Abstract
Twist1, a basic helix-loop-helix transcription factor, plays a key role during development and is a master regulator of the epithelial-mesenchymal transition (EMT) that promotes cancer metastasis. Structure-function relationships of Twist1 to cancer-related phenotypes are underappreciated, so we studied the requirement of the conserved Twist box domain for metastatic phenotypes in prostate cancer (PCa). Evidence suggests that Twist1 is overexpressed in clinical specimens and correlated with aggressive/metastatic disease. Therefore, we examined a transactivation mutant, Twist1-F191G, in PCa cells using in vitro assays which mimic various stages of metastasis. Twist1 overexpression led to elevated cytoskeletal stiffness and cell traction forces at the migratory edge of cells based on biophysical single-cell measurements. Twist1 conferred additional cellular properties associated with cancer cell metastasis including increased migration, invasion, anoikis resistance, and anchorage-independent growth. The Twist box mutant was defective for these Twist1 phenotypes in vitro. Importantly, we observed a high frequency of Twist1-induced metastatic lung tumors and extra-thoracic metastases in vivo using the experimental lung metastasis assay. The Twist box was required for PCa cells to colonize metastatic lung lesions and extra-thoracic metastases. Comparative genomic profiling revealed transcriptional programs directed by the Twist box that were associated with cancer progression, such as Hoxa9. Mechanistically, Twist1 bound to the Hoxa9 promoter and positively regulated Hoxa9 expression in PCa cells. Finally, Hoxa9 was important for Twist1-induced cellular phenotypes associated with metastasis. These data suggest that the Twist box domain is required for Twist1 transcriptional programs and PCa metastasis.
Keywords: Twist1, Twist box, epithelial-mesenchymal transition, prostate cancer, metastasis, Hoxa9
Introduction
Prostate cancer is the most common cancer diagnosed in men in the United States and is responsible for the second most cancer deaths in men (1). Patterns of disease failure in prostate cancer suggest understanding the determinants that confer progression of localized presentations to metastatic disease will result in the largest therapeutic gains (2).
One mechanism by which cancer cells may acquire the characteristics necessary for metastasis is the epithelial-mesenchymal transition (EMT). EMT is a transcriptional program, crucial in early embryonic development that is co-opted by some cancer cells to facilitate aggressive and metastatic behavior (3). Twist1 is a basic helix-loop-helix (bHLH) multi-domain transcription factor which directly mediates EMT by transcriptional activation and repression of E-box regulated target genes (4, 5). A role for TWIST in prostate cancer pathogenesis has been suggested (6, 7), but the role of EMT and Twist1 in prostate cancer disease progression and metastasis is just now being explored (8, 9). The critical domains of Twist1 and the crucial Twist1 downstream transcriptional targets required for increased tumorigenicity and aggressive metastatic phenotypes in prostate cancer are unknown. The carboxyl-terminal Twist box is a highly conserved domain among Twist1 orthologues for which little functional information in the context of cancer phenotypes is known (5). A greater understanding of the structure-function relationships and downstream targets of Twist1 may allow for an increased appreciation of the mechanisms responsible for Twist1-induced metastasis and may facilitate more precise inhibitory strategies of Twist1 as a therapeutic maneuver in cancer.
Here we used a single amino acid substitution mutation, Twist1 codon 191 phenylalanine-to-glycine (F191G), to study the role of the Twist box for Twist1-induced aggressive cellular and metastatic phenotypes in prostate cancer cells. Isogenic androgen-dependent, Myc-CaP (10), and androgen-independent, PC3, cell lines overexpressing Twist1 or the Twist box mutant demonstrated specific requirements for the Twist box during Twist1-induced metastasis of prostate cancer cells. Gene expression profiling revealed transcriptional programs directed by the Twist box that were associated with cancer metastasis. Finally, we show that Twist1 directly regulates one such target, Hoxa9, which is partially required for Twist1-induced prostate cancer pro-metastatic phenotypes.
Materials and Methods
Plasmids, Antibodies and Reagents
pBABE-Twist1-puro or –hygro (42) was used to construct the Twist1-F191G mutant using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, CA) and confirmed by sequencing. Antibodies used were: Twist (Twist2C1a) (sc-81417, Santa Cruz Biotech), E-cadherin (ab53033, Abcam), vimentin (ab92547), ZO-1 (5406, Cell Signaling Tech.), beta-actin (A5316, Santa Cruz Biotech), c-Myc (N-term) (1472-1, Epitomics), HRP conjugated secondary antibodies (Invitrogen) and Alexa flour 488 conjugated secondary antibodies (Invitrogen). Hoxa9 shRNA retroviral constructs were purchased and used as directed by Origene (cat #TG500979).
Cell Line and Culture Conditions
PC3 and 22RV1 were obtained from American Type Culture Collection (Manassas, VA). Myc-CaP was a kind gift from Dr. John Isaacs (JHU) (10). Growth media: Myc-CaP, DMEM (Invitrogen); PC3, Hams F12K (Invitrogen); and 22RV1, RPMI-1640 (Invitrogen). Cell line identity confirmed by short tandem repeat profiling and mycoplasma tested. All media was supplemented with 10% fetal bovine serum (FBS) and penicillin (100units/ml), streptomycin (0.1 mg/ml). Cells were maintained at 37°C in a humidified incubator with 5% CO2 in air.
Retroviral Experiments
Retroviral production used ecotropic and amphotropic Phoenix packaging lines. Myc-CaP cells were transduced with pGFP-V-RS based shRNA contructs from Origene as described above or with scrambled control vector for two successive times over a 36-h period followed by selection with 1 mg/ml puromycin and passaged once 80% confluent.
Luciferase Promoter Reporter Assay
Sub-confluent cells were transfected using Lipofectamine 2000 (Invitrogen) with 200 ng of firefly luciferase reporter gene construct (100 ng was used for SNAI2 reporter assays), 100 ng of the pRL-SV40 Renilla luciferase construct and 500 ng of the Twist1 or Twist1-F191G mutant expression construct. Cell extracts were prepared 36h after transfection in passive lysis buffer and the reporter activity was measured using the Dual-Luciferase Reporter Assay System (Promega).
Wound-Healing Migration Assay
Two-dimensional migration assay was performed using a scratch/wound model. Cells were grown in 6 well plates for 24 hr to confluence. PC3 cells were treated with 500 pM TGF-beta at the time of wounding. Multiple scratch wounds were created using a P-20 micropipette tip and cells fed with fresh complete media. Five representative fields of the wound were marked and images were taken at 0 and 24-h after wounding. Relative wound closure is calculated from the remaining wound area normalized to the initial wound area using ImageJ software (NIH Image, Bethesda, USA).
Biophysical Assays
Fourier transform traction microscopy (FTTM) was used to measure the contractile stress arising at the interface between each adherent cell and its substrate as described (47). Briefly, cells were plated sparsely on elastic collagen type I coated gel blocks. Images of fluorescent microbeads (0.2 μm in diameter, Molecular Probes, Eugene, OR) embedded near the gel apical surface was taken at different times with cell-free reference (traction-free) images. The displacement field between a pair of images was then obtained by identifying the coordinates of the peak of the cross-correlation function (31, 32). From the displacement field and known elastic properties of the gel (Young's modulus of 1 kPa with a Poisson's ratio of 0.48), the cell traction field was computed. The computed traction field was used to obtain net contractile moment, which is a scalar measure of the cell's contractile strength, expressed in pico-Newton meters (pNm).
Magnetic twisting cytometry (MTC) was used to measure material properties of the cytoskeleton as described (28, 29). In brief, cells were plated at 150,000 cells/cm2 on coated collagen type I plastic wells (96-well Removawell, Immulon II: Dynetech) at 500 ng/cm2. After scratching with a 200 μl pipette tip and the indicated time, ferrimagnetic microbeads were functionalized to the CSK, and both stiffness g’ and loss modulus g” were measured over a physiological range of frequency (f) expressed in Pascal per nm (Pa/nm).
Matrigel Invasion Assay
The invasion potential was assessed using Chemicon cell invasion assay kit (Millipore, USA) as direct by the manufacturer. 8 μM transwells with Matrigel were used for the assay. Serum starved 0.5-1× 106 cells (12-16 hr) in 300 μl were seeded in upper chambers while lower wells were filled with 500 μl of 10% FBS complete medium. Invading cells on the lower surface were fixed and stained. The stain is dissolved in 200 μl of 10% acetic acid and measured at 570 nm. Invasive potential is derived by normalizing with the readings from blank transwell inserts.
Immunohistochemistry (IHC), Immunofluorescence (IF) and Western Blotting
IHC, IF and Western blotting was performed as described previously (48).
Anoikis Assay and Apoptosis Assessment
Anoikis resistance was measured using a modified protocol (49). Cells were grown in normal attachment and ultra-low attachment (Corning, NY) 6 well plates. 24-hr later cells were blocked in 5% FBS and stained with Alexa Fluor 488 conjugated AnnexinV followed by propidium iodide (PI) staining (50μg/ml) (Invitrogen). Cells were enumerated on a BD FACS Caliber (BD Biosciences, San Jose, CA) and analysis was done using FloJo analysis software. All conditions were n=4 and two replicates per experiment.
Clonogenic Survival and Soft Agar Colony Formation Assays
Clonogenic survival was performed as previously described (50). Soft agar clonogenic assays used six well plates pre-coated with 1ml of basal 0.6% agarose in complete media and overlaid with 2 ml of cells (5 × 103 cells/ml) mixed with 0.3% agarose in complete media and allowed to solidify. The wells were constantly fed with complete media to prevent drying of agarose and then after 10-15 days of incubation colonies were scored under phase contrast microscopy. All conditions were repeated at least twice with 3 wells per experiment.
Animal Models and Histology
All procedures were carried out in accordance with the Johns Hopkins Animal Care and Use Committee, maintained under pathogen-free conditions and given food and water ad libitum. For the subcutaneous tumor graft assay, 100 μl of PBS and Matrigel (BD Biosciences) mixed 1:1 containing 0.5-2 ×106 cells were injected subcutaneously into both the flanks of 8 week old male FVB/N or athymic nude mice. Tumors measured 3 times weekly and volume calculated: length × width × height × π/6. Tumor growth delay is the difference between the quadrupling times of untreated versus treated tumors. For the experimental lung metastasis assay, 100 μl of PBS containing 5×105 cells were injected into athymic nude mice via the tail vein. After 4 weeks the mice were sacrificed, necropsies performed to score surface lung tumors and extra-thoracic metastases.
Microarray Data Acquisition and Analysis
Microarrays were performed using GeneChip WT cDNA Synthesis and amplification Kit and WT terminal labeling Kit (Affymetrix, Santa Clara, CA). The labeled ssDNA was hybridized to the GeneChip Mouse Gene 1.0 ST array (Affymetrix, Santa Clara, CA), washed with the Fluidics station 450 and array scanning was performed as previously described. Arrays were normalized using the Robust Multichip Average (RMA) in the oligo Bioconductor package at the transcript level. Genes and gene sets with Benjamini-Hochberg p-values below 0.05 were considered statistically significant. GSEA was performed using the C2 Curated Gene Sets collection from the Molecular Signature Database 3.0 and statistical comparisons by Fisher's Exact Test. More detailed description of the analysis and R code used for this analysis is included as Supplementary Materials.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed using a SimpleChIP Enzymatic IP Kit (Cell Signaling Technology, Danvers, MA). See Supplementary Materials for details.
SYBR-green Quantitative RT-PCR and Prostate Cancer cDNA Arrays
The iTaq Universal SybrGreen Master Mix (BioRad, Hercules, CA) was used according to the manufacturer's instructions. Human normal prostate and prostate cancer qPCR tissue arrays and TWIST1 qPCR oligos were purchased from OriGene. All relevant clinical information can be found (http://www.origene.com/qPCR/Tissue-qPCR-Arrays.aspx).
Statistical Analyses
Statistical analysis was carried out using Graph Pad Prism v5.04 (GraphPad Software, La Jolla CA). Paired comparisons were tested using the Mann-Whitney test or Fisher's exact test. Throughout this study: *, p < 0.05; **, p < 0.01; and ***, p <0.001.
Results
TWIST1 is overexpressed in prostate cancer and correlates with aggressive and metastatic disease
TWIST1 expression in prostate cancers was analyzed from 14 independent microarray datasets constituting 1,013 prostate samples (11-23) using Oncomine. Ten of the 14 datasets and the aggregate analysis showed TWIST1 overexpression in prostate cancers (Figure 1a, p =0.002 for aggregate). Further analysis of one of these microarray studies (11) showed that TWIST1 overexpression correlated with metastatic disease (Figure 1b; p<0.000001).
Figure 1.
TWIST1 is overexpressed in human prostate cancers and correlates with more aggressive and metastatic disease. (a) Human prostate cancer samples (n=700) compared against normal prostate (n=313) from 14 independent microarray datasets for TWIST1 expression using Oncomine. The heatmap contains individual studies (11-23). The heat map intensity corresponds to percentile overexpression (red) or repression (blue). The median rank across all 14 datasets demonstrates TWIST1 is overexpressed in human prostate cancer, p=0.002. (b) Analyzing study #2 (11) from (a) demonstrated that TWIST1 overexpression correlates with metastatic disease, p<0.000001. (c) We validated this microarray analysis by performing qPCR on primary human prostate samples for TWIST1. TWIST1 mRNA is overexpressed in human prostate cancer (n=107) compared to normal prostate (n=24), p<0.0001 by Mann-Whitney t-test. (d) Analysis of data from (c) broken down by Gleason score shows that TWIST1 overexpression correlates with increasing Gleason score, p<0.0001 using one-way ANOVA.
This microarray data was validated using quantitative PCR (qPCR) for TWIST1 on human prostate cancer samples. Cancer samples (n=107) screened by qPCR confirmed that TWIST1 was overexpressed in prostate cancer (40/107 or 37% demonstrated ≥3-fold upregulation, 18/107 or 17% ≥10-fold overexpression and some cases ≥50-fold overexpression; Figure 1c, p<0.0001). Similar to the microarray data, TWIST1 overexpression was directly correlated with prostate cancer aggressiveness as determined by Gleason score (Figure 1d, p <0.0001). These data agreed with prior studies that demonstrated TWIST1 overexpression in human prostate cancer and correlation with prostate cancer disease aggressiveness and metastasis (7, 24).
The Twist box domain is required for full transcriptional activity of Twist1 in prostate cancer cells
Twist1 has four domains: (1) a Twist1 domain that is highly conserved among human and mice; (2) a glycine rich region; (3) bHLH domain; and (4) the Twist box (or WR domain). The Twist box is identical between mouse, human, frog, zebra fish and jellyfish species and is located in the last 23 residues of the mouse polypeptide. The Twist box has been shown to be necessary and sufficient to transactivate E-box containing heterologous reporter constructs in vitro (25). We generated a site specific Twist box mutant by mutating a critical Phe-191 to Gly, referred to as Twist1-F191G (Figure 2a). This Twist1-F191G mutant had been shown previously to be deficient for transactivation of E-box containing reporter constructs in mesenchymal cells (25), but has not been examined in epithelial cancer cells. In Myc-CaP androgen-dependent prostate cancer cells, Twist1 overexpression significantly repressed CDH1 (Figure 2b, left panel, p<0.001) promoter activity (26) and increased SNAI2 (Figure 2b, right panel, p<0.001) promoter activity. Twist1-F191G was found to be defective for both repression and activation in these assays and significantly different from Twist1 (Figure 2b, p <0.05 for both). There was some suggestion that Twist1-F191G was more defective for activation than repression, as Twist1-F191G was still able to repress the CDH1 promoter to some extent but could not activate the SNAI2 promoter as compared to Vector control (Figure 2b, left panel p<0.05 and right panel p=0.0952). Similarly, in HEK 293 cells, Twist1 repressed CDH1 promoter activity whereas Twist1-F191G only partially repressed the CDH1 promoter activity compared to Twist1 wildtype (Supplementary Figure S1a, both p<0.001). Neither Twist1 nor Twist1-F191G seemed to alter expression from the SNAI2 promoter in HEK 293 cells, which is not surprising as this cell line is of likely mesenchymal origin (Supplementary Figure S1b, all p>0.05). These reporter assay data were concordant with levels of the endogenous Cdh1 and Snai2 genes and respective gene products when Myc-CaP cells overexpressed Twist1 or Twist1-F191G stably (Figure 2c and Supplementary Figure S1c-e). Twist1-F191G bound the Cdh1 promoter as well as Twist1 wildtype in Myc-CaP cells according to ChIP-qPCR (Supplementary Figure S1f). These results suggest that the Twist box domain is required for the full transcriptional activity of Twist1.
Figure 2.

The Twist box mutant is deficient for Twist1 transcriptional activity and displays an attenuated EMT cellular marker profile in prostate cancer cells. (a) A schematic of Twist1 protein structure and the position 191 phenylalanine site specific mutant examined (this schematic is not to scale). Key functional residues required for transcriptional transactivation in the Twist box, L-187, F-191 and R-195, are shown in green. We created constructs overexpressing the Twist1-F191G mutant which has the substitution of phenylalanine-191 for glycine. (b) Twist1 promoter reporter assays show that the Twist1-F191G mutant is defective for transcriptional activity. Myc-CaP cells were transiently transfected with expression vectors for the firefly luciferase-linked human E-cadherin gene (CDH1) promoter construct and a Renilla luciferase reporter vector for normalization of transfection efficiency. After 36 hours, cell extracts were assayed for luciferase and Renilla activity and showed Twist1 overexpression repressed transcription from the E-cadherin gene promoter, but the Twist1-F191G mutant was attenuated for this function (*** - p < 0.001 by Mann-Whitney test). Similar reporter assays were performed using a SLUG gene (SNAI2) promoter construct and showed that the Twist1-F191G mutant had no ability to transactivate transcription compared to wildtype Twist1 overexpression (*** - p < 0.001 by Mann-Whitney test). Each bar represents values from five-six independent experiments performed in triplicate. Bars represent column mean; error bars ± SEM. Western blot analysis was performed for Twist1 expression in (c) Myc-CaP (left panels) and PC3 (right panels) cells stably expressing Vector and overexpressing similar levels of Twist1 or Twist1-F191G with β-actin used as a loading control. Epithelial and mesenchymal markers were also assessed by (c) Western blotting and (d) immunofluorescence for Twist1, E-cadherin, ZO-1 and Vimentin in Myc-CaP (left panels) and PC3 (right panels) cells.
The Twist box mutant is partially defective for induction of EMT markers in prostate cancer cells
Metastasis is a complex series of discrete events that a neoplastic cell must traverse (27). These serial events include: loss of cell-to-cell adhesion, migration and invasion into the local extracellular matrix, intravasation into the vasculature, resistance to anoikis, extravasation into the parenchyma of distant tissues and then colonization into a macroscopic metastatic tumor. To ascertain the role of the Twist box domain in a subset of these metastatic steps in vitro and in vivo, stable isogenic cell lines expressing Twist1 and Twist1-F191G in Myc-CaP and PC3 prostate cancer cells were established (Western blot, Figures 2c; and immunofluorescence, Figures 2d, top row panels and Supplementary Figure S2a & b). Consistent with an EMT marker profile, stable Twist1 overexpression led to downregulation of epithelial markers E-cadherin and ZO-1 in Myc-CaP cells and in PC3 cells and upregulation of the mesenchymal marker vimentin in both Myc-CaP and PC3 cells (Western and IF shown in Figures 2c & d and qPCR shown in Supplementary Figure S1c and S3a). The Twist box mutation, Twist1-F191G, resulted in a reduced ability to downregulate E-cadherin and ZO-1 and upregulate vimentin in Myc-CaP cells (Figures 2c & d and Supplementary Figure S1c and S3a). A similar, but less dramatic loss of an EMT marker profile was seen with PC3 cells stably overexpressing Twist1-F191G (Figures 2c & d, Supplementary Figure S2c & d show IF quantification of PC3 cells and S3b & c show qPCR for CDH1 and VIM). The findings from these prostate cancer cell lines overexpressing Twist1 and Twist1-F191G suggest that the Twist1 box domain is required for Twist1 to induce a full EMT marker profile.
Twist1 overexpression increases cellular motility that is partially defective in the Twist1-F191G mutant
We next examined Twist1-induced cell migration and correlates of cell mechanics using the scratch/wound healing model. For this study, we made a scratch into an ensemble of confluent Myc-CaP cells (Figure 3a) and measured the spatiotemporal changes in forced motions of microbeads anchored to the cytoskeleton (CSK) through integrin cell adhesion receptors (28, 29). Using magnetic twisting cytometry (MTC), we measured cytoskeletal stiffness (g’) and internal friction (g”) before, immediately after and 24-hour after making a scratch. Over five decades of frequency, we found no differences in stiffness g’ and friction g” between isogenic Myc-CaP cell ensembles (Twist1 versus Vector) before or immediately after a scratch (Supplementary Figure S4a & b). By 24-hours, however, Twist1 overexpressing cells infiltrated more into the site of the scratch wound than Vector control cells (Figure 3a) and showed appreciably higher cytoskeletal stiffness (Figure 3b and Supplementary Figure S4c) and friction (Figure 3c and Supplementary Figure S4c). At 24-hour after making a scratch, Twist1 overexpressing cell ensembles exhibited a 1.6-fold higher stiffness than Vector control ensembles (Figure 3d). Most striking was the greatest differences of cytoskeletal stiffness between Twist1 and Vector control cell ensembles were localized at the leading migratory front (Figure 3a & e). Twist1 overexpressing cell ensembles had progressively decreasing cytoskeletal stiffness with increasing distance from the leading migratory edge (1st > 2nd > 3rd > 4th cell layers, Figure 3a & e).
Figure 3.
Twist1 overexpression induces spatiotemporal changes in the material properties of the living cytoskeleton (CSK) during cellular migration of prostate cancer cells. (a) Representative bright field images of Myc-CaP cells 24 hr after wound scratch. Using magnetic twisting cytometry (MTC), stiffness g’ (b) and loss modulus g” (c) were measured over a physiological range of frequency (f). Open and closed squares represent g’ and g” of Myc-CaP overexpressing Twist1 cells. Open and closed circles represent g’ and g” of Vector control cells. The lines are the fit of experimental data to the structural damping equation with addition of a Newtonian viscous term as previously described (28). Fitting was carried out by non-linear regression analysis. The colors indicate the respective cell layer from the migrating front (as shown in a). Data are presented as Median (1st layer: Vector n=73, Twist1 n=77; 2nd layer: Vector n=119, Twist1 n=168: 3rd layer: Vector n=101, Twist1 n=87; 4th layer: Vector n=44, Twist1 n=64). (d) Stiffness of Vector and Twist1 expressing Myc-CaP cells probed at 0.75 Hz, following scratch wound (T, 0 hr) and 24-hr after. Data are presented as Geometric Mean + SE (Vector: T 0 hr n=169, T 24 hr n=337; Twist1: T 0 hr n=240, T 24 hr n=396). (e) Spatial distribution of cell stiffness 24-hr after making a scratch in Vector and Twist expressing Myc-CaP cells [data are presented as Median and are same as in (b)]. (f) Scratch wound healing assay was performed in Myc-CaP isogenic cell lines and representative images shown at 0-hr and 24-hr. (g) Relative wound closure is calculated by the remaining wound area normalized to the initial wound area (n=3, 3 fields; * - p <0.05 by Mann-Whitney test) by ImageJ software (NIH) and showed that Myc-CaP cells overexpressing Twist1-F191 cells were less migratory than wildtype Twist1 cells. (h) Twist1 overexpression increases single prostate cancer cell traction forces on the substratum which is attenuated by the Twist box mutation Twist1-F191G. The cell traction forces for individual cells (n=20-21) is measured by using Fourier transform traction microscopy (FTTM). The top panel shows representative phase contrast images of Myc-CaP isogenic cell lines. The bottom panel shows the traction maps; the colors within the cells represent the absolute magnitude of tractions in Pascals, and the arrows represent the relative magnitude and directions. (i) Twist1 overexpression increases the mean of the projected area represented in bar graph format and the Twist box mutant isogenic cell line is attenuated for this phenotype. (j) Twist1 overexpression increases cell traction force exerted by a single living cell, or net contractile moment, and the Twist1-F191G mutant is completely deficient for this function. Bars represent column mean; error bars are ± SEM. The values are significant by Mann Whitney test: *, p < 0.05; **, p < 0.01; and ***, p <0.001.
We then directly assessed the requirement of the Twist box domain for Twist1-induced cell invasive potential using the scratch/wound healing assay in vitro. Importantly, Twist1 overexpression did not increase the proliferative potential of Myc-CaP or PC3 cells (Supplementary Figure S5). Myc-CaP cells overexpressing Twist1 migrated 2.5-fold faster than Vector control Myc-CaP cells (Figure 3f & 3g, p <0.05). The Twist1-F191G overexpressing cells were significantly less migratory than Twist1 overexpressing cells, but still migrated more than the Vector (1.5-fold) (Figure 3f & g, both comparisons p<0.05). The same trends for cell migration were also observed in PC3 cells (Supplementary Figure S6).
Cancer cell migration and invasion entail the ability of malignant cells to exercise contractile force upon their surroundings (30). Using traction microscopy (31, 32), we interrogated the force generating capacity of single Myc-CaP and PC3 cells overexpressing Twist1 (Figure 3h-j and Supplementary Figure S7). Compared with Vector, the Twist1 overexpressing Myc-CaP and PC3 cells showed increased cell spreading area and net contractile moment, a scalar measure of the cell's contractile strength (Figure 3i & j, p<0.01 both measurements and Supplementary Figure S7b & c, p<0.001 both measurements). The Twist box mutant displayed less cell spreading and lower net contractile moment compared to Twist1 (Figure 3i & j, p<0.05 for both measurements). These single cell biophysical data corroborated our results with bulk migration assays. Collectively, these data suggested that the Twist box domain was required for increasing cytoskeletal force generation in prostate cancer cells that was associated with the full migratory potential of Twist1.
The Twist box is required for Twist1-induced prostate cancer invasion, cell death resistance and anchorage-independent growth in vitro
Both Myc-CaP and PC3 cells overexpressing Twist1 showed an increased invasiveness compared to Vector control cells using a Matrigel coated transwell invasion assay (Figure 4a & b, both p<0.05). The Twist box mutant was completely defective for invasion in Myc-CaP cells compared to Twist1 wildtype (Figure 4a, p<0.05) and trended towards being less invasive in PC3 cells (Figure 4b, p=0.156). Similar to motility, the Twist box is at least partially required for Twist1-induced prostate cancer invasion.
Figure 4.
The Twist box mutant is defective for Twist1-induced invasion, anoikis resistance and soft agar tumorigenicity. Transwell invasion assays with Matrigel were performed with isogenic (a) Myc-CaP (n=7) and (b) PC3 cells (n=6). The Myc-CaP cells were allowed to invade for 24 hrs and PC3 cells for 60 hrs. Twist1 overexpression increased invasion into Matrigel for both Myc-CaP and PC3 cells, but the Twist1-F191G mutant conferred less invasive ability to these cells (represented by column mean ± SEM, * - p <0.05 by Mann-Whitney test). Cells were grown adherent or in suspension using ultra low attachment dishes. The amount of apoptotic cell death or anoikis for the ultra-low attachment conditions was quantified by AnnexinV- AlexaFluor 488 and propidium iodide staining followed by flow cytometric analysis. Representative dot plots of (c) Myc-CaP and (e) PC3 Twist1 isogenic cell lines are shown. Percent of cells in quadrants II (early apoptotic) and III (late apoptotic) that constitute apoptotic fractions are in bold. Percent apoptosis was calculated by normalizing total apoptotic fraction in ultra-low attachment conditions to that of adherent cells and plotted as bar graph ± SEM for (d) Myc-CaP (n=8) and (f) PC3 (n=6) Twist1 isogenic cell lines (*, p < 0.05; and **, p < 0.01 by Student t-test). 5×105 cells were embedded in soft agar and incubated for 2 weeks. Colonies containing above 50 cells were scored in at least 5 random fields. Representative phase contrast images of (g) Myc-CaP and (i) PC3 Twist1 isogenic cell lines at 40 × magnifications are shown. The percent clonogenicity in soft agar is calculated by normalizing the number of colonies to the total number of cells and represented as bar graphs ± SEM for (h) Myc-CaP (n=6) and (j) PC3 (n=6) (*, p < 0.05; **, p < 0.01; and ***, p <0.001 by Mann-Whitney test).
The resistance to anoikis facilitates metastasis of cancer cells to distant organs. Both Twist1 overexpressing Myc-CaP and PC3 cells showed decreased apoptosis when grown in suspension compared to their isogenic Vector control cells (Figure 4c-f, both cell lines p<0.05). The Twist1-F191G mutant in Myc-CaP and PC3 cells were similar to their isogenic Vector control cells (Figure 4c-f, both cells p>0.47) for anoikis resistance. We also observed that the Twist box was required to confer radioresistance to prostate cancer cells (Supplementary Figure S8). Altogether, these data show that Twist1 overexpression can confer resistance to multiple cell death stimuli and that the Twist box domain is required for these Twist1 activities in prostate cancer cells.
The in vitro anchorage-independent growth of Myc-CaP and PC3 cells stably overexpressing Twist1 and the Twist box mutant was performed (Figure 4g-j). Both Twist1 overexpressing Myc-CaP and PC3 cells showed increased frequency of colonies in soft agar compared to their isogenic Vector control cells (Figure 4g-j, both cells p<0.01). In addition, Myc-CaP cells overexpressing Twist1 had colonies of larger size (Figure 4g). Myc-CaP and PC3 Twist box mutant cells had a similar frequency of colonies in soft agar compared to their isogenic Vector control cells (Figure 4g-j, both cells p>0.20). These general results were repeated and confirmed in a third prostate cancer cell line, 22Rv1, stably overexpressing Twist1 and Twist1-F191G (Supplementary Figure S9). These data further confirm the importance of the Twist box domain for aggressive in vitro prostate cancer cell behavior induced by Twist1.
The Twist box is required for Twist1-induced prostate cancer metastasis in vivo
Using a subcutaneous tumor graft assay we did not observe Twist1 or Twist1-F191G overexpression increasing the in vivo primary tumorigenic potential or primary tumor growth of Myc-CaP, PC3 or 22Rv1 cells (Supplementary Figure S10). The metastatic potential of Twist1 and Twist1-F191G overexpressing Myc-CaP cells was assessed using the experimental lung metastasis assay. Twist1 overexpression significantly increased the ability of Myc-CaP cells to colonize the lungs and form macroscopic metastases in vivo (Figure 6a & b, 10/18 mice with Twist1 overexpressing Myc-CaP cells versus 2/17 mice with isogenic Vector control cells, p=0.0116). The Twist box mutant overexpressing Myc-CaP cells lost some potential to form macroscopic lung metastases in vivo and had an intermediate phenotype to Vector and Twist1 Myc-CaP cells (Figure 6b, 4/12 mice with Twist1-F191G overexpressing Myc-CaP cells, p>0.2). The tumor cell morphology from Twist1 and Twist1-F191G overexpressing cells was not different (Figure 6c). Interestingly, mice injected tail vein with Twist1 overexpressing Myc-CaP cells showed extra-thoracic metastases to distant subcutaneous tissues, abdominal organs and distant lymph nodes (Figure 6d-f). Cells injected in the venous circulation seed the lungs and therefore must undergo the full metastatic pathway in order to produce extra-thoracic metastases. Twist1 overexpression significantly increased the frequency of mice with extra-thoracic metastases (Figure 6e, 11/18 mice with Twist1 overexpressing Myc-CaP cells compared to 1/17 mice with isogenic Vector control cells, p=0.0009). The Twist box was required for Myc-CaP cells to undergo the full metastatic pathway and give rise to extra-thoracic metastases (Figure 6e, 2/12 mice injected with Twist1-F191G overexpressing Myc-CaP cells, p=0.0256 compared to Twist1; and p=0.553 compared to Vector control cells). The Myc-CaP identity of lung tumors and extra-thoracic metastases was confirmed by Myc IHC (Figure 6g). These results show that the Twist box domain is required for Twist1-induced metastasis of prostate cancer cells in vivo.
Figure 6.
The Twist1 global gene expression profile in Myc-CaP prostate cancer cells is greatly attenuated by a mutation in the Twist1 box domain. (a) Gene expression analysis of Myc-CaP cells stably expressing Vector, Twist1 (WT) and Twist1-F191G by microarray revealed a larger set of genes that were differentially expressed between Twist1 overexpressing cells and Vector control compared to the Twist1-F191G mutant and Vector control cells. Gene expression between groups was performed by empirical Bayesian moderated ANOVA, and genes were considered differentially expressed if B > 0 following Benjamini-Hochberg FDR. (b) Heatmap visualization of supervised clustering analysis of gene expression from Myc-CaP cells expressing Vector, Twist1 (WT) and Twist-1-F191G show the Twist1 box mutant Twist1-F191G has a gene expression profile more similar to the Vector control cells. Each column represents a Myc-CaP microarray sample, and each row represents median-centered expression values for a single gene. High expression is indicated in green, intermediate expression in black and low expression in red. (c) Selected gene sets from the curated Molecular Signatures database that were overrepresented (p < 0.05, one-way Fisher's exact test, Benjamini-Hochberg FDR) in the set of genes differentially regulated by Twist1 but not by Twist1-F191G that are relevant to phenotypic differences between prostate cancer cells over expressing Twist1 versus Twist1-F191G as demonstrated in this study (relative overexpression is indicated in green and relative repression by red). Twist1 but not Twist1-F191G overexpression resulted in Hoxa9/HOXA9 overexpression in (d) Myc-CaP and (e) PC3 cells. (f) Schematic of the promoter region of Hoxa9 with E-box containing regions (ER). Twist1 and Twist1-F191G binds to ER4 and ER5 by ChIP-qPCR. Inverted triangles are E-box sequences and arrows represent qPCR oligo sets flanking each ER. * - indicates p<0.05 as compared to Vector; and # - indicates p<0.05 as compared to Twist1. (g) Summary of phenotypes for Twist1 and the Twist box mutant in Myc-CaP and PC3 prostate cancer cells. T – Twist1, F – Twist1-F191G mutant and V – Vector control.
Gene expression profiling reveals Hoxa9 as a direct target of Twist1 that is partially required for Twist1-induced pro-metastatic phenotypes
Global gene expression analysis on the isogenic Myc-CaP lines revealed several genes differentially expressed in each pair-wise comparison. Compared with Vector control, Twist1 overexpression altered the expression of 424 genes. Twist1-F191G overexpression compared against Vector control altered the expression of much fewer genes, 53 genes, and the majority (28/53) of these genes were also observed with Twist1 overexpression. Between Twist1 and Twist1-F191G, 158 genes were altered, of which the majority were also altered in Twist1 (81/158) (Figure 6a and Supplementary Tables S1 & S2). This expression pattern is consistent with Twist1-F191G having a greatly attenuated transcriptional program compared to wildtype Twist1 (Figure 6b). These global gene expression profiling data are consistent with our promoter reporter assays performed above suggesting that the Twist box domain is required for the full transcriptional activity of Twist1 (Figure 2b and Supplementary Figure S1). These findings are highly suggestive of a transcriptional mechanism for the phenotypic differences observed between prostate cancer cells overexpressing Twist1 and those overexpressing the Twist box mutant.
Gene set enrichment analysis (GSEA) (33) was used to identify gene sets which were overrepresented in Twist1 but not Twist1-F191G. Many of the overrepresented gene sets were related to phenotypes which we directly assayed, aggressive cellular behavior and metastasis, and were observed with overexpression of Twist1 but not Twist1-F191G (Figure 6c). One gene set of interest was directed by the homeobox transcription factor Hoxa9, which is strongly implicated in leukemogenesis. Furthermore, the Hox homolog HOXA5 had been shown previously to interact physically with Twist and antagonize repression of p53 to genotoxic stressors. Thus, we confirmed our microarray data by qPCR and Western showing that Twist1 overexpression resulted in Hoxa9/HOXA9 overexpression in Myc-CaP and PC3 (Figure 6d & e, both p<0.01 by qPCR and Supplementary Figure S11a & b by Western). Twist1 also bound to the Hoxa9 promoter in a region containing canonical E-box sequences as shown by chromatin immunoprecipitation (ChIP)-qPCR (Figure 6f). Consistent with our global gene expression data the Twist box mutant was unable to upregulate the expression of Hoxa9/HOXA9 overexpression in Myc-CaP and PC3 and was similar to Vector control (Figure 6d & e, both p<0.05 by qPCR and Supplementary Figure S11a & b by Western). However, the Twist1-F191G mutant was still capable of binding to the Hoxa9 promoter by ChIP-qPCR, suggesting the Twist box was required for the full transcriptional activity of Twist1 (Figure 6f and summarized differences between in vitro and in vivo phenotypes of Twist1 and Twist1-F191G in Figure 6g).
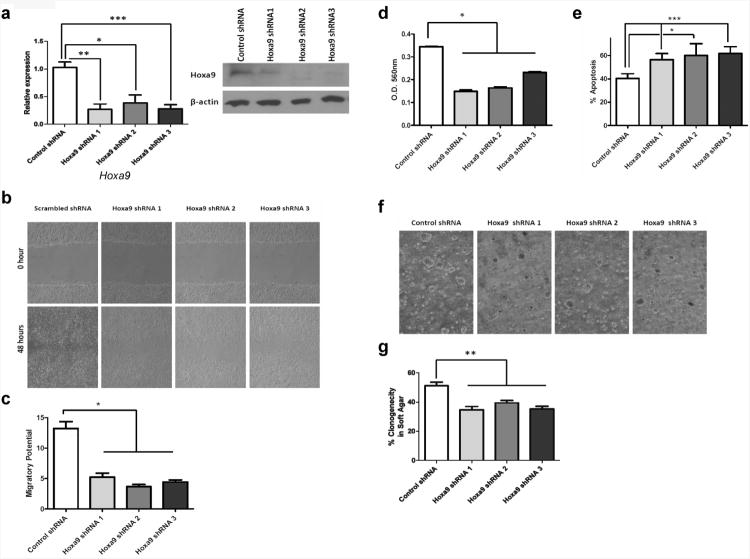
Interestingly, we found that many of the in vitro pro-metastatic phenotypes of Twist1 overexpression in Myc-CaP cells were significantly blunted following shRNA mediated knockdown of Hoxa9 (Figure 7). Three separate shRNA constructs against Hoxa9 were each able to knockdown Hoxa9 mRNA and protein expression in Myc-CaP cells overexpressing Twist1 (Figure 7a, p<0.05 for qPCR). Hoxa9 knockdown in these Twist1 overexpressing cells resulted in a reduction in Twist1-induced cellular migration, invasion, anoikis resistance and soft agar clonogenicity (Figure b-g, p<0.05 all at least). Collectively, these data suggest that Twist1 imparts pro-metastatic phenotypes on prostate cancer cells in part by directly upregulating Hoxa9 expression.
Figure 7.
Hoxa9 is partly required for Twist1-induced migration, invasion, anoikis resistance and soft agar tumorigenicity of Myc-CaP cells. (a) Myc-CaP cell stably overexpressing Twist1 were transduced with shRNA constructs against Hoxa9 or scrambled control and relative expression determined by Hoxa9 qPCR (left panel, n≥3) and Hoxa9 Western blotting (right panel). (b) Scratch wound healing assay of Myc-CaP + Twist1 isogenic cell lines and representative images shown at 0-hr and 48-hr. (c) Relative wound closure calculations (as in Figure 3; all n=3, 3 fields). (d) Transwell invasion assays with Matrigel were performed with isogenic Myc-CaP + Twist1 cells (n=6, represented by column mean ± SEM). (e) Cells were grown adherent or in suspension using ultra low attachment dishes and anoikis quantified by AnnexinV- AlexaFluor 488 and propidium iodide staining (as in Figure 4) plotted as bar graphs ± SEM. 5X105 cells were embedded in soft agar and incubated for 2 weeks. Colonies containing above 50 cells were scored in at least 5 random fields. (f) Representative phase contrast images 40 × magnifications are shown. (g) The percent clonogenicity in soft agar is calculated by normalizing the number of colonies to the total number of cells and represented as bar graphs ± SEM (n≥4). All comparisons, *, p < 0.05; **, p < 0.01; and ***, p <0.001 by Mann-Whitney test.
Discussion
Our study shows that Twist1 overexpression in prostate cancer cells induces an EMT phenotype, augments migration, invasion, resistance to anoikis and metastasis. We show that the highly conserved Twist box domain is required for many of these properties of Twist1 associated with aggressive tumor cell behavior in vitro and most importantly for metastasis in vivo. We also demonstrate that the Twist box domain is required for the full transcriptional activity of Twist1 and facilitates these pro-metastatic cellular functions by directing specific transcriptional programs. We show that Twist1 directly regulates the transcriptional pro-metastatic target Hoxa9 which is at least partially required for Twist1-induced pro-metastatic phenotypes in prostate cancer cells.
The Twist box is highly conserved among vertebrates and is critical for the role of Twist1 in development as demonstrated by inactivating mutations in this region of the human gene resulting in the Saethre-Chotzen syndrome, characterized primarily by craniosynostosis (5, 34). Consistent with humans, the Charlie Chaplin mouse strain with craniosynostosis and hind-limb abnormalities results from a S192P substitution mutation in the Twist box domain (35). Mechanistically, Twist1 binds to the Runx2 transcription factor via the Twist box and inhibits the Runx2 transcriptional program necessary for osteoblast differentiation. Similarly, Twist1 binds Sox9 via the Twist box and inhibits Sox9-dependent transcriptional programs required for chondrocyte differentiation (36). Twist1 may also directly modulate transcription of target genes and the Twist box was shown to be both necessary and sufficient for this transactivation activity (25). The Twist box transactivation domain likely adopts an α-helical structure and the three amino acids Leu-187; Phe-191; Arg-195 are essential for transactivation function and may occupy the same three-dimensional surface of this α-helix. Twist1 has been shown to directly upregulate the expression of several target genes important for cancer progression like Akt2 expression, which enhances cell migration, invasion and resistance to chemotherapy (37). In agreement with these findings in breast cancer, our expression profiling of Twist1 overexpressing prostate cancer cells were similar to gene signatures consistent with increased cell migration, invasion and resistance to apoptosis. However, whether the Twist box mediates a transcriptional program required for Twist1-induced metastasis in prostate cancer cells by actively inhibiting another transcription factor or by directly regulating downstream target genes requires further study.
The role of the Twist box in cancer related functions have only recently been appreciated. In a recent study, the Twist box was required for Twist1 binding to the NF-kB subunit RELA to activate transcriptional activity, increased DNA binding affinity to the interleukin 8 (IL-8) promoter and transcriptional activation that was required for breast cancer cell invasion in vitro (38). We did not observe direct Twist box-dependent regulation of IL-8 by Twist1 in our system, but in agreement with this study, we did observe Twist box-dependent gene sets consistent with NF-kB regulated inflammatory genes. The Twist box has also recently been shown to bind p53 and induce destabilization via MDM2-mediated degradation in sarcoma cells (39). This mechanism is separate from Twist1 indirect regulation of p53 by modulating the ARF/MDM2/p53 pathway (40). We do not believe that Twist box-dependent p53 regulation explains the Twist1-induced metastatic phenotypes we observed in prostate cancer cells as PC3 cells are TP53-null and a similar Twist1-F191L substitution mutation in the Twist box did not affect Twist1-p53 interaction (39). Our study findings require validation in other cancer histologies before our results can be generalized further and confirmation using autochthonous transgenic models of tumorigenesis and spontaneous metastasis models are needed. Despite these limitations, our current study confirms the recent in vitro data demonstrating the importance of the Twist box domain for the pro-tumorigenic activities of Twist1. Importantly, our study shows for the first time the requirement of the Twist box for the pro-metastatic functions of Twist1 in vitro and in vivo.
TWIST1 expression levels appear to be correlated with prostate cancer aggressiveness and factors associated with lethal metastatic disease [Figure 1 and (7, 24)]. The down-regulation of Twist1 in androgen independent prostate cancer cells increased their sensitivity to anticancer drugs and suppressed their migration and invasion abilities, suggesting Twist1 inactivation as a potential therapeutic strategy (7). TWIST1 expression during postnatal life is restricted tightly to a subpopulation of mesoderm derived tissues and limited studies suggest that Twist1 inhibition systemically may be well tolerated (41). Furthermore, our previous study suggested that suppression of Twist1 to physiological levels in vivo is sufficient for anti-cancer effects (42). However, the direct inhibition of Twist1 as a therapeutic maneuver still poses a few potential challenges. First, Twist1 is a pleiotropic transcription factor essential for mammalian development (43). Secondly, bHLH transcription factors have been difficult to target directly with small molecules (44). A solution to these issues are dissecting what are the critical domains of Twist1 and what are the crucial Twist1 downstream transcriptional targets that are required for Twist1-dependent tumorigenicity and pro-metastatic functions.
Using comparative gene expression profiling of Twist1 and Twist box mutant cells we discovered Hoxa9 as a novel direct gene target of Twist1 that was required in part for many Twist1-induced prostate cancer pro-metastatic phenotypes in vitro. Although there is a rich literature on the oncogenic role of Hoxa9 in leukemia (45), only one recent report has suggested a role for Hoxa9 in prostate cancer (46). Thus, we have uncovered a novel mechanism involving Twist1 and Hoxa9 oncogenes collaborating to facilitate prostate cancer progression and metastatic cellular behavior.
In conclusion, these data herein have increased our insight into the structure-function relationships of the Twist1 oncoprotein in cancer and point to the Twist box as a critical domain required for directing transcriptional pro-metastatic programs in prostate cancer cells. Our findings suggest therapeutic measures against TWIST1 overexpressing prostate cancer cells should be minimally directed against the Twist box domain and Twist1 regulated transcriptional targets such as Hoxa9.
Supplementary Material
Figure 5.
Twist1 overexpression confers metastatic ability to Myc-CaP prostate cancer cells in vivo that is dependent on the Twist box domain. The experimental lung metastasis assay was performed with Myc-CaP Twist1 isogenic cell lines. 5 × 105 cells were tail vein injected into 8 week old athymic nude male mice, sacrificed 4 weeks later and inspected for lung colonization and extra-thoracic metastases. Cohorts of 4-6 mice were used for each cell line and experiments performed three times. (a) Representative necropsy photographs of the lungs with lung tumors distinguished by black arrows. (b) A table comparing the ability of the three isogenic cell lines to colonize lung tumors in vivo from (a). Twist1 overexpressing Myc-CaP cells are able to form macroscopic lung tumors in vivo in a much higher frequency of mice (10/18 or 55.6%) than Vector control cells (2/17 or 11.8%; p= 0.0116 by Fisher's exact test). The Twist1 box mutant Myc-CaP cells had an intermediate phenotype in vivo (4/12 or 33.3% of mice; p= 0.198 compared to Vector and p= 0.2839 compared to wildtype Twist1 by Fisher's exact test). (c) Representative H&E images of lung samples from (a) with insets showing magnified views of lung tumors. (d) Representative necropsy photographs of extra-thoracic metastases from mice injected with Twist1 isogenic cells with metastases indicated by black arrows. These extra-thoracic metastases represent the consequence of prostate cancer cells undergoing the full metastatic pathway following tail vein injection. (e) A table comparing the ability of the three isogenic Myc-CaP cell lines to form extra-thoracic metastases from (d). Twist1 overexpression conferred Myc-CaP cells with the ability to form extra-thoracic metastases at a higher frequency in mice (11/18 or 61.1%) than Vector control cells (1/17 or 5.9%) and the Twist1 box mutant overexpressing cells (2/12 or 16.7%; p= 0.0009 for Twist1 versus Vector and p=0.0256 for Twist1 versus Twist1-F191G by Fisher's exact test). (f) Representative H&E image of a Twist1-induced extra-thoracic metastasis with the inset showing a magnified image. (g) Representative anti-Myc immunohistochemistry images of lungs isolated from mice tail vein injected with Myc-CaP + Vector cells (left) or Myc-CaP + Twist1 cells (right). The lung tumor (and extra-thoracic metastases not shown) stained positive for c-Myc, confirming the tumor cells were Myc-CaP cells.
Acknowledgments
Funding: RSNA Research Medical Student Grant (ATW); Johns Hopkins Laboratory Radiation Oncology Training Fellow NIH-T32CA121937 (RDW); NIH P50CA103175 and U54CA141868 (SSA); and Patrick C. Walsh Prostate Cancer Research Fund, DoD Prostate Cancer Physician Research Training Award W81XWH-11-1-0272, ACS Scholar 122688-RSG-12-196-01-TBG and the NIH P50CA103175 (PTT).
Sources of funding: AT Wild was funded by an RSNA Research Medical Student Grant. RD Williams was a Johns Hopkins Laboratory Radiation Oncology Training Fellow (NIH-T32CA121937). SS An was funded by the NIH (P50CA103175 and U54CA141868). PT Tran was funded by the Phyllis and Brian L. Harvey Scholar Award from the Patrick C. Walsh Prostate Cancer Research Fund, a DoD Prostate Cancer Physician Research Training Award (W81XWH-11-1-0272), an ACS Scholar award (122688-RSG-12-196-01-TBG) and the NIH (P50CA103175).
Footnotes
Implicaiton statement: Targeting the Twist box domain of Twist1 may effectively limit prostate cancer metastatic potential.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999 May 5;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009 Nov 25;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Ansieau S, Morel AP, Hinkal G, Bastid J, Puisieux A. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010 Jun 3;29(22):3173–84. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 5.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2011 Jan;22(1):90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005 Jun 15;65(12):5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 7.Shiota M, Yokomizo A, Tada Y, Inokuchi J, Kashiwagi E, Masubuchi D, et al. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene. 2009 Oct 5; doi: 10.1038/onc.2009.322. [DOI] [PubMed] [Google Scholar]
- 8.Shiota M, Zardan A, Takeuchi A, Kumano M, Beraldi E, Naito S, et al. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012 Oct 15;72(20):5261–72. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 9.Xie D, Gore C, Liu J, Pong RC, Mason R, Hao G, et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2010 Feb 9;107(6):2485–90. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson PA, Ellwood-Yen K, King JC, Wongvipat J, Lebeau MM, Sawyers CL. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005 Dec 15;65(24):11565–71. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- 11.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2004 Jan 20;101(3):811–6. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arredouani MS, Lu B, Bhasin M, Eljanne M, Yue W, Mosquera JM, et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin Cancer Res. 2009 Sep 15;15(18):5794–802. doi: 10.1158/1078-0432.CCR-09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, et al. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001 Aug 1;61(15):5692–6. [PubMed] [Google Scholar]
- 14.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004 Jul 15;22(14):2790–9. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 15.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, et al. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001 Aug 15;61(16):5974–8. [PubMed] [Google Scholar]
- 16.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003 Jul 15;63(14):3877–82. [PubMed] [Google Scholar]
- 17.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer cell. 2005 Nov;8(5):393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010 Jul 13;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, et al. Gene expression correlates of clinical prostate cancer behavior. Cancer cell. 2002 Mar;1(2):203–9. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 20.Luo JH, Yu YP, Cieply K, Lin F, Deflavia P, Dhir R, et al. Gene expression analysis of prostate cancers. Mol Carcinog. 2002 Jan;33(1):25–35. doi: 10.1002/mc.10018. [DOI] [PubMed] [Google Scholar]
- 21.Liu P, Ramachandran S, Ali Seyed M, Scharer CD, Laycock N, Dalton WB, et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006 Apr 15;66(8):4011–9. doi: 10.1158/0008-5472.CAN-05-3055. [DOI] [PubMed] [Google Scholar]
- 22.LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, et al. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002 Aug 1;62(15):4499–506. [PubMed] [Google Scholar]
- 23.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008 Feb 1;68(3):927–36. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 24.Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007 Apr;50(5):648–58. doi: 10.1111/j.1365-2559.2007.02665.x. [DOI] [PubMed] [Google Scholar]
- 25.Laursen KB, Mielke E, Iannaccone P, Fuchtbauer EM. Mechanism of transcriptional activation by the proto-oncogene Twist1. The Journal of biological chemistry. 2007 Nov 30;282(48):34623–33. doi: 10.1074/jbc.M707085200. [DOI] [PubMed] [Google Scholar]
- 26.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochemical and biophysical research communications. 2008 Mar 7;367(2):235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011 Mar 25;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 28.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001 Oct 1;87(14):148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 29.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? American journal of respiratory cell and molecular biology. 2006 Jul;35(1):55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mierke CT, Rosel D, Fabry B, Brabek J. Contractile forces in tumor cell migration. European journal of cell biology. 2008 Sep;87(8-9):669–76. doi: 10.1016/j.ejcb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. American journal of physiology. 2002 Mar;282(3):C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American journal of physiology. 2002 Mar;282(3):C606–16. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005 Oct 25;102(43):15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nature genetics. 1997 Jan;15(1):36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 35.Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, et al. A twist code determines the onset of osteoblast differentiation. Developmental cell. 2004 Mar;6(3):423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 36.Gu S, Boyer TG, Naski MC. Basic helix-loop-helix transcription factor Twist1 inhibits transactivator function of master chondrogenic regulator Sox9. The Journal of biological chemistry. 2012 Jun 15;287(25):21082–92. doi: 10.1074/jbc.M111.328567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007 Mar 1;67(5):1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Kendall SE, Raices R, Finlay J, Covarrubias M, Liu Z, et al. TWIST1 associates with NF-kappaB subunit RELA via carboxyl-terminal WR domain to promote cell autonomous invasion through IL8 production. BMC Biol. 2012;10:73. doi: 10.1186/1741-7007-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccinin S, Tonin E, Sessa S, Demontis S, Rossi S, Pecciarini L, et al. A “twist box” code of p53 inactivation: twist box: p53 interaction promotes p53 degradation. Cancer cell. 2012 Sep 11;22(3):404–15. doi: 10.1016/j.ccr.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, et al. Twist is a potential oncogene that inhibits apoptosis. Genes & development. 1999 Sep 1;13(17):2207–17. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009 Apr 3;137(1):73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran PT, Shroff EH, Burns TF, Thiyagarajan S, Das ST, Zabuawala T, et al. Twist1 suppresses senescence programs and thereby accelerates and maintains mutant kras-induced lung tumorigenesis. PLoS Genet. 2012 May;8(5):e1002650. doi: 10.1371/journal.pgen.1002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes & development. 1995 Mar 15;9(6):686–99. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 44.Verdine GL, Walensky LD. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. Clin Cancer Res. 2007 Dec 15;13(24):7264–70. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 45.Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011 Dec 8;118(24):6247–57. doi: 10.1182/blood-2011-07-328880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen JL, Li J, Kiriluk KJ, Rosen AM, Paner GP, Antic T, et al. Deregulation of a Hox protein regulatory network spanning prostate cancer initiation and progression. Clin Cancer Res. 2012 Aug 15;18(16):4291–302. doi: 10.1158/1078-0432.CCR-12-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garzon-Muvdi T, Schiapparelli P, ap Rhys C, Guerrero-Cazares H, Smith C, Kim DH, et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biology. 2012;10(5):e1001320. doi: 10.1371/journal.pbio.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran PT, Fan AC, Bendapudi PK, Koh S, Komatsubara K, Chen J, et al. Combined Inactivation of MYC and K-Ras Oncogenes Reverses Tumorigenesis in Lung Adenocarcinomas and Lymphomas. PLoS ONE. 2008;3(5):e2125. doi: 10.1371/journal.pone.0002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002 Apr 4;21(15):2365–75. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 50.Zeng J, See AP, Aziz K, Thiyagarajan S, Salih T, Gajula RP, et al. Nelfinavir induces radiation sensitization in pituitary adenoma cells. Cancer biology & therapy. 2011 Oct 1;12(7):657–63. doi: 10.4161/cbt.12.7.17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.