Abstract
Objective
We determined where and when category-preferential augmentation of gamma activity took place during naming of animal or non-animal pictures.
Methods
We studied 41 patients with focal epilepsy who underwent measurement of naming-related gamma-augmentation50–120 Hz during extraoperative electrocorticography. The assigned task consisted of naming of a visually-presented object classified as either ‘animal’ or ‘non-animal’.
Results
Within 80 ms following the onset of picture presentation, regardless of stimulus type, gamma-activity in bilateral occipital regions began to be augmented compared to the resting period. Initially in the occipital poles (at 140 ms and after) and subsequently in the lateral, inferior and medial occipital regions (at 320 ms and after), the degree of gamma-augmentation elicited by ‘animal naming’ became larger (by up to 52%) than that by ‘non-animal naming’. Immediately prior to the overt response, left inferior frontal gamma-augmentation became modestly larger during ‘animal naming’ compared to ‘non-animal naming’.
Conclusions
Animal category-preferential gamma-augmentation sequentially involved the lower- and higher-order visual areas. Relatively larger occipital gamma-augmentation during ‘animal naming’ can be attributed to the more attentive analysis of animal stimuli including the face. Animal-preferential gamma-augmentation in the left inferior frontal region could be attributed to a need for selective semantic retrieval during ‘animal naming’.
Significance
A specific program of cortical processing to distinguish an animal (or face) from other objects might be initiated in the lower-order visual cortex.
Keywords: Epilepsy, Intracranial ECoG recording, Ripples, Gamma activity, High-frequency oscillations (HFOs)
1. INTRODUCTION
Behavioral studies of healthy adults, children, and newborns consistently reported that humans rapidly detect and intensely gaze at the face when they are presented a pictorial representation of a living object possessing such features (Johnson et al., 1991; Itier and Batty, 2009; Drewes et al., 2011). Functional neuroimaging studies demonstrated that larger hemodynamic responses occurred within portions of the occipital and temporal lobes, bilaterally, during viewing of pictures of human faces or common animals, compared to that during non-animal objects (Sergent et al., 1992; Chao et al., 1999; Whatmough et al., 2002). Such face- and animal-preferential hemodynamic activation was reported to commonly involve the higher-order visual cortices including Brodmann areas (BA) 19 and 37 (Sergent et al., 1992; Chao et al., 1999; Gauthier et al., 2000; Whatmough et al., 2002; Pitcher et al., 2011). It has been hypothesized that the first stage in a hierarchical perception of the face occurs in the lateral occipital regions (often referred to as the “occipital face area” [Gauthier et al., 2000]), followed by subsequent complex processing in the inferior occipital-temporal regions including the fusiform gyri (Pitcher et al., 2011).
In the present study using intracranial electrocorticography (ECoG) recording, we determined where and when augmentation of gamma activity at 50–120 Hz (Kojima et al., 2013a) became larger during a picture naming task including trials with animals compared to those with non-animals. Event-related gamma-augmentation, tightly correlated to increased firing rates on single neuron recording (Ray et al., 2008), is considered an excellent summary measure of in situ neuronal activation (Lachaux et al., 2012). The advantages of ECoG recording include (i) a spatial resolution of 1 cm or less, (ii) a temporal resolution on the millisecond scale as well as (iii) direct signal sampling, via implanted electrodes, from deep brain regions such as the medial occipital region. We believe that external validation of animal-preferential hemodynamic activation is warranted using invasive, direct electrophysiological measures, which do not suffer from the electromagnetic inverse problem in source reconstruction of electrical signals from the brain. Furthermore, some discrepancy in the localization of neural activation between event-related electrophysiological responses and blood oxygen level dependent (BOLD) responses on functional MRI (fMRI) has been reported (Logothetis, 2003; Brown et al., 2012).
Based on the results of previous neuroimaging studies (Pitcher et al., 2011), we hypothesized that sites showing animal-preferential gamma-augmentation would be concentrated in the higher-order visual areas (including the lateral occipital and inferior occipital-temporal regions; BA 19 and 37) rather than in the lower-order visual areas (including the medial and polar occipital regions; BA 17 and 18). We also hypothesized that the onset of animal-preferential gamma-augmentation would range 100 – 200 ms, since scalp EEG and magnetoencephalography (MEG) recordings showed face/animal-preferential event-related potentials/fields (ERPs/ERFs) in the posterior head region at this range (often referred to as N170 or M170; Bentin et al., 1996; Liu et al., 2000).
We utilized clinical macro-electrodes, each of which supposedly records electrical activities from on the order of 100,000 neurons (Modolo et al., 2010). Thus, our study is not designed to detect category-specific neural activities generated by single or few neurons. Previous studies of monkeys using single neuron recording demonstrated that subsets of neurons in the lower-order visual areas (such as V1) specifically responded to a bar in a certain orientation (Knierim and van Essen, 1992). Other studies of monkeys as well as patients with focal epilepsy demonstrated that small subsets of neurons in the medial-temporal regions as well as anterior-inferior temporal regions showed neural activations specific to a category including animals (Suzuki et al., 1997; Quiroga et al., 2005; Chan et al., 2011; Mormann et al., 2011).
2. METHODS
2.1. Patients
This study has been approved by the Institutional Review Board at Wayne State University. The inclusion criteria consisted of: (i) patients with focal epilepsy who underwent extraoperative subdural ECoG recording as a part of presurgical evaluation in Children’s Hospital of Michigan or Harper University Hospital in Detroit between January 2007 and May 2012; (ii) written informed consent obtained from patients or their guardians; (iii) language mapping using measurement of gamma-augmentation elicited by picture-naming tasks (Wu et al., 2011); and (iv) verbal intelligence adequate to correctly name at least 60% of the 30 pictures of each category (Supplementary Table S1). The exclusion criteria consisted of: (i) presence of massive brain malformations (such as large porencephaly, perisylvian polymicrogyria or hemimegalencephaly) which are known to confound the anatomical landmarks for the calcarine sulci; (ii) presence of epileptic seizures or frequent interictal spikes (three spikes per second) involving the occipital lobe during the picture naming task; and (iii) left-handedness with early-onset neocortical lesions (for example cortical dysplasia) in the left-hemisphere preoperatively suggested on neuroimaging, because such patients are likely to have essential language function reorganized to the right hemisphere (Rasmussen and Milner, 1977; Akanuma et al., 2003; Möddel et al., 2009; see the detailed discussion in Kojima et al., 2013a). This cohort study included a consecutive series of 41 English-speaking patients who satisfied these criteria (age range: 4–56 years; median age: 15 years) (Tables 1 and 2).
Table 1.
Patient demographics.
| Age-year | |
| Median | 15 |
| Range | 4–56 |
| Gender-number (%) | |
| Male-number (%) | 23 (56.1) |
| Female-number (%) | 18 (43.9) |
| Antiepileptic drugs | |
| 1-number (%) | 12 (29.3) |
| 2-number (%) | 21 (51.2) |
| 3-number (%) | 8 (19.5) |
| VCI score (30 patients) | |
| Median (Standard deviation) | 87 (21) |
| VIQ score (3 patients) | |
| Median (Standard deviation) | 82 (4) |
| PPVT score (32 patients) | |
| Median (Standard deviation) | 89 (16) |
| CELF score (16 patients) | |
| Median (Standard deviation) | 81 (25) |
| Handedness | |
| Right-number (%) | 36 (87.8) |
| Left-number (%) | 4 (9.8) |
| Ambidextrous-number (%) | 1 (2.4) |
VCI: verbal comprehension index. VIQ: verbal IQ. PPVT: peabody picture vocabulary test. CELF: clinical evaluation of language fundamentals.
Oxcarbazepine: 21 patients. Levetiracetam: 17. Lacosamide: 10. Lamotrigine: 9. Valproic acid: 5. Carbamazepine: 5. Phenytoin: 5. Topiramate: 4. Zonisamide: 1. Clobazam: 1.
Table 2.
Patient profile.
| Patient/Gender | Age (years) | Seizure onset zone on ECoG | MRI diagnosis | Location of subdural electrodes placement |
|---|---|---|---|---|
| 1/M | 4 | *: Spiking in Lt T | Multiple cavernous angioma | Lt FTPO |
| 2/M | 5 | Lt F | Normal | Lt FTPO |
| 3/F | 6 | *: Spiking in Rt F | Normal | Rt FTPO |
| 4/M | 8 | Rt F | Normal | Rt FTPO; Lt FP |
| 5/M | 9 | Lt P | Gliotic changes in Lt PFT | Lt FTPO |
| 6/M | 10 | Rt FT | Tumor | Rt FTPO |
| 7/M | 10 | *: Spiking in Rt FP | Arachnoid cyst in Rt T | Rt FTPO |
| 8/M | 10 | Rt P | Dysplasia in Rt P | Rt FTPO |
| 9/M | 10 | Lt FTP; Rt F | Normal | Lt FTPO; Rt F |
| 10/M | 11 | Rt FP | Gliotic changes or dysplasia in Rt FP | Rt FTPO |
| 11/F | 11 | Rt FP | Normal | Rt FTPO |
| 12/M | 12 | Lt FTP | Normal | Lt FTPO |
| 13/M | 12 | Rt T | Rt mesial temporal sclerosis | Rt FTPO |
| 14/F | 13 | Lt F | Dysplasia or inflammation in Lt F | Lt FTPO |
| 15/F | 13 | *: Spiking in Rt TPO | Normal | Rt FTPO |
| 16/M | 13 | Rt FP | Tumor in Rt P | Rt FTPO |
| 17/F | 14 | Lt T | Un-identified neurofibromatosis object in Lt F | Lt FTPO |
| 18/M | 14 | Lt FP; Rt FP | Normal | Rt FTP; Lt FTP |
| 19/F | 14 | Rt T | Focal atrophy of Rt T | Rt FTPO; Lt F |
| 20/F | 14 | *: No spikes | Possible ulegyria in Lt P | Lt FTPO |
| 21/M | 15 | Lt T | Tumor in Lt T | Lt FTPO |
| 22/F | 16 | Lt OP | Normal | Lt FTPO; Rt T |
| 23/M | 16 | Rt TP | Normal | Rt FTPO |
| 24/F | 16 | Rt FTPO | Normal | Rt FTPO; Lt FP |
| 25/M | 17 | Lt T | Normal | Lt FTPO |
| 26/M | 17 | Lt F | Post-traumatic changes in Lt F | Lt FTPO; Rt FP |
| 27/M | 17 | Lt T | Tumor in Lt T | Lt FTPO |
| 28/M | 17 | Rt TPO | Small ulegyria in Rt P | Rt FTPO |
| 29/F | 17 | Lt F | Normal | |
| 30/M | 20 | Lt T | Normal | Lt FTPO |
| 31/F | 21 | Lt O | Small tumor or dysplasia in Lt O | Lt TPO |
| 32/M | 23 | Lt F | Normal | Lt FTP; Rt FP |
| 33/F | 27 | Lt F | Tumor or dysplasia in Lt F | Lt FTPO |
| 34/F | 28 | Lt T | Normal | Lt FTPO |
| 35/M | 28 | Rt T | Tumor in Rt T | Rt FTP |
| 36/F | 34 | Lt T | Normal | Lt FTPO; Rt T |
| 37/F | 37 | Lt T | Tumor or dysplasia in Lt P | Lt FTPO |
| 38/F | 37 | *: No spikes | Tumor in Lt P | Lt FTPO |
| 39/F | 40 | Lt T | Normal | Lt FTPO |
| 40/M | 44 | Rt TPO | Ulegyria in Rt T | Rt FTPO |
| 41/F | 56 | Rt T | Small lacuna infarctions | Rt FTPO |
Our ECoG study inevitably suffered from sampling limitations. The locations of subdural electrode placement were guided as a part of clinical management of patients with focal epilepsy. The presence of large bridging veins generally prevents us from placing large subdural grid electrode arrays on the occipital lobe surfaces. Lt: Left. Rt: Right. F: Frontal. P: Parietal. T: Temporal. O: Occipital.
2.2. Subdural electrode placement
Platinum macro-electrodes were placed in the subdural space over left, right, or bilateral cortical regions (intercontact distance: 10 mm; diameter: 4 mm; median: 112 electrodes per patient [standard deviation: 21]). Placement of subdural electrodes was clinically guided by the results of Phase-I presurgical evaluation including: scalp video-EEG recording, MRI, and 2-deoxy-2-[18F] fluoro-D-glucose (FDG) positron emission tomography (PET) (Asano et al., 2009a). All electrode plates were stitched to adjacent plates or the edge of dura mater, to avoid movement of subdural electrodes after intracranial implantation. In all patients, intraoperative photographs were taken with a digital camera before dural closure as well as after re-opening during the second stage of surgery. All electrodes were displayed on the three-dimensional brain surface reconstructed from high-resolution MRI (Alkonyi et al., 2009; Wu et al., 2011). The spatial accuracy of electrode display on the three-dimensional brain surface was confirmed by intraoperative digital photographs (Dalal et al., 2008).
2.3. Extraoperative video-ECoG recording
ECoG signals were obtained for 3–5 days with a sampling rate of 1,000 Hz and amplifier band pass at 0.08–300 Hz, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA). The averaged voltage of ECoG signals derived from the fifth and sixth intracranial electrodes on the amplifier was used as the original reference; ECoG signals were then re-montaged to a common average reference. Channels contaminated with large interictal epileptiform discharges or artifacts were visually identified and excluded from the average (Laufs et al., 2006), in order to minimize their contamination on ECoG signals. Usage of a common average reference is a widely-accepted practice in assessment of event-related gamma-augmentation recorded on subdural grid electrodes; its advantages and limitations were previously discussed (Crone et al., 2001; Asano et al., 2009b; Nagasawa et al., 2011; Kojima et al., 2013a). Surface electromyography electrodes were placed on the left and right deltoid muscles, and electrooculography electrodes were placed 2.5 cm below and 2.5 cm lateral to the left and right outer canthi. ECoG traces were visually inspected with a time constant of 0.003 s and a sensitivity of 20 μV/mm; thereby, irregular broadband signals synchronized with facial and ocular muscle activities seen on electrooculography electrodes were treated as artifacts (Nagasawa et al., 2011; Kojima et al., 2013a).
Sites involved by the seizure onset zone (Asano et al., 2009a; Jacobs et al., 2009) or structural lesions were excluded from further analysis (Caplan et al., 2001), because our previous ECoG study of language-related gamma activity reported that the chance of significant amplitude augmentation in a given region of interest was lower in the seizure onset zone compared to the other regions (Kojima et al., 2013a). One may claim that ECoG results derived from patients with focal epilepsy cannot be generalized to healthy individuals, but the presence of a seizure focus does not simply indicate that the remaining cortices are also abnormal. It has been reported that the spatial-temporal-spectral characteristics of sensory-related gamma-band responses were similar between healthy monkeys and patients with focal epilepsy (Ray et al., 2008; Fukuda et al., 2008; Asano et al., 2009b).
2.4. Picture naming task
The task employed to measure picture-naming-related gamma-augmentation on ECoG was previously described (Wu et al., 2011). Patients were comfortably seated on the bed in a dimly lit room. Patients were instructed to overtly name objects presented sequentially in the picture naming task. Neither target nor non-target stimuli were defined in the present study. Stimuli were presented sequentially on a 19-inch LCD monitor placed 60 cm in front of patients. Picture stimuli consisted of 60 common grayscale objects (Rossion and Pourtois, 2004) of which size ranged from 11 to 16 cm in height and width. Each object was categorized as either animal (e.g. ‘cat’ or ‘rabbit’) or non-animal (e.g. ‘car’ or ‘pineapple’) (Supplementary Table S1). No group difference in size, spatial frequency, or contrast was noted between animal and non-animal stimuli (p ≥ 0.2 on Mann-Whitney U test). Picture stimuli were binocularly presented in a pseudo-random order at the center of the monitor, in grayscale on a black background, for 5,000 ms with an inter-stimulus interval randomly ranging 2,000 – 2,500 ms. TTL trigger signals synchronized with the onset and offset of each stimulus presentation were delivered to the ECoG recording system. These audible visual-language sessions were recorded using a Digital Voice Recorder (WS-300M, Olympus America Inc, Hauppauge, NY, USA) concurrently with ECoG recording, and the amplified audio waveform was integrated into the Digital ECoG Recording System (Brown et al., 2008). ECoG traces were aligned to: (i) stimulus (picture) onset and (ii) response (answer) onset. The response time was defined as the period between onset of stimulus presentation and onset of overt responses.
2.5. Time-frequency analysis
Each ECoG trial was transformed into the time-frequency domain using complex demodulation (Papp and Ktonas, 1977) via BESA® software (BESA GmbH, Gräfelfing, Germany; Hoechstetter et al., 2004). A given ECoG signal was assigned an amplitude (a measure proportional to the square root of power) as a function of time and frequency (in steps of 10 ms and 5 Hz). The time-frequency transform was obtained by multiplication of the time-domain signal with a complex exponential, followed by a band-pass filter. The band-pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform; the band-pass filter had a full width at half maximum of 2 × 15.8 ms in the temporal domain and 2 × 7.1 Hz in the frequency domain. Thus, the corresponding time-frequency resolution was ±15.8 ms and ±7.1 Hz (defined as the 50% power drop of the finite impulse response filter).
We then determined ‘when,’ ‘where,’ and ‘how much’ gamma activity at 50–120 Hz averaged across trials were augmented compared to the resting periods (Kojima et al., 2013a). Further methodological details (including duration of the reference period) are described in Supplementary Figure S1.
2.6. Individual-level statistical analysis to localize sites showing category-preferential gamma-augmentation
In each time-frequency bin at each individual site, we determined whether the amplitude differed between ‘animal naming’ and ‘non-animal naming’, using a studentized bootstrap statistic followed by Simes’ correction (Fukuda et al., 2010; Koga et al., 2011). A given site was defined as that showing category-preferential gamma-augmentation (Figures 1, 2 as well as Supplementary Figure S2) only when surviving correction with a significant difference spanning (i) at least 20-Hz in width and (ii) at least 20-ms in duration. We previously discussed the advantage and limitation of this analytic approach (Wu et al., 2011; Brown et al., 2012; Kojima et al., 2013a).
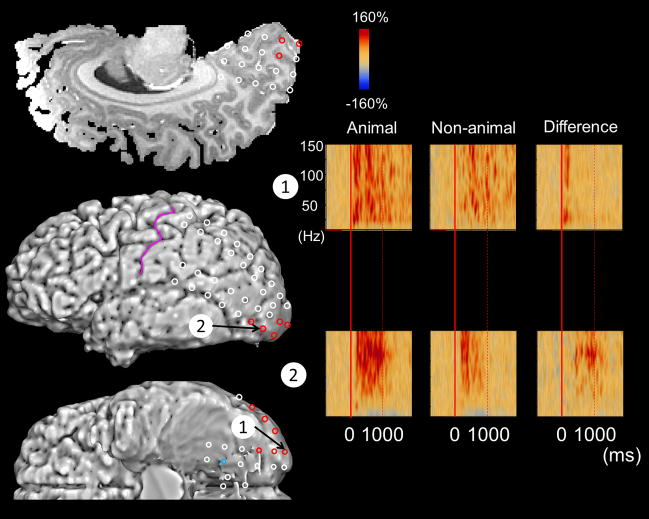
Figure 1. Category-preferential gamma responses in a 21-year-old female with epilepsy.
(A) Red circles indicate the sites showing animal-preferential gamma-augmentation (significant amplitude augmentation at 50–120 Hz, spanning at least 20-Hz in width and at least 20-msec in duration), while blue ones indicate those showing non-animal-preferential gamma-augmentation. (B) The results of time-frequency analyses in this patient are shown. Red: significant animal-preferential gamma augmentation. Blue: significant non-animal-preferential amplitude-attenuation. At channel #1 in the left polar occipital region, the degree of gamma-augmentation was larger during picture naming of animals compared to that of non-animals. At channel #2 in the left lateral occipital region, the degree of gamma-augmentation was likewise larger during picture naming of animals compared to that of non-animals, but the onset of such differential gamma-augmentation was delayed compared to that at channel #1.
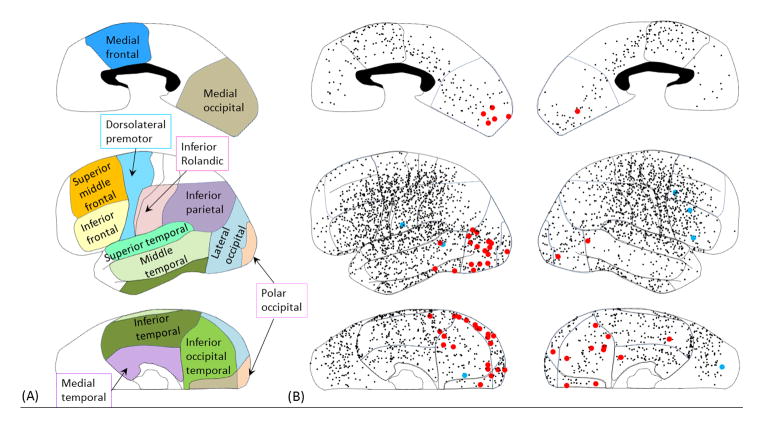
Figure 2. Category-preferential gamma responses.
(A) Presented is the definition of the anatomical regions of interest; this was employed in our previous study (Matsuzaki et al., 2012; Kojima et al., 2013b). Medial occipital region: medial portion of BA 17/18. Polar occipital region: polar portion of BA 17/18. Lateral occipital region: lateral portion of BA 19/37. Inferior occipital-temporal region: inferior portion of BA 19/37. Medial temporal region: BA 27/28/34/35/36. Inferior temporal region: inferior temporal gyrus involving BA 20/37. Middle temporal region: middle temporal gyrus involving BA 21/37. Superior temporal region: BA 22/41/42. Inferior parietal region: BA 39/40. Inferior Rolandic region: BA 4/3/1/2 not more than 4 cm superior from the sylvian fissure. Dorsolateral premotor region: dorsolateral portion of BA 6. Inferior frontal region: inferior frontal gyrus involving BA 44/45. Middle/superior frontal region: lateral portion of BA 46/9/8. Medial frontal region: medial portion of BA 6/8 and posterior portion of BA 24/32/33. Red dots: electrode sites showing significantly larger gamma-augmentation during ‘animal naming’ compared to ‘non-animal naming’. Blue dots: electrode sites showing significantly larger gamma-augmentation during ‘non-animal naming’ compared to ‘animal naming’. Black dots: electrode sites showing no category-preferential gamma-augmentation. (B) The results of time-frequency analyses in all individual sites are summarized. Sites showing animal-preferential gamma-augmentation involved the polar-, lateral-, medial-occipital, as well as inferior occipital-temporal regions bilaterally, and outnumbered those showing non-animal-preferential gamma-augmentation.
2.7. Group-level statistical analysis to localize sites showing category-preferential gamma-augmentation
We employed group statistics on each region of interest (Figures 2–4). We recognize that group-level statistics have both advantage and disadvantage compared with individual-level ones. The advantage of group analysis is a potentially increased sensitivity to detect significant category-preferential gamma-augmentation which might not be detected at the individual level. In this study, each region of interest contained a large number of analyzed electrode sites (Figure 2; Table 3). The statistical power to detect a significant difference in amplitudes between categories was dependent on the number of analyzed electrodes in each region of interest and not on the number of trials in each naming category. The disadvantage of group-level analysis is that variability in functional organization within each region is inevitably underestimated. To avoid circular analysis, which is flawed (Kriegeskorte et al., 2009), we did not define regions of interest based on the results of afore-mentioned individual-level analysis, but strictly employed the same set of anatomical regions of interest utilized in our previous studies (Matsuzaki et al., 2012; Kojima et al., 2013a). The grand-average of ECoG amplitudes in each time-frequency bin across all electrodes in each region of interest in each hemisphere was calculated for each naming category (Figures 3 and 4). We determined whether ECoG amplitudes in each region of interest differed between ‘animal naming’ and ‘non-animal naming’, using a studentized bootstrap statistic (McIntosh et al., 1998, Efron and Tibshirani, 1986; Zhou et al., 1997) followed by Simes’ correction (Simes, 1986). A p-value < 0.05 after the correction was considered significant in all analyses.
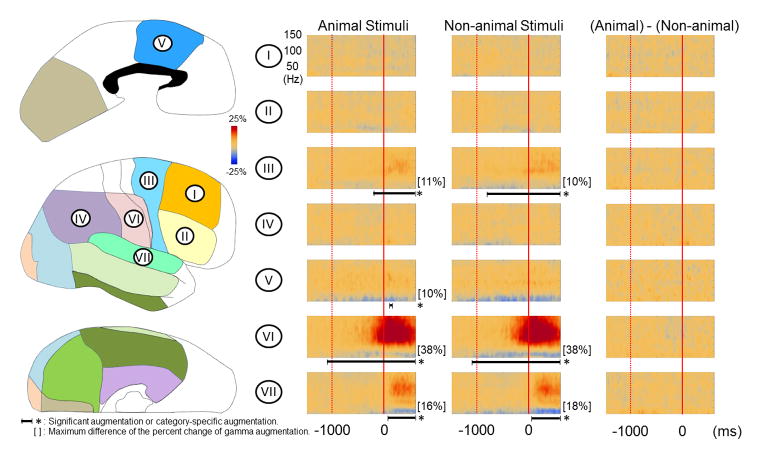
Figure 4. Grand-average of ECoG amplitudes relative to response onset.
(A) The results of group time-frequency analyses relative to response onset are shown. Prior to the onset of overt responses, regardless of category, gamma-activity in the left frontal regions (regions II, III, and V) and the left inferior Rolandic region (region VI) began to be augmented compared to the preceding resting period. Gamma activity in the left superior temporal region (region VII) was augmented during responses. The degree of gamma-augmentation in the left inferior frontal region was modestly larger during ‘animal naming’ compared to ‘non-animal naming’ (corrected p < 0.05; Onset: 70 ms prior to the onset of overt responses). (B) Regardless of category of pictures, gamma-augmentation involved the right dorsolateral premotor, medial frontal, inferior Rolandic and superior temporal regions. No category-preferential gamma-augmentation was noted in these regions of the right hemisphere.
Table 3.
Proportion of sites showing category-preferential gamma-augmentation.
| Total number of analyzed electrode sites | Number of sites showing animal-preferential gamma-augmentation | Proportion of sites showing animal-category-preferential gamma-augmentation (95% CI) | Number of sites showing non-animal-preferential gamma-augmentation | Proportion of sites showing non-animal-category-preferential gamma-augmentation (95% CI) | |
|---|---|---|---|---|---|
| Left hemisphere | |||||
| Medial-occipital | 64 | 6 | 0.09 (0.04 to 0.19) | 0 | 0.0 |
| Polar-occipital | 41 | 3 | 0.07 (0.02 to 0.20) | 0 | 0.0 |
| Lateral-occipital | 107 | 15 | 0.14 (0.08 to 0.22) | 0 | 0.0 |
| Inferior-occipital-temporal | 79 | 5 | 0.06 (0.02 to 0.14) | 1 | 0.01 (0.00 to 0.07) |
| Medial-temporal | 54 | 0 | 0.0 | 0 | 0.0 |
| Inferior-temporal | 180 | 3 | 0.02 (0.00 to 0.05) | 0 | 0.0 |
| Middle-temporal | 159 | 1 | 0.01 (0.00 to 0.03) | 1 | 0.01 (0.00 to 0.03) |
| Superior-temporal | 223 | 0 | 0.0 | 0 | 0.0 |
| Superior/middle-frontal | 121 | 0 | 0.0 | 0 | 0.0 |
| Inferior-frontal | 201 | 0 | 0.0 | 0 | 0.0 |
| Dorsolateral-premotor | 185 | 0 | 0.0 | 0 | 0.0 |
| Inferior-parietal | 161 | 0 | 0.0 | 0 | 0.0 |
| Medial-frontal | 70 | 0 | 0.0 | 0 | 0.0 |
| Inferior-Rolandic | 165 | 0 | 0.0 | 1 | 0.01 (0.00 to 0.03) |
| ‘Other’ | 319 | 0 | 0.0 | 0 | 0.0 |
| Right hemisphere | |||||
| Medial-occipital | 62 | 3 | 0.05 (0.01 to 0.14) | 0 | 0.0 |
| Polar-occipital | 32 | 0 | 0.0 | 0 | 0.0 |
| Lateral-occipital | 69 | 1 | 0.01 (0.00 to 0.08) | 0 | 0.0 |
| Inferior-occipital-temporal | 61 | 6 | 0.10 (0.04 to 0.20) | 0 | 0.0 |
| Medial-temporal | 53 | 1 | 0.02 (0.00 to 0.10) | 0 | 0.0 |
| Inferior-temporal | 137 | 1 | 0.01 (0.00 to 0.04) | 0 | 0.0 |
| Middle-temporal | 42 | 1 | 0.02 (0.00 to 0.13) | 0 | 0.0 |
| Superior-temporal | 145 | 0 | 0.0 | 0 | 0.0 |
| Superior/middle-frontal | 102 | 0 | 0.0 | 1 | 0.01 (0.00 to 0.05) |
| Inferior-frontal | 120 | 0 | 0.0 | 2 | 0.02 (0.00 to 0.06) |
| Dorsolateral-premotor | 191 | 0 | 0.0 | 0 | 0.0 |
| Inferior-parietal | 133 | 0 | 0.0 | 0 | 0.0 |
| Medial-frontal | 62 | 0 | 0.0 | 0 | 0.0 |
| Inferior-Rolandic | 155 | 0 | 0.0 | 0 | 0.0 |
| ‘Other’ | 244 | 0 | 0.0 | 1 | 0.004 (0.00 to 0.02) |
The results of individual-level statistics are shown. The advantage of such individual-level analysis is that inter-individual variability in location of sites showing category-preferential gamma-augmentation can be highlighted (Supplementary Figure S2). Conversely, statistical power for detecting significant category-preferential gamma-augmentation was lower at the individual-level statistics, compared to that at the group-level. Absence of statistically significant category-preferential gamma-augmentation at the individual-level does not infer that such a contrast in cortical processing between the categories did not occur, but only that it was not found. Regions outside of the aforementioned 14 regions were collectively defined as ‘Other’, in each hemisphere.
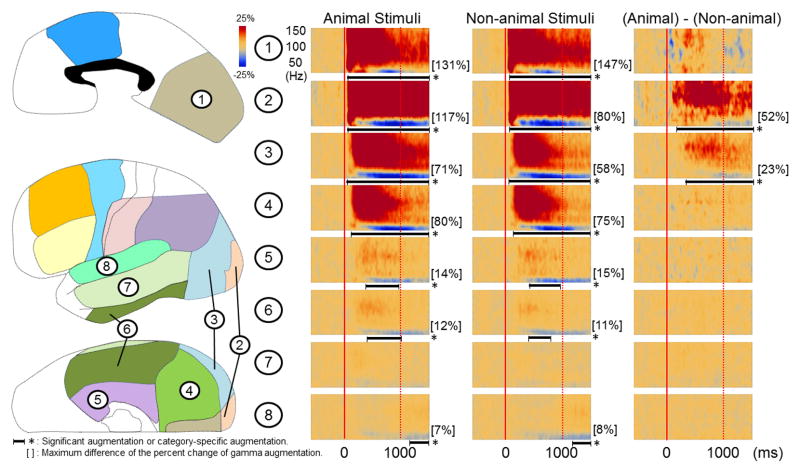
Figure 3. Grand-average of ECoG amplitudes relative to stimulus onset.
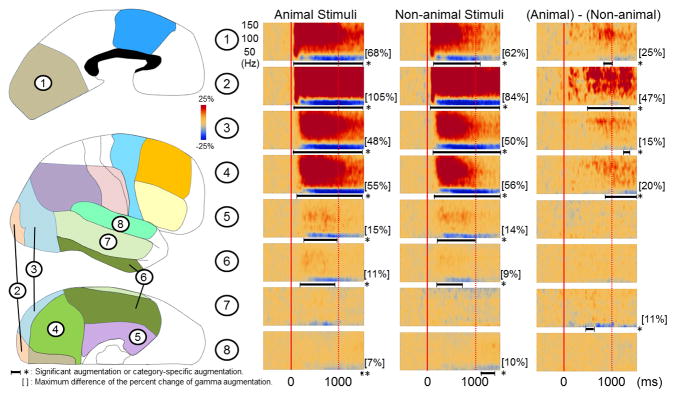
(A) The results of group time-frequency analyses relative to the stimulus onset are shown. Within 80 ms following the onset of picture presentation, regardless of category, gamma-activity in the left occipital regions (regions #1 to #4) began to be augmented compared to the preceding resting period. Gamma-activity in bilateral inferior and medial temporal regions (regions #5 and #6) began to be augmented at 170–230 ms. The degree of gamma-augmentation in the left polar and lateral occipital regions (regions #2 and #3) was larger during ‘animal naming’ compared to ‘non-animal naming’ (corrected p < 0.05). The onset of animal-preferential gamma-augmentation was 140 ms in the left polar occipital region and 320 ms in the left lateral occipital region. (B) Gamma-augmentation likewise involved the right occipital lobe as well as medial and inferior temporal regions. The degree of gamma-augmentation in the right polar, medial, lateral occipital regions as well as inferior occipital-temporal region (regions #1 to #4) was larger during ‘animal naming’ compared to ‘non-animal naming’ (corrected p < 0.05). The onsets of animal-preferential gamma-augmentation in the right occipital regions were 480 ms and after.
3. RESULTS
3.1. Behavioral results
The response time for the animal stimuli was modestly but still significantly longer than that for the non-animal stimuli (median across all patients: 1660 ms [animal] and 1580 ms [non-animal]; p = 0.02 on Wilcoxon Signed Rank Test) (Supplementary Figure S3).
3.2. The spatial-temporal characteristics of category-preferential gamma-augmentation
Individual sites showing category-preferential gamma-augmentation are presented in Figures 1 and 2, as well as Supplementary Figure S2. Individual-level statistics suggested that 23 patients had at least one electrode site showing category-preferential gamma-augmentation, while the remaining 18 failed to show such a site. A total of 46 sites showed animal-preferential gamma-augmentation, while 7 showed non-animal-preferential gamma-augmentation. Table 3 summarizes the proportion of sites showing category-preferential gamma-augmentation among all sites in each region of interest. In short, animal-preferential gamma-augmentation was observed in widespread occipital regions bilaterally across a wide range of ages (4 to 44 years). Five out of the nine patients at age <11 years (56%) and 16 out of the 32 patients at age ≥11 years (50%) had a site showing animal-preferential gamma-augmentation; we failed to find a difference in the proportion of number of patients showing animal-preferential gamma-augmentation between younger and older groups (p = 1.0 on the Fisher’s exact probability test). Based on the individual-level statistics, the left lateral occipital region showed the largest proportion of sites showing animal-preferential gamma-augmentation among all analyzed ones (15/107 sites [14%]). The Fisher’s exact probability test employed to individual electrodes data suggested that the proportion of occipital sites showing animal-preferential gamma-augmentation was modestly but still significantly larger on the left side compared to the right (10% vs 4%; p = 0.02), but failed to show a difference in the proportion of sites showing animal-preferential gamma-augmentation between the lower- and higher-order visual areas (medial/polar-occipital vs lateral/inferior-occipital regions; p > 0.3; Table 3).
Group-level statistics on each region of interest showed that within 80 ms following the onset of picture presentation, gamma-activity in bilateral occipital regions, regardless of stimulus type, began to be augmented compared to the resting period (Figure 3). The degree of gamma-augmentation elicited by ‘animal naming’ was larger than that by ‘non-animal naming’ initially in the occipital poles (at 140 ms and after) and subsequently in the lateral and medial occipital regions as well as inferior occipital-temporal region (at 320 ms and after). Immediately prior to the overt response, left inferior frontal gamma-augmentation became modestly but still significantly larger during ‘animal naming’ compared to ‘non-animal naming’ (Figure 4).
4. DISCUSSION
4.1. Significance of animal-preferential gamma-augmentation in the occipital lobes
Both individual- and group-level analyses in the present study demonstrated that animal-preferential gamma-augmentation involved subsets of occipital sites across lower- and higher-order visual areas with slight predominance in the left hemisphere. Such animal-preferential gamma-augmentation took place in occipital lobes constantly across a wide range of ages including the youngest 4-year-old boy. This observation is consistent with the notion that the occipital lobe of young children is already capable to exert animal- or face-preferential processing (Quinn and Eimas, 1998; Quinn et al., 2009). The onset of such category preferential gamma-augmentation in the left polar occipital region was as early as 140 ms following the stimulus presentation. Thus, our ECoG study using macro-electrodes failed to support a model suggesting lower-order visual areas to preprocess visual stimuli nonspecifically to material categories while specific sites within the higher-order visual areas play a primary role in distinguishing animals from other objects. Indeed, unlike previous observations with fMRI (Pitcher et al., 2011), the greatest group difference in gamma augmentation occurred in polar occipital regions, exerting lower-order visual processing for the central visual field. Our ECoG observation supports the notion that mammalian vision is so highly conserved that a rapid response to the face of another species is built in as a potentially protective reflex to improve survival. Since animal-preferential gamma-augmentation involved widespread occipital regions, the present study failed to warrant the usage of functionally-defined terms such as “occipital animal areas”.
Greater attentiveness to animal stimuli containing a face could have contributed to larger animal-preferential gamma augmentation in the occipital lobes. A number of studies have shown that humans and monkeys most intensely gaze at the face when a picture of a living object was presented (Perrett et al., 1982; Johnson et al., 1991; Fletcher-Watson et al., 2008; Langton et al., 2008; Itier and Batty, 2009; Drewes et al., 2011; Goodman et al., 2012). A behavioral study of healthy adults, using natural scenes with and without a person presented side by side, showed that participants preferentially gazed at the scene containing a person (Fletcher-Watson et al., 2008). Thereby, the mean onset of initial gaze to the person was 276 ms in a free-viewing task and 229 ms in a gender discrimination task. The initial gaze was face-preferentially distributed at 150 ms and after, but randomly distributed at 100 ms and before (Fletcher-Watson et al., 2008). Another behavioral study using a forced-choice saccade task showed that adult participants reliably made saccades to the side showing an animal containing the face in 240 ms, on average (Kirchner and Thorpe, 2006). A behavioral study of 3 to 4-month-old infants showed that there was no spontaneous gaze preference for human over non-human animal stimuli (Quinn and Eimas, 1998). Another behavioral study of 6 to 7-month-old infants showed that they fixated on the head more than the body of dogs and cats, only when a novel picture of such an animal was presented in the upright orientation (Quinn et al., 2009). Thus, it is plausible to hypothesize that animal-preferential gamma-augmentation at 200 ms or earlier reflects the first stage in a distributed network for perception of animals. Such early animal-preferential gamma-augmentation was found in the polar occipital regions in our ECoG study. It is difficult to attribute early animal-preferential occipital gamma augmentation solely to differences in the physical properties between two categories of stimuli, since there was no group difference in size, spatial frequency, or contrast.
Late animal-preferential gamma-augmentation in widespread occipital regions can be attributed to multiple factors, including differences in eye movements during a task. A behavioral study of 13 healthy adults showed that the saccade rate within the facial contour was greater by 60% compared to that outside the face, when assigned a gender discrimination task (Andari et al., 2010). It has been suggested that saccades were associated with gamma-augmentation involving widespread occipital regions (Nagasawa et al., 2011). Further studies along with systematic eye-tracking measures are warranted to determine the effects of overt eye movements on category-specific neural activation in the occipital lobes.
We cannot completely rule out the effects of task demands on late animal-preferential gamma-augmentation. The present study showed that the response time was slightly longer for ‘animal naming’ than ‘non-animal naming’. Left inferior frontal gamma-augmentation became modestly larger during ‘animal naming’ compared to ‘non-animal naming’ immediately prior to the overt response. Animals are structurally similar; thus, ‘animal naming’ may require more intense visual analysis and controlled semantic retrieval (Gaffan and Heywood, 1993; Humphreys and Forde, 2001; Wagner et al., 2001). A previous fMRI study of healthy adults demonstrated that a task requiring selective semantic retrieval was associated with increased BOLD responses in the left inferior frontal region (Wagner et al., 2001). Repetitive transcranial magnetic stimulation of the left inferior frontal region induced a transient impairment in semantic retrieval (Whitney et al., 2011). Thus, animal-preferential gamma-augmentation in the left inferior frontal region immediately prior to the overt responses could be attributed to the enhanced need for selective semantic retrieval during ‘animal naming’.
4.2. Future Prospects
The present study focused on the contrast between ‘animal’ and ‘non-animal’ stimuli, and did not explore the effects of another category on ECoG gamma activity. Further ECoG studies are warranted to determine the spatial-temporal characteristics of gamma-augmentation preferentially elicited by a given category or class. It has been reported that picture naming, compared to auditory naming, has a smaller sensitivity to localize the primary language areas (Cervenka et al., 2013; Kojima et al., 2013b). It remains to be determined if presentation of pictures of animals rather than common objects can improve the accuracy of language mapping.
Supplementary Material
HIGHLIGHTS.
Animals, compared to non-animal objects, elicited greater occipital gamma-augmentation.
Occipital poles began to show such animal-preferential gamma-augmentation at 140 ms.
Animal-preferential gamma-augmentation also involved the left inferior frontal gyrus.
Acknowledgments
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano) as well as Japan Foundation for Neuroscience & Mental Health, Japan Epilepsy Research Foundation, and Japan-North America Medical Exchange Foundation (to K. Kojima). We are grateful to Harry T. Chugani, MD, Sandeep Sood, MD, Csaba Juhász, MD, PhD, Sarah Minarik, RN, BSN, and Carol Pawlak, REEG/EPT at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akanuma N, Alarcón G, Lum F, Kissani N, Koutroumanidis M, Adachi N, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–18. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–94. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009a;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009b;45:477–89. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological Studies of Face Perception in Humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, et al. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–31. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Muzik O, Rothermel R, Matsuzaki N, Juhász C, Shah AK, et al. Evaluating reverse speech as a control task with language-related gamma activity on electrocorticography. Neuroimage. 2012;60:2335–45. doi: 10.1016/j.neuroimage.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–80. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Cervenka MC, Corines J, Boatman-Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, et al. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage. 2013;69:267–76. doi: 10.1016/j.neuroimage.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, et al. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci. 2011;31:18119–29. doi: 10.1523/JNEUROSCI.3122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–9. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–15. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes J, Trommershäuser J, Gegenfurtner KR. Parallel visual search and rapid animal detection in natural scenes. J Vis. 2011 doi: 10.1167/11.2.20. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75. [Google Scholar]
- Gaffan D, Heywood CA. A spurious category-specific visual agnosia for living things in normal human and nonhuman primates. J Cogn Neurosci. 1993;5:118–28. doi: 10.1162/jocn.1993.5.1.118. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Findlay JM, Leekam SR, Benson V. Rapid detection of person information in a naturalistic scene. Perception. 2008;37:571–83. doi: 10.1068/p5705. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–45. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Goodman LR, Phelan HL, Johnson SA. Sex differences for the recognition of direct versus averted gaze faces. Memory. 2012;20:199–209. doi: 10.1080/09658211.2011.651089. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neuropsychological deficits. Behav Brain Sci. 2001;24:453–76. [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. 2009;33:843–63. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–37. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Thorpe SJ. Ultra-rapid object detection with saccadic eye movements: visual processing speed revisited. Vision Res. 2006;46:1762–76. doi: 10.1016/j.visres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J Neurophysiol. 1992;67:961–80. doi: 10.1152/jn.1992.67.4.961. [DOI] [PubMed] [Google Scholar]
- Koga S, Rothermel R, Juhasz C, Nagasawa T, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a stroop task-intracranial recording in epileptic patients. Hum Brain Mapp. 2011;32:1580–91. doi: 10.1002/hbm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki N, et al. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2013a;124:857–69. doi: 10.1016/j.clinph.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki M, et al. Gamma activity modulated by picture and auditory naming tasks: intracranial recording in patients with focal epilepsy. Clin Neurophysiol. 2013b doi: 10.1016/j.clinph.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton SR, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107:330–42. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, et al. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31:1408–18. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Higuchi M, Marantz A, Kanwisher N. The selectivity of the occipitotemporal M170 for faces. Neuroreport. 2000;11:337–41. doi: 10.1097/00001756-200002070-00023. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–71. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki N, Nagasawa T, Juhász C, Sood S, Asano E. Independent predictors of neuronal adaptation in human primary visual cortex measured with high-gamma activity. Neuroimage. 2012;59:1639–46. doi: 10.1016/j.neuroimage.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J Neurophysiol. 1998;80:2790–6. doi: 10.1152/jn.1998.80.5.2790. [DOI] [PubMed] [Google Scholar]
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Modolo J, Bhattacharya B, Edwards R, Campagnaud J, Legros A, Beuter A. Using a virtual cortical module implementing a neural field model to modulate brain rhythms in Parkinson’s disease. Front Neurosci. 2010 doi: 10.3389/fnins.2010.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Dubois J, Kornblith S, Milosavljevic M, Cerf M, Ison M, et al. A category-specific response to animals in the right human amygdala. Nat Neurosci. 2011;14:1247–9. doi: 10.1038/nn.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhász C, Hanazawa A, Shah A, Mittal S, et al. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. Neuroimage. 2011;58:1101–9. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–45. [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209:481–493. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res. 1982;47:329–42. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Eimas PD. Evidence for a global categorical representation of humans by young infants. J Exp Child Psychol. 1998;69:151–74. doi: 10.1006/jecp.1998.2443. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Doran MM, Reiss JE, Hoffman JE. Time course of visual attention in infant categorization of cats versus dogs: evidence for a head bias as revealed through eye tracking. Child Dev. 2009;80:151–61. doi: 10.1111/j.1467-8624.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–7. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci. 2008;28:7334–43. doi: 10.1523/JNEUROSCI.1588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–36. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–4. [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–81. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40:174–86. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex. 2011;21:1066–75. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Nagasawa T, Brown EC, Juhasz C, Rothermel R, Hoechstetter K, et al. γoscillations modulated by picture naming and word reading: intracranial recording in epileptic patients. Clin Neurophysiol. 2011;122:1929–42. doi: 10.1016/j.clinph.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Gao S, Hui SL. Methods for comparing the means of two independent log-normal samples. Biometrics. 1997;53:1129–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.