Abstract
Rats selectively bred for low (LCR) or high (HCR) intrinsic running capacity simultaneously present with contrasting risk factors for cardiovascular and metabolic disease. However, the impact of these phenotypes on left ventricular (LV) morphology and microvascular function, and their progression with aging, remains unresolved. We tested the hypothesis that the LCR phenotype induces progressive age-dependent LV remodeling and impairments in microvascular function, glucose utilization, and β-adrenergic responsiveness, compared with HCR. Hearts and vessels isolated from female LCR (n = 22) or HCR (n = 26) were studied at 12 and 35 wk. Nonselected N:NIH founder rats (11 wk) were also investigated (n = 12). LCR had impaired glucose tolerance and elevated plasma insulin (but not glucose) and body-mass at 12 wk compared with HCR, with early LV remodeling. By 35 wk, LV prohypertrophic and glucose transporter GLUT4 gene expression were up- and downregulated, respectively. No differences in LV β-adrenoceptor expression or cAMP content between phenotypes were observed. Macrovascular endothelial function was predominantly nitric oxide (NO)-mediated in both phenotypes and remained intact in LCR for both age-groups. In contrast, mesenteric arteries microvascular endothelial function, which was impaired in LCR rats regardless of age. At 35 wk, endothelial-derived hyperpolarizing factor-mediated relaxation was impaired whereas the NO contribution to relaxation is intact. Furthermore, there was reduced β2-adrenoceptor responsiveness in both aorta and mesenteric LCR arteries. In conclusion, diminished intrinsic exercise capacity impairs systemic glucose tolerance and is accompanied by progressive development of LV remodeling. Impaired microvascular perfusion is a likely contributing factor to the cardiac phenotype.
Keywords: cardiomyocyte hypertrophy, cardiac fibrosis, insulin resistance, EDHF, low-capacity runner, metabolic syndrome, resistance arteries
the metabolic syndrome is a polygenic disorder that includes obesity, insulin resistance, type 2 diabetes, dyslipidemia, hypertension, and impaired glycemic control. The prevalence of this disorder is dramatically increasing and is strongly linked to cardiovascular diseases (23). The presence of cardiovascular risk factors that constitute the metabolic syndrome is correlated with impairments in aerobic capacity and vascular endothelial function, as well as increased heart failure risk, all of which are strong independent predictors of mortality (13, 30, 37, 48). Furthermore, the myocardial and vascular abnormalities associated with the metabolic syndrome in general, and the associated impairments in insulin signaling, likely include alterations in myocardial structure (through cardiomyocyte hypertrophy, cardiac fibrosis, and/or impairments in myocardial perfusion). These cardiac changes, in combination with induction of systemic endothelial dysfunction (potentially elevating afterload), may well be contributing factors to later development of cardiac dysfunction and failure (48). How these cardiovascular abnormalities progress in the context of a reduction in habitual physical activity, however, remain unresolved.
In 2001, Koch and Britton (20) developed a novel rodent model based on differences in intrinsic exercise capacity. Rats were selectively bred to yield divergent phenotypes with either low (LCR) or high (HCR) intrinsic running capacity. When compared with HCR, LCR rats have reduced whole body insulin sensitivity, glucose tolerance, and hyperlipidemia, indicative of poor metabolic health (26, 43, 46, 49). Furthermore, LCR rats exhibit modest but significantly higher systolic blood pressure (SBP) compared with HCR, particularly in males (6, 49). While this may be a result of concomitant impairments in endothelial nitric oxide (NO)-dependent relaxation (49), endothelial function has yet to be studied in the LCR microvasculature. Skeletal muscle impairments are also evident in LCR with regard to insulin sensitivity, responsiveness to β-adrenoceptor activation, and levels of key proteins for mitochondrial function (3, 20, 26). Modest but significant impairments in myocardial systolic and diastolic function are observed in cardiomyocytes isolated from LCR compared with HCR rats (49). Presumably, these differences are attributable to abnormalities in myocardial mitochondrial protein content (3), similar to those previously seen in skeletal muscle in LCR rats, in addition to a relative myocardial downregulated expression of genes controlling lipid versus glucose as an energy substrate (4).
Taken collectively, it is evident that there is a close association between impaired muscle metabolic function and low intrinsic exercise capacity and that this relationship increases cardiovascular risk. In the context of the LCR phenotype, it remains to be resolved whether the myocardial and/or skeletal muscle metabolic defects precede the impairment in exercise capacity or vice versa. Whether these differences in both the heart and vasculature between the LCR and HCR phenotypes are evident at the onset of early adulthood or further develop with age and the extent to which they differ from the nonselected founder rats from which the two selected lines were derived have not been fully elucidated. In the present study, we thus tested the hypotheses that the cardiovascular phenotype of the young adult LCR rat is 1) impaired relative to both control N:NIH and HCR animals and 2) worsens with aging. Regional differences in vascular reactivity and the relative contribution of endothelial-derived NO versus endothelial-derived hyperpolarizing factor (EDHF) to vasorelaxation were also investigated.
METHODS
Animal model.
LCR and HCR rats were bred from genetically heterogeneous N:NIH stock rats by artificial selection as previously reported (20). The present study used female rats from generations 16, 20, and 22, as the body weight differences between phenotypes are less marked in females than in males at the same age (16), and hence body weight is less likely to represent a confounding influence to our observations. Rats were phenotyped for intrinsic running capacity at 11 wk of age (26, 43, 49) and were studied in two age groups, young (∼12 wk) and mature adulthood (∼35 wk). Conscious fasting glucose tolerance tests were determined (26, 43, 49) before euthanasia. All animal experimentation procedures were carried out with the approval of animal ethics committees from Royal Melbourne Institute of Technology University and the University of Michigan. The investigation conformed to the Guidelines for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All reagents were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in distilled water except where indicated.
Tissue collection and histology.
Following a 5-h fast, animals were weighed, anesthetized with pentobarbital sodium (60 mg/kg), and killed by exsanguination. Blood samples (∼60 μl, via tail cut) were collected for determination of glucose (One Touch glucometer, Roche, Sydney, NSW, Australia) and plasma insulin (Rat insulin ELISA, ALPCO Immunoassays, No. 80-INSRT-E01). Hearts were removed and rinsed in ice-cold Krebs buffer, consisting of (in mM) 118 NaCl, 4.7 KCl, 1.18 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11.1 d-glucose, and 1.6 CaCl2, and lightly blotted and wet weight determined. Hearts were cut at the horizontal short-axis plane, and the middle portion of the left ventricle was fixed in 10% neutral buffered formalin (Australian Biostain, Melbourne, Australia) and paraffin-embedded for histological analysis of 4-μm cross sections via hematoxylin-eosin and Sirius red staining (17, 18, 41). Aorta and mesenteric arteries were also promptly removed on euthanasia and immediately placed in ice-cold Krebs containing indomethacin (10 μM, dissolved in 0.1 M sodium carbonate), a nonselective cyclooxygenase (COX) inhibitor, to limit prostanoid synthesis. Superoxide generation was quantified using lucigenin (5 μM)-enhanced chemiluminescence in fresh left ventricular (LV) and aortic tissues (17, 35, 41, 42). Results were normalized to tissue weight and expressed as counts per milligram of tissue. Remaining basal and apical portions of the ventricle were snap frozen in liquid nitrogen and stored at −80°C for biochemical analysis.
Assessment of cardiac remodeling.
Cardiomyocyte hypertrophy was determined using cell width from hematoxylin-eosin sections and on β-myosin heavy chain expression; cardiac fibrosis was assessed on collagen deposition in Sirius red-stained sections (17, 18, 41). Cardiac gene expression of glucose transporters (GLUT1, GLUT4) and β1-adrenoceptors (via real-time PCR), as well as cardiac content of the β-adrenoceptor second messenger cAMP [via enzyme immunoassay, Cayman Chemical, Ann Arbor, MI (28)], were also determined in addition to markers of cardiac remodeling. RNA was extracted from frozen ventricle as previously described (42). DNase-treated RNA was reverse transcribed (Taqman Reverse Transcription reagent, Applied Biosystems, Mulgrave, VIC, Australia) using the Gene-Amp PCR system 9700 (Applied Biosystems) to produce cDNA template at 20 ng/μl (42, 44). Gene expression was quantitated using SYBR Green chemistry with the FAST 7500 v. 2.0.5 sequence detection system with ribosomal 18s as the endogenous control (Applied Biosystems). All primers were generated from rat-specific sequences published on GenBank at previously determined optimal concentrations (24, 44). The comparative Δ-Δ cycle threshold method was used to analyze changes in gene expression as a relative fold change to HCR rats (42).
Vascular function experiments.
Aortae and third-order branches of rat mesenteric artery (internal diameter, ∼200–300 μm) were cleared of fat and connective tissue and cut into ∼2 mm-long ring segments. Aortic rings were mounted in standard organ baths and allowed to equilibrate for 60 min at a resting tension of 1 g. Mesenteric artery rings were mounted in a Mulvany-style small vessel myograph (Danish Myo Technology, Aarhus, Denmark) and allowed to stabilize at zero tension for 15 min. After stabilization, the mesenteric arteries were normalized to 90–100 mmHg and all vascular reactivity experiments were performed at 37°C and bubbled with carbogen (95% O2 and 5% CO2) as previously described (25, 31, 36). Briefly, after maximum contraction and assessment of endothelial integrity were established, arteries were precontracted to ∼50% of maximum with phenylephrine (0.1–3 μM) and cumulative concentration-response curves to acetylcholine (ACh, 0.1 nM–10 μM, BDH Chemicals, Poole, Dorset, UK) and sodium nitroprusside (SNP, 0.01 nM–10 μM) obtained in vessels from both age groups were studied.
The contribution of endothelial-derived NO to ACh responses was examined after 20 min incubation with NG-nitro-l-arginine [l-NNA, 100 μM, nonselective NO synthase (NOS) inhibitor] and 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ, 10 μM, soluble guanylate cyclase inhibitor, Cayman Chemical). The role of EDHF in ACh responses was examined using the combination of 1-[(2-chlorophenyl)(diphenyl)methyl]-1H-pyrazole (TRAM-34, 1 μM, selective blocker of intermediate-conductance Ca2+-activated K+ channels) and apamin (1 μM, inhibitor of small-conductance Ca2+-activated K+ channels). Stock solutions of both ODQ and TRAM-34 were prepared in dimethyl sulfoxide before dilution in Krebs buffer. Cumulative concentration-response curves to the selective β2-adrenoceptor agonist fenoterol (1 nM–100 μM) were constructed in precontracted mesenteric artery and aorta as described above.
Data and statistical analyses.
All results are expressed as means ± SE, where n represents the number of animals per group. The area under the curve for glucose tolerance was calculated using Prism version 5.0 (GraphPad Software, San Diego, CA). Concentration-response curves from rat isolated arteries were computer fitted to a sigmoidal curve using nonlinear regression (Prism version 5.0) to calculate the sensitivity of each agonist (EC50). Maximum relaxation (Rmax) to ACh or SNP was measured as a percentage of precontraction to phenylephrine. Group data for pEC50, Rmax, glucose tolerance area under the curve, cardiac histology, gene expression, and cAMP were compared via one-way ANOVA (with post hoc analysis using Dunnett's test) or Student's unpaired t-test, as appropriate. Two-way ANOVA was also used to compare differences in cardiomyocyte size, cardiac collagen deposition, and microvascular sensitivity to ACh across phenotype and age. P < 0.05 was considered statistically significant.
RESULTS
Systemic characteristics.
The systemic characteristics of the three cohorts under investigation are shown in Table 1. At ∼12 wk of age, LCR rats are similar in body mass and heart mass to founder animals. HCR rats were significantly smaller than either LCR or founders, and this difference was maintained at 35 wk of age. No significant differences in heart mass or heart mass index were observed. Elevated fasting plasma insulin (but not blood glucose) and impaired glucose tolerance were evident in the cohort of young LCR compared with HCR rats (Fig. 1), indicative of some degree of systemic insulin resistance. By 35 wk of age, blood glucose levels in LCR rats were significantly elevated compared with their HCR counterparts.
Table 1.
Systemic characteristics of HCR, control founder, and LCR rats at 12 and 35 wk of age
| 12-wk-Old Rats |
35-wk-Old Rats |
||||
|---|---|---|---|---|---|
| HCR | Founders | LCR | HCR | LCR | |
| n | 12 | 12 | 12 | 14 | 10 |
| Age, wk | 12 ± 0.1 | 11 ± 0.1 | 12 ± 0.1 | 34.5 ± 0.5 | 34.5 ± 1.0 |
| Body weight, g | 168 ± 4 | 199 ± 4***,† | 214 ± 4*** | 237 ± 6 | 268 ± 5# |
| Heart weight, mg | 621 ± 16 | 720 ± 57 | 717 ± 18 | 760 ± 22 | 821 ± 19 |
| Heart weight:body weight, mg/g | 3.71 ± 0.07 | 3.60 ± 0.25 | 3.34 ± 0.05 | 3.21 ± 0.06 | 3.06 ± 0.06 |
| Fasting blood glucose, mM | 6.48 ± 0.16 | 6.34 ± 0.12 | 6.54 ± 0.14 | 3.87 ± 0.22 | 4.87 ± 0.18## |
| Fasting plasma insulin, ng/ml | 0.39 ± 0.01§ | 0.41§§ | 0.62 ± 0.07§* | ND | ND |
Values are means ± SE. HCR, high capacity runner; LCR, low capacity runner;
P < 0.05 and
P < 0.001 vs. HCR;
P < 0.05 vs. LCR in 12-wk-old rats (one-way ANOVA; Dunnett's multiple comparison) and
P < 0.05 and
P < 0.005 vs. HCR in 35-wk-old rats (unpaired t-test). ND, not determined.
Data for n = 7/group only.
Samples from all rats combined to yield sufficient serum for analysis.
Fig. 1.
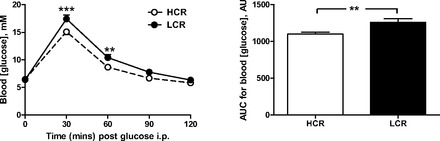
Low intrinsic running capacity (LCR) exhibit impaired systemic glucose tolerance. Both the time course of glucose utilization and the area under the curve (AUC) for this time course were significantly impaired in female LCR rats relative to aged-matched high intrinsic running capacity (HCR) rats at 12 wk of age, following a glucose load (2 g/kg ip) in fasted animals (n = 7 per group). AU, arbitrary units. ***P < 0.001 and **P < 0.01, LCR vs. HCR (at the same time point on two-way ANOVA or on AUC on unpaired t-test).
LCR is associated with progressive cardiac structural remodeling.
At ∼12 wk of age, LCR rats exhibit cardiomyocyte hypertrophy in the intact heart relative to age-matched HCR counterparts (Fig. 2). Cardiomyocyte size increased with age to a similar extent in both LCR and HCR rats, such that the relative increase in cardiomyocyte width for the LCR rat was similar at 12 and 35 wk (Fig. 2B, right). Furthermore, on two-way ANOVA, both age and phenotype were associated with significant differences in cardiomyocyte size (both, P < 0.0001), although the interaction was not significant. Hypertrophic gene expression progressively increased with age in LCR rats relative to their HCR counterparts. At 12 wk, β-myosin heavy chain expression in LCR rats was not significantly different to HCR or founder control rats (P = 0.1, Fig. 3, left). By 35 wk, β-myosin heavy chain expression in LCR rats was markedly elevated compared with age-matched HCR rats (P < 0.005, Fig. 3). Cardiac collagen deposition was elevated in LCR compared with HCR rats, at both ages studied. At 12 wk of age, cardiac collagen deposition was 2.7 ± 0.2-fold in LCR relative to age-matched HCR rats, with some suggestion of age-dependent progression of cardiac fibrosis (Fig. 4). On two-way ANOVA, however, only phenotype (and not age) tended to remain associated with differences in cardiac collagen deposition (P = 0.07). Despite this clear cardiac remodeling evident in LCR rats, no phenotype differences in cardiac generation of superoxide were seen (Table 2).
Fig. 2.
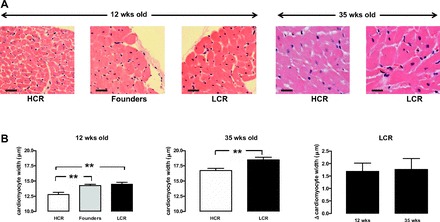
LCR exhibit cardiomyocyte hypertrophy. Increased cardiomyocyte width is evident on histological analysis of left ventricular (LV) cross sections stained with hematoxylin-eosin in the intact LCR heart relative to HCR rats at both 12 and 35 wk of age. A: representative sections (hematoxylin-eosin stain; magnification, ×400). Scale bars show 20 μm. B: cardiomyocyte width pooled data. The LCR-induced increase in cardiomyocyte size (Δcardiomyocyte size relative to age-matched HCR) is comparable at both ages (n = 9–14 per group). **P < 0.01 vs. age-matched HCR rats (on one-way ANOVA at 12 wk and unpaired t-test at 35 wk of age).
Fig. 3.
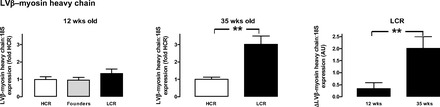
Hypertrophic gene expression in LCR rats progressively increases with age. LV β-myosin heavy chain expression relative to the housekeeping gene 18S in either LCR or control founder rats relative to age-matched HCR rats was not significantly different at 12 wk of age (left), but LCR upregulated β-myosin heavy chain expression at 35 wk of age (middle). The relative increase in LCR above age-matched HCRs is shown (right) (n = 5–12 per group). **P < 0.005 vs age-matched HCR rats (unpaired t-test).
Fig. 4.
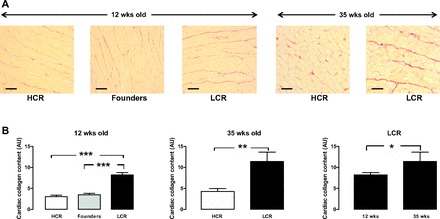
LCR exhibit cardiac fibrosis. Increased cardiac collagen deposition is evident on histological analysis of ventricular cross sections stained with Sirius red in the intact LCR heart relative to HCR rats at both 12 and 35 wk of age. A: representative sections (magnification, ×200). Scale bars show 40 μm. B: quantitation of collagen deposition pooled data. The LCR-induced increase in cardiac collagen deposition is evident at both ages but was further increased in LCR at 35 wk of age (n = 8–14 per group). ***P < 0.001, **P < 0.01, and *P < 0.05 (on one-way ANOVA at 12 wk and unpaired t-test at 35 wk of age).
Table 2.
Impact of low vs. high inherent running capacity on cardiac β-adrenergic signaling and superoxide generation in 35-wk-old rats
| HCR (n) | LCR (n) | |
|---|---|---|
| LV β1-adrenoceptor expression, AU | 0.86 ± 0.1 (7) | 1.23 ± 0.3 (6) |
| LV cAMP content, pmol/ml | 42.6 ± 3.1 (8) | 36.4 ± 1.6 (9) |
| LV superoxide, counts/mg tissue | 530 ± 70 (15) | 540 ± 80 (11) |
| Aortic superoxide, counts/mg tissue | 1,360 ± 220 (17) | 1,380 ± 150 (14) |
Values are means ± SE. LV, left ventricular; AU, arbitrary units.
Cardiac GLUT4 is downregulated with inherent LCR.
LCR rats exhibit a degree of systemic insulin resistance (Table 1 and Fig. 1). There was no impact of the LCR phenotype on GLUT4 expression at 12 wk (Fig. 5A, left), but GLUT4 was significantly downregulated in the LCR myocardium by 35 wk (Fig. 5A, middle and right). In contrast, the LCR phenotype does not impact on cardiac GLUT1 expression at either 12 or 35 wk (Fig. 5B). GLUT1 and GLUT4 expression in founders mimicked that seen in the HCR phenotype.
Fig. 5.
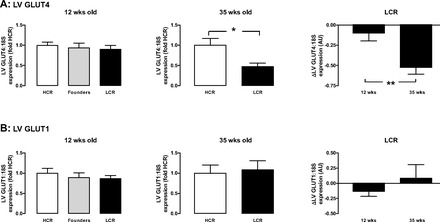
Cardiac glucose transporter GLUT4 gene expression in LCR rats progressively increases with age. A: LV GLUT4 expression relative to the housekeeping gene 18S in LCR relative to age-matched HCR or control founder rats was not significantly different at 12 wk of age (left), but LCR upregulated GLUT4 expression at 35 wk of age (middle). The relative increase in LCR above age-matched HCRs is shown (right). B: LV GLUT1 expression relative to the housekeeping gene 18S in LCR relative to age-matched HCR or control founder rats was not significantly different at either 12 wk (left) or 35 wk of age (middle). The relative increase in LCR above age-matched HCRs is shown (right) (n = 8–12 per group). *P <0.05 and **P <0.005 vs. age-matched HCR rats (unpaired t-test).
LCR selectively impairs endothelial function in resistance vessels.
For both selected lines and age-groups studied, ACh elicited concentration-dependent vasorelaxation in isolated aorta, a large conduit vessel, and in mesenteric artery, a smaller resistance vessel (Fig. 6). Vascular sensitivity to ACh was significantly impaired in LCR mesenteric arteries, at 12 and 35 wk (Fig. 6A), compared with HCR arteries. Furthermore, on two-way ANOVA, phenotype remained associated with significant differences in sensitivity to ACh (P < 0.0005); a similar but nonsignificant trend for age to influence sensitivity to ACh was also seen (P = 0.09). In contrast, endothelial function was intact in LCR aorta (Fig. 6B), and superoxide generation was not different (Table 2). No differences in maximal relaxation elicited by ACh in either vessel were observed. No phenotype differences were evident on responses to the endothelium-independent vasorelaxant SNP in either vessel regardless of age (n = 3, P = 0.087, Fig. 7).
Fig. 6.
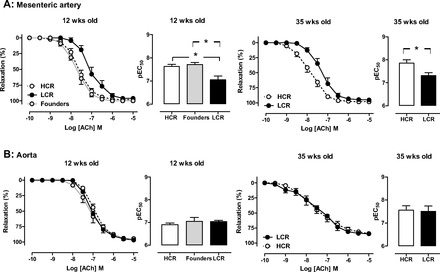
Endothelial function is impaired in LCR rat mesenteric arteries. Concentration-response curves and sensitivity (pEC50) to the endothelium-dependent dilator ACh in both mesenteric (A) and aortic (B) rings from HCR, LCR, and control founder rats at either at 12 wk of age (left) or 35 wk of age (right). Endothelial function is selectively impaired in mesenteric arteries (but not aorta) in LCR relative to HCR, regardless of age (n = 4–12 per group). *P < 0.05.
Fig. 7.
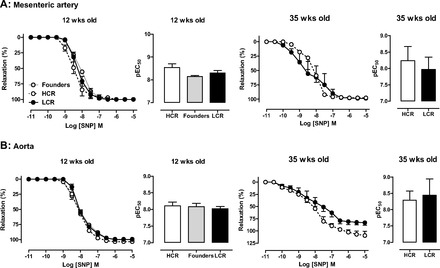
Vascular nitric oxide (NO) responsiveness is intact in LCR rat arteries. Concentration-response curves and sensitivity (pEC50) to the endothelium-independent dilator sodium nitroprusside (SNP) in both mesenteric (A) and aortic (B) rings from HCR, LCR, and control founder rats at either at 12 wk of age (left) or 35 wk of age (right) (n = 3–6 per group).
EDHF is impaired in LCR resistance vessels.
The relative roles of endothelium-derived NO and EDHF to endothelium-dependent vasorelaxation were determined in rat isolated aorta and mesenteric artery at 35 wk (Fig. 8). In both HCR (Fig. 8A) and LCR (Fig. 8B) mesenteric arteries, the presence of l-NNA + ODQ did not significantly affect either the sensitivity (pEC50, Fig. 8C) or Rmax (Fig. 8D) to ACh. Furthermore, the impairment in microvascular endothelial function evident in LCR rats persisted in the presence of l-NNA + ODQ, indicating that EDHF-type relaxation is impaired in LCR mesenteric arteries. In contrast, blockade of Ca2+-activated K+ channels with TRAM-34 + apamin markedly shifted the concentration-response curve to ACh rightward in both HCR (Fig. 8A) and LCR (Fig. 8B) mesenteric arteries, revealing a significant contribution of EDHF in resistance vessels of both phenotypes. As also shown in Fig. 8C, mesenteric sensitivity to ACh in the presence of TRAM-34 + apamin is similar in HCR and LCR, indicating that NO makes a similar contribution to mesenteric relaxation in the two phenotypes. As shown in Fig. 8, E and F, in direct contrast to results obtained in the microvasculature, concentration-response curves to ACh in aortic rings from both HCR and LCR rats at 35 wk of age are abolished by l-NNA, implicating a predominant role for NO in macrovascular function regardless of phenotype.
Fig. 8.
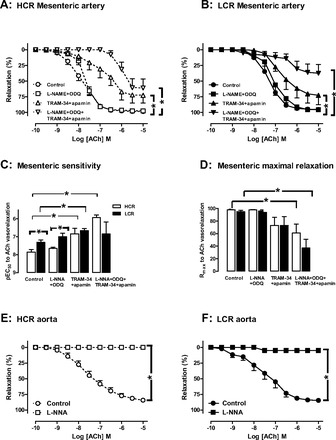
Endothelium-derived hyperpolarizing factor (EDHF) is impaired in LCR resistance vessels. Concentration-response curves to the endothelium-dependent vasodilator ACh in mesenteric rings from both HCR (A) and LCR (B) rats at 35 wk of age are unaffected by NG-nitro-l-arginine (l-NNA) + 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ; inhibitors of NO synthase and soluble guanylyl cyclase) but are significantly impaired by 1-[(2-chlorophenyl)(diphenyl)methyl]-1H-pyrazole (TRAM-34) + apamin (Ca2+-activated K+ channel inhibitors), implicating a role for EDHF in microvascular function (n = 4 per group). C: mesenteric sensitivity to ACh (pEC50). D: maximal relaxation to ACh (Rmax). Concentration-response curves to ACh in aortic rings from both HCR (E) and LCR (F) rats at 35 wk of age are abolished by l-NNA, implicating a predominant role for NO in macrovascular function, regardless of phenotype (n = 3–11 per group). *P < 0.05.
β-Adrenergic signaling is impaired in LCR vasculature.
The selective β2-adrenoceptor agonist fenoterol elicited concentration-dependent vasorelaxation in both rat isolated mesenteric artery (Fig. 9A) and aorta (Fig. 9B) at 12 wk. The sensitivity (pEC50) to fenoterol, however, was significantly impaired in both micro- and macrovasculature in LCR animals. In contrast, no differences in either expression of the predominant cardiac subtype β1-adrenoceptors or content of its second messenger cAMP were observed (Table 2).
Fig. 9.
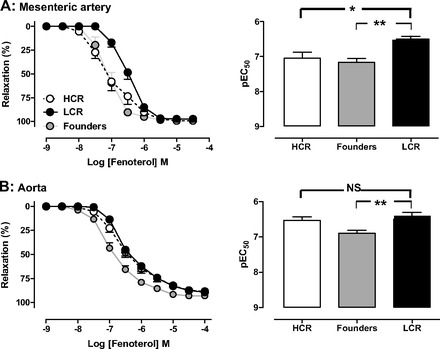
β-Adrenergic signaling is impaired in LCR vasculature. Concentration-response curves to the β2-adrenoceptor agonist fenoterol in mesenteric (A) and aortic (B) rings from HCR, LCR, and control founder rats at 12 wk of age (n = 7–11 per group). *P < 0.05 and **P < 0.01 (one-way ANOVA). NS, not significant.
DISCUSSION
Much of the recent evidence suggesting that low intrinsic aerobic capacity is associated with increased risk factors for cardiovascular disease has emerged from a novel rodent model of inherited LCR and impaired metabolic health. Using this model, we now provide new information regarding the impact of these phenotypes on LV morphology and microvascular function and how this is affected with age in young adult females. Our key findings provide evidence that impaired intrinsic exercise capacity is accompanied by early LV remodeling (evident at 12 wk of age, Figs. 2 and 4) with concomitant early microvascular endothelial dysfunction (Figs. 6A and 8). This cardiovascular phenotype was associated with impaired glucose tolerance and elevated plasma insulin and body weight but precedes elevation in blood glucose (Table 1 and Fig. 1). Subsequent progression to upregulated LV hypertrophic gene expression and downregulated myocardial GLUT4 expression was evident with age (Figs. 3 and 5). Macrovascular function, however, remained intact (Fig. 6B). Although microvascular β2-adrenoceptor responsiveness was reduced in LCR rats (Fig. 9), no phenotypic differences in LV β-adrenoceptor expression or activity (as estimated on LV cAMP content) were apparent (Table 2). These findings raise the possibility that impaired microvascular perfusion is a contributing factor to the cardiac phenotype associated with an inherited low capacity for exercise.
Previous proteomic and array studies (3, 4) suggested that LCR may exhibit a predisposition for LV remodeling, but those studies focused on older animals (30–50 wk of age). Confirmation of morphological changes specifically on LV histology, protein content, or gene expression using real-time PCR or other semiquantitative approaches was not available. Recent work has now suggested that more mature LCR rats (∼30 wk of age) exhibit upregulated cardiac collagen deposition (6). Following enzymatic isolation, cardiomyocytes from older LCR also tend to be shorter and wider than those from their HCR counterparts (4, 19). Here we demonstrate that the onset of cardiac fibrosis in the LCR phenotype occurs considerably earlier and is evident already by 12 wk. In addition, we demonstrate that concomitant cardiomyocyte hypertrophy is not an artefact of enzymatic isolation but is manifest in the heart in situ (Fig. 2). Interestingly, increased LV cardiomyocyte width is also manifest at the same early time point as collagen deposition (Fig. 4). Furthermore, the temporal upregulation of both hypertrophic gene expression (Fig. 3) and LV collagen content (Fig. 4) suggests that cardiac remodeling in the LCR continues to progress with age. The LV remodeling evident in even relatively young LCR rats (at 12 wk of age) is associated with significant cardiac fibrosis (Figs. 2 and 4), with subsequent alterations at the level of hypertrophic and GLUT4 gene expression evident by 35 wk of age (Fig. 3). Thus the cardiomyocyte hypertrophy observed in this phenotype more closely resembles a pathological (as opposed to a physiological) cardiac growth response (31), even at 12 wk of age. This is also in agreement with the loss of myocardial transverse T tubules (19, 21) and blunted cardiomyocyte contractile function (15) evident in older LCR rats, as well as the cardiac phenotype of rodent models of insulin resistance/type 2 diabetes (15, 39, 42).
There is strong evidence that maintaining an increased level of physical activity is a key intervention favoring health benefits and longevity in patients with insulin resistance and diabetes (5, 14). The current data suggest that a low capacity for aerobic exercise can be inherited (20), and that this inherited trait per se represents a setting of metabolic syndrome (26). Insulin resistance is an antecedent of type 2 diabetes, and both scenarios are associated with cardiac upregulation of reactive oxygen species (ROS) generation and systemic oxidative stress (17, 33, 35, 39, 42). Given that superoxide is a key trigger of both cardiomyocyte hypertrophy (24, 40, 41) and impaired vasoreactivity (9), its upregulation is widely regarded as a contributing factor to cardiovascular abnormalities in insulin resistance (9, 12, 22, 34, 39). In the present study, impaired systemic glucose tolerance and hyperinsulinemia were already evident at 12 wk of age in LCR rats, relative to their HCR counterparts (Table 1 and Fig. 1), but sensitivity to insulin was not specifically investigated. Insulin resistance is clearly evident in LCR rats by 30 wk of age (26). Surprisingly, LV and vascular superoxide generation were not different between LCR and HCR rats at 35 wk of age (Table 2), suggesting that the cardiovascular phenotype evident in mature LCR may not be a downstream consequence of ROS upregulation. We are unable to preclude a transient increase in LV and vascular superoxide generation at an earlier age as a potential trigger of early remodeling, as this was not determined at 12 wk of age in the present study. At the level of endogenous cardiovascular antioxidant enzyme activity, the counterbalance to ROS generation, is similarly lacking from current knowledge, although trends for reduced antioxidant enzyme activity have been reported in LCR skeletal muscle (47).
In the present study, macrovascular function (i.e., in large conduit vessels) was determined ex vivo in rat isolated aortae. Contrary to a previous report from carotid arteries, a moderate-sized conduit vessel (49), we did not observe any differences in endothelium-dependent (i.e., in response to ACh, Fig. 6B) or -independent vasorelaxation (i.e., in response to SNP, Fig. 7B) in aortae isolated from LCR rats relative to HCR in either age group. We were thus unable to attribute the changes in cardiac remodeling to macrovascular abnormalities. Given that these studies were performed in the presence of the COX inhibitor indomethacin, the abolition of ACh vasodilatation responses by the nonselective inhibition of NOS indicates that NO is the sole endothelium-derived macrovascular vasorelaxant mediator in both phenotypes (29).
This study provides the first insights into endothelial function in smaller LCR arteries. In direct contrast to the macrovasculature, microvascular endothelial function (determined ex vivo in third-order mesenteric arteries) was significantly impaired in LCR rats at both ages studied (Fig. 6A) without impact on endothelium-independent vasorelaxation (Fig. 7A). NO and EDHF both contribute to vascular tone in vivo (1, 7, 25, 29, 50); our observations that the contribution of EDHF was greater in the rat microvasculature than the macrovasculature ex vivo (Fig. 8) are consistent with the mouse mesenteric microvasculature in vivo (50). Our studies revealed a significant contribution of EDHF to endothelium-dependent vasorelaxation in these smaller vessels (considerably greater than that attributable to NO), as indicated by the significantly greater sensitivity to inhibition of Ca2+-activated K+ channels [with TRAM-34 + apamin (29)] than to inhibition of NO/cGMP signaling (with l-NNA + ODQ, Fig. 8). This greater reliance on EDHF over NO was observed in both HCR and LCR mesenteric arteries. Indeed, the role of endothelial-derived NO in smaller resistance vessels is revealed once the EDHF component of ACh vasodilatation is removed (by the combination of TRAM-34 + apamin)—the residual vasodilatation response is attributed to endothelial-derived NO, as we can confirm by the combined addition of l-NNA + ODQ and TRAM-34 + apamin (Fig. 8C), confirming that endothelial-derived NO and EDHF account for all of the microvascular sensitivity to ACh in both phenotypes studied. Furthermore, the sensitivity to ACh in mesenteric arteries in the face of combined NOS, COX, and cGMP inhibition was significantly greater in microvessels from HCR to that seen from LCR, indicative of reduced EDHF responses in LCR microvasculature. NO, particularly that derived from the endothelium, is a negative regulator of cardiomyocyte hypertrophy [for review, see Ritchie et al. (40)], in addition to its well-known role as a critical regulator of vascular tone (10). Although differences in NOS expression were not determined in the present study, our findings indicate that there were no phenotype differences in vascular NOS activity. Rather, these data suggest that impairment at the level of EDHF-type relaxation mediates microvascular dysfunction in LCR rats, which is in accordance with settings of type 2 diabetes, where EDHF-dependent responses are similarly impaired in resistance arteries (2, 51).
An increase in afterload as a result of hypertension is an important driver of LV hypertrophy (40). Based on previous evidence of only a modest level of hypertension in female LCR rats, SBP was not measured in the present study. Differences in SBP female LCR relative to HCR rats range from negligible (6) to about 10 mmHg (49) at 24–30 wk of age, considerably older that the young adult females studied here, unlikely to be a sufficiently large enough stimulus to drive cardiac remodeling. For comparison, male spontaneously hypertensive rats exhibit an ∼40 mmHg greater SBP than their normotensive counterparts even at 12 wk of age (8); such a substantial pressure gradient in contrast is clearly sufficient to drive a cardiac hypertrophic response (45). As both phenotypes age, this difference becomes more marked but still does not exceed ∼20 mmHg, as shown in female LCR relative to female HCR rats at both 2 yr of age (21). Together, these findings suggest that only modest effects on SBP are evident in LCR and that these emerge later in life and may be more pronounced in males than females.
Although macrovascular endothelial function was intact in LCR rats (Fig. 6), we demonstrate that β-adrenergic responsiveness is impaired in LCR aorta and mesenteric artery at 12 wk of age (Fig. 9). This finding is consistent with previous observations in skeletal muscle (26, 27). In contrast, no phenotypic differences in mesenteric responses to isoproterenol were observed (results not shown). Despite comparable net microvascular β-adrenergic responsiveness in LCR and HCR rats, differences were observed on the relative contribution of different β-adrenoceptor subtypes to vasorelaxation. No corresponding phenotypic differences in basal myocardial β-adrenoceptor actions were apparent (Table 2), although LV responses to β-adrenoceptor stimulation was beyond the scope of the current study.
LV systolic and diastolic function has previously been reported to be impaired in sedentary LCR rats compared with their HCR counterparts, both in vivo and at the level of the isolated cardiomyocyte (4, 6, 15, 49). This evidence, like that for cardiac remodeling, has largely been obtained in older animals (20–30 wk of age) and whether LV dysfunction is impaired in younger animals remains to be determined. Often, changes at the level of LV diastolic function are more marked than systolic parameters (6, 15), suggesting that LCR, like other models of metabolic syndrome and type 2 diabetes, may predispose to earlier onset of abnormalities in myocardial relaxation rather than contractile dysfunction (39). If permitted to proceed to senescence (≥2 yr of age), marked dysfunction in both systolic and diastolic function are evident (21). Interestingly, the NOS1 isoform of NOS (as distinct from the NOS3 isoform likely mediating macrovascular function) has been implicated in both the systolic and diastolic abnormalities in LCR cardiomyocyte mechanical function (15), using the selective NOS1 inhibitor N5-(1-imino-3-butenyl)-l-ornitine. Given the extent of cardiac remodeling already evident at 12 wk of age in LCR rats, we speculate that the age of onset of cardiac dysfunction may occur earlier than first thought, particularly if the extent of microvascular dysfunction evident in the coronary vascular bed at this age mirrors that in the mesenteric circulation. The reduced cardiac index and coronary flow rates reported in LCR even in the absence of a significant LV systolic fraction (6, 16) indicate that interrogation of myocardial perfusion abnormalities particularly at the level of the microvasculature (analogous to our work here in mesenteric arteries) is warranted. While our findings of impaired mesenteric microvascular function cannot necessarily be extrapolated to changes at the level of coronary microvascular function, such abnormalities could account for the impairments in myocardial perfusion often evident in metabolic syndrome.
The prevalence of the metabolic syndrome is dramatically increasing and is strongly linked to cardiovascular diseases (23). This link persists in females, where it represents a powerful risk factors for cardiovascular disease, even before the onset of menopause (38). Our findings in the present study suggest that the cardiovascular abnormalities evident in the LCR rat model of metabolic syndrome are evident at a relatively young age, in young adult females at 12 wk of age, of relevance to the impairments in insulin sensitivity evident in young women. In conclusion, an inherited impairment in intrinsic exercise capacity per se is associated with progressive development of LV remodeling in rats, in parallel with early microvascular dysfunction and impaired systemic glucose utilization. Impaired microvascular perfusion is a likely contributing factor to the cardiac phenotype. These cardiovascular abnormalities are evident even in young adult female animals. Understanding the potential for exercise training and/or pharmacological insulin-sensitizing approaches to ameliorate these cardiovascular abnormalities over the longer term, alone or in combination with standard care (e.g., targeting the renin-angiotensin and/or β-adrenergic systems), could uncover new translational clues.
GRANTS
This work was supported by National Health and Medical Research Council (NHMRC) of Australia Project Grant ID526638 (to R. H. Ritchie) and National Heart Foundation Grant-in-Aid G-09M-4348 (to J. A. Hawley and O. L. Woodman) and supported in part by the Victorian Government's Operational Infrastructure Support Program. R. H. Ritchie is an NHMRC Senior Research Fellow (ID472673). The LCR-HCR rat model system was funded by the National Center for Research Resources Grant R24 RR017718 and is currently supported by the Office of Research Infrastructure Programs/OD Grant ROD012098A from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.H.R., J.A.H., and O.L.W. conception and design of research; R.H.R., C.H.L., C.Q., M.A.B., K.D.B., and O.L.W. analyzed data; R.H.R., C.H.L., J.A.H., and O.L.W. interpreted results of experiments; R.H.R. prepared figures; R.H.R. and O.L.W. drafted manuscript; R.H.R., C.H.L., C.Q., E.J.S., L.G.K., S.L.B., J.A.H., and O.L.W. edited and revised manuscript; R.H.R., C.H.L., C.Q., E.J.S., M.A.B., K.D.B., S.J.L., D.A.R., L.G.K., S.L.B., J.A.H., and O.L.W. approved final version of manuscript; C.H.L., C.Q., E.J.S., M.A.B., K.D.B., S.J.L., and D.A.R. performed experiments.
ACKNOWLEDGMENTS
We thank Ritu Bala and Amy Alexander for technical assistance. The LCR and HCR model is maintained at the University of Michigan and can be made available for collaborative study (contact: brittons@umich.edu or lgkoch@umich.edu).
Present addresses: S. J. Lessard, Joslin Diabetes Center, 1 Joslin Pl., Boston, MA 02215; D. A. Rivas, John Mayer USDA HNRCA at Tufts University, 711 Washington St., Boston, MA 02111-1524.
REFERENCES
- 1. Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R. Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation. Am J Physiol Heart Circ Physiol 297: H1337–H1346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in type II ZDF rats. Br J Pharmacol 148: 434–441, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burniston JG, Kenyani J, Wastling JM, Burant CF, Qi NR, Koch LG, Britton SL. Proteomic analysis reveals perturbed energy metabolism and elevated oxidative stress in hearts of rats with inborn low aerobic capacity. Proteomics 11: 3369–3379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics 33: 100–109, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Colberg SR. Being active: a commentary. Diabetes Educ 33: 989–990, 2007 [Google Scholar]
- 6. DeMarco VG, Johnson MS, Ma L, Pulakat L, Mugerfeld I, Hayden MR, Garro M, Knight W, Britton SL, Koch LG, Sowers JR. Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol 302: H1667–H1682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edgley AJ, Tare M, Evans RG, Skordilis C, Parkington HC. In vivo regulation of endothelium-dependent vasodilation in the rat renal circulation and the effect of streptozotocin-induced diabetes. Am J Physiol Regul Integr Comp Physiol 295: R829–R839, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Ely DL, Turner ME. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension 16: 277–281, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Goh SS, Woodman OL, Pepe S, Cao AH, Qin CX, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antiox Redox Signal 9: 101–113, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol 4: 215–227, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev 20: 383–393, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hoydal MA, Wisloff U, Kemi OJ, Britton SL, Koch LG, Smith GL, Ellingsen O. Nitric oxide synthase type-1 modulates cardiomyocyte contractility and calcium handling: association with low intrinsic aerobic capacity. Eur J Cardiovasc Prev Rehabil 14: 319–325, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hussain SO, Barbato JC, Koch LG, Metting PJ, Britton SL. Cardiac function in rats selectively bred for low- and high-capacity running. Am J Physiol Regul Integr Comp Physiol 281: R1787–R1791, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Huynh K, Kiriazis H, Du XJ, Love JE, Jandeleit-Dahm KA, Forbes JM, McMullen JR, Ritchie RH. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia 55: 1544–1553, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Huynh K, McMullen JR, Julius TL, Tan JW, Love JE, Cemerlang N, Kiriazis H, Du XJ, Ritchie RH. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes 59: 1512–1520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kemi OJ, Hoydal MA, Macquaide N, Haram PM, Koch LG, Britton SL, Ellingsen O, Smith GL, Wisloff U. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J Cell Physiol 226: 2235–2243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 109: 1162–1172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kowluru RA, Engerman RL, Kern TS. Diabetes-induced metabolic abnormalities in myocardium: effect of antioxidant therapy. Free Radic Res 32: 67–74, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Laskowski A, Woodman OL, Cao AH, Drummond GR, Marshall T, Kaye DM, Ritchie RH. Antioxidant actions contribute to the antihypertrophic effects of atrial natriuretic peptide in neonatal rat cardiomyocytes. Cardiovasc Res 72: 112–123, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Leo CH, Hart JL, Woodman OL. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol 162: 365–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology 150: 4883–4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lessard SJ, Rivas DA, Stephenson EJ, Yaspelkis BB, Koch LG, Britton SL, Hawley JA. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol 300: R175–R182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin EQ, Irvine JC, Cao AH, Alexander AE, Love JE, Patel R, McMullen JR, Kaye DM, Kemp-Harper BK, Ritchie RH. Nitroxyl (HNO) stimulates soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PLoS One 7: 34891–34810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 202: 330–344, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Mamas MA, Deaton C, Rutter MK, Yuille M, Williams SG, Ray SG, New J, Gibson JM, Neyses L. Impaired glucose tolerance and insulin resistance in heart failure: underrecognized and undertreated? J Card Fail 16: 761–768, 2010 [DOI] [PubMed] [Google Scholar]
- 31. McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 32. McPherson GA. Assessing vascular reactivity of arteries in the small vessel myograph. Clin Exp Pharmacol Physiol 19: 815–825, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Mellor K, Ritchie RH, Meredith G, Woodman OL, Morris MJ, Delbridge LM. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition 26: 842–848, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Mellor KM, Ritchie RH, Delbridge LM. Reactive oxygen species and insulin-resistant cardiomyopathy. Clin Exp Pharmacol Physiol 37: 222–228, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Mellor KM, Young MJ, Bell JR, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 50: 1035–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Ritchie RH. Evidence for a causal role of oxidative stress in the myocardial complications of insulin resistance. Heart Lung Circ 18: 11–18, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Ritchie RH, Irvine JC, Rosenkranz AC, Patel R, Wendt IR, Horowitz JD, Kemp-Harper BK. Exploiting cGMP-based therapies for the prevention of left ventricular hypertrophy: NO and beyond. Pharmacol Ther 124: 279–300, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Ritchie RH, Love JE, Huynh K, Bernardo BC, Henstridge D, Kiriazis H, Tham YK, Sapra G, Qin C, Cemerlang N, Boey EJH, Jandeleit-Dahm KA, Du XJ, McMullen JR. Enhanced phosphoinositide 3-kinase (p110α) activity prevents diabetes-induced cardiomyopathy and superoxide generation in a mouse model of diabetes. Diabetologia 55: 3369–3381, 2012 [DOI] [PubMed] [Google Scholar]
- 42. Ritchie RH, Quinn JM, Cao AH, Drummond GR, Kaye DM, Favaloro JM, Proietto J, Delbridge LM. The antioxidant tempol inhibits cardiac hypertrophy in the insulin-resistant GLUT4-deficient mouse in vivo. J Mol Cell Cardiol 42: 1119–1128, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R835–R843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenkranz AC, Hood SG, Woods RL, Dusting GJ, Ritchie RH. B-type natriuretic peptide prevents acute hypertrophic responses in the diabetic rat heart: importance of cyclic GMP. Diabetes 52: 2389–2395, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sen S, Tarazi RC, Khairallah PA, Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Circ Res 35: 775–781, 1974 [DOI] [PubMed] [Google Scholar]
- 46. Stephenson EJ, Stepto NK, Koch LG, Britton SL, Hawley JA. Divergent skeletal muscle respiratory capacities in rats artificially selected for high and low running ability: a role for Nor1? J Appl Physiol 113: 1403–1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am J Physiol Regul Integr Comp Physiol 300: R544–R553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Bibra H, St. John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia 53: 1033–1045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Yada T, Shimokawa H, Morikawa K, Takaki A, Goto M, Ogasawara Y, Kajiya F. Role of Cu/Zn-SOD in the synthesis of endothelium-derived hyperpolarizing factor during reactive hyperemia in mouse mesenteric microvessels in vivo. IFMBE Proc 14: 3496–3499, 2007 [Google Scholar]
- 51. Young EJ, Hill MA, Wiehler WB, Triggle CR, Reid JJ. Reduced EDHF responses and connexin activity in mesenteric arteries from insulin-resistant obese zucker rats. Diabetologia 51: 872–881, 2008 [DOI] [PubMed] [Google Scholar]


