmid DNA per well, using a high efficiency cationic scaffold method (GenePorter II, Gene Therapy Systems). Five hours after beginning the transfection, culture medium was replaced and cells were further cultured for 22 or 48 h.
Perturbation of secretory protein trafficking by SGCD: Chemiluminescent detection of secretory protein/embryonic alkaline phosphatase (EAP) chimera secretion by PC12 cells
The protocol describing the chemiluminescent assay used to detect secretagogue-stimulated secretion of CHGA-EAP from PC12 cells, co-transfected with pCMV-SGCD and pCMV-CHGA-EAP, was previously published (Taupenot 2007). Briefly, detection of EAP enzymatic activity release from CHGA–EAP chimera-expressing PC12 cells was achieved using the chemiluminescent substrate CSPD (Phospha-Light, Applied Biosystems, Foster City, California) in a Luminometer Autolumat 953 (EG&G Berthold). EAP activity was measured in the culture supernatant and cell lysate. Exocytotic secretion of the transfected/expressed EAP chimeras was provoked by the potent regulated pathway stimulus Ba2+ (2 mmol/L), which acts by blocking efflux of cellular K+ and hence cell membrane repolarization. The secretion rate was calculated from supernatant EAP activity as a percentage of total enzymatic activity (cell plus supernatant). The “sorting index” was calculated as a function of increase in secretion rate after stimulation of the regulated exocytotic pathway by Ba2+: [stimulated minus basal]/basal.
RESULTS
Role of heredity and pleiotropy in norepinephrine secretion
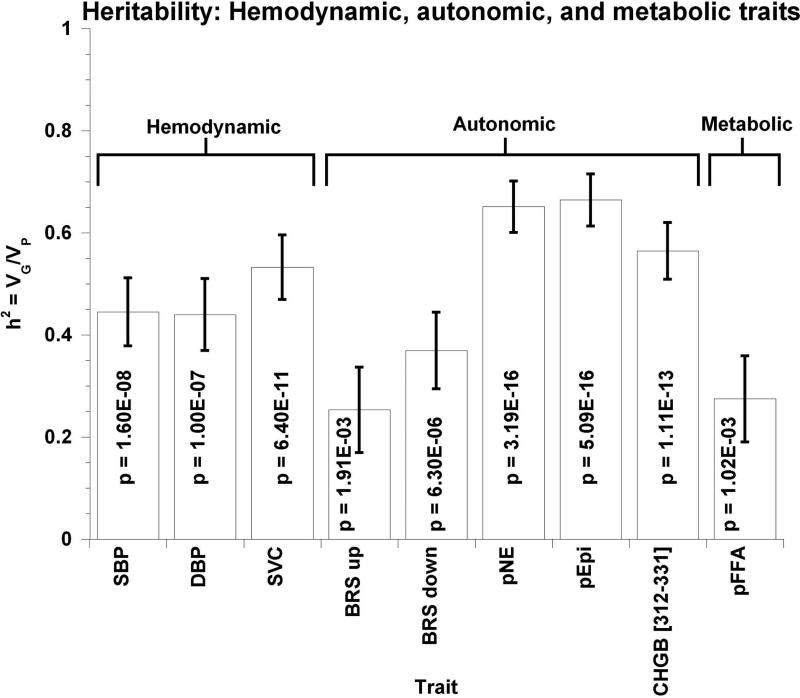
Table S1 presents heritability (h2) for each trait estimated from twin and sibling pair variance components, expressed as a % of trait variation (h2 scaled from 0 to 100%). Circulating NE heritability is also exhibited graphically, along with several cardio-metabolic traits, in Figure 1a. Heritability of NE secretion was substantial (P=3.19E-16), at 65.2±5.0% of trait variance. Heritability was also substantial for cardio-metabolic traits listed in Table S1, including BP and cardiovascular risk factors, as well as physical (i.e., body mass index (BMI)) and a number of biochemical (e.g., catecholamine) traits.
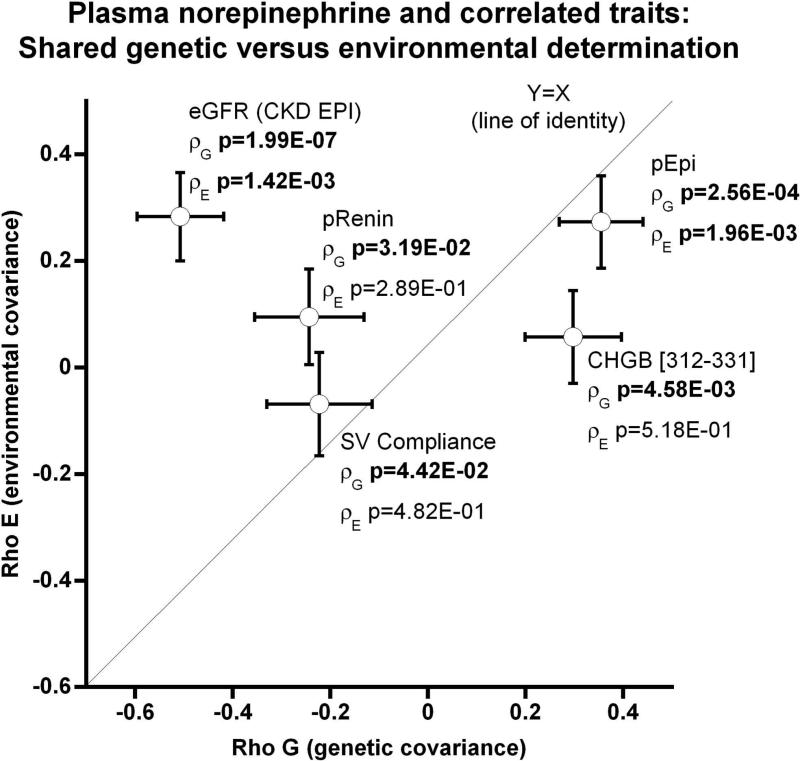
Figure 1. Trait determination: Relative roles of heredity and environment, estimated by variance components in twin pairs.
Results are shown as the mean ± SEM for traits. Significant differences (P<0.05) are bold. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; SVC/SV Compliance, systemic vascular compliance; BRS up, baroreceptor slope upward deflection, BRS down, baroreceptor slop downward deflection; pEPI, plasma epinephrine; CHGB, chomogranin B; pFFA, plasma free fatty acids; eGFR (CKD-EPI), estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration algorithm; pRenin, plasma active renin.
(a) Heritability (h2) of hemodynamic, autonomic, and metabolic traits.
(b) Genetic pleiotropy for plasma norepinephrine (pNE) with correlated traits, plotting RhoG (genetic covariance) as a function of RhoE (environmental covariance). The diagonal line is the theoretical line of identity (Y = X).
Correlations (Pearson) of NE secretion with other specific traits are also shown in Table S1. Hemodynamic/autonomic and biochemical traits displayed several correlating phenotypes with pNE. Correlations of significance were found for a variety of traits, including SBP and DBP, vascular compliance and resistance, baroreceptor slope (during both upward and downward BP deflections), circulating and urinary catecholamines, CHGB312-331, active renin, free fatty acids (pFFA), and estimated GFR (eGFR, by CKD-EPI algorithm).
Heritability varied among traits correlating with circulating NE (Table S1); we then examined these correlated traits for shared genetic determination (pleiotropy, RhoG) with pNE (Table S1, Figure 1b). Results indicate that pNE shares significant genetic determination (RhoG) with BMI, SVC, SVR, circulating and urinary catecholamines, CHGB312-331, eGFR, and plasma active renin. By contrast, several traits shared significant environmental determination (environmental codetermination, RhoE) with pNE: DBP, circulating and urinary catecholamines, pFFA, and eGFR. Circulating and urinary catecholamines and eGFR traits displayed both shared hereditary and environmental co-determination with pNE.
Figure 1b displays the shared hereditary co-determination, without significant environmental co-determination, for circulating NE with plasma EPI and CHGB312-331. Environmental co-determination, without significant shared hereditary co-determination, was noted for eGFR (by CKD-EPI algorithm), plasma active renin, and SVC, as displayed in Figure 1b.
Trait aggregation with norepinephrine secretion: Norepinephrine quantiles
Table S2 provides a description of the twin/sibling cohort, divided into 2 groups (quantiles, upper and lower) stratified around the pNE median. Plasma NE increased with age and was higher in men than women. Participants with higher pNE showed significant (P<0.05) differences across several traits, including increased BP levels and hypertension risk factors: baroreceptor slope, catecholamines, pulse pressure, plasma insulin, pFFA, total cholesterol, triglycerides, plasma neuropeptide Y (pNPY), and eGFR (by CKD-EPI algorithm). However, subjects with higher pNE values did not show evidence of intrinsic cardiac pathology. Several cardiac traits, including cardiac output, cardiac index, stroke volume, and stroke volume index, were not affected by pNE level increases.
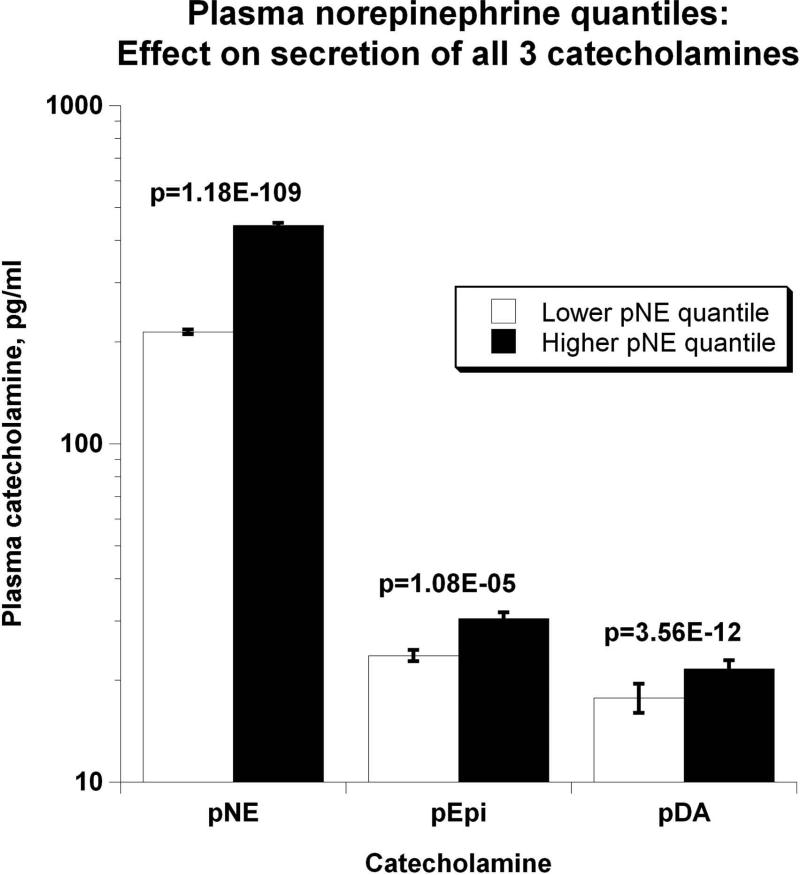
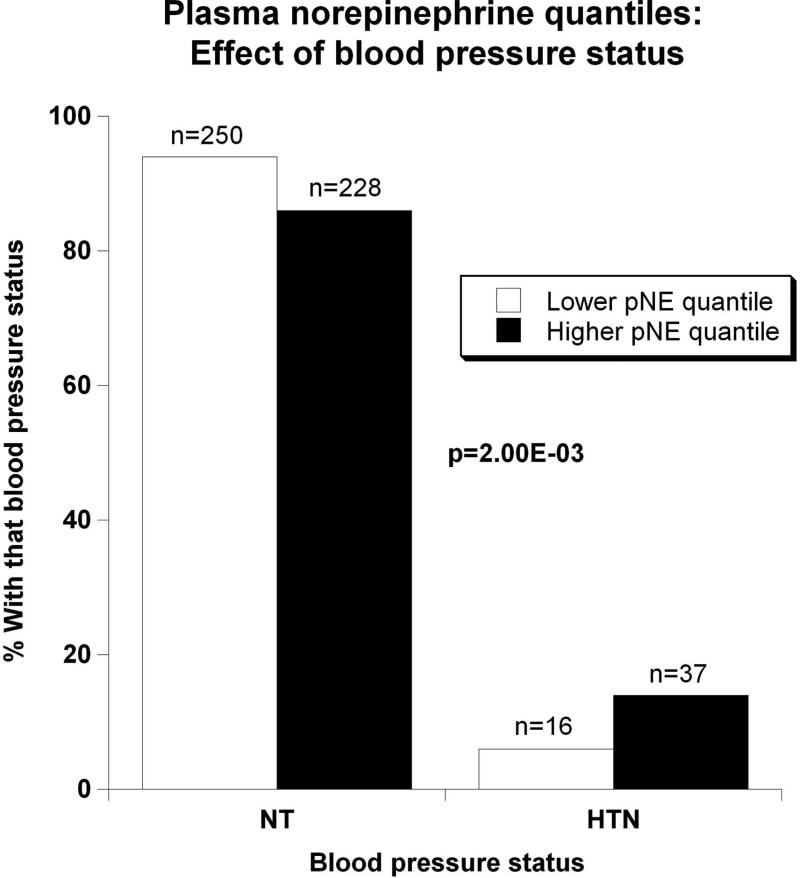
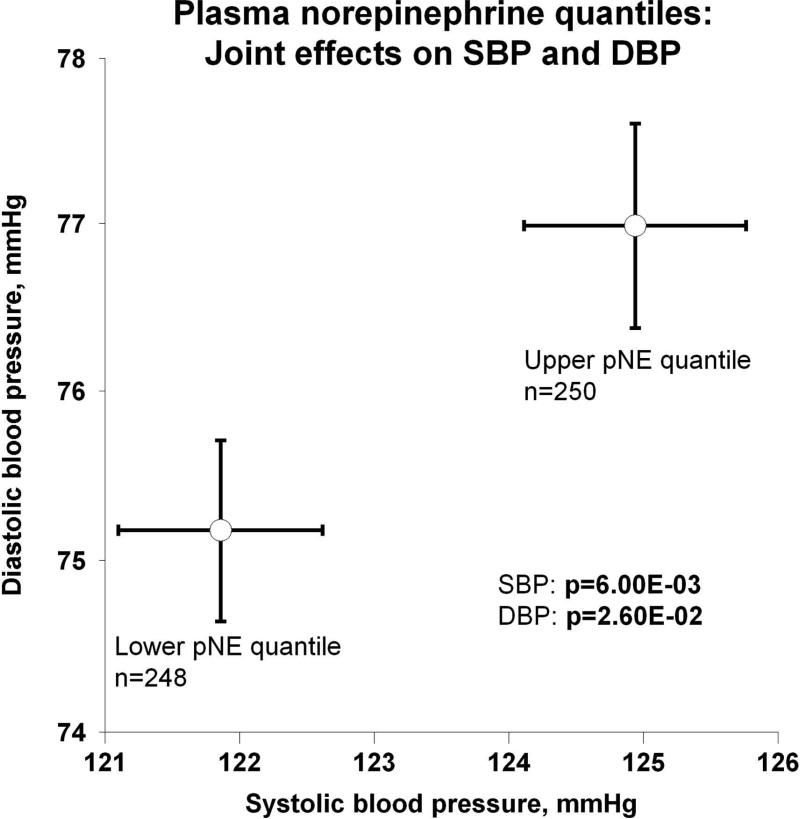
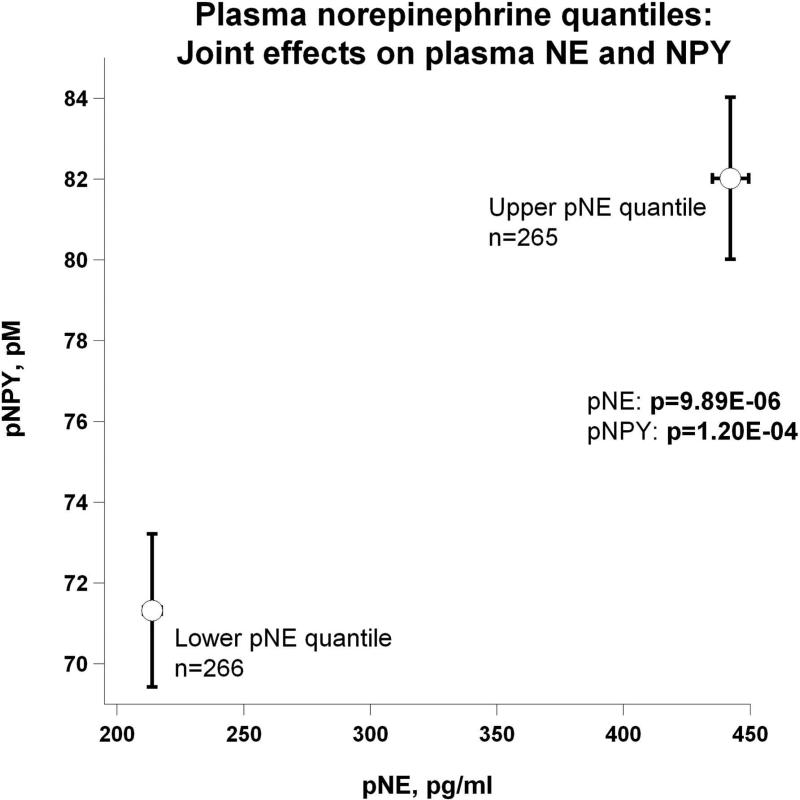
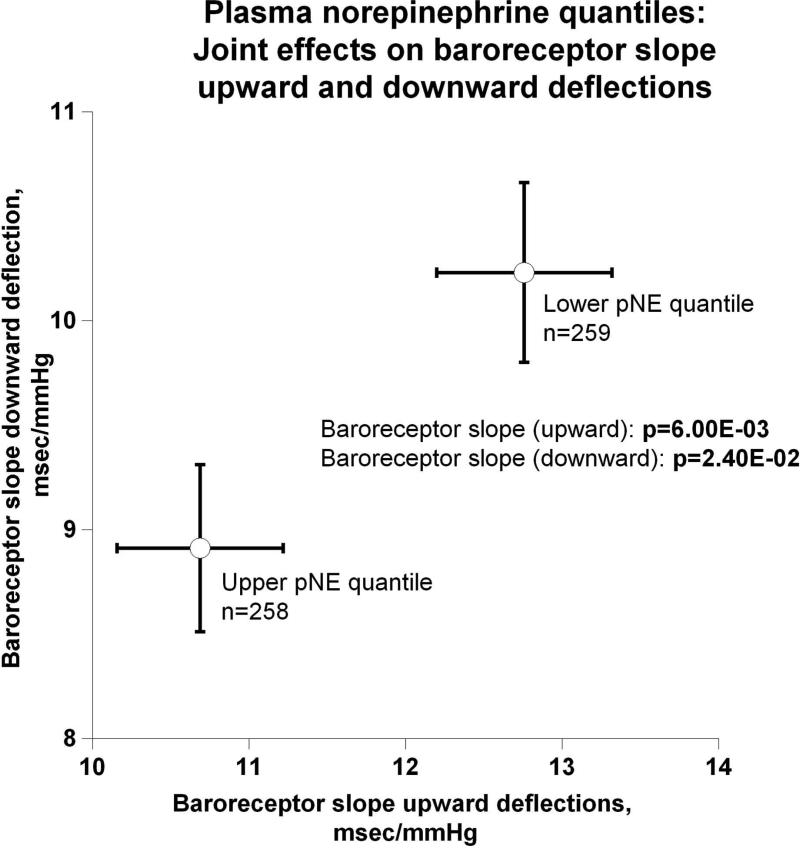
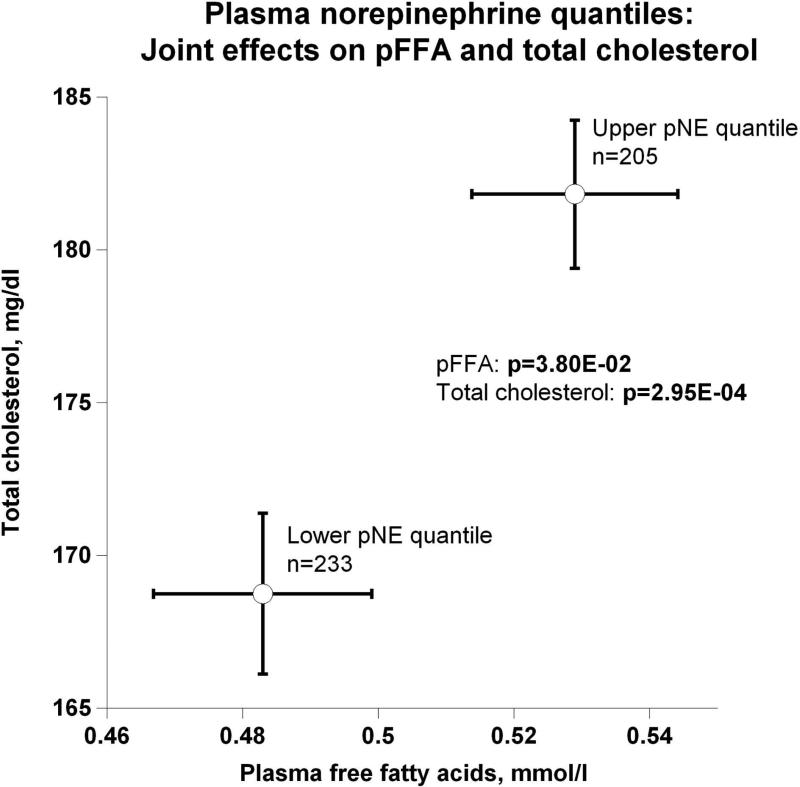
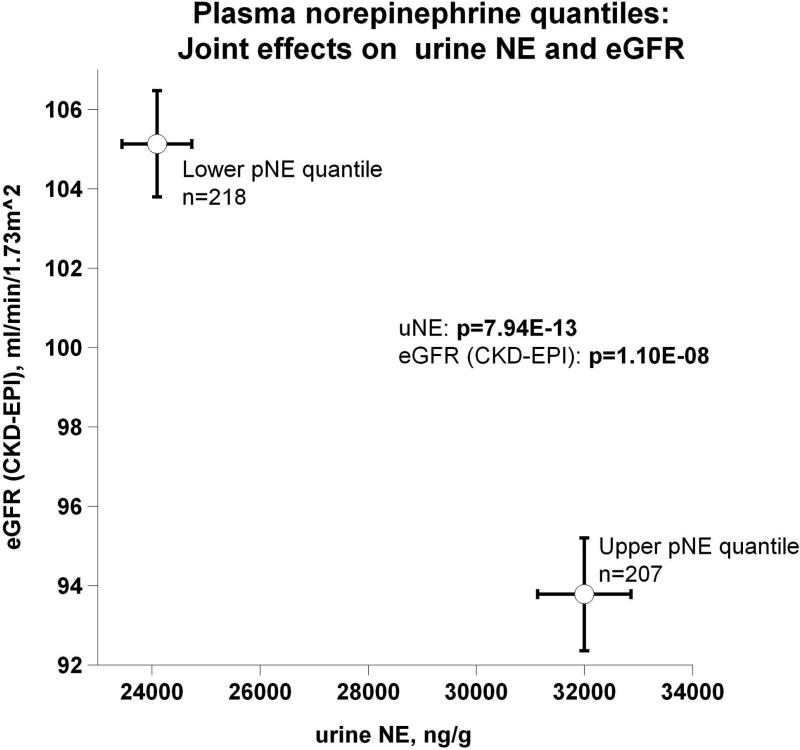
Evidence showing significant aggregation of other secreted catecholamines (EPI and DA) with increased circulation of NE is found in Figure 2a. Correspondingly, Figure 2b displays pNE effects on BP of hypertensive versus normotensive subjects, with significant elevation in hypertension (P=2.00E-03). Figure 2c further shows the effects of pNE distribution for the two major BP characteristics: SBP and DBP. Figure 2d illustrates that pNE and pNPY increase coordinately. Baroreceptor slope (upward and downward deflections) aggregated with pNE (Figure 2e), such that elevated NE secretion was found in subjects with lower/impaired baroreflex function. Metabolic traits, pFFA and total cholesterol, also rose with pNE level (Figure 2f). Figure 2g demonstrates the direct correspondence of pNE with urinary excreted NE, though an inverse effect of pNE level on eGFR (by CKD-EPI algorithm).
Figure 2. Trait aggregation of plasma norepinephrine (pNE) with physiological traits.
Results (shown as mean ± SEM) were analyzed by independent sample t-test. Significant differences (P<0.05) are bold.
(a) Effects on the secretion of all three catecholamines pNE, epinephrine (pEPI), and dopamine (pDA);
(b) Effects on blood pressure status (NT indicates normotensive; HTN, hypertensive);
(c) Joint effects on systolic (SBP) and diastolic blood pressure (DBP);
(d) Joint effects on pNE and plasma neuropeptide Y (pNPY);
(e) Joint effects on baroreceptor slope upward and downward deflections;
(f) Joint effects on plasma free fatty acids (pFFA) and total cholesterol;
(g) Joint effects on urine norepinephrine (uNE) and estimated glomerular filtration (eGFR) rate (by CKD-EPI, Chronic Kidney Disease EPIdemiology collaboration algorithm).
Human SGCD genomic region and norepinephrine secretion
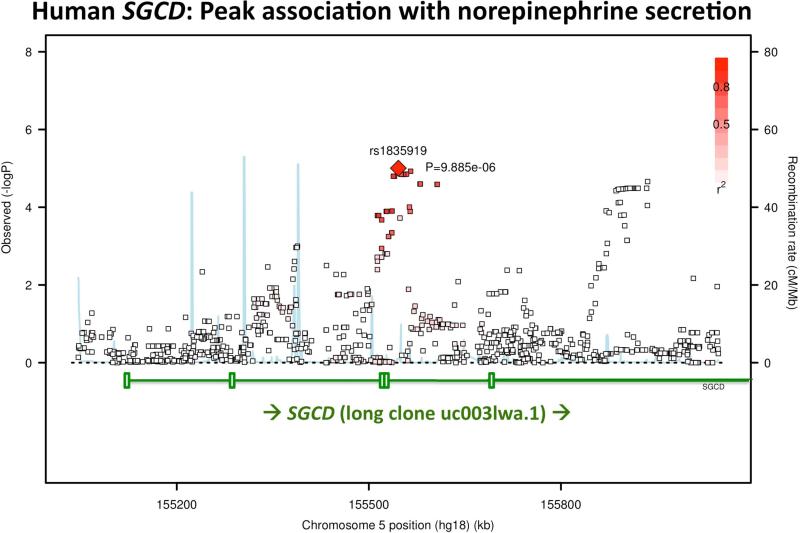
Genetic markers were typed across the local SGCD region on chromosome 5q33; a SNAP plot, displaying local significance for genetic determination of NE secretion, is displayed in Figure 3a. The ~100 kbp peak association occurred for SNP rs1835919 in the SGCD region, located in intron-4 of long genomic clone uc0031wa.1; alternatively, the same SNP peak is found in the distal promoter of RefSeq clone NM_000337, ~200 kbp upstream of exon-1.
Figure 3. Genetic determination of traits by variation at the delta-sarcoglycan (SGCD) locus: Effects upon norepinephrine (NE) secretion as well as other physical, physiological, and biochemical traits.
Results are shown as mean ± SEM. Significant differences (P<0.05) are bold.
(a) Peak effect in the SGCD local region on chromosome 5q33. Local region of the Manhattan plot displayed by SNAP (SNP Annotation and Proxy Search) plot <http://www.broadinstitute.org/mpg/snap/ldplot.php>. The peak associated with NE secretion is at SGCD (intron-4, rs1835919, in long clone uc0031wa.1).
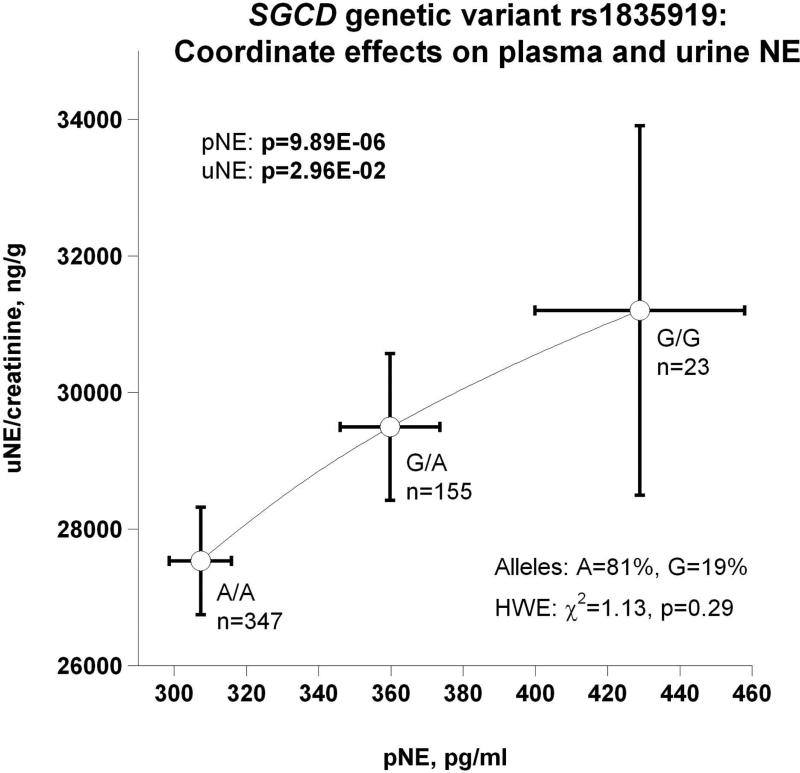
(b) Coordinate effects on plasma and urine NE (uNE indicates urine norepinephrine; uUA urine uric acid, pUA, plasma uric acid; associated allele frequency and Hardy-Weinberg equilibrium (HWE) test results displayed);
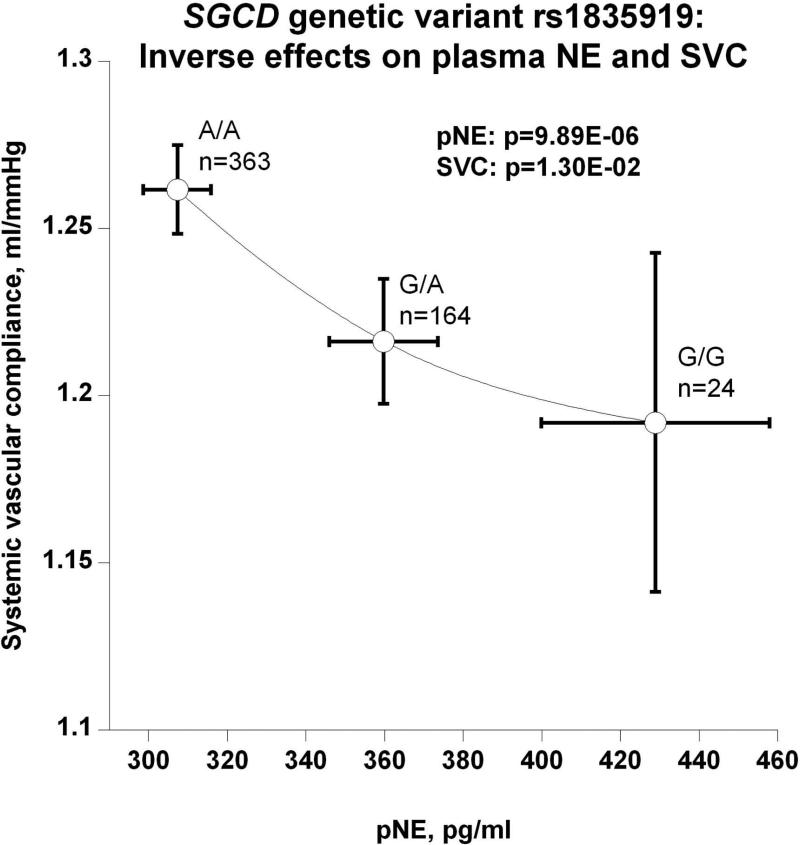
(c) Inverse effects on plasma NE (pNE) and systemic vascular compliance (SVC);
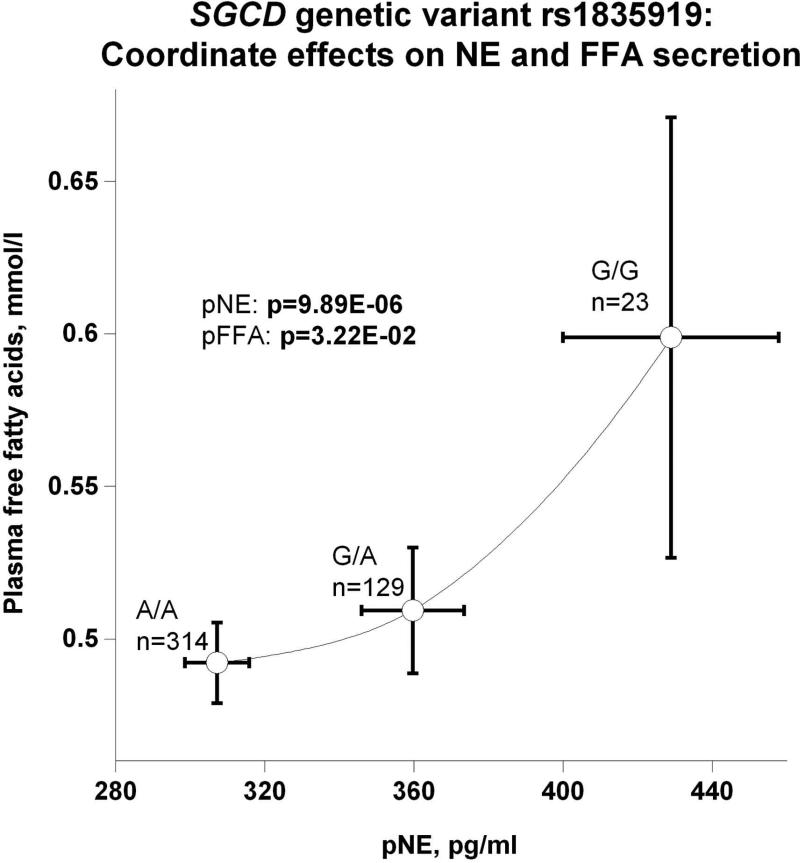
(d) Coordinate effects on pNE and plasma free fatty acids (pFFA) secretion;
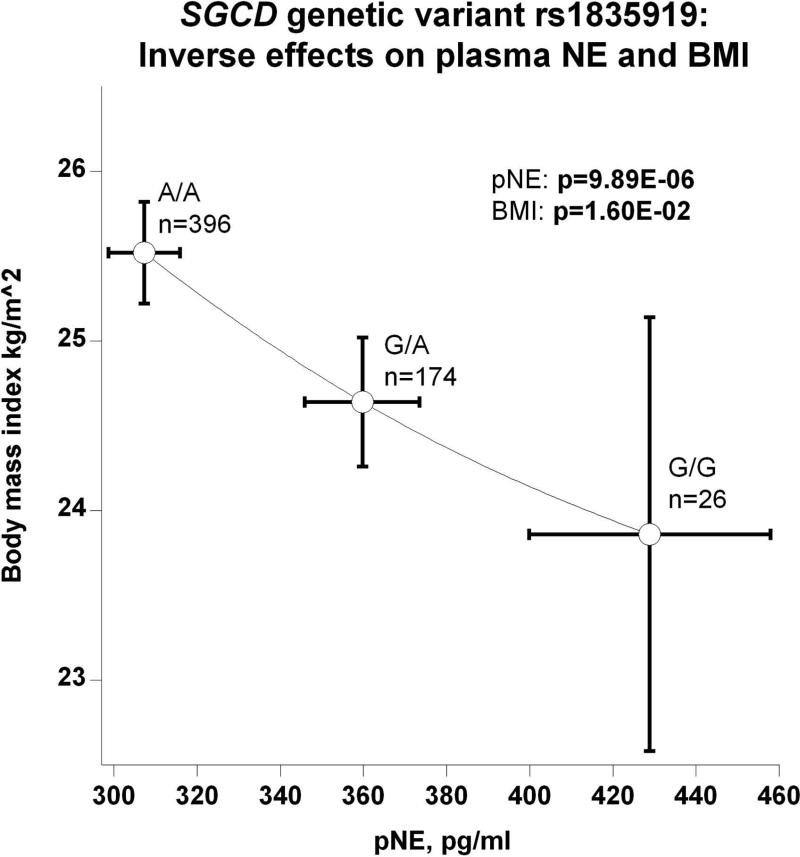
(e) Inverse effects on pNE and body mass index (BMI);
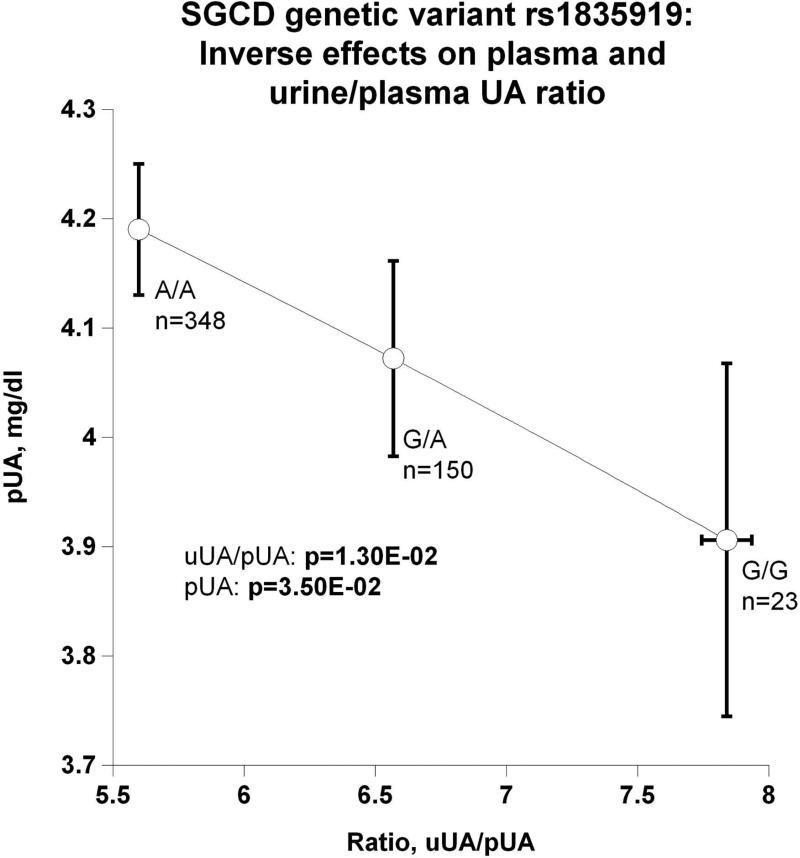
(f) Inverse effects on plasma and urine/plasma uric acid (UA) ratio;
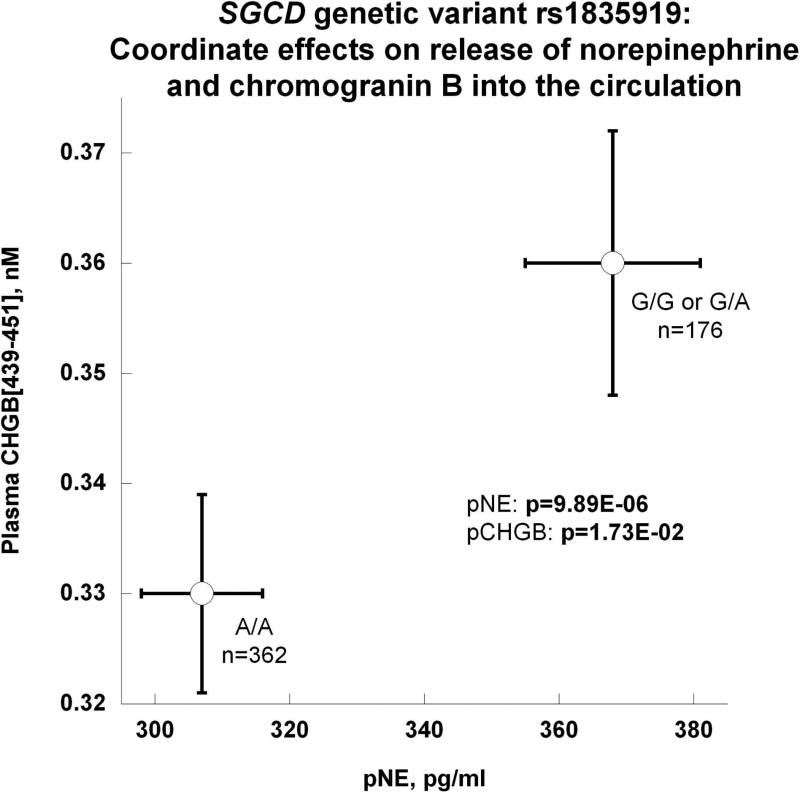
(g) Coordinate effects on release of NE and chromogranin B (CHGB [439-451]) into the circulation.
The minor (G) allele frequency of the peak trait-associated variant (rs1835919) was 19%, and the 3 diploid genotypes (A/A, A/G, G/G) were in Hardy-Weinberg equilibrium (χ2=1.13, p=0.29). The phenotypic effect of heterozygosity (A/G genotype) was intermediate between major (A/A) and minor (G/G) allele homozygotes, indicating that an additive genetic model was appropriate (see below).
Pleiotropic effects of SGCD genetic variation on multiple human traits
An additive genetic model for SGCD diploid genotype effects on traits demonstrated pleiotropic effects on multiple phenotypes (Table S3, Figure 3), and the effects were confirmed by FDR procedures. The variant/minor G allele, heterozygosity (G/A) or homozygosity (G/G), influenced several biochemical, physical or cardio-metabolic traits: BMI, brachial artery compliance (BAC), SVC, plasma CHGB439-451, pFFA, plasma and urinary (excreted) catecholamines, urine/plasma uric acid ratio, and uric acid in plasma (Table S3).
The SGCD peak genetic variant's (rs1835919) directionally coordinate effects on plasma and urine NE are depicted in Figure 3b. The most parsimonious explanation is that the minor allele G gives rise to higher circulating NE, which in turn is excreted, raising urine NE. However, genetic variation inversely affected pNE and SVC: as genotype shifted from major allele homozygotes (A/A) through A/G heterozygotes to G/G minor allele homozygotes, pNE increased while SVC decreased (Figure 3c), suggesting an adverse effect of NE on vascular stiffness. Also as a function of SGCD genotype, increased secretion of NE provoked increased circulating FFA (Figure 3d). Figures 3e and 3f display reciprocal trait effects of the SGCD genetic variant: with increased pNE, BMI decreases as shown in Figure 3e. Figure 3f indicates, as a function of SGCD genotype, urinary uric acid increases as plasma uric acid decreases. The most parsimonious explanation for this occurrence is that elevated NE increases renal tubular secretion of uric acid, in turn lowering plasma uric acid.
The G (minor) allele (by either heterozygosity or homozygosity) elevated secretion of both NE and CHGB439-451 into plasma (Figure 3g), indicating exocytosis as the mechanism of catecholamine release; FDR confirmed the effects.
SGCD genetic variation: Potential mechanisms to influence expression and human traits
Regulated secretory pathway exocytosis
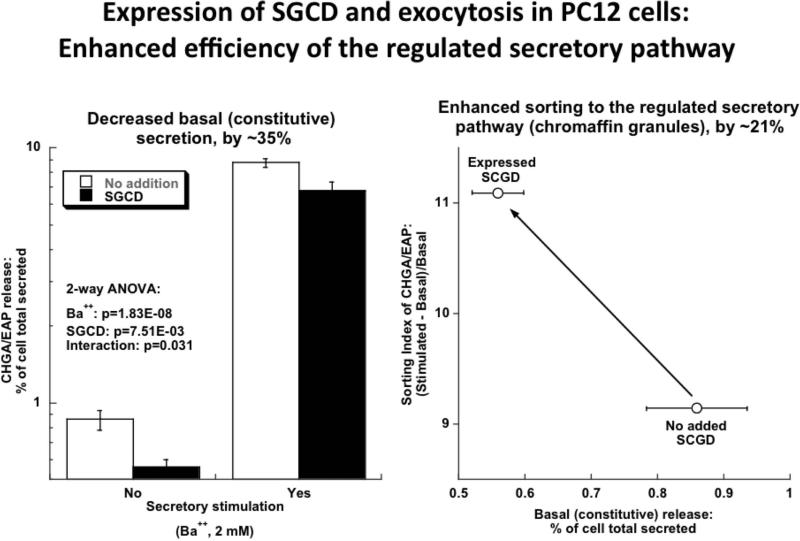
SGCD is one of 4 sarcoglycan apparatus components, contributing to the dystrophin-glycoprotein complex linking the F-actin cytoskeleton with the extracellular matrix. How might perturbation of this complex influence NE secretion? In a catecholaminergic cellular model of the regulated secretory pathway, trafficking into and out of that pathway can be monitored by storage and secretion of an enzymatically “tagged” version of the regulated secretory protein CHGA, with provocation of exocytosis by the potent depolarizing secretagogue Ba2+. When this system is established in PC12 cells, co-expression of SGCD, representing SGCD over-expression, diminished basal secretion of a CHGA-EAP chimera by ~35%, while preserving stimulated secretion, with an overall enhancement of “sorting index” (i.e., efficiency of entry into the regulated secretory pathway) for the chimera, by ~21% (Figure 4a).
Figure 4. Delta-sarcoglycan (SGCD): Potential mechanisms influencing trait variation.
Results are shown as mean ± SEM. Significant differences (P <0.05) are bold.
(a) Protein trafficking into the regulated secretory pathway in PC12 cells, co-transfected with pCMV-SGCD and pCMV-CHGA-EAP chimera. Decreased stimulated secretion of Chromogranin A (CHGA) following SGCD exposure in PC12 cells, with and without secretory stimulation. In this system, co-expression of SGCD enhances CHGA secretory protein sorting to the regulated pathway.
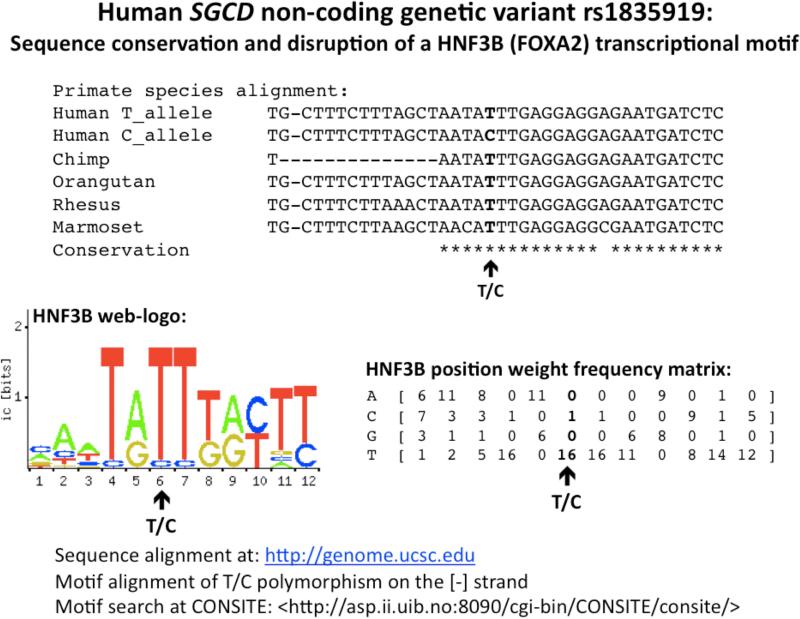
(b) Transcription factor binding. The peak associated SGCD variant disrupts a potential Hepatocyte nuclear factor 3-beta (HNF3B; aka Forkhead box protein A2 (FOXA2)) transcriptional motif. The SGCD transcriptional motif is shown to maintain conservation through inter-species sequence homology; polymorphism in bold, SGCD promoter SNP rs1835919 ([-] strand, as T>C). Also displayed is the graphical representation of HNF3B motif multiple sequence alignment and the position frequency and weight matrix representing known variation at the HNF3B alignment.
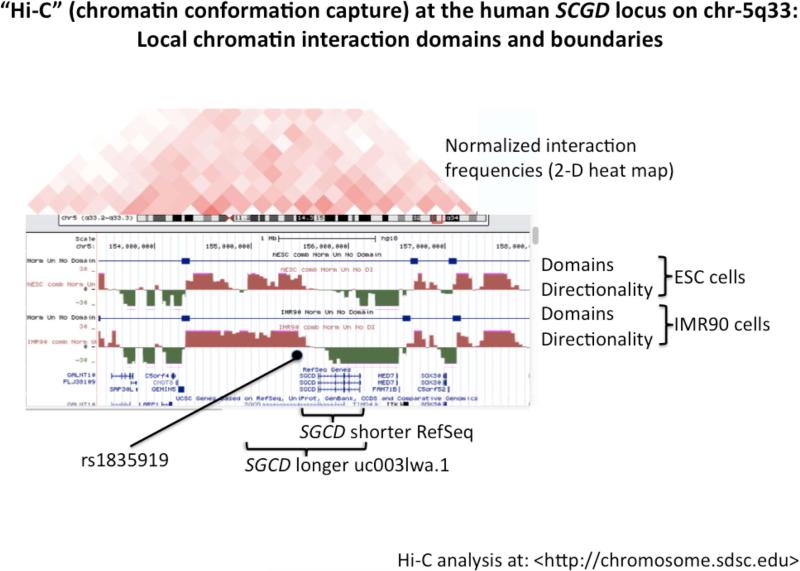
(c) Chromatin Conformation Capture. The plot indicates that the local interaction domain <http://chromosome.sdsc.edu> accessible to the rs1835919 region includes the entire SGCD gene, in both the longer (uc0031wa.1) or RefSeq (NM_000337) versions.
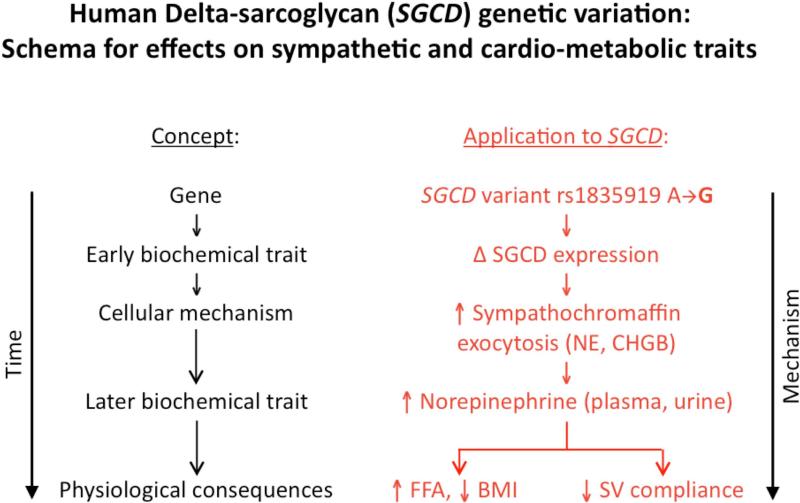
(d) The schematic presents a hypothetical framework integrating the overall rationale and experimental results of our study, and suggests future questions for exploration. CHGB indicates chromogranin B; all other abbreviations as in Figures 1 and 3. Ba2+ indicates barium; EAP, embryonic alkaline phosphatase.
Transcriptional motifs at the peak
The SGCD rs1835919 peak genetic variant is located in a region of conserved sequence homology. Computationally, the local region encompassing rs1835919 displays a 10/12 bp match for the Hepatocyte Nuclear Factor 3-Beta (HNF3B; aka Forkhead box protein A2 [FOXA2]) transcriptional motif (Figure 4b), with disruption of the motif by the minor allele. In addition, the more extended local region around rs1835919 harbors additional transcriptional and epigenetic motifs, as demonstrated by Chip-Seq and archived in the ENCODE elements at <genome.ucsc.edu/ENCODE>.
Chromatin Conformation Capture (”Hi-C”) domain across SGCD
Figure 4c illustrates the local Chromatin Conformation Capture domain structure in the region of rs1835919, with data derived from human embryonic stem cells and IMR90 fibroblasts, and analysis accomplished at <http://chromosome.sdsc.edu>. The plot indicates that the local transcription factor protein/protein interaction domain accessible to the rs1835919 region includes the entire SGCD gene, in both the longer (uc0031wa.1) and RefSeq (NM_000337) versions.
cis-eQTL at SGCD
Documentation that cis-genetic variation at/near the human SGCD locus can influence SGCD transcript expression is also available as an SGCD eQTL at <http://eqtl.uchicago.edu/>, determined for SGCD variant rs140615 (p=3.08E-10, with Bonferroni threshold p=3.95E-08), in a dataset coupling expression levels of 39,280 transcripts with 782,476 genotyped SNPs in 427 human liver samples (Schadt et al. 2008). SGCD variant rs140615 lies in intron-3 of RefSeq SGCD isoform-1 gene NM_000337.
DISCUSSION
Overview
A principal finding of our study is that human NE secretion is under substantial genetic control, including a major contribution by the SGCD locus on chromosome 5q33, with a relatively common minor allele frequency of the trait-associated variant at ~19%. In carriers of the trait-associated allele, plasma elevations of both NE and CHGB suggest exocytosis as the mechanism of induced NE release, a process that can be directly perturbed by SGCD expression. Thus, not only does rare Mendelian variation at SGCD yield human disease, but even common variation gives rise to phenotypic consequences, albeit milder, and without apparent myocardial involvement.
Heritability and pleiotropy in twins
By variance components in twin pairs, we found that the heritability (Table S1) of NE secretion is substantial (at h2 = 65.2±5.0%, P=3.19E-16) and the trait correlates with hemodynamic/autonomic and biochemical phenotypes in addition to cardio-metabolic and physical traits, many of which contribute to the metabolic syndrome. Circulating NE increased with age and was higher in men than women. Additionally, we found participants with higher pNE levels showed significant differences in other adrenergic traits, such as increased BP and hypertension risk factors.
Once again, trait-on-trait correlations could be decomposed into shared genetic (RhoG, pleiotropy) versus environmental (RhoE) determination, by variance components (Table S1). Inspection of the genetic correlations in the biochemical realm revealed pleiotropy of pNE with urine NE and plasma CHGB, suggesting exocytosis as the mechanism of catecholamine release. Other genetically co-regulated metabolic traits with pNE included renin secretion. Genetically co-regulated physiological traits included BMI and eGFR. Among cardiovascular traits, SVC displayed pleiotropy with pNE.
Role of SGCD
Mendelian cardiomyopathy in the SCH results from a naturally occurring major deletion in the SGCD gene (Nigro et al. 1997). In the SCH, SGCD affects not only cardiac, but also skeletal muscle (Nigro et al. 1997, Vainzof et al. 2008). Hamsters born with the SGCD variant, and consequent SGCD mRNA and protein deficiency, experience a pleiotropic spectrum of cardiac pathologies: cardiac myolysis, hypertrophy and dilation, congestive failure, as well as focal lesions with calcification, necrosis, and fibrosis in the left ventricle as early as 30 to 40 days of life (Nigro et al. 1997, Vainzof et al. 2008, Yamada et al. 1997).
SCH circulating NE levels exceed those of controls as the animals age. Elevated sympathetic tone in rodents and humans can augment or even provoke cardiac pathology (Yamada et al. 1997). Correspondingly, previous studies of patients suffering from chronic heart failure documented the adverse effects of increased levels of circulating NE, and the corresponding therapeutic benefits of adrenergic blockade in patients, particularly with beta-adrenergic antagonists (Schroeder & Jordan 2012).
However, on examination of our subjects, including those with SGCD-associated pNE elevation, intrinsic cardiac dysfunction was not observed (Table S3), with the exception of a ~6% decline in SVC (i.e., a modest increment in vascular stiffness), even in the absence of elevated BP. Given that even risk allele homozygotes (G/G) displayed essentially normal mean BP values (127.2±2.9 / 76.0±0.5 mmHg), the pertinence of such modest SVC increments for disease risk is uncertain.
Cell and molecular biology of catecholamine secretion
How might quantitative changes in SGCD perturb NE release or its exocytotic process (suggested by co-release of CHGB, Table S3)? Here we turned to a secretory model of functional catecholaminergic cells, in which we expressed SGCD and then evaluated consequences on exocytotic release of a CHGA-EAP chimera. Indeed, co-expression of SGCD diminished basal secretion of a CHGA-EAP chimera by ~35%, while preserving stimulated secretion, with an overall enhancement of the chimera's “sorting index”, by ~21% (Figure 4a). Thus, SGCD is sufficient to perturb exocytosis substantially, tightening the trafficking of the chimera into the regulated pathway. While SGCD is primarily known for expression in the myocardium, a variety of sources document its physiological (or endogenous) expression in neuronal and endocrine cells <http://www.ncbi.nlm.nih.gov/geo>, and indeed in PC12 cells used in this experiment (NCBI-GEO dataset GSE-37564), as well as healthy adrenal medulla (NCBI-GEO datasets GDS-3777, GDS-3779). Effects of SGCD on protein trafficking are already documented in the myocardium (Heydemann et al. 2007), in that the S151A SGCD dominant negative mutation seems to yield protein trafficking defects that disrupt nuclear localization of lamin A/C, emerin, and membrane sarcoglycan.
Do transcriptional alterations underlie the mechanism by which SGCD variation changes expression? Using bioinformatic approaches, we were able to show that the peak trait-associated variant disrupts a potential transcription factor binding motif (Figure 4b), and that local patterns of chromosomal conformation yield the potential for the trait-associated region in SGCD intron-4 to interact with other domains across the locus (Figure 4c).
Trait-on-trait aggregation: Quantiles
Another factor correlating with the rise in circulating NE, documented previously (Steinberg et al. 1964), is the increased circulation of FFA. Steinberg et al. reported that catecholamines, specifically NE, stimulated the mobilization of triglyceride deposits into FFA. Increased FFA, even independent of NE, was found in subjects with high BP, as an indication of sympathetic activity.
Advantages and limitations of this study
We describe an initial link between SGCD and sympathetic activity in human subjects, and then document functional connections between SGCD and the exocytotic secretory process. In addition, the evaluation of twins and their families permits us to also determine the heritability of traits to then assess the proportion of phenotypic variation that is attributable to genetic variation. Whether our results in predominantly healthy individuals can be extrapolated to disease populations is not yet certain.
CONCLUSIONS AND PERSPECTIVES
A schematic formulating our results into a global hypothesis is presented in Figure 4d, outlining the role of intermediate phenotypes and the SGCD gene influence on sympathetic and cardio-metabolic traits. By focusing on naturally occurring genetic variation at SGCD, we discovered a novel determinant of human NE secretion. Similar SGCD effects were also observed on a number of biochemical, physical, and cardio-metabolic traits; indeed, coordinate elevation of NE and CHGB suggested exocytosis as the mechanism of co-release, a process that could be directly perturbed by SGCD expression. However, in our predominantly healthy subject group, common variation at SGCD exhibited no evidence of myocardial pathology. Plasma NE concentration and several of the elevated physical and cardio-metabolic traits are highly heritable and known cardiovascular risk factors; secreted NE displayed joint genetic determination (pleiotropy) with hypertension risk factors such as the other catecholamines (DA and EPI) and SVC. Our results document a novel trans-QTL for sympathetic activity, in addition to suggesting new possibilities for its regulation. Further investigation may confirm the current results and establish specific molecular mechanisms for human adrenergic trait variation.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NHLBI and NIDDK), and the Department of Veterans Affairs.
GLOSSARY OF ABBREVIATIONS
- BAC
Brachial artery compliance
- BMI
Body mass index
- BP
Blood pressure
- BSA
Body surface area
- CHGA
Chromogranin A
- CHGB
Chromogranin B
- ChIP-seq
Chromatin immunoprecipitation sequencing
- CKD-EPI algorithm
Chronic Kidney Disease EPIdemiology collaboration algorithm
- COMT
catechol-O-methyltransferase
- CSPD
3-(4-methoxyspiro [1,2-dioxetane-3,2-(5chloro)tricyclo[3.3.1.1(3,7)]decan] 4-yl) phenyl phosphate
- DA, pDA
Dopamine, plasma dopamine
- DGC
Dystrophin-glycoprotein complex
- DPB
Diastolic blood pressure
- DZ
Dizygotic
- EAP
Embryonic alkaline phosphatase
- eGFR, eGFR
Estimated glomerular filtration rate
- EPI, pEPI
Epinephrine, plasma epinephrine
- eQTL
Expression quantitative trait loci
- FDR
False Discovery Rate
- FFA, pFFA
plasma free fatty acids
- GEE
Generalized estimating equations
- GWAS
Genome-wide association study
- HiC
Chromatin Conformation Capture
- MZ
Monozygotic
- NE, pNE
Norepinephrine, plasma norepinephrine
- NPY, pNPY
Neuropeptide Y, plasma neuropeptide Y
- QUICKI
QUantitative Insulin sensitivity ChecK Index
- RefSeq
Reference sequence
- RhoG (ρG)
Genetic covariance
- RhoE (ρE)
Environmental covariance
- SBP
Systolic blood pressure
- SCH
Syrian Cardiomyopathic Hamster
- SEAP
Secreted embryonic alkaline phosphatase
- SGCD
Delta-sarcoglycan
- SNAP
SNP Annotation and Proxy Search
- SNP
Single nucleotide polymorphism
- SOLAR
Sequential Oligogenic Linkage Analysis Routines
- SVC
Systemic vascular compliance
- SVR
Systemic vascular resistance
Footnotes
CONFLICTS OF INTEREST DISCLOSURE.
The authors have no conflicts of interest to disclose.
REFERENCES
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–181. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- Brinton TJ, Kailasam MT, Wu RA, Cervenka JH, Chio SS, Parmer RJ, DeMaria AN, O'Connor DT. Arterial compliance by cuff sphygmomanometer. Application to hypertension and early changes in subjects at genetic risk. Hypertension. 1996;28:599–603. doi: 10.1161/01.hyp.28.4.599. [DOI] [PubMed] [Google Scholar]
- Cockburn M, Hamilton A, Zadnick J, Cozen W, Mack TM. The occurrence of chronic disease and other conditions in a large population-based cohort of native Californian twins. Twin Res. 2002;5:460–467. doi: 10.1375/136905202320906282. [DOI] [PubMed] [Google Scholar]
- Coral-Vazquez R, Cohn RD, Moore SA, et al. Disruption of the sarcoglycansarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- Davis JT, Rao F, Naqshbandi D, Fung MM, Zhang K, Schork AJ, Nievergelt CM, Ziegler MG, O'Connor DT. Autonomic and hemodynamic origins of pre-hypertension: central role of heredity. J Am Coll Cardiol. 2012;59:2206–2216. doi: 10.1016/j.jacc.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobales N, Crespo MJ. Early pathophysiological alterations in experimental cardiomyopathy: the Syrian cardiomyopathic hamster. P R Health Sci J. 2008;27:307–314. [PubMed] [Google Scholar]
- Falconer DS, Mackay Trudy F.C. Introduction to Quantitative Genetics. Longman Pub Group; London: 1996. [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Demonbreun A, Hadhazy M, Earley JU, McNally EM. Nuclear sequestration of delta-sarcoglycan disrupts the nuclear localization of lamin A/C and emerin in cardiomyocytes. Hum Mol Genet. 2007;16:355–363. doi: 10.1093/hmg/ddl453. [DOI] [PubMed] [Google Scholar]
- Ziegler MG, Kennedy B, Elayan H. A sensitive radioenzymatic assay for epinephrine forming enzymes. Life Sci. 1988;43:2117–2122. doi: 10.1016/0024-3205(88)90361-x. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro V, Okazaki Y, Belsito A, et al. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6:601–607. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Okuizumi H, Ohsumi T, et al. A genetic linkage map of the Syrian hamster and localization of cardiomyopathy locus on chromosome 9qa2.1-b1 using RLGS spot-mapping. Nat Genet. 1996;13:87–90. doi: 10.1038/ng0596-87. [DOI] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32:W249–252. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Molony C, Chudin E, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273–1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- Sole MJ, Lo CM, Laird CW, Sonnenblick EH, Wurtman RJ. Norepinephrine turnover in the heart and spleen of the cardiomyopathic Syrian hamster. Circ Res. 1975;37:855–862. doi: 10.1161/01.res.37.6.855. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Nestel PJ, Buskirk ER, Thompson RH. Calorigenic Effect Of Norepinephrine Correlated With Plasma Free Fatty Acid Turnover And Oxidation. J Clin Invest. 1964;43:167–176. doi: 10.1172/JCI104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupenot L. Analysis of regulated secretion using PC12 cells. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb1512s36. Chapter 15, Unit 15 12. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, Mahapatra NR, Parmer RJ, Mahata SK, O'Connor DT. Identification of a novel sorting determinant for the regulated pathway in the secretory protein chromogranin A. J Cell Sci. 2002;115:4827–4841. doi: 10.1242/jcs.00140. [DOI] [PubMed] [Google Scholar]
- Vainzof M, Ayub-Guerrieri D, Onofre PC, Martins PC, Lopes VF, Zilberztajn D, Maia LS, Sell K, Yamamoto LU. Animal models for genetic neuromuscular diseases. J Mol Neurosci. 2008;34:241–248. doi: 10.1007/s12031-007-9023-9. [DOI] [PubMed] [Google Scholar]
- Wessel J, Moratorio G, Rao F, et al. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- Yamada S, Ohkura T, Yamadera T, Ito O, Kimura R, Nozawa Y, Hayashi S, Miyake H. Abnormality in plasma catecholamines and myocardial adrenoceptors in cardiomyopathic BIO 53.58 Syrian hamsters and improvement by metoprolol treatment. J Pharmacol Exp Ther. 1997;283:1389–1395. [PubMed] [Google Scholar]
- Zhang L, Rao F, Wessel J, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.