Abstract
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat model of obesity (a spontaneous CCK1 receptor knockout) has been extensively studied as model of hyperphagia-induced obesity. In previous studies, young OLETF rats presented abnormal eating patterns [compared with Long-Evans Tokushima Otsuka (LETO) controls] in a variety of independent ingestion and nursing tests during the suckling period. The aim of the present study was to characterize the early emergence of abnormal adiposity in the pups. Moreover, because both the dams and the pups present the genetic mutation, a close follow-up of the dams' body weight and intake during pregnancy and lactation was performed to examine the circumstances that contribute to build up the pups' early adiposity. Compared with controls, OLETF pups presented higher fat levels, larger adipocytes, and increased waist circumference as early as postnatal day 7 and this profile persisted to the age of weaning. While LETO dams gained weight throughout pregnancy and lactation, OLETF dams were obese and hyperphagic during pregnancy but lost weight during lactation, probably as a result of rearing hyperphagic pups. Current and previous results suggest a possible influence of the dams' obesity during gestation and a high investment in nursing time during lactation on the pups' obesity levels during childhood. This, combined with the innate hyperphagia repeatedly observed in the pups at these early ages, makes the OLETF strain a useful tool in the research of childhood-onset obesity.
Keywords: overeating, animal models, gestation, feeding
obesity is a growing public health concern that has reached epidemic proportions in Western societies in the last few decades (27). Although much research has already been conducted on obesity by using animal models, investigations have usually focused on adult males, leaving a gap regarding childhood and female obesity. In many cases, early symptoms can be detected during “preobese” stages, where interventions could be more successful in reaching perdurable outcomes. Given the permanent upward resetting of body weight set point that takes place when genetically predisposed individuals become obese, the identification of the early factors that can help understand and prevent obesity might be the key to control the spread of the obesity epidemic (1, 5, 6, 33, 36).
Maternal obesity during pregnancy and lactation have been found to influence the offspring in the long term, predisposing them to develop larger fat pads and higher leptin and glucose levels later in life, especially in the presence of a genetic tendency (23, 30). Maternal obesity can also predispose offspring to obesity for generations to come (15, 47). In a study on mice, females fed a palatable obesogenic diet throughout pregnancy and lactation produced obese offspring presenting hyperphagia during adolescence and adulthood, with reduced locomotor activity and increased adiposity. Abdominal obesity in this model was associated with adipocyte hypertrophy (38). Exposure to a high-energy diet specifically during pregnancy (and not lactation) has been found to cause hyperadiposity and reduced glucose tolerance in the neonatal/weanling offspring (14). Another study suggested that maternal obesity at conception leads to fetal programming of the offspring, which results in obesity later in life (44). In a further study, rat dams provided with a “cafeteria diet” (a variety of high fat/palatable food) from the first day of pregnancy exhibited hyperphagia throughout pregnancy and lactation, compared with chow-fed controls. Despite feeding on a cafeteria diet, these dams showed no body mass increase during lactation. This perhaps suggests a higher energy investment in milk production, which might have also been richer in energy and fat. Rats born to mothers fed a cafeteria diet developed an exacerbated preference for fat, sugar, and salty foods and also exhibited increased body weight and body mass index compared with other offspring. This study shows that a maternal high-fat diet during pregnancy and lactation may be an important contributing factor in the development of obesity (7). Altogether, the results of these studies expose some consequences of the mothers' overeating (and probably of a high body fat percentage) at the time of fetal development and lactation on their offspring's' short- and long-term obesity development.
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat model lacks the CCK1 receptor due to a spontaneous genetic mutation, and represents a broadly established model of noninsulin-dependent diabetes mellitus (NIDDM) (28, 29) and hyperphagia-induced obesity (34). CCK is a brain-gut peptide that acts as a peripheral satiety molecule through CCK receptors of type 1, as proved by extensive research with exogenous CCK, specific CCK1 receptor antagonists and with the OLETF CCK1 knockout model of obesity (for a review, see Ref. 45). Adult OLETF males are hyperphagic, obese, hyperleptinemic, completely resistant to CCK administration, hyperglycemic, develop NIDDM, and present upregulation of neuropeptide Y mRNA in the dorsomedial hypothalamus (10, 34, 35). OLETF females typically do not develop diabetes (50).
We have recently dedicated much effort into understanding the behavioral aspects of the “preobese” stages in the OLETF strain and demonstrated that OLETF rats are heavier from birth (39) and show hyperphagic characteristics in independent ingestion tests as early as postnatal day 2 (PND2) (12). OLETF pups also consume more milk during nursing bouts whether they are suckling from an OLETF or a Long-Evans Tokushima Otsuka (LETO) control dam (41) and also initiate more nursing bouts, especially during the third postnatal week (40). Overall, we concluded that an abnormal intake profile is observed very early during development, showing that obesity in this strain develops following lifelong abnormalities in eating behavior.
In addition to these differences between the pups, there is a disparity in the behavior of OLETF and LETO dams that may contribute to the difference in the rate of weight gain of the offspring (39, 40). Beyond the increased nursing time found in the OLETF strain, we have recently reported a detailed physiological characterization of the changes dams of both strains undergo throughout lactation (49). OLETF females appear to gradually loose weight and body fat after delivery, an outcome that hypothetically might be a consequence of larger milk production to feed hyperphagic pups. This contrasted with the pattern of weight gain over lactation that was apparent in control LETO dams. That study, however, was cross sectional, so our conclusions result from group comparisons rather than from individual follow-up data. Furthermore, that study did not include pregnant rats, starting the follow-up postpartum. Therefore, in the present study, it was of special interest to closely follow up the dams' changes in body weight and intake throughout pregnancy and lactation to identify further contributing factors to the overweight of the pups at birth and during the suckling period.
In the present study, we aimed first, to characterize the early adiposity-related development in the offspring from birth until weaning. Examination of this potentially critical time for obesity development when fat stores, body weight, and intake change dramatically, may help to identify possible markers that could successfully predict later obesity and provide time windows where interventions could be more successful in the treatment of long-term obesity (36) (see Barker hypothesis, Refs. 5 and 6). Second, we aimed to further explore the dams' contribution to the early development of obesity in the OLETF strain, by characterizing their intake and body weight during pregnancy and lactation.
MATERIALS AND METHODS
Subjects
Twenty-seven nulli- and primiparous OLETF and LETO dams and their offspring were raised in the specific pathogen-free facility of the Gonda Brain Research Center at Bar-Ilan University, Ramat-Gan, Israel. The original animals were from the Tokushima Research Institute, Japan. Females were mated with males from their own strain. For the pregnancy follow-up, adult females were mated with males for only one night during the proestrous phase of the estrous cycle (determined by vaginal smears). If sperm was identified by a further vaginal smear the morning after, it was considered as gestation day 0 (GD0). Pregnant females were housed in pairs until 3–4 days before parturition to avoid isolation stress. After parturition, OLETF and LETO dams and their offspring were housed together until weaning. Controls for the dams were group housed, not mated, and followed up at comparable ages. Clean polycarbonate cages (18.5 cm height × 26.5 cm width × 43 cm length) were used, with stainless-steel wire lids and wood shavings as bedding material. Food [Harlan 2018SC+F (6% fat)] and water were freely available. The animals were on a 12:12-h light-dark cycle, with lights on at 07:00. Room temperature was maintained at 22 ± 2°C. Newborn litters were culled on PND1, between 11:00 AM and 2:00 PM, to 10 pups (minimum 7), with sex distribution kept as equal as possible in each litter.
The research protocol was approved by the Institutional Animal Care and Use Committee, and it adhered to the guidelines of the APS's Guiding Principles in the Care and Use of Animals and of the Society for Neuroscience.
Body Weight and Intake
Dams were weighed every fifth day from GD0 until GD20 and then from PND1 until the time of weaning, and intake was measured daily. Pups were weighed every fifth day from PND1 until weaning (PND22 and -23). Body mass index [body wt in grams/(body length in cm2)] and waist circumference were also examined as further obesity parameters in the offspring on PND1, -7, -15, -23.
Tissue Collection
Four death time points were chosen throughout lactation to obtain a good coverage of the pup's development. PND1 intended to examine the influence of an “overweight” or “lean” pregnancy on the pups' obesity levels at birth, but it was impossible to extract fat pads from such small and lean animals, and only plasma and external parameters were collected. PND6, -7, -15, -16, -22, and -23 were chosen to characterize each week of the suckling period. On the experimental day, rats were weighed, measured and killed between 11:00 AM and 2:00 PM. For tissue collection on PND1, all the litter was culled. For the rest of the ages, one male and one female were culled for tissue collection, always taking care that at least seven pups remained in the litter. To be able to maintain large, similar-size litters after culling pups for tissue collection, while reaching a reasonable number of subjects per measure, we collected data from pups from a larger number of litters than the ones whose dams were studied during pregnancy and lactation. Thus, we also collected pups culled from additional litters (that were larger than 7 pups) in our breeding colony. Interscapular brown adipose tissue (BAT), retroperitoneal, inguinal adipose tissue, and epydidimal white fat (only males) tissues were collected from decapitated animals, weighed, and immediately frozen on dry ice. Measurable amounts of epydidimal white fat tissue were only available at PND23. Inguinal white fat samples were preserved at −80°C until analyzed. Trunk blood for leptin analysis was collected in chilled heparinized vacutainer tubes coated with EDTA. On PND1 plasma from 2–5 same-sex siblings was pooled in each tube. At all other ages n = 1 per tube. Plasma was stored at −80°C until assayed.
Leptin
Plasma leptin levels were assessed using a commercial ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturers' instructions.
Histology
Samples of the inguinal white adipose tissue were used for the characterization of the adipocyte cross-sectional area. The parameters were measured by an observer blind to the experimental group. Tissues were sectioned to 8 mm by a Cryostat (Leyca) at −35°C and mounted on glass slides. Digital photographs were taken using the ACT1 program, at ×200 magnification. For each inguinal fat pad examined, between 10–20 pictures were taken from three different zones of the sample, with at least 100 mm distance from each other. Adipocyte size parameters were derived from three to six representative cells from each picture (depending on the size of the cell) using the public domain National Institutes of Health Scion image program. For each animal, at least 50 cells were analyzed. Representative cells chosen presented a smooth and clear membrane, with no granulation around. A similar methodological approach was described elsewhere (31, 49). The estimated number of cells per fat pad was calculated with the average cell diameter, a density conversion factor (0.915 g/cc), and the mass of the fat pads, as previously described (3, 32).
Statistical Analysis
Maternal weight gain and intake data were analyzed by repeated-measures ANOVA with strain (OLETF-LETO) as the independent variable and measurement day as the repeated measure. Group differences on each of the days of gestation and lactation were analyzed by one-way ANOVAs followed by post hoc Scheffé tests. Offspring data were analyzed by univariate ANOVA with strain (OLETF-LETO), sex, and measurement day as the three independent variables. Significant interactions were followed up by post hoc ANOVA within each group with Duncan's test for pairwise comparisons (P < 0.05) or by comparisons of OLETF vs. LETO rats at particular ages.
RESULTS
Gestation
As shown in Fig. 1, the patterns of body weight change over gestation were similar, but significantly greater in OLETF compared with LETO dams [F(5,15) = 12.58, P < 0.001, interaction effect]. From GD10 and on, the percent of body weight gain of the OLETF females was significantly greater than that of the LETO females (P < 0.05). Furthermore, on postpartum day 1 OLETF dams still had body weight levels that were significantly higher than at gestation onset (P < 0.001). In contrast, LETO dams returned to body weight levels that were not significantly different from GD1 (Fig. 1). Mean raw body weights are presented in Table 1. OLETF dams weighed significantly more than LETO controls over all points measured during gestation and on postpartum day 1 [F(1,19) = 23.31, P < 0.001 overall; P < 0.05 at different days of comparison].
Fig. 1.
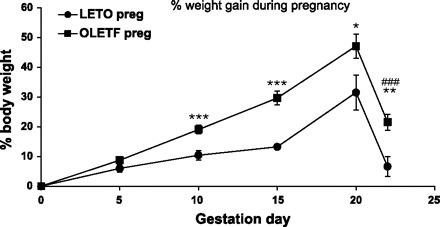
Percentage body weight change in Otsuka Long Evans Tokushima Fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) pregnant females from the day of conception. Data are presented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 [for differences between the strains at each gestation day (GD)] and ###P < 0.001 (significant difference in %body weight retention compared with GD0 within the OLETF strain) (n = 9 LETO, 12 OLETF).
Table 1.
Raw body weight of OLETF and LETO females throughout pregnancy and lactation
| LETO | OLETF | |
|---|---|---|
| GD0 | 234.67±8.54 | 276.5±23.80* |
| GD5 | 248.33±8.04 | 300.8±25.43† |
| GD10 | 258.56±8.03 | 329.1±26.99‡ |
| GD15 | 271.33±7.44 | 357.5±28.40‡ |
| GD20 | 305.33±6.98 | 404.9±30.84‡ |
| PND1 | 253.89±10.75 | 342.9±13.80‡ |
| PND5 | 265.22±12.89 | 353.7±13.84‡ |
| PND10 | 271.11±12.29 | 345.7±9.40‡ |
| PND15 | 275.00±10.87 | 334.1±10.43‡ |
| PND20 | 277.67±11.18 | 322.5±9.27† |
| PND22 | 274.11±10.72 | 313.4±8.11* |
Data are means ± SE in grams; OLETF, Otsuka Long-Evans Tokushima Fatty; LETO, Long-Evans Tokushima Otsuka; GD, gestation day, PND, postnatal day. LETO, n = 9 (lactation and pregnancy); OLETF, n = 8 (lactation), n = 12 (pregnancy).
P < 0.05,
P < 0.01 and
P < 0.001 for differences between the strains.
The patterns of caloric intake were significantly different among the four groups [F(44, 66) = 8.14, P < 0.001; Fig. 2], with a significant main effect of strain [OLETF females ate significantly more than LETO females F(3,37) = 118.99, P < 0.001]. On all 22 days of gestation, there were significant differences among the groups (P < 0.01). Post hoc Scheffé tests showed that control nonpregnant OLETF rats consumed significantly more kcal than control LETO rats. The OLETF pregnant dams ate consistently more than their within-strain nonpregnant controls, starting from GD5 and until GD20. Interestingly, pregnant LETO dams starting overeating later, from GD9 until GD20. The daily intake differences were significant on GD9, -10, -12, -16, and -17 (Scheffé tests). From GD20 until birth, pregnant females consumed similar amounts as controls. Pregnant OLETF dams ate significantly more than pregnant LETO controls on all gestation days (P < 0.05) with two exceptions: GD20 and -22.
Fig. 2.
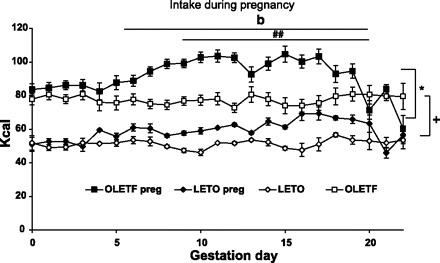
Daily intake of OLETF and LETO pregnant (preg.) and control (◊, □) females from the day of conception until parturition. Intake is presented in kilo-calories (kcal). Data are presented as means ± SE. *P < 0.05, for OLETF pregnant vs. LETO pregnant; ##P < 0.01 for LETO pregnant vs. LETO control and bP < 0.01 for OLETF pregnant vs. OLETF control. There was also a significant effect between OLETF and LETO controls (+P < 0.05) (n = 9 LETO, 12 OLETF).
Lactation
The number of pups born to LETO (8 ± 0.73, mean ± SE) and OLETF (10 ± 0.90, mean ± SE) dams was not significantly different [t(19) = 1.41; NS, not significant].
The patterns of body weight change during the postpartum period were opposite and significantly different in the two strains [F(5,12) = 8.30, P < 0.001, interaction effect], LETO dams significantly gained weight (%PND1 body wt) from the earliest age measured (PND5) and continued to do so (P < 0.05). In contrast, the weight of OLETF dams significantly decreased starting from PND15 and on (P < 0.05). Starting from PND10, strain differences in percent body weight change were statistically significant (P < 0.01) (Fig. 3). Mean raw body weights are presented in Table 1. OLETF dams weighed significantly more than LETO controls over all points measured during lactation [F(1,15) = 26.07, P < 0.001 overall; P < 0.01 at different days of comparison, with one exception: P < 0.05 on PND22, when strain differences were at minimum].
Fig. 3.
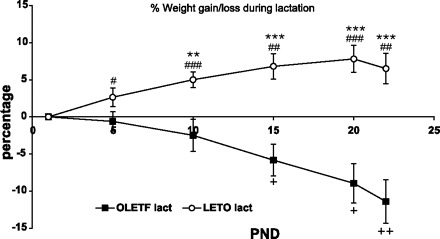
Percentage body weight change of lactating OLETF and LETO females from birth until weaning. Data are presented as means ± SE. **P < 0.01, ***P < 0.001, for differences between the strains at each postnatal day (PND); #P < 0.05, ##P < 0.01, ###P < 0.001 for the differences in body weight change compared with PND1 within the LETO strain; and +P < 0.05, ++P < 0.01 for the differences in body weight change compared with PND1 within the OLETF strain (n = 9 LETO, 8 OLETF).
The pattern of food intake was significantly different among the four groups [F(66,40) = 4.54, P < 0.001, interaction effect]. A significant main effect of strain was found, with OLETF females consuming more kcal than LETO females [F(3,34) = 136.24, P < 0.001]. Control nonlactating groups showed stable levels of intake over the assessment period, whereas the lactating groups increased intake significantly over time (P < 0.001). Post hoc Scheffé tests showed that the two lactating groups ate significantly less than control nonlactating female rats on the day of birth (PND0). From PND1 and on, both lactating groups ate significantly more than LETO controls and from PND4 and on, ate significantly more also than nonlactating OLETF controls (Fig. 4). Intake of lactating rats from both strains did not differ significantly over the 23 days of assessment. We would like to point out that the intake levels presented here in the third postnatal week include both the dams and the pups, because they shared the source of food from the emergence of independent ingestion, and over the third week of lactation, the pups increasingly feed independently.
Fig. 4.
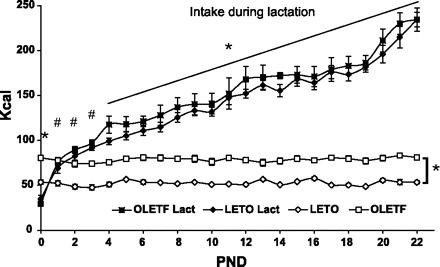
Daily intake of lactating (Lact) OLETF and LETO females from birth until weaning and same aged OLETF and LETO controls (◊, □). Intake is presented in kcal. Data are presented as means ± SE. *Significantly different from both nonlactating groups; #significantly different from the LETO nonlactating group (n = 9 LETO, 8 OLETF).
Pups
OLETF and LETO pups' weight differed significantly across time points [F(3,233) = 15.06, P < 0.001, for strain × age interaction effect], regardless of the sex of the pups (sex main effect and interactions with sex, NS). At all ages and within each sex, OLETF pups weighed significantly more than LETO pups (P < 0.01) (Fig. 5).
Fig. 5.
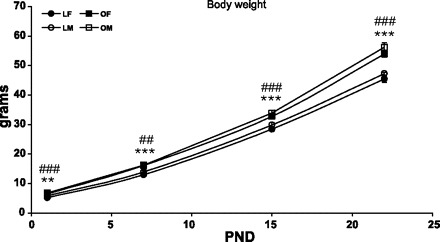
OLETF and LETO pups' body weight in grams on PND1, -7, -15, and -23. Data are presented as means ± SE. **P < 0.01, ***P < 0.001 for the females, ##P < 0.01, ###P < 0.001 for the males (for strain differences at this age) (n = 10–22 per group).
The weight of the excised white fat pads (expressed as %rat body weight) was significantly greater overall in OLETF vs. LETO rats [F(1,91) = 121.38, P < 0.001], in females vs. males [F(1,91) = 5.12, P < 0.05], and in 7-day-old vs. 15- and 22-day-old rats [F(2,91) = 17.96, P < 0.001; post hoc Duncan's test (P < 0.05)]. Furthermore, LETO pups had significantly less white fat on PND15, compared with older and younger ages; and 7-day-old OLETF pups had significantly higher levels of white fat compared with older OLETFs [PND15 and -22; F(2,91) = 13.88, P < 0.001, for the strain × age interaction]. Other interactions were not statistically significant (see Fig. 6). Group differences in different fat pads (expressed as %body wt) are presented in Table 2.
Fig. 6.
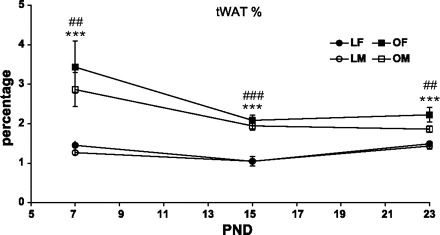
Total white adipose tissue (tWAT; expressed as %body weight) of OLETF and LETO males and females on PND7, -15, and -23. LF, LETO female; LM, LETO male; OF, OLETH female; OM, OLETF male. Data are presented as means ± SE. ***P < 0.001 for the females, ##P < 0.01, ###P < 0.001 for the males (for strain differences at each age) (n = 6–14 per group).
Table 2.
Weight of different fat pads collected from the pups
| PND | LETO M | OLETF M | LETO F | OLETF F | ||||
|---|---|---|---|---|---|---|---|---|
| IAT | ||||||||
| 1 | ||||||||
| 7 | 0.95±0.03 | 1.81±0.25* | 0.95±0.06 | 2.18±0.43* | ||||
| 15 | 0.93±0.09 | 1.43±0.05* | 0.89±0.04 | 1.55±0.08* | ||||
| 22 | 1.02±0.05 | 1.39±0.05*† | 1.19±0.04 | 1.71±0.12* | ||||
| Retro | ||||||||
| 1 | ||||||||
| 7 | 0.33±0.03 | 1.06±0.18* | 0.50±0.07 | 1.25±0.30* | ||||
| 15 | 0.15±0.03 | 0.49±0.05* | 0.15±0.02 | 0.53±0.06* | ||||
| 22 | 0.32±0.03 | 0.35±0.03 | 0.30±0.04 | 0.51±0.07 | ||||
| BAT | ||||||||
| 1 | ||||||||
| 7 | 0.47±0.03 | 0.69±0.06*† | 0.58±0.05 | 0.74±0.12* | ||||
| 15 | 0.46±0.02 | 0.38±0.02 | 0.50±0.03 | 0.47±0.07 | ||||
| 22 | 0.46±0.05† | 0.29±0.01 | 0.40±0.03 | 0.31±0.01 | ||||
Data are presented as means ± SE, expressed as %body wt; n = 6–14 per group. IAT, inguinal adipose tissue; Retro, retroperitoneal adipose tissue; BAT, brown adipose tissue; M, males; F, females.
Significantly different from same-sex and same-age LETO controls;
significantly different from the same-strain opposite sex. All significant differences derived from post hoc Duncan's tests, P < 0.05.
Inguinal adipose tissues.
Inguinal fat as a percentage of body weight was significantly greater in OLETF compared with LETO pups at all ages without sex differences. The only exception was at PND23; at this age, female OLETF rats had more inguinal adipose tissue than male OLETFs [F(3,24) = 15.57, P < 0.001 on PND7; F(3,38) = 24.50, P < 0.001 on PND15; F(3,33) = 9.20, P < 0.001 on PND23].
Retroperitoneal fat pads.
On PND7 and -15, retroperitoneal fat pads were heavier in OLETF than in LETO pups, with no sex differences [F(3,24) = 12.44, P < 0.001 on PND7; F(3,35) = 14.41, P < 0.001 on PND15]. On PND23, there were no group differences (NS).
Brown adipose tissue.
On PND7, brown adipose fat pad was significantly larger in OLETF than in LETO pups, with no sex differences [F(3,24) = 4.97, P < 0.01]. On PND15 there were no significant group differences, whereas on PND23, LETO males had significantly more BAT than OLETF males [F(3,33) = 4.09; P < 0.05; the difference in the females was NS].
Adipocyte Size and Number
Mean inguinal adipocyte size was greater in OLETF than in LETO pups [F(1,41) = 54.97, P < 0.001], males had larger adipocytes than females on PND22 but not earlier [F(1,41) = 7.27, P < 0.001 for sex main effect; F(2,41) = 3.97, P < 0.05 for the sex × age interaction] and this was true in both LETO and OLETF strains (P < 0.05, see Fig. 7). The estimated adipocyte number was larger in OLETF compared with LETO pups [F(1,41) = 9.0, P < 0.01] and in females compared with males [F(1,41) = 24.81, P < 0.001] (sex main effect), with a significant sex × age interaction [F(2,41) = 3.86, P < 0.05]. Estimated adipocyte number increased across development in females of both strains, while males' adipocyte number remained almost constant. Post doc Duncan's tests revealed that females had a greater estimated adipocyte number on PND7 and -22 in the LETO strain and at all ages in the OLETF strain (P < 0.05), compared with males. Significant strain effects were only observed between males on PND7 (Fig. 8).
Fig. 7.
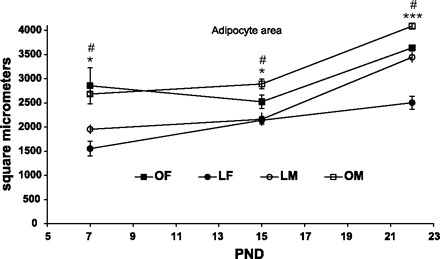
Adipocyte size of OLETF and LETO males and females on PND7, -15, and -23. Data are presented as means ± SE. *P < 0.05, ***P < 0.001 for the females, #P < 0.05 for the males (for strain differences at each age) (n = 4–6 per group).
Fig. 8.
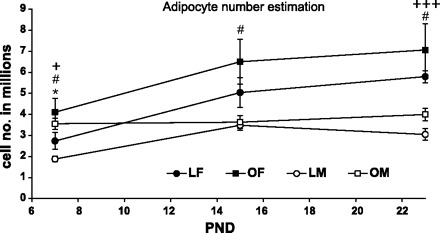
Estimation of the inguinal adipocyte number of OLETF and LETO males and females on PND7, -15, and -23. Data are presented as means ± SE. +P < 0.05, +++P < 0.001 for LETO females vs. LETO males, #P < 0.05 for OLETF females vs. OLETF males, and *P < 0.05, for strain differences between the males (n = 4–6 per group).
Leptin
OLETF pups had significantly higher leptin levels on PND7, compared with LETO pups, within each sex; on the other days the differences were NS [F(3,52) = 12.38, P < 0.001, for the strain × age interaction]. However, the strain difference on PND1 is based on samples pooled from several newborn pups, thus the total number is greater than the number of samples analyzed. Student's t-tests within each sex showed that male and female OLETF newborns had higher levels of leptin at this age, compared with same-sex LETO newborns (P < 0.05), see Fig. 9. Overall, leptin levels were higher on PND7 and -22, compared with PND1 and -15 [F(3,52) = 11.14, P < 0.001].
Fig. 9.
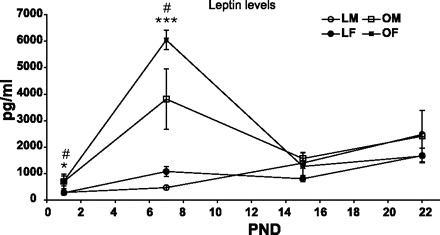
Plasma leptin levels of OLETF and LETO males and females on PND1, -7, -15, and -23. Data are presented as means ± SE. *P < 0.05, ***P < 0.001 for the females, #P < 0.05 for the males (for strain differences at each age) (n = 4–7 per group).
Body Mass Index and Waist Circumference
Group differences in body mass index were as follows: on PND1, LETO females presented lower body mass index than OLETF females (in the males the strain difference was NS), and on PND15 LETO males presented lower body mass index than the OLETF males (in the females the strain difference was NS). On PND7 no significant differences were observed; while at weaning age, a significant strain effect (OLETF>LETO) was found in both sexes [F(3,109) = 3.68, P < 0.05, Fig. 10].
Fig. 10.
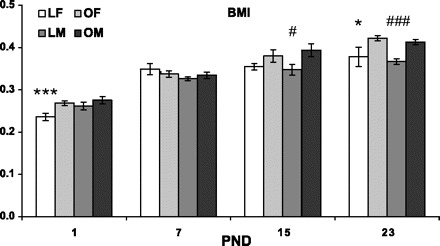
Body mass index (BMI) of OLETF and LETO males and females on PND1, -7, -15, and -23. Data are presented as means ± SE. *P < 0.05, ***P < 0.001 for the females, #P < 0.05, ###P < 0.001 for the males (for strain differences at each age) (n = 6–10 per group).
Regarding waist circumference, LETO females presented lower waist circumference than OLETF females on PND1 (in the males the strain difference was NS); from PND7 on a significant strain effect was found in both sexes with OLETF rats presenting consistently greater waist circumference than LETO controls [F(3,109) = 22.86, P < 0.001, for the strain × age interaction; Fig. 11].
Fig. 11.
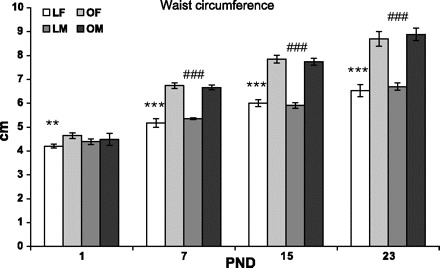
Waist circumference of OLETF and LETO males and females on PND1, -7, -15, and -23. Data are presented as means ± SE. **P < 0.01, ***P < 0.001 for the females, ###P < 0.001 for the males (for strain differences at this age) (n = 6–10 per group).
DISCUSSION
In the present study, we explored the short-term consequences of maternal obesity during pregnancy and lactation on several measures of adiposity and growth in infant OLETF and LETO males and females. In the last few years, researchers have focused their attention on the consequences of maternal obesity on food preferences and long-term obesity levels of the offspring, mostly using rodent animal models. In these studies, females are usually given access to high fat or highly palatable foods to induce obesity and/or hyperphagia. The OLETF rats' development of obesity is secondary to their hyperphagia (34), which is a direct consequence of their genetic mutation. Therefore, OLETF females (and males) are obese and hyperphagic even when consuming regular chow. Some studies have shown that giving the OLETF rats access to high fat or palatable food leads to an even more morbid obesity (9, 26), but this is not necessary to induce moderate obesity in this strain. Therefore, the results presented here are related to the hyperphagia and obesity levels of the dam during pregnancy and lactation (and to the pups' genetic tendency) and not to any specific kind of “junk food.”
OLETF dams were obese at the time they became pregnant, and exhibited hyperphagia resulting in significantly higher rates of weight gain than controls during gestation. It should be mentioned, that OLETF females weighing between 23–30% more than controls were primarily used for these studies. At this level of obesity they became pregnant, while OLETF females weighing more than that usually did not. Moreover, OLETF females in this study were on average younger (8–12 wk old) than LETO females (8–16 wk of age), because only the youngest became pregnant and gave birth to the expected number of pups.
OLETF females were hyperphagic compared with LETO females during pregnancy, but this difference was not maintained after delivery. Lactating OLETF dams consumed the same amounts of food as lactating LETO dams (note though that a portion of the intake recorded in the third postnatal week in both strains was pup consumption, increasing as the offspring matured toward weaning age). Interestingly, whereas OLETF dams gained significantly more weight during pregnancy, the lactating period represented a time point where OLETF females gradually lost weight, almost reaching weight levels of LETO dams at the time of weaning. We have recently reported that this weight loss throughout lactation is accompanied by a decrease in fat stores, adipocyte cell size, and leptin levels (49). We can only speculate about why OLETF females do not maintain a relative hyperphagia beyond that shown by lactating controls during such an energy-demanding period. It appears that overall the OLETF strain has difficulties in compensating to energy challenges. OLETF rats do not compensate by reducing their intake of standard chow after intake of a palatable food (43). OLETF preweanling pups do not reduce their milk intake in independent ingestion tests after a fat preload (13). There is the same lack of compensation to increased energy demands. Male and female OLETF do not increase their intake to compensate for increased energy expenditure when they exercise, whereas LETO females do (11, 42). Whereas the OLETF females in the current study were hyperphagic compared with nonlactating OLETF females, the relative (normalized to body weight) degree of increase was not as great as that found in lactating LETO females. This difference, together with the demands of rearing hyperphagic pups, may account for their weight loss. A higher energy investment in milk production may also contribute to the weight loss of OLETF females during lactation. This is an issue still to be elucidated.
As we have already reported, OLETF pups are born significantly heavier than controls (39). This might be a consequence of the exposure to an obese dam during pregnancy, the genetic mutation itself, or both. In the present study, we characterized their obesity development and identified preobese features in this strain that suggest that this disorder develops early in life in the OLETF strain, making them a valid model of childhood-onset obesity.
During lactation, the increased amount of nursing and, consequently, of milk that the OLETF pups receive from their obese mother, may be at least partially responsible for the high body fat percentages observed during suckling. As early as PND7, OLETF pups had increased waist circumference, fat levels, and larger adipocytes. These differences between the strains remained throughout lactation in both sexes. However, at the end of the second prenatal week (PND15) there was an obvious decrease in body fat and leptin levels in the OLETF strain. At this point, OLETF pups also show a temporary lack of increase in adipocyte hypertrophy. We have previously studied each prenatal week separately, and the second prenatal week frequently failed to present differences between the strains in maternal and nursing behavior (39) and sometimes also in pup suckling characteristics (42). It seems that this prenatal week represents a “transition time,” where the very significant differences observed in the first and third prenatal weeks become moderate. This second prenatal week appears to be a period of time where differences between the strains diminish, and could therefore be an appropriate target for future interventions.
The profile of ontogenetic changes in BAT (levels decreasing) vs. white adipose tissue (increasing) found in this study, is consistent with previous descriptions in other strains (17). It is interesting that the BAT was significantly larger in OLETF pups compared with controls only on PND7. BAT has been reported to become hypertrophic under several conditions, with overfeeding being one of them (4, 25, 37). At this age, OLETF pups also presented greater white fat percentages and adipocytes and higher leptin levels compared with controls, a profile resembling adult-like differences. In previous studies, hyperphagic characteristics were systematically observed in the preobese strain around this age, both in independent ingestion tests and nursing (with increased suckling rate) (12, 41). Nevertheless, changes in BAT weight do not necessarily reflect changes in its activity or in mitochondrial thermogenic capacity (16, 46). By PND15, BAT in the OLETF pups was normalized to LETO levels. Thus it would appear that this transitory neonatal increase in BAT may be in response to the high amounts of food consumed in the first postnatal week.
The body mass index and waist circumference measurements were added as noninvasive parameters for identifying further obesity features, as these are similar to measures regularly used in humans. In the case of body mass index, it seems that even when body weight and fat are relatively high in the pups, this is not always reflected by this parameter. The classic body mass index also fails to identify obesity in children and teenagers in a reliable manner because of their constant physiological changes; and to correctly diagnose overweight in that population (<20 years of age), the original body mass index was modified by including sex and age parameters together with body weight and height (18). While the original body mass index appears to be an effective tool for identifying obesity levels in adult rats (2), an improved index has not yet been created for young animals, making it a less accurate method for diagnosing obesity in rat pups. In contrast, measuring waist circumference appears to be a more effective way of detecting the different pattern of adiposity development taking place in the OLETF pups as early as PND7 in both sexes.
Females of both strains, although weighing less, exhibited greater percentages of body fat than the males, and this is particularly true for the OLETF females. Interestingly, this finding is not accompanied by adipocyte hypertrophy, with OLETF females presenting smaller adipocytes than OLETF males on PND15 and -22. The analysis of adipocyte number estimated the presence of more cells at these ages in both strains, suggesting that adipocyte hyperplasia is more pronounced in the females than in the males in the preweaning period. In rats, the phase of highest hyperplasia occurs between birth and PND40 and from then on cell number remains more or less constant. From PND40 until PND80, adipocyte hypertrophy is the main process responsible for the growth of the fat pads (24). Unlike many tissues, adipose tissue has the potential for almost unlimited growth, but importantly, diet-induced increases in fat cell number are apparently irreversible (19, 22). The comparison between OLETF and LETO rats shows a marked hypertrophy of the fat cells of the inguinal fat pad at all these early ages in the preobese OLETF in both sexes. Adipocyte hypertrophy has also been reported in weanling rats from dams fed a highly palatable diet in pregnancy and lactation (8) and in pups that underwent postnatal overfeeding due to a reduction in litter size (21). One of the few studies performed on adipose tissues of developing rats further reported a general pattern of increase in fat cell size in males and increased fat cell number in females as a consequence of postnatal overfeeding (20). A high amount of fat cells may be an important determinant responsible for the differences in fat depot sizes observed in the (obese) females compared with the males. Overall, it seems that obesity development in the OLETF strain undergoes similar paths in both sexes regarding adipocyte hypertrophy, but sharply different paths regarding adipocyte hyperplasia, at least in the preweaning period. It appears that, whereas OLETF males' increase in fat levels relies mainly on exaggerated fat cell enlargement, OLETF females present both high increase in cell number and size, predisposing them to a potentially worse profile of long-term obesity.
The profile of decreases in leptin levels, cell size, and fat percentages observed from PND7 to -15 in the OLETF strain in both sexes, is similar to what has been previously described in another model of early-onset obesity; where litter size was reduced to four to induce early obesity appearance in the offspring (21). Here, early obesity was characterized by adipocyte hypertrophy during the first 10 days of life and by a combination of adipocyte hypertrophy and hyperplasia from then on. Even though that study did not differentiate between males and females during the suckling period, their results appear to be in accordance with ours, with a sharp transitory increase in cell size (and in leptin and % fats) during the first phase of the lactating period as a consequence of early overeating, which was followed by an hyperplastic and hypertrophic period until adolescence. The significant surge in leptin levels observed around PND7 both in male and female OLETF pups may also be relevant to long-term obesity risk. In a study performed on mice, neonatally leptin-treated offspring exhibited an impaired response to acute peripheral leptin administration on a regular chow diet at around 8 wk of age, with impaired leptin transport to the brain, as well as an increased density of hypothalamic nerve terminals (48). Whereas OLETF females' sensitivity to leptin has never been examined, adult OLETF males have been reported to be resistant to peripheral (but not icv) administration of leptin already at 8 wk of age. (35). Whether this is a consequence of this early leptin surge or of their overall high leptin levels remains to be elucidated.
In summary, OLETF dams are hyperphagic and obese during pregnancy, leading to overweight pups at the time of birth. According to an investigation recently published, this alone might be enough to ensure an overweight adulthood (44). During lactation, OLETF dams eat like normal LETO lactating females, but in contrast to the weight gain observed in the LETO strain; normal intake leads OLETF females to gradually lose weight. The gradual increase in the nutritional demands of the OLETF pups, reflected by increased nursing time and initiative, especially in the third postnatal week (40, 41), is accompanied by a sharper body weight loss in the dams. It appears that the pups consume their dams' fat stores beyond the dam's normal levels, because the same energy intake in the LETO dams leads to transitory body weight gain during lactation and normal-weight pups. A cross-fostering study currently being performed will shed light on whether raising pups of the opposite strain will have different consequences on the obesity levels of the dams or the pups during lactation and will help with the understanding of critical periods of obesity development in this strain.
Perspectives and Significance
OLETF rats present preobese characteristics during the suckling period beyond abnormal eating behavior, such as increased body fat; larger adipocyte size, number (in the females), and waist circumference; and develop obesity as a consequence of chronic hyperphagia. On the basis of these new physiological and the previous behavioral findings, we propose the OLETF strain as a valid genetic model of childhood-onset obesity.
GRANT
This work was supported by the US-Israel Binational Research Foundation (to A. Weller and T. H. Moran), and by a President's Fellowship (Bar-Ilan University) to M. Schroeder.
Acknowledgments
We thank Dr. Kawano of the Tokushima Research Institute (Otsuka Pharmaceutical, Japan) for the generous gift of the OLETF and LETO rats.
Research reported in this paper was completed as part of the PhD dissertation of M. Schroeder in the Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel.
A portion of this research was presented at the 13th Annual Meeting of the Society for the Study of Ingestive Behavior, Pittsburgh, PA.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res 61: 653–659, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Altunkaynak BZ, Altunkaynak ME. Relationship of body weight and volume of liver. A morphometrical and stereological study. Saudi Med J 28: 891–895, 2007 [PubMed] [Google Scholar]
- 3.Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing–a quick, simple method. J Lipid Res 17: 190–192, 1976 [PubMed] [Google Scholar]
- 4.Ashwell M, Holt S, Jennings G, Stirling DM, Trayhurn P, York DA. Measurement by radioimmunoassay of the mitochondrial uncoupling protein from brown adipose tissue of obese (ob/ob) mice and Zucker (fa/fa) rats at different ages. FEBS Lett 179: 233–237, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Barker DJP. In utero programming of chronic disease. Clin Sci 95: 115–128, 1998 [PubMed] [Google Scholar]
- 6.Barker DJP. Fetal programming of coronary heart disease. Trends Endocrinol Metab 13: 364–368, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bayol SA, Farrington SJ, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. Br J Nutr 98: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567: 951–961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol 293: R55–R63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol 281: R254–R260, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Blumberg S, Haba D, Schroeder M, Smith GP, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol 290: R208–R218, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Blumberg S, Schroeder M, Tsitolovskya L, Torregrossa AM, Smith GP, Hurwitz I, Weller A. Post-preload feeding and brain c-fos immunoreactivity in Long Evans and OLETF preweanling rats. Paper presented at the 11th Annual Meeting of the Society for the Study of Ingestive Behavior. Groningen, The Netherlands. Appetite 40: 318–319, 2003 [Google Scholar]
- 14.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism 56: 1431–1438, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Campos KE, Volpato GT, Calderon IMP, Rudge MVC, Damasceno DC. Effect of obesity on rat reproduction and on the development of their adult offspring. Braz J Med Biol Res 41: 122–125, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Cannon B, Nedergaard J. Biochemical aspects of acclimation to cold. J Them Biol 8: 85–90, 1983 [Google Scholar]
- 17.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for mild overweight and obesity worldwide: international survey. BMJ 320: 1240–1243, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr 44: 173–180, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Cryer A, Jones HM. The development of white adipose tissue. Biochem J 86: 805–815, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dugail I, Quignard-Boulangé A, Dupuy F. Role of adipocyte precursors in the onset of obesity induced by overfeeding in suckling rats. J Nutr 116: 524–535, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol Endocrinol Metab 235, E279–E286, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 292: R1782–R1791, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Herrera E, Amusquivar W. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 16: 202–210, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Holloway BR. Reactivation of brown adipose tissue. Proc Nutr Soc 48: 225–230, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Ishida K, Mizuno A, Murakami T, Shima K. Obesity is necessary but not sufficient for the development of diabetes mellitus. Metabolism 45: 1288–1295, 1996 [DOI] [PubMed] [Google Scholar]
- 27.James WP. The epidemiology of obesity: the size of the problem. J Intern Med 263: 336–352, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Diabetes 41: 1422–1428, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract Supplement 24: S317–S320, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Govek. E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol Regul Integr Comp Physiol 275: R1374–R1379, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Yu X, Pan W, Unger RH. Expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am J Physiol Endocrinol Metab 282: E1334–E1341, 2002 [DOI] [PubMed] [Google Scholar]
- 32.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577–R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Miller JL, Silverstein JH. Management approaches for pediatric obesity. Nat Clin Pract Endocrinol Metab 3: 810–818, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci 29: 361: 1211–1218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol 11: 605–611, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res 65 Suppl 3: 83–89, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Ansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Schroeder M, Zagoory-Sharon O, Lavi-Avnon Y, Moran TH, Weller A. Weight gain and maternal behavior in CCK1 deficient rats. Physiol Behav 89: 402–409, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Schroeder M, Lavi-Avnon Y, Dagan M, Zagoory-Sharon O, Moran TH, Weller A. Diurnal and nocturnal nursing behavior in the OLETF rat. Dev Psychobiol 49: 323–333, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Schroeder M, Lavi-Avnon Y, Zagoory-Sharon O, Moran TH, Weller A. Pre obesity in the infant OLETF rat: The role of suckling. Dev Psychobiol 49: 685–691, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Schroeder M, Shbiro L, Gelber V, Moran TH, Weller A. Gender differences in anti-obesity programming achieved by early interventions in OLETF rats (Abstract). Dev Psychobiol 49: 740, 2007 [Google Scholar]
- 43.Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol Regul Integr Comp Physiol 277: R1144–R1151, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity (Silver Spring) 14, Suppl 4: 168S–170S, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tyzbir RS. Altered brown adipose tissue mitochondrial function in neonates born to rats overfed foods of various protein content. J Nutr 114: 234–237, 1984 [DOI] [PubMed] [Google Scholar]
- 47.Vadlamudi S, Kalhan SC, Patel MS. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol Endocrinol Metab 269: E731–E738, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1: 371–378, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Zagoory-Sharon O, Schroeder M, Levine A, Moran TH, Weller A. Adaptation to lactation in OLETF rats lacking CCK1 receptors: body weight, fat tissues, leptin and Oxytocin. Int J Obes 32: 1211–1221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu M, Mizuno A, Kuwajima M, Ogino T, Murakami T, Noma Y, Sano T, Shima K. Ovarian hormone-induced beta-cell hypertrophy contributes to the homeostatic control of beta-cell mass in OLETF female rat, a model of Type II diabetes. Diabetologia 41: 799–805, 1998 [DOI] [PubMed] [Google Scholar]


