Abstract
Recent data suggest that both nocturnal and diurnal mammals generate circadian rhythms using similarly phased feedback loops involving Period genes in the suprachiasmatic nuclei (SCN) of the hypothalamus. These molecular oscillations also exist in the brain outside of the SCN, but the relationship between SCN and extra-SCN oscillations is unclear. We hypothesized that a comparison of “diurnal” and “nocturnal” central nervous system Per rhythms would uncover differences in the underlying circadian mechanisms between these two chronotypes. Therefore, this study compared the 24-h oscillatory patterns of Per1 and Per2 mRNA in the SCN and putative striatum and cortex of Octodon degus (degu), a diurnal hystricognath rodent, with those of the nocturnal laboratory rat, Rattus norvegicus. The brains of adult male degus and rats were collected at 2-h intervals across 24 h in entrained light-dark and constant darkness conditions, and sections were analyzed via in situ hybridization. In the SCN, degu Per1 and Per2 hybridization signal exhibited 24-h oscillatory patterns similar in phasing to those seen in other rodents, with peaks occurring during the light period and troughs during the dark period. However, Per1 remained elevated for five fewer hours in the degu than in the rat, and Per2 remained elevated for two fewer hours in the degu. In brain areas outside of the SCN, the phase of Per2 hybridization signal rhythms in the degu were 180° out of phase with those found in the rat, and Per1 hybridization signal lacked significant rhythmicity. These results suggest that, while certain basic components of the transcriptional-translational feedback loop generating circadian rhythms are similar in diurnal and nocturnal mammals, there are variations that may reflect adaptations to circadian niche.
Keywords: clock genes, Period, diurnality, chronotype, striatum, cortex, suprachiasmatic nucleus
while the suprachiasmatic nuclei (SCN) of the hypothalamus are integral both for generating and synchronizing circadian rhythms in mammals, their role in influencing chronotype is not well understood. In the few diurnal mammals that have been systematically compared with nocturnal mammals, the phasing of SCN activity appears surprisingly similar (52). This has led many researchers to believe that, instead of a “diurnal signal” coming from the SCN, there are “diurnal interpretations” of the SCN's signal that may account for mammalian diurnality (13, 29, 43, 56). However, a growing number of studies indicate consistent differences between the SCN neural activity of diurnal and nocturnal species (25–26, 34, 40, 57). Therefore, continued research may yet elucidate a relationship between SCN function and chronotype.
The discovery of a self-sustaining molecular feedback loop within SCN cells has provided a direct means to examine the circadian pacemaker's phase in relation to physiological and behavioral chronotype. Among the players in this feedback loop, the period genes Per1 and Per2 are of particular interest in relation to circadian niche because of their integral involvement in generating rhythmicity under constant conditions (9, 68) and in entraining circadian phase to time cues (zeitgebers) such as light (4–5, 35, 42, 53, 55). Another period gene, Per3, does not play a direct role in the circadian clock mechanism regulating the locomotor activity of nocturnal mice (9, 53); however, polymorphisms in this gene have been linked to extreme diurnal preference in humans (6, 16, 48), suggesting that it may play a significant role in the circadian organization of diurnal species.
To date, there have been no studies directly comparing Period gene expression in diurnal and nocturnal species. There have been several studies that have independently examined Period mRNA rhythms in species of both chronotypes (e.g., Refs. 10, 29, 35, 37, 40, 55, 59). However, the methodology used in these studies varied greatly, particularly in terms of lighting condition and tissue collection interval (which were typically large, e.g., 4–6 h). Therefore, although we can confidently argue that in both nocturnal and diurnal chronotypes, Per1 and Per2 mRNA in the SCN peak during midsubjective day and fall to baseline levels during the night, it is difficult to assess less robust differences in peak duration or timing, or the precise phase of the rise and fall. It is important to investigate these possible differences, since SCN zeitgeber sensitivity is thought to reflect Period gene expression levels (21, 32). Furthermore, PER induction in response to light has been hypothesized to mediate photic phase-shifts of behavioral rhythms (4, 5, 55, 62), and so differences in Per transcript levels may reflect differences observed in daytime zeitgeber sensitivity and “dead zones” in the phase response curves (PRC) between diurnal and nocturnal mammalian species (24, 56).
Daily rhythms in clock gene expression also occur in brain areas besides the SCN, and the phase relationship between SCN and extra-SCN oscillators may reflect differences in chronotype. Previous work in rats and mice has indicated that the phase of Per mRNA expression in brain areas outside of the SCN closely relates to the phase of behavioral activity rhythms (3, 31, 37–38, 63). In the diurnal ground squirrel, Per1 rhythms in the motor cortex peak during the light period, a complete phase-reversal compared with Per1 rhythms in brain areas outside the SCN in nocturnal rodents (37). Therefore, the differences in phasing of the molecular clocks within the brain might also be an important factor in organizing a mammal's temporal phenotype.
To better address the relationship between the circadian pacemaker and chronotype, we systematically compared Per1 and Per2 expression profiles inside and outside of the SCN in the diurnal Octodon degus (degu) and nocturnal Rattus norvegicus (rat) using frequent sampling intervals (2 h), species-specific probes, and identical in situ and analysis methodology. Within the SCN, we hypothesized that subtle differences would exist between degus and rats in Per1 and Per2 peak timing and duration, so as to account for the lack of a daytime dead zone in the PRC of the diurnal species. However, we expected that the basic waveforms of mRNA expression would still be quite similar. In contrast, outside of the SCN, we hypothesized that the Per1 and Per2 mRNA waveforms in degus and rats would be 180° out of phase with each other, reflecting the differential phasing of their behavioral rhythms. Additionally, because no study had examined the circadian expression pattern of Per3 in a diurnal mammal, we sought to characterize its expression in the degu.
MATERIALS AND METHODS
Animals and Housing
Adult male degus (n = 82, age 2–4 yr; average lifespan 5–7 yr) were obtained from a breeding colony at the University of Michigan (Ann Arbor, MI), and adult male Sprague-Dawley rats (n = 56, age 3–7 mo) were obtained from Charles River Laboratories (Wilmington, MA). All animals were housed in 48 × 26.8 × 20.3 cm opaque plastic cages under a 12:12-h light-dark (LD) cycle (250 lux, lights on from 0600 to 1800) with food and water available ad libitum. Room temperature was maintained at 18°C. Degus were defined as diurnal and included in the study if screening showed that the majority of their daily activity occurred during the light period of the LD cycle, as analyzed using Vitalview and Actiview software (Minimitter, Bend, OR).
Degus (n = 55) and rats (n = 56) were killed by decapitation within 15 min of the following zeitgeber times (ZT): 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 22. Additional degus (n = 27) were placed in constant darkness (DD) 24 h before the day of death and killed at circadian times (CT) 0, 4, 8, 10, 14, and 20. Each group in both conditions contained brains from three to six animals, with the exception of the rat groups at ZT16 (n = 0) and ZT18 (n = 1) for the Per2 in situ hybridization. Animals were rapidly anesthetized using isoflurane anesthetic (MinRad, Buffalo, NY). Brains were removed, immediately flash-frozen using a 20- to 30-s immersion in acetone on dry ice, and stored at −80°C until use. During the dark phase of the LD cycle or DD conditions, brains were collected under dim red light. All procedures were approved by the University Committee for the Care and Use of Animals at the University of Michigan.
In Situ Hybridization
The in situ hybridization method was adapted from the protocol described elsewhere (8). Degu Per1, Per2, and Per3 antisense and sense probes were used to separately label degu SCN tissue collected in LD and DD. Rat Per1 and Per2 antisense probes and sense probes were used to label rat SCN tissue collected in LD. A series of tissue from all time points was processed identically and in parallel in preparation for in situ labeling by any particular riboprobe.
Isolation of degu Per1, Per2, and Per3 cDNA sequences.
Primers were used in RT-PCR to isolate fragments from the Per cDNA sequences. The DePer1 cDNA sequence (GenBank accession no. EU715821) is a 892-bp fragment 89% identical to the corresponding region of mouse Per1 (GenBank NM_011065). The DePer2 cDNA sequence (GenBank EU590918) is a 532-bp fragment 87% identical to the corresponding region of mouse Per2 (GenBank NM_011066). The DePer3 cDNA sequence (GenBank EU590919) is a 204-bp fragment 84% identical to the corresponding region of mouse Per3 (GenBank NM_011067).
Production of radioactive riboprobes.
Degu Per1, Per2, and Per3 fragment-containing vectors (courtesy of Dr. Jeremiah Shepard and Dr. Steven McKnight of Southwest Medical School) and rat Per1 and Per2 fragment-containing vectors (courtesy of Dr. Stanley Watson and Dr. Huda Akil, University of Michigan) were linearized with restriction enzymes and used as templates for hybridized RNA probes. The sequences of the degu DNA fragments used to produce the antisense RNA probes corresponded to nucleotides 194-1082 of mPer1, 491-1022 of mPer2, and 1057-1260 of mPer3. The sequences for the rat DNA fragments used to produce the antisense RNA probes can be found elsewhere (54). Linearized plasmid was purified and incubated at 37°C for 1.5 h in a transcription buffer that included RNA polymerase and 35S-labeled UTP and CTP (for all reagents, see Ref. 8). The resulting probe was treated with DNase and separated from free nucleotides using Micro Bio-Spin Chromatography Columns (Bio-Rad Laboratories, Hercules, CA).
Tissue preparation.
Brain sections (16 μm) were sliced on a cryostat into four series of coronal sections through the SCN and stored on slides at −80°C until hybridization. Tissue sections were fixed with 4% paraformaldehyde for 1 h, rinsed, and incubated in 0.1 M triethanolamine with 0.25% acetic acid to reduce nonspecific binding. The tissue was then dehydrated through a series of alcohols and allowed to air-dry for 1–3 h.
Hybridization.
The 35S-labeled probe was diluted in hybridization buffer to yield a concentration of 1.5–3 million counts·min−1·100 μl−1. This diluted probe (100 μl) was applied to cover slips that were placed on the slides. The slides were incubated overnight at 55°C in sealed boxes containing filter paper soaked in 50% formamide. After incubation, cover slips were removed, and slides were rinsed. To reduce nonspecific binding, sections were incubated in RNase A solution, briefly washed, and then incubated in a high-stringency saline solution at 65°C for 1 h. Tissue was finally dehydrated through a series of alcohols and exposed to Biomax film (Kodak, Rochester, NY) in light-tight boxes for 14–72 h (depending on the probe). To verify the absence of nonspecific binding, sense probes were run using tissue from several representative time points.
Analysis
Autoradiographs were digitized using MCID software (Imaging Research, St. Catharines, Ontario), and the magnitude of the hybridization signal was determined by experimenters blind to the group identity of the tissue using Scion Image software. This software analyzed the density of grayscale units for any particular brain region of interest relative to the background threshold signal measured from the corpus callosum (macro written by Dr. S. Campeau, University of Michigan). This measurement was then divided by the total area of the region of interest to produce a mean hybridization signal per unit area (we refer to this as the “hybridization signal” for any particular brain region).
Measurements taken for each animal were represented by the average hybridization signal of three mid-SCN sections (Fig. 1). The location of the SCN was determined by comparing the shape of the optic chiasm and other ventral landmarks with a published brain atlas (rat, see Ref. 47) or with our own previous anatomical characterization of the SCN (degu, see Ref. 18). Outside of the SCN, measurements taken for each animal were represented by the average hybridization signal of two sections using 1.3-mm-diameter bilateral samples (Fig. 2). In the rat, these samples were taken from the striatum, parietal cortices, and cingulate cortices at 0.30–1.30 mm posterior to bregma (47). In the degu, samples were taken from regions showing analogous structure (e.g., cortical layering) or similar placement in relationship to local landmarks (e.g., the lateral ventricles, the corpus callosum, the optic chiasm). Because these regions have not been rigorously anatomically verified in this species, we refer to them as “putative”. These particular brain regions outside the SCN were selected for analysis because of 1) previous evidence that they contain rhythmic Period gene expression (31, 36) and 2) reliable presence in coronal tissue sections containing the SCN.
Fig. 1.

Representative autoradiographs showing daily Period gene mRNA levels in the suprachiasmatic nucleus (SCN) of the diurnal degu. Brains were sampled every 2 h from animals housed in 12:12-h light-dark (LD) periods. Period gene mRNA levels in the SCN at each time point were visualized using in situ hybridization and autoradiography. Depicted are coronal slices labeled for degu Per1, Per2, and Per3 mRNA.
Fig. 2.
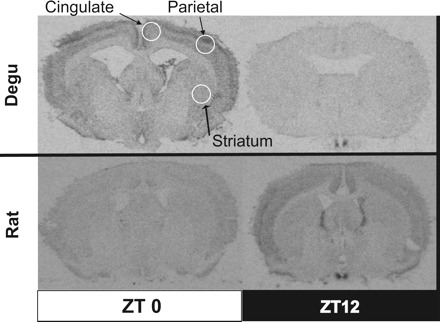
Representative autoradiographs showing Per2 mRNA levels outside of the SCN in the diurnal degu and nocturnal rat. Per2 mRNA levels in the putative striatum and cingulate and parietal cortices at each time point were visualized using in situ hybridization and autoradiography. The circles illustrate the specific locations on coronal slices that were used to sample hybridization signal from each brain region.
Statistics
Daily changes in the hybridization signal for mRNA probes were determined statistically using between-subject analyses. For within-species comparisons, nonparametric analyses were performed using the k-independent samples Kruskal-Wallis H Test because some data did not meet the parametric statistical assumptions of normal distribution and homogeneous variance. For between-species comparisons, we normalized the amplitude of the Per1 and Per2 hybridization signal rhythms because each species required a separate in situ hybridization. To perform this normalization, we defined “0” as the average hybridization signal of the trough time point and “100” as the average hybridization signal of the peak time point for each species. Between-subjects ANOVA was used to determine if there was a main effect of species on normalized hybridization signal in the SCN or an interaction between time point and species. Post hoc independent-samples Student's t-tests compared the normalized hybridization signal in the SCN between species at the 12 individual time points (using a Sidak correction for multiple comparisons).
Outside of the SCN, we hypothesized that Per1 and Per2 hybridization signal in degus would show elevation during the light period of the LD cycle, whereas Per1 and Per2 hybridization signal in rats would show elevation during the dark period of the LD cycle. To test this hypothesis, mean hybridization signals during the light and dark periods of the LD cycle were compared using either an independent-samples Student's t-test or the Mann-Whitney U-Test (if the design was unbalanced or the assumption of normality violated). The light period was defined as ZT0–ZT10, and the dark period was defined as ZT12–ZT22.
RESULTS
Period Gene mRNA in the SCN of the Degu
A strong hybridization signal for antisense Per1, Per2, and Per3 mRNA probes was observed in the SCN of the degu under 12:12-h LD and DD conditions (Figs. 1 and 3). No hybridization signal for sense Per1, Per2, and Per3 mRNA probes was observed in any brain region (data not shown).
Fig. 3.
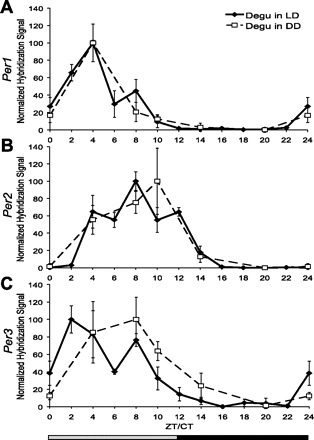
Period gene mRNA levels in the SCN of the degu show similar daily fluctuation under 12:12-h LD and constant darkness (DD) conditions. Relative mRNA levels in the SCN at each time point were compared using in situ hybridization. Separate in situ hybridizations were performed on LD and DD tissue; therefore, the mean hybridization signal for each time point (n = 3–5, ±SE) is normalized as %peak, with trough given the value of “0.” Zeitgeber time (ZT)/circadian time (CT) 0 is double-plotted as CT/ZT24. Each of the three Period genes, Per1 (A), Per2 (B), and Per3 (C), exhibits significant daily fluctuation (P < 0.02) in transcript level in the degu SCN under both conditions.
Degu Per1.
Per1 hybridization signal under LD and DD conditions showed a significant change over time [LD: n = 54, χ2(11) = 37.479, P < 0.001; DD: n = 27, χ2(5) = 15.154, P = 0.010; Fig. 3A]. Per1 was elevated during the light phase of the LD cycle [n = 54, T(23.064) = 6.322, P < 0.001] and the subjective day of the DD cycle (n = 27, U = 21, P = 0.003). Both conditions produced peak Per1 levels at ZT4/CT4.
Degu Per2.
Per2 hybridization signal under LD and DD conditions showed a significant change over time [LD: n = 55, χ2(11) = 49.205, P < 0.001; DD: n = 26, χ2(5) = 14.571, P = 0.012; Fig. 3B]. Per2 was elevated during the light phase of the LD cycle [n = 55, T(38.013) = 3.252, P = 0.002] and demonstrated a nonsignificant trend toward elevation during the subjective day of the DD cycle (n = 26, U = 37, P = 0.088). The LD condition produced peak Per2 at ZT8.
Degu Per3.
Per3 hybridization signal under LD and DD conditions showed a significant change over time [LD: n = 51, χ2(11) = 37.029, P < 0.001; DD: n = 5, χ2(5) = 16.038, P = 0.007; Fig. 3C]. Per3 was elevated during the light phase of the LD cycle [n = 51, T(23.368) = 6.853, P < 0.001] and the subjective day of the DD cycle [n = 25, T(22.794) = 3.464, P = 0.002]. The LD condition produced peak Per3 at ZT2. Under DD conditions, CT2 was not sampled, and a peak was found at CT8.
Period Gene mRNA in the SCN of the Rat
A strong hybridization signal for antisense Per1 and Per2 mRNA probes was observed in the SCN of the rat under 12:12-h LD conditions (Fig. 4). No hybridization signal for sense Per1 and Per2 mRNA probes was observed in any brain region (data not shown).
Fig. 4.
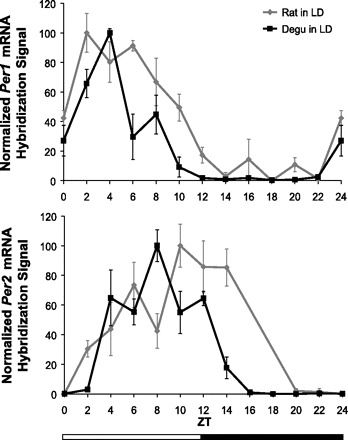
Comparison of daily Period gene mRNA levels in the SCN of the nocturnal rat and diurnal degu. Relative Period gene mRNA levels in the SCN at each time point were compared using in situ hybridization. The mean hybridization signal for each time point (n = 3–5, ±SE) is normalized as %peak, with trough given the value of 0. ZT0 is double-plotted as ZT24. Both species demonstrate daily fluctuation (degu: P < 0.001, rat: P < 0.001) in Per1 (top) and Per2 (B) mRNA levels in the SCN that differed between the two species (P < 0.05) and varied by time point (P = 0.001). In the rat Per2 dataset (bottom), ZT16 is absent, and ZT18 is not shown due to only having data from one animal.
Rat Per1.
Per1 hybridization signal under LD conditions showed a significant change over time [n = 55, χ2(11) = 43.404, P < 0.001; Fig. 4A]. Per1 was elevated during the light period of the LD cycle [n = 55, T(35.081) = 10.097, P < 0.001], with the peak occurring at ZT2.
Rat Per2.
Per2 hybridization signal under LD conditions showed a significant change over time [n = 46, χ2(9) = 34.908, P < 0.001; Fig. 4B]. Unlike in the degu, Per2 in the rat was not significantly elevated during the light period of the LD cycle [n = 46, T(33.793) = 0.381, P = 0.705]. Peak Per2 occurred at ZT10.
Comparison of Period Gene mRNA in the SCN of the Degu and Rat
The monophasic rhythms of Per1 and Per2 hybridization signal in the SCN of degus and rats were grossly similar. However, differences existed in peak duration and timing. We found a significant main effect of species on normalized Per1 hybridization signal [n = 109, F(1, 85) = 24.907, P < 0.001; Fig. 4A], as well as an interaction between species and time point [n = 109, F(11, 85) = 3.402, P = 0.001; Fig. 4A]. Following correction for multiple comparisons, post hoc analyses did not have enough power to reveal which specific time points were responsible for these species differences (P > 0.05). However, the species differences at several time points surrounding the peak had large (r > 0.80, ZT6) or moderate (r > 0.50, ZT2, -10, -12, and -20) effect sizes. Using 50% peak values as a relative measure of elevated mRNA levels, these results suggest that Per1 in the rat is elevated for twice as long as Per1 in the degu.
Similarly, we found a significant main effect of species on normalized Per2 hybridization signal [n = 91, F(1, 71) = 4.650, P = 0.034] as well as an interaction between species and time point [n = 91, F(9, 71) = 5.358, P < 0.001]. Post hoc analyses revealed that these species differences were most significant at ZT14 (P = 0.020), and several time points surrounding the peak had large (r > 0.80, ZT2 and -14) or moderate (r > 0.50, ZT8 and -10) effect sizes. These results suggest that Per2 in the rat peaks later in the day than Per2 in the degu and is elevated above 50% of peak for at least two hours longer. Because of data loss, rat Per2 was unable to be characterized at ZT16 and ZT18; therefore, it is possible that species differences in Per elevation could be greater than depicted.
Period Gene mRNA Outside of the SCN in the Degu
Outside of the SCN in the degu, strong hybridization signals for Per1 and Per2 were observed in the putative cortex, striatum, lateral septal nuclei, several thalamic nuclei, and the cornus ammonis (CA)1, CA2, CA3 and dentate gyrus regions of the hippocampus (Fig. 2). Strong hybridization signals for Per3 were observed in the putative cortex, striatum, and the CA1, CA2, CA3, and dentate gyrus regions of the hippocampus (data not shown).
Putative cingulate cortex.
Per2 hybridization signal under LD conditions showed a significant change over time [n = 49, χ2(11) = 22.037, P = 0.024; Fig. 5A]. Per2 was elevated during the light period of the LD cycle [n = 49, T(30.75) = 2.754, P = 0.010; Fig. 5B]. Peak Per2 occurred at ZT0. Per1 and Per3 hybridization signals under LD conditions did not show a significant change over time [Per1: n = 46, χ2(11) = 6.524, P = 0.836; Fig. 6, Per3: n = 47, χ2(11) = 13.200, P = 0.280; data not shown] nor elevation during the light period of the LD cycle [Per1: n = 46, T(44) = 0.206, P = 0.838; Fig. 6, Per3: n = 47, T(45) = 0.841, P = 0.405; data not shown].
Fig. 5.
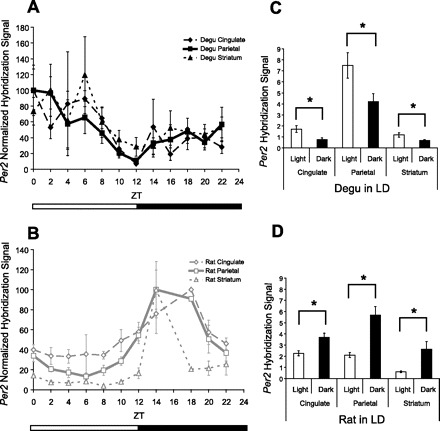
Comparison of daily Per2 mRNA levels outside of the SCN of the nocturnal rat and diurnal degu. Relative Per2 mRNA levels at each time point were compared using in situ hybridization. The mean hybridization signal for each time point (n = 3–5, ±SE, LD 12:12) is normalized as %peak, with trough given the value of 0 (A and B). A: the degu demonstrated daily fluctuation of Per2 mRNA levels in the putative parietal cortex and cingulate cortex (P < 0.05). B: the rat demonstrated daily fluctuation of Per2 in the parietal cortex and striatum (P < 0.05). C: in the degu, Per2 mRNA levels were found to be elevated during the light period of the LD cycle compared with the dark period in the putative cingulate cortex, parietal cortex, and striatum (P < 0.05). D: in the rat, Per2 mRNA levels were found to be elevated during the dark period of the LD cycle compared with the light period in the cingulate cortex, parietal cortex, and striatum (P < 0.001).
Fig. 6.
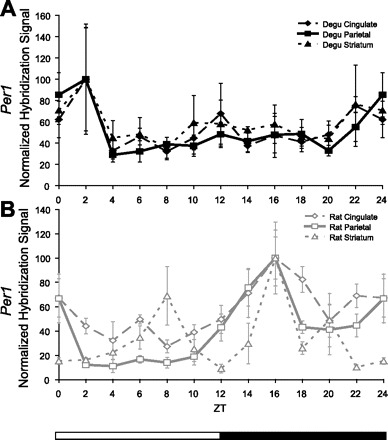
Comparison of daily Per1 mRNA levels outside of the SCN of the nocturnal rat and diurnal degu. The mean hybridization signal for each time point (n = 3–5, ±SE, 12:12-h LD) is normalized as %peak, with trough given the value of 0. A: daily fluctuation in the 24-h profile of Per1 mRNA levels in the cingulate cortex, parietal cortex, and striatum of the degu was not found to be significant (P > 0.05). B: the rat demonstrated daily fluctuation of Per1 mRNA levels in the parietal cortex and striatum (P < 0.01) and elevation of Per1 mRNA levels during the dark period of the LD cycle compared with the light period in the cingulate and parietal cortices (P < 0.01).
Putative parietal cortex.
Per2 levels under LD conditions showed a nonsignificant trend toward change over time [n = 54, χ2(11) = 19.391, P = 0.054; Fig. 5A]. Per2 was elevated during the light period of the LD cycle [n = 54, T(40.953) = 2.405, P = 0.021; Fig. 5B]. Peak Per2 occurred at ZT0, and post hoc analysis indicated that ZT0 was significantly different from ZT12 (P < 0.05). Per1 and Per3 levels under LD conditions did not show a significant change over time [Per1: n = 53, χ2(11) = 11.946, P = 0.368; Fig. 6, Per3: n = 47, χ2(11) = 5.882, P = 0.881; data not shown] nor elevation during the light period of the LD cycle [Per1: n = 53, T(51) = 0.849, P = 0.400; Fig. 6, Per3: n = 47, T(45) = 0.266, P = 0.791; data not shown].
Putative striatum.
Per2 levels under LD conditions did not show a significant change over time [n = 54, χ2(11) = 11.088, P = 0.436; Fig. 5A]. However, Per2 was elevated during the light period of the LD cycle [n = 54, T(31.728) = 2.367, P = 0.024; Fig. 5B]. Peak Per2 occurred at ZT6. Per1 and Per3 levels under LD conditions did not show a significant change over time [Per1: n = 53, χ2(11) = 9.286, P = 0.596; Fig. 6, Per3: n = 49, χ2(11) = 5.622, P = 0.897; data not shown] nor elevation during the light period of the LD cycle [Per1: n = 53, T(51) = 0.393, P = 0.696; Fig. 6, Per3: n = 49, T(25.408) = 1.232, P = 0.229; data not shown].
Period Gene mRNA Outside of the SCN in the Rat
Outside of the SCN in the rat, strong hybridization signals for Per1 and Per2 were observed in the cortex, striatum, several thalamic nuclei, and the CA1, CA2, CA3, and dentate gyrus regions of the hippocampus (Fig. 5). Unlike the degu, no strong hybridization signal for Per1 and Per2 was observed in the lateral septal nuclei (data not shown).
Cingulate cortex.
Per2 levels under LD conditions did not show a significant change over time [n = 38, χ2(9) = 13.817, P = 0.129; Fig. 5A] but were elevated during the dark period of the LD cycle [n = 39, T(37) = −3.493, P = 0.001; Fig. 5D]. Peak Per2 occurred at ZT18. Per1 mRNA under LD conditions did not show significant change over time [n = 45, χ2(11) = 16.643, P = 0.119] but was similarly elevated during the dark period of the LD cycle [n = 45, T(37.340) = −2.697, P = 0.010; Fig. 6] and showed a peak at CT16.
Parietal cortex.
Per2 levels under LD conditions showed a significant change over time [n = 38, χ2(9) = 23.959, P = 0.004; Fig. 5A] and were elevated during the dark period of the LD cycle [n = 39, T(16.307) = −4.576, P < 0.001 Fig. 5D]. Peak Per2 occurred at ZT14. Similarly, Per1 levels under LD conditions showed a significant change over time [n = 45, χ2(11) = 29.737, P = 0.002] and were elevated during the dark period of the LD cycle [n = 45, T(43) = −3.111, P = 0.003; Fig. 6], showing a peak at CT16.
Striatum.
Per2 levels under LD conditions showed a significant change over time [n = 39, χ2(9) = 23.959, P = 0.004; Fig. 5A] and were elevated during the dark period of the LD cycle [n = 39, T(16.307) = −4.576, P < 0.001; Fig. 5D]. Peak Per2 occurred at ZT14. Per1 levels under LD conditions showed a significant change over time [n = 45, χ2(11) = 25.359, P = 0.008], showing a peak at CT16 but were not significantly elevated during the dark period of the LD cycle [n = 45, T(33.966) = −0.774, P = 0.444; Fig. 6].
DISCUSSION
To date, this is the only study to document Period gene expression in the brain of the O. degus, a useful diurnal laboratory species, as well as the first to examine Per3 expression in any diurnal animal. Examination of Period gene expression in the degu under both a 12:12-h LD cycle and constant conditions suggests that these rhythms are endogenously generated, as in other mammals. Within the SCN, degu Per1 and Per2 rhythms appear similar to those reported for the diurnal ground squirrel (Spermophilus tridecimlineatus), grass rat ansorgei (Arvicanthis ansorgei), and grass rat niloticus (Arvicanthis niloticus), (10, 37, 40, although see Ref. 29). Each of these species shows a peak in Per1 expression during the early subjective day and a peak in Per2 expression during the late subjective day.
We had originally hypothesized that differences in Per3 might be related to diurnal chronotype because polymorphisms in Per3 in humans correlate with delayed sleep phase syndrome and extreme diurnal preference (6, 16, 48). Indeed, the daily pattern of Per3 mRNA in the SCN of the diurnal degu was several hours phase-advanced compared with previously published studies of Per3 mRNA in the SCN of the nocturnal laboratory rat (12, 49), showing transcript levels that peaked during the day (ZT/CT2–8) and declined between ZT/CT10-14. However, we did not explicitly compare Per3 mRNA in the two species, and the timing of the degu Per3 peak and trough is similar to Per3 rhythms in the nocturnal mouse (27, 60). Therefore, any differences in Per3 waveform that may exist between the degu and rat are unlikely to be related to chronotype.
A detailed comparison of Per1 and Per2 waveforms in the SCN of the degu and rat revealed several differences. Rat Per1 mRNA levels in the SCN peaked early in the day and maintained elevated levels for nearly two times as long as found in the degu (Fig. 4). Per2 levels in the rat SCN also appeared to fall noticeably later in the evening than Per2 in the degu SCN, although the full extent of this duration difference was not completely captured because of missed evening sample points. These trends are corroborated by our data from degus kept under DD conditions, as well as data from other rat studies (7, 39, 66–67). Furthermore, a review of current literature suggests that our data may identify a consistent difference in the duration of Per elevation in the SCN of diurnal and nocturnal mammals (Fig. 7 and Refs. 7, 9, 10, 22, 29, 33, 36–37, 39–40, 45, 61, 64, and 66–67). Therefore, we believe it is unlikely that these species differences are due to random chance or an artifact of the thresholding procedure used in our analysis. However, replication is desirable using additional diurnal and nocturnal species.
Fig. 7.
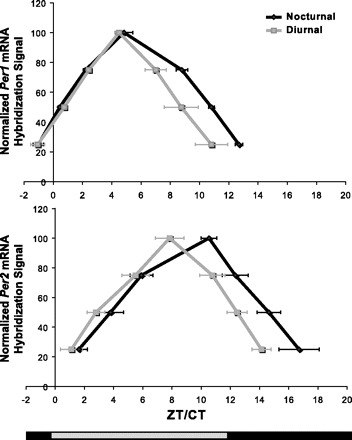
Comparative analysis of published Per1 and Per2 rhythms in the SCN of diurnal and nocturnal species. The timing and shape of Per1 and Per2 mRNA rhythms were compared from published studies of adult animals with clearly defined chronotypes (7, 9, 10, 20, 27, 31, 34–35, 37–38, 43, 59, 62, 64–65). Papers that were excluded from the analysis included studies of Period gene expression in the sheep (28) because of unusual photoperiod, the blind mole rat (44) because of ambiguous chronotype (48), and studies of the juvenile rat and rabbit (11, 56) because of possible developmental confounds. To compare Period gene expression patterns, the published rhythms were normalized as previously described. Based on this normalization, mRNA hybridization levels of 25, 50, and 75% of peak expression were estimated and averaged to create a “typical” Per1 and Per2 waveform for each species. To compare the two chronotypes, the species’ means were averaged to produce typical diurnal and nocturnal Per1 and Per2 waveforms. This method prevented well-studied species from being overrepresented.
Outside of the SCN, the phasing of Per2 rhythms of degus and rats differed by 180°, correlating with the differential phasing of their behavioral rhythms. Degu Per2 mRNA in the putative cingulate cortex, parietal cortex, and striatum was found to be elevated during the early part of the light period. In contrast, Per2 mRNA in these same regions in the rat was found to be elevated during the early part of the dark period. These results are similar to those found in previous studies using rats in which Per2 mRNA rhythms were shown to peak at ZT16 in the parietal cortex (31), striatum (31), and cortex (38). Other studies of nocturnal rodents have similarly observed Per2 mRNA peaking during the early part of the dark period in these brain regions (2–3, 15, 63, 65).
Similarly, in a previous study, Per1 mRNA levels in the motor cortex of the diurnal ground squirrel (S. tridecemlineatus) were elevated during the lighted period of the LD cycle (37). We did not observe a similar rhythm in Per1 in the putative cortex or striatum of the degu. This difference may be explained by a decreased sensitivity for detecting lower amplitude rhythms using our techniques. Alternatively, Per1 and Per2 may be differentially expressed in brain regions outside of the SCN (17) in a manner that varies by species. Our current rat data, as well as previous studies of Per1 expression in the cortical and striatal regions of nocturnal rodents, show elevated mRNA levels during the dark period (2–3, 31, 38, 63).
The function of period genes outside the SCN is currently a matter of speculation. Masubuchi et al. (31) have argued that the Per mRNA rhythms outside of the SCN are unlikely to be the result of locomotor activity, since the rhythms anticipate the beginning of activity onset and gradually decrease throughout the day while activity remains elevated. Our findings are similarly incongruent with the idea that Per outside of the SCN is a result of activity, since the trough in Per2 mRNA rhythms occurred at a time point (ZT10–ZT12) that is typically a period of vigorous activity in the degu (44). Therefore, our data support the proposition that Period gene expression outside of the SCN may represent slave oscillators that under normal conditions are driven by the master circadian oscillator in the SCN (19, 31). We propose that these slave oscillators are differentially phased in diurnal and nocturnal species.
In conclusion, the aim of this study was to correlate chronotype with the daily pattern of core clock gene expression within the SCN and external to the core circadian pacemaker. Because we considered relative mRNA levels in the SCN to reflect the phase of the circadian pacemaker, we believe that our study indirectly compared clock phase between a diurnal and nocturnal mammal. However, before we can draw any strong conclusions regarding the functional significance of these results, several parameters remain to be compared between diurnal and nocturnal species. For instance, although the mRNA level of analysis is useful, protein expression profiles are ultimately needed. Furthermore, a more careful characterization of mRNA expression in the core and shell regions of the SCN could yield further differences between diurnal and nocturnal rodents. Last, the in situ hybridization technique may not be sensitive enough to detect the low amplitude rhythms outside of the SCN. Replication of these results using quantitative RT-PCR may be a useful alternative.
Perspectives and Significance
Our study adds to growing evidence that the SCN may be adapted for a diurnal or nocturnal lifestyle (14, 25–26, 28, 34, 40, 51, 57). However, whether these differences in SCN function are sufficient to “cause” diurnal phasing of behavioral output or reflect an adaptation to a light-intense niche remains unresolved (20). Diurnal species contain many adaptations to their light-saturated niche that go beyond the production of circadian rhythms with a particular phase. For example, the degu has color vision and an increased threshold for circadian photic sensitivity (as reviewed in Ref. 20). Interestingly, both Per1 and Per2 are hypothesized to be insensitive to photic induction during midday elevation (32, 21). Therefore, our observation that the degu SCN shows a shorter duration of Per1 elevation could be related to the shorter duration of midday photic insensitivity that arguably characterizes diurnal mammals (23–24, 56).
Our data indicating that Per2 rhythms outside of the degu SCN are 180° out of phase with those rhythms in the rat are more likely to relate to questions regarding the origin of diurnal phasing of behavioral output. Nocturnal species with diurnally phased activity rhythms have been shown to exhibit similar changes in extra-SCN phasing (31, 63).
Finally, it is important to acknowledge that the direct application of information about mammalian core circadian mechanisms taken from nocturnal animal models may not be entirely translatable to human circadian systems. Diurnal mammalian circadian systems have been found to be highly variable (54); therefore, it is vital that more species be examined to reach generalizable conclusions.
GRANTS
These investigations were supported by National Science Foundation Grant IBN-0212322 (T. M. Lee), a Reproductive Science Program T32 Training Grant (HD07048 to M. H. Hagenauer and D. L. Hummer), and by a grant from the College of Literature, Science, and Arts at the University of Michigan (T. M. Lee).
Acknowledgments
We thank Dr. Stephen McKnight, Dr. Jeremiah Shepard, Dr. Stan Watson, and Dr. Huda Akil for supplying the cDNA necessary for producing the Period gene mRNA probes. We are deeply indebted to Dr. Robert Thompson, Dr. Megan Mahoney, and Jennifer Lapierre for thoughtful consultation on technical matters. We also thank Hailey Hines, Jenny Agrusa, William S. Shield, Anna de Caneva, Jenna Stelzer, and Aimee Roby for assistance with data collection and analysis. Finally, we acknowledge Kathy Gimson, Julie Stewlow, and Jim Donner for work managing the degu colony.
Present address of D. L. Hummer: Morehouse College, Dept. of Psychology, Atlanta, GA 30314.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe H, Honma S, Honma K. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol Regul Integr Comp Physiol 292: R607–R615, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Abe H, Honma S, Ohtsu H, Honma K. Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Brain Res Mol Brain Res 124: 178–187, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Abe H, Honma S, Namihira M, Masubuchi S, Honma K. Behavioural rhythm splitting in the CS mouse is related to clock gene expression outside the suprachiasmatic nucleus. Eur J Neurosci 14: 1121–1128, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19: 1115–1121, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J Biol Rhythms 16: 100–104, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26: 413–415, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66: 1133–1139, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci 18: 10579–10593, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Caldelas I, Poirel VJ, Sicard B, Pevet P, Challet E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei Neuroscience 116: 583–591, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Caldelas I, Tejadilla D, González B, Montúfar R, Hudson R. Diurnal pattern of clock gene expression in the hypothalamus of the newborn rabbit. Neuroscience 144: 395–401, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem 88: 1547–1554, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dardente H, Klosen P, Caldelas I, Pevet P, Masson-Pevet M. Phenotype of Per1- and Per2-expressing neurons in the suprachiasmatic nucleus of a diurnal rodent (Arvicanthis ansorgei): comparison with a nocturnal species, the rat. Cell Tissue Res 310: 85–92, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res 124: 143–151, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Dey J, Carr AJ, Cagampang FR, Semikhodskii AS, Loudon AS, Hastings MH, Maywood ES. The tau mutation in the Syrian hamster differentially reprograms the circadian clock in the SCN and peripheral tissues. J Biol Rhythms 20: 99–110, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, Watanabe T, Sekimoto M, Shibui K, Kim K, Kudo Y, Ozeki Y, Sugishita M, Toyoshima R, Inoue Y, Yamada N, Nagase T, Ozaki N, Ohara O, Ishida N, Okawa M, Takahashi K, Yamauchi T. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep 2: 342–346, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci 37: 209–221, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Goel N, Lee TM, Smale L. Suprachiasmatic nucleus and intergeniculate leaflet in the diurnal rodent Octodon degus: retinal projections and immunocytochemical characterization. Neuroscience 92: 1491–1509, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25: 3195–3216, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hagenauer MH, Lee TM. Circadian organization of the diurnal Caviomorph rodent, Octodon degus Biol Rhythm Res 39: 269–289, 2008 [Google Scholar]
- 21.Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms 19: 400–413, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H, Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J Neurosci 20: 5867–5873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hut RA, Mrosovsky N, Daan S. Nonphotic entrainment in a diurnal mammal, the European ground squirrel (Spermophilus citellus). J Biol Rhythms 14: 409–419, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol Regul Integr Comp Physiol 273: R1800–R1809, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Jiao YY, Rusak B. Electrophysiology of optic nerve input to suprachiasmatic nucleus neurons in rats and degus. Brain Res 960: 142–151, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Jiao YY, Lee TM, Rusak B. Photic responses of suprachiasmatic area neurons in diurnal degus (Octodon degus) and nocturnal rats (Rattus norvegicus). Brain Res 817: 93–103, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96: 57–68, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Krajnak K, Dickenson L, Lee TM. The induction of Fos-like proteins in the suprachiasmatic nuclei and intergeniculate leaflet by light pulses in degus (Octodon degus) and rats. J Biol Rhythms 12: 401–412, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus). J Biol Rhythms 20: 206–218, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc Natl Acad Sci USA 99: 13890–13895, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci 12: 4206–4214, 2000 [PubMed] [Google Scholar]
- 32.Maywood ES, Mrosovsky N. A molecular explanation of interactions between photic and non-photic circadian clock-resetting stimuli. Brain Res Gene Expr Patterns 1: 27–31, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA 96: 15211–15216, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijer JH, Rusak B, Harrington ME. Photically responsive neurons in the hypothalamus of a diurnal ground squirrel. Brain Res 501: 315–323, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, Okamura H. Phase-dependent responses of Per1 and Per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett 294: 41–44, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Moriya T, Horikawa K, Akiyama M, Shibata S. Correlative association between N-methyl-d-aspartate receptor-mediated expression of period genes in the suprachiasmatic nucleus and phase shifts in behavior with photic entrainment of clock in hamsters. Mol Pharmacol 58: 1554–1562, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Mrosovsky N, Edelstein K, Hastings MH, Maywood ES. Cycle of period gene expression in a diurnal mammal (Spermophilus tridecemlineatus): implications for nonphotic phase shifting. J Biol Rhythms 16: 471–478, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res 82: 622–630, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen HS, Hannibal J, Knudsen SM, Fahrenkrug J. Pituitary adenylate cyclase-activating polypeptide induces Period1 and Period2 gene expression in the rat suprachiasmatic nucleus during late night. Neuroscience 103: 433–441, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Novak CM, Albers HE. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am J Physiol Regul Integr Comp Physiol 286: R820–R825, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Novak CM, Ehlen JC, Paul KN, Fukuhara C, Albers HE. Light and GABA(A) receptor activation alter period mRNA levels in the SCN of diurnal Nile grass rats. Eur J Neurosci 24: 2843–2852, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Numano R, Yamazaki S, Umeda N, Samura T, Sujino M, Takahashi R, Ueda M, Mori A, Yamada K, Sakaki Y, Inouye ST, Menaker M, Tei H. Constitutive expression of the Period1 gene impairs behavioral and molecular circadian rhythms. Proc Natl Acad Sci USA 103: 3716–3721, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunez AA, Bult A, McElhinny TL, Smale L. Daily rhythms of Fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. J Biol Rhythms 14: 300–306, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Ocampo-Garces A, Mena W, Hernandez F, Cortes N, Palacios AG. Circadian chronotypes among wild-captured west Andean octodontids. Biol Res 39: 209–220, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Oishi K, Fukui H, Sakamoto K, Miyazaki K, Kobayashi H, Ishida N. Differential expressions of mPer1 and mPer2 mRNAs under a skeleton photoperiod and a complete light-dark cycle. Brain Res Mol Brain Res 109: 11–17, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Oster H, Avivi A, Joel A, Albrecht U, Nevo E. A switch from diurnal to nocturnal activity in S. ehrenbergi is accompanied by an uncoupling of light input and the circadian clock. Curr Biol 12: 1919–1922, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set (5th ed.). Burlington, MA: Elsevier, 2005
- 48.Pereira DS, Tufik S, Louzada FM, Benedito-Silva AA, Lopez AR, Lemos NA, Korczak AL, D'Almeida V, Pedrazzoli M. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep 28: 29–32, 2005 [PubMed] [Google Scholar]
- 49.Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120: 745–755, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Rado R, Terkel J. Circadian activity of the blind mole rat, Spalax ehrenbergi, monitored by radio telemetry, in seminatural and natural conditions. In: Environmental Quality and Ecosystem Stability Vol. IV/B, edited by Spanier E, Steinberger Y, and Luria M. Jerusalem, Israel: Israel Society for Ecology and Environmental Quality Sciences, 1989, p. 391–400.
- 51.Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience 127: 13–23, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: a comparative study. Brain Res 274: 184–187, 1983 [DOI] [PubMed] [Google Scholar]
- 53.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19: 1261–1269, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Shieh KR, Yang SC, Lu XY, Akil H, Watson SJ. Diurnal rhythmic expression of the rhythm-related genes, rPeriod1, rPeriod2, and rClock, in the rat brain. J Biomed Sci 12: 209–217, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms 18: 356–366, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Smith RD, Inouye S, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol [A] 164: 805–814, 1989 [DOI] [PubMed] [Google Scholar]
- 58.Sumová A, Bendová Z, Sládek M, Kováciková Z, El-Hennamy R, Laurinová K, Illnerová H. The rat circadian clockwork and its photoperiodic entrainment during development. Chronobiol Int 23: 237–243, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Takumi T, Matsubara C, Shigeyoshi Y, Taguchi K, Yagita K, Maebayashi Y, Sakakida Y, Okumura K, Takashima N, Okamura H. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells 3: 167–176, 1998a [DOI] [PubMed] [Google Scholar]
- 60.Takumi T, Taguchi K, Miyake S, Sakakida Y, Takashima N, Matsubara C, Maebayashi Y, Okumura K, Takekida S, Yamamoto S, Yagita K, Yan L, Young MW, Okamura H. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. Embo J 17: 4753–4759, 1998b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389: 512–516, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem 278: 718–723, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13: 1190–1196, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol 430: 518–532, 2001 [PubMed] [Google Scholar]
- 65.Yamamoto Y, Yagita K, Okamura H. Role of cyclic mPer2 expression in the mammalian cellular clock. Mol Cell Biol 25: 1912–1921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 16: 1531–1540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience 94: 141–150, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105: 683–694, 2001 [DOI] [PubMed] [Google Scholar]


