Abstract
Purpose
Prognostic factors for distal bile duct cancer are contentious. This study was conducted to analyze the prognostic factors of distal bile duct cancer after surgery with the aim of identifying those associated with diminished survival.
Methods
Two hundred forty-one patients who underwent pylorus-preserving pancreaticoduodenectomy (PPPD) or Whipple procedure in our tertiary hospital from February 1995 to June 2011 were retrospectively analyzed. All patients were pathologically proven to have distal bile duct adenocarcinoma. Postoperative complications, survival, and well-known prognostic factors after resection for distal bile duct cancer were investigated.
Results
Preoperative elevated carbohydrate antigen 19-9 (CA 19-9) level (P = 0.006), positive resection margin (P < 0.001), advanced T stage (P = 0.043), and lymph node metastasis (P = 0.002) were significantly independent worse prognostic indicators by multivariate analysis of resectable distal bile duct cancer.
Conclusion
R0 resection is the most important so that frozen sections should be utilized aggressively during each operation. For the distal bile duct cancer with elevated preoperative CA 19-9 level or advanced stage, further study on postoperative adjuvant treatment may be warranted.
Keywords: Bile duct cancer, CA-19-9 antigen, Pancreaticoduodenectomy
INTRODUCTION
Malignant neoplasms of the bile duct are relatively rare. The annual incidence rate in western countries is 1-2 cases per 100,000. In Korea, the incidence rate was 3-4 cases per 100,000 in 2009, according to data from the National Cancer Information Center [1]. Incidence of bile duct cancer increases with the age. However, it grows slowly so that patients and physicians can be unaware of its presence in its early stages [2]. With growth, infiltration of adjacent vessels can easily occur, making a patient unresectable.
Bile duct cancer is anatomically classified as intrahepatic, perihilar and distal. Among them, distal bile duct cancer is the second most frequent [3]. However, its prognosis is better than the others, which can present symptoms including jaundice and cholangitis relatively early [4]. This can allow patients to be diagnosed early and surgically resected before the cancer advances.
Potential risk factors of distal bile duct cancer include age, sex, serum bilirubin, tumor markers, biliary drainage, operation time, amount of transfusion, surgeon's experience, resection margin, tumor depth, perineural infiltration, tumor cell differentiation, stage of tumor, lymph node metastasis and complications [5-13]. To clarify the risk factors affecting survival from distal bile duct cancer, we reviewed 16 years surgeries of our facility.
METHODS
Selection criteria
Six hundred eighty-six patients were diagnosed with extrahepatic bile duct cancer at Samsung Medical Center between February 1995 and June 2011. At first 138 patients who had overlapping midportion or perihilar bile duct cancer and 14 patients whose cell type were not ductal adenocarcinoma of distal bile duct were excluded. To avoid confusion between middle and distal bile duct, the authors defined distal bile duct cancer that originating below the superior margin of pancreas head. 14 who were diagnosed other primary cancer earlier and four patients who were diagnosed another malignancy during the follow-up period were excluded. Inclusion criteria were pylorus-preserving pancreaticoduodenectomy (PPPD) and Whipple procedure for distal bile duct cancer, which were performed as curative intent and were confirmed as ductal adenocarcinoma by specialized pathologists in our hospital. One patient with distant metastasis operated with palliative intent was excluded consequently. Finally, 241 patients were enrolled.
Data collection
Data collection was achieved by reviewing electric medical records retrospectively. Follow-up day began on the operation day and ended on the last treatment day. We analyzed age, sex, preoperative carbohydrate antigen 19-9 (CA 19-9) level, preoperative serum total bilirubin, preoperative biliary drainage, types of biliary drainage, transfusion, tumor size, TNM staging and differentiation of cancer cell for recognized risk factors affecting survival postoperatively.
Preoperative biliary drainage such as percutaneous transhepatic biliary drainage (PTBD), endoscopic nasobiliary drainage (ENBD), and percutaneous transhepatic biliary drainage (PTBD) were performed for the patients with hyperbilirubinemia caused by biliary obstruction which were identified with preoperative imaging tools.
Adjuvant therapy was applied as concurrent chemoradiation manner. 5-Fluorouracil or gemcitabine or capecitabine were used as main chemotherapeutic agent and 44 Gy in 22 fractions were used for radiation. In palliative therapy, three ways such as chemotherapy only, radiation only and concurrent chemoradiation were carried out. The majority of them underwent chemotherapy with GEMOX (gemcitabine plus oxaliplatin) regimen or GP (gemcitabine plus cisplatin) regimen with or without radiation.
Resection margin was classified as R0, R1 and R2 whether tumor exists or not observed by macroscopically or microscopically. Macroscopic and microscopic tumor negative resection margin is considered as R0 resection. Macroscopic tumor negative and microscopic tumor positive resection margin is regarded as R1 resection. Macroscopic tumor positive resection margin is termed R2 resection. Transfusion was performed when patients' hemoglobin level was under 8.5 g/dL or hypoxia induced by bleeding or unstable vital sign caused by sustained hemorrhage. We defined complication and mortality as which appeared within a month after the surgery. Postoperative pancreatic fistula was graded as A, B, or C according to an international study group on pancreatic fistula definition [14]. TNM staging of distal bile duct cancer based on American Joint Committee on Cancer (AJCC) 7th edition.
Statistical analyses
Overall survival and survival of each variable were calculated with Kaplan-Meier formula. Univariate analysis of risk factors affecting survival was calculated with log-rank test and multivariate analysis was calculated with Cox-regression model. We defined significant data as a P-value < 0.05 and used IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) as a tool of statistical analysis.
RESULTS
Demographics
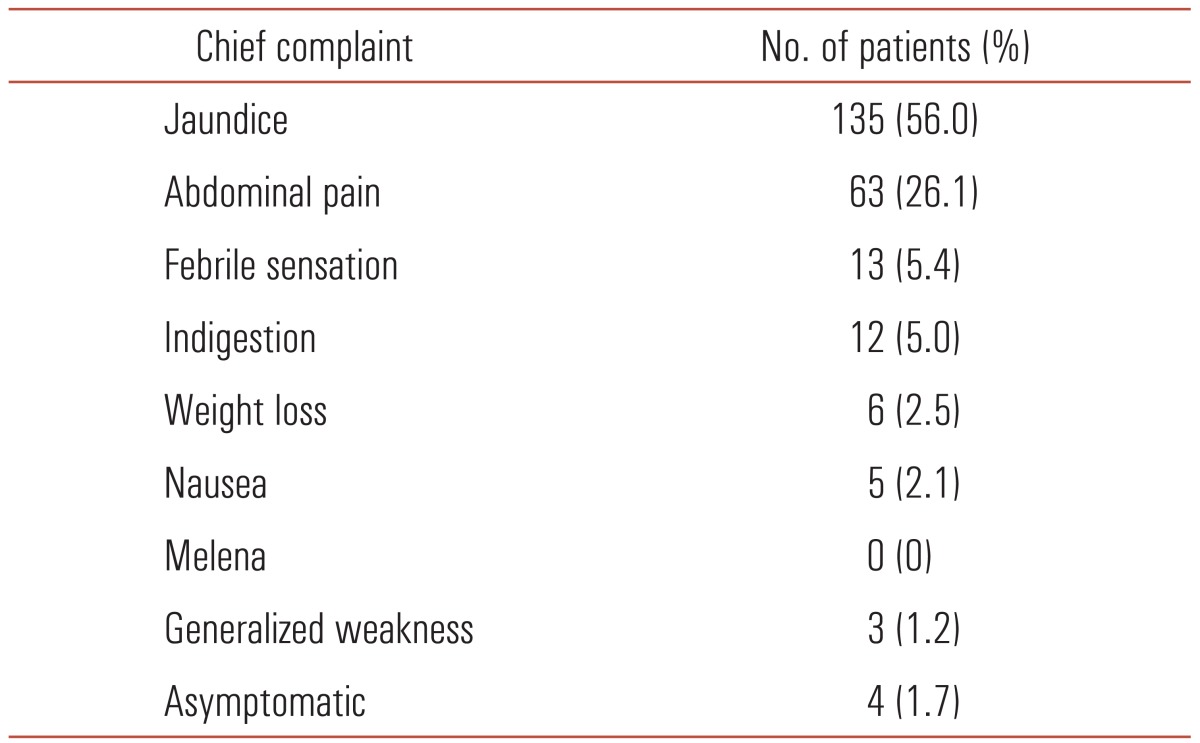
Average age of the 241 patients was 62.4 years (range, 31 to 88 years). There were 166 males (68.9%) and 75 females (31.1%). Table 1 shows that the patient's chief complaint at the time of their first visit to our clinic. Jaundice was the most frequent symptom and was evident in 135 patients (56.0%), followed by abdominal pain (26.1%) and fever (5.4%). Preoperative biliary drainage was performed in 219 cases (90.9%). There were 115 cases of PTBD (52.5%), 80 cases of ENBD (36.5%), and 24 cases of ERBD (11.0%).
Table 1.
Chief complaints at presentation of 244 cases of distal bile duct adenocarcinoma
Pathologic characteristics
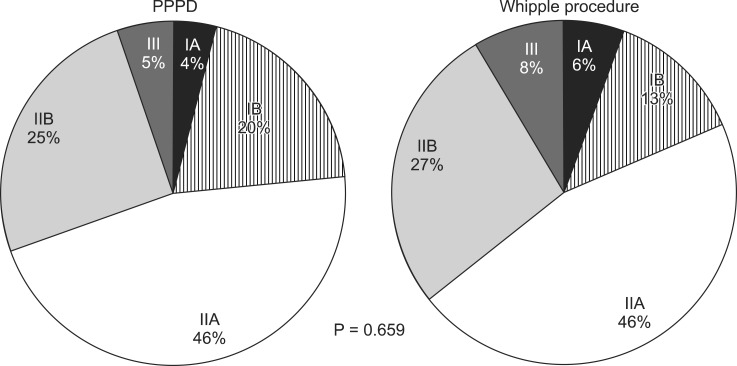
PPPD were performed in 171 patients and Whipple procedure were performed in 70 patients. 73 patients (30.3%) had lymph node metastasis. 6 patients were revealed to be R1 resection. Cancer cell was identified at the proximal margin of bile duct in five cases and at the soft tissue around superior mesenteric artery (SMA) in one case. R2 resection cases were four. One of them had portal vein invasion, one had SMA invasion, one had portal vein and SMA invasion and the last one showed proximal margin of bile duct invasion. Forty-one patients (17.5%) were pathologically confirmed as well differentiated form of ductal adenocarcinoma, 119 patients (50.9%) were moderate and 74 patients (31.6%) were poorly differentiated form. The last 7 patients had no pathological reports about tumor cell differentiation. According to AJCC 7th edition, stage IA were 11 cases (4.6%), stage IB were 42 cases (17.4%), stage IIA were 111 cases (46.1%), stage IIB were 62 cases (25.7%), stage III were 15 cases (6.2%), and stage IV was only one case but excluded as mentioned above.
Follow-up and complications
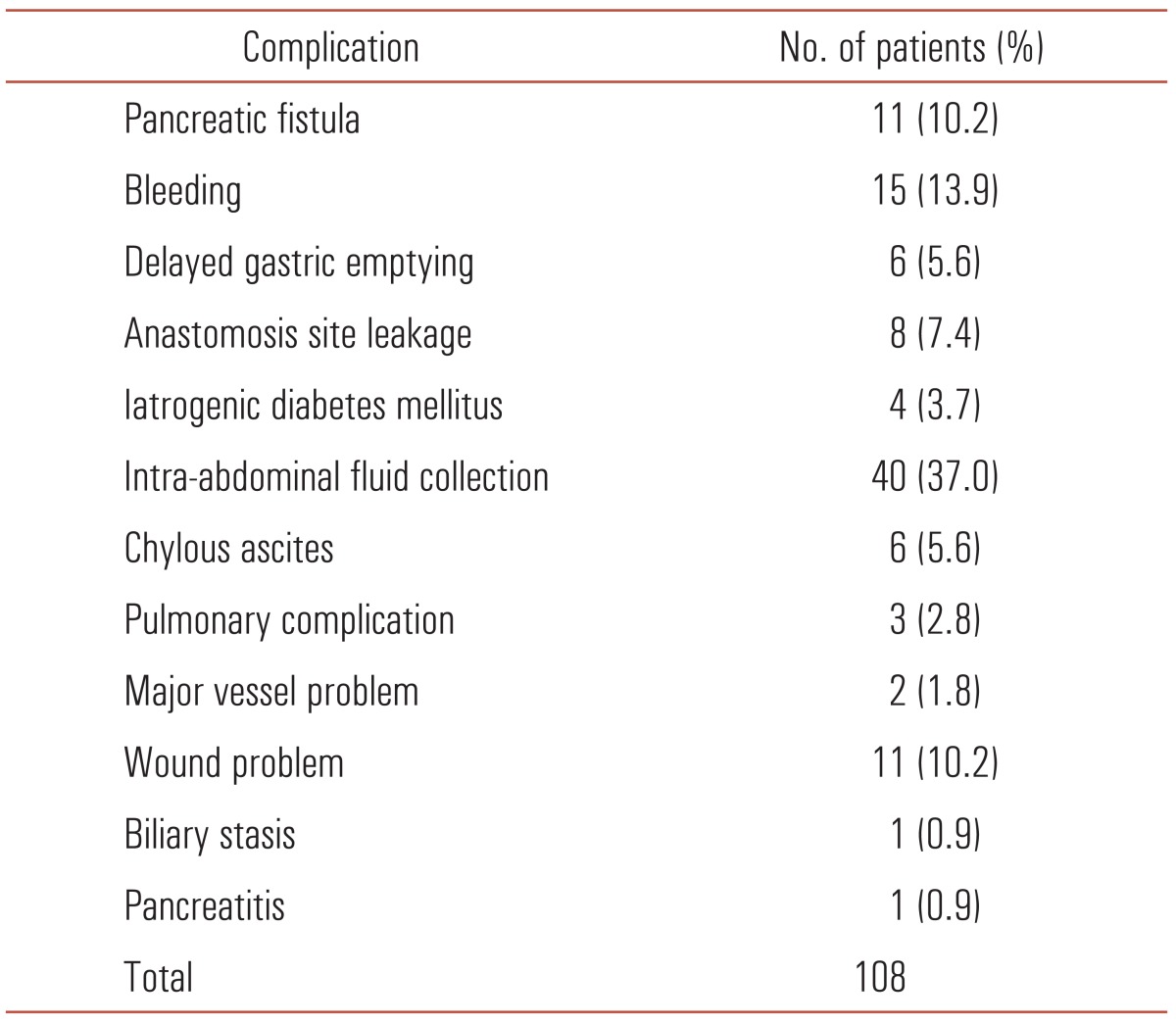
Complications within a month from the operation date were recorded (Table 2). One hundred eight patients (44.8%) experienced various types of complications. The most frequent complication was intra-abdominal fluid collection (40 cases, 37.0%), which needed intervention or prolonged conservative treatment, followed by bleeding (15 cases, 13.9%). Three mortalities occurred as a consequence of postoperative bleeding and one mortality was caused by liver failure after left hepatic artery sacrifice during surgery.
Table 2.
Complications after major resection of distal bile duct cancer
The average follow-up period of 237 patients, during which four immediate postoperative mortality cases were excluded, was 31.3 months (range, 2 to 177 months). One hundred twelve patients (47.3%) experienced recurrence during the follow-up and one hundred twenty five patients (52.7%) had no follow-up recurrence. Among the 112 recurrent patients, local recurrence were 53 (47.3%), intrahepatic recurrence were 33 (29.5%), and systemic recurrence were 26 (23.2%). Eventually, 86 patients died because of the recurrence.
Adjuvant therapy was performed in 6 patients with stage IIA, 14 patients with stage IIB, and 3 patients with stage III. Despite of the adjuvant therapy, 13 patients recurred and died. Palliative therapy was conducted in 44 recurrent patients and 2 patients with R2 resection. Twenty-two patients, including one R2 resection case, showed partial response and survived during the follow-up. However 24 patients died after or during the palliative therapy. Only one patient was lost to follow-up.
Risk factors and survival
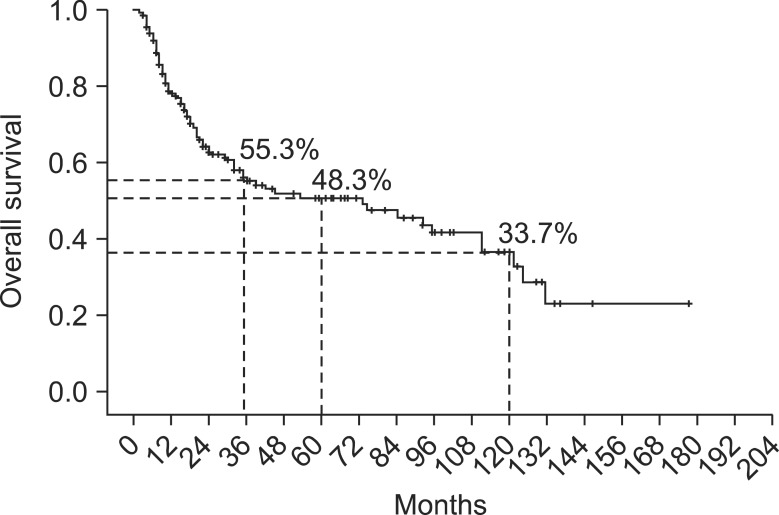
Four postoperative mortality cases were also excluded for survival analysis. The 3-, 5- and 10-year survival was 55.3%, 48.3% and 33.7%, respectively, after curative resection of distal bile duct cancer in our hospital (Fig. 1). Median survival duration was 73.0 months (range, 30.8 to 115.1 months). Univariate analysis revealed preoperative CA 19-9 >35 U/mL, tumor size >2 cm, presence of biliary drainage, complications, advanced stage and tumor cell differentiation and positive resection margin as poor prognostic factors (Table 3). In multivariate analysis, only CA 19-9, resection margin, T stage and N stage were significant factors (Table 4). Among them, positive resection margin was the strongest factor (hazard ratio, 5.078) indicating a negative influence on survival.
Fig. 1.
Overall survival curve.
Table 3.
Univariate analysis of predictors for survival in resected distal bile duct cancer
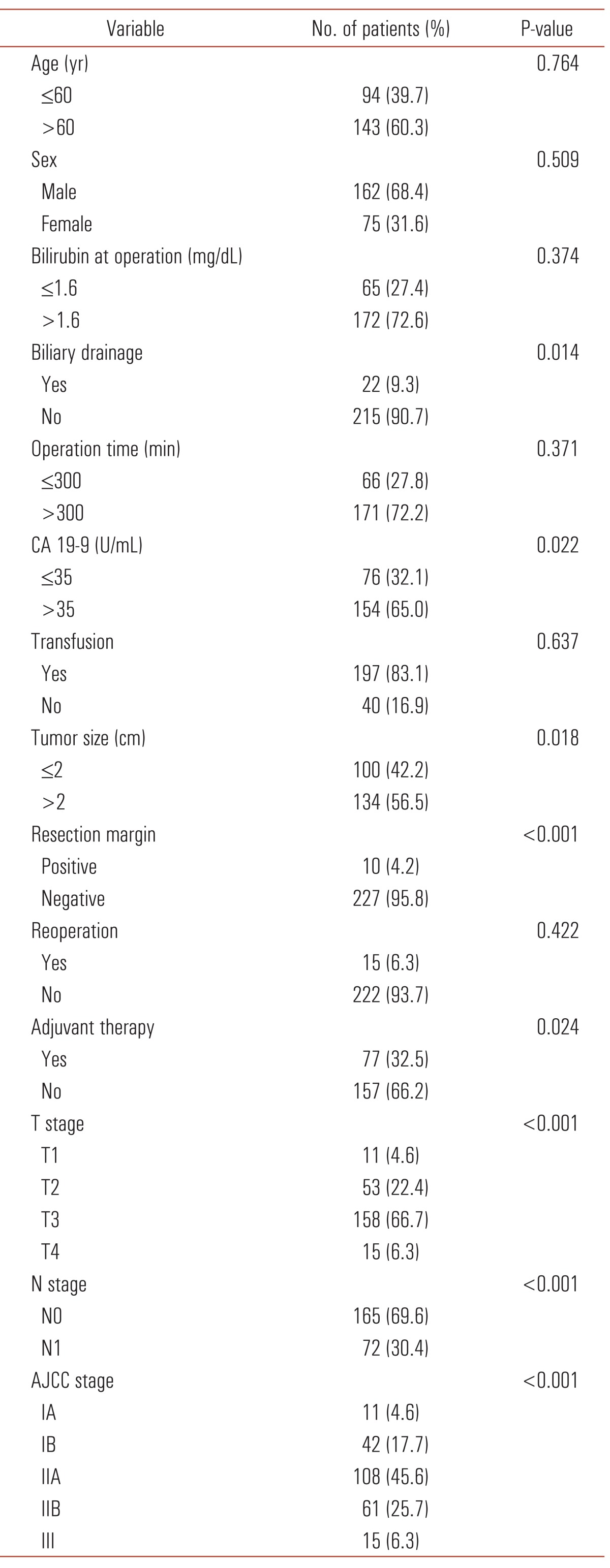
CA 19-9, carbohydrate antigen 19-9; AJCC, American Joint Committee on Cancer.
Table 4.
Multivariate analysis of prognostic factors after major resection for distal bile duct cancer
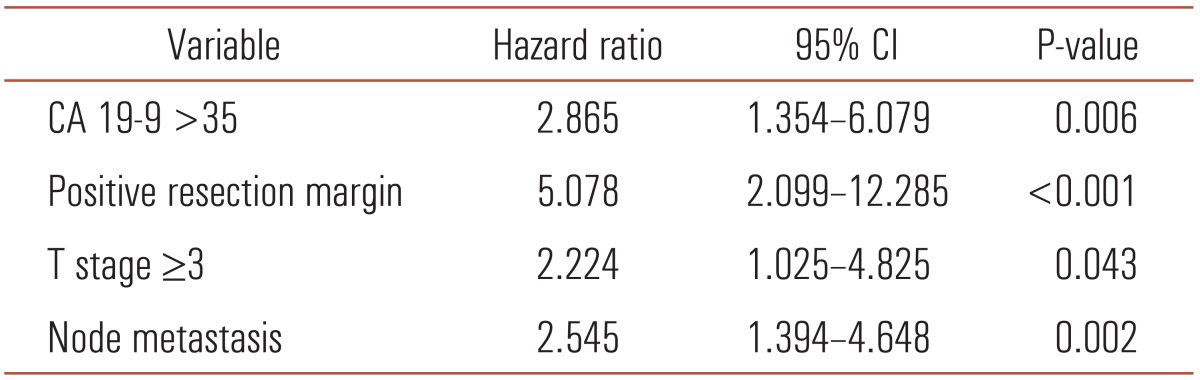
CA 19-9, carbohydrate antigen 19-9; CI, confidence interval.
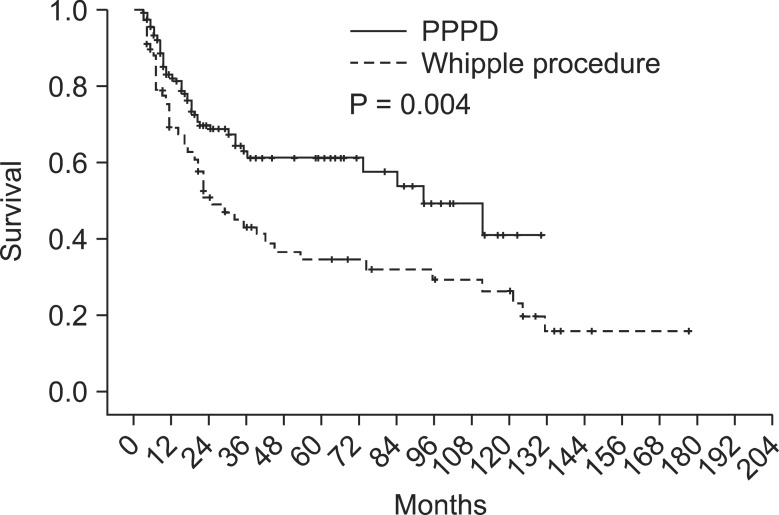
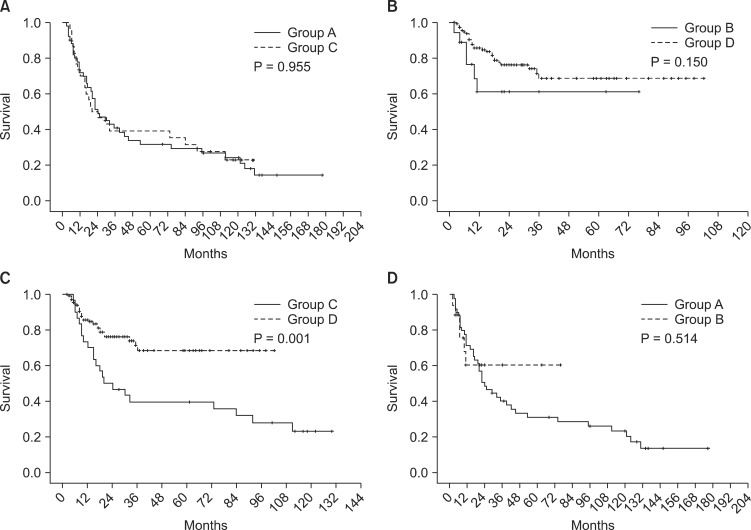
Survival difference between Whipple procedure and PPPD was presently evident (P = 0.006) (Fig. 2), despite the fact that their proportion of tumor staging was not significantly different (P = 0.659) (Fig. 3). For that reason, to rule out a cohort effect, we divided patients into four groups by period and by operation type. Patients who underwent Whipple procedure before 2003 were classified as group A and those who underwent the procedure after 2002 constituted group B. Patients underwent PPPD before 2003 were classified as group C and after 2002 were group D. When comparing groups A and B, it is obvious that the use of Whipple procedure had declined from 51 to 18 cases. On the other hand, PPPD had increased from 30 to 138 cases. There were no survival differences between groups A and C (P = 0.955). Groups B and D also showed no survival differences (P = 0.150). However, survival of group D patients was significantly better than group C patients (P = 0.001) (Fig. 4).
Fig. 2.
Survival difference according to preoperative serum carbohydrate antigen 19-9. PPPD, pancreaticoduodenectomy.
Fig. 3.
Survival difference according to lymph node metastasis. PPPD, pancreaticoduodenectomy.
Fig. 4.
Survival difference according to resection margin.
DISCUSSION
In 1976, a classification of distal bile duct cancer in an anatomical order was proposed by Longmire [15]. He defined proximal bile duct cancer as that originating above the bifurcation of the cystic duct, middle bile duct cancer as that growing between the bifurcation of the cystic duct and the superior margin of pancreas and distal bile duct cancer as that emerging below the superior margin of the pancreas. It is difficult to discern middle bile duct cancer. Often they are not localized to the middle portion of the bile duct, but are otherwise spread to proximal or distal portion of the bile duct. Recently, concerning its extent of invasion, middle bile duct cancer was divided into proximal bile duct cancer and distal bile duct cancer [16]. The authors decided to include patients with distal bile duct cancer only, by excluding those proximal and middle ones.
The representative operations for curative resection of distal bile duct cancer are Whipple procedure and PPPD. To presently eliminate any influence caused by surgical technique and extent of cancer, we excluded patients who underwent other operations than those two major operations.
Several studies have reported appreciably different 5-year overall survival rates ranging from 16-52% of surgically resected distal bile duct cancer patients [17]. In our study, the overall 5-year survival rate was 48.3% and the median survival duration was 73 months, which is comparable to other reports.
Serum CA19-9 is a well-known tumor marker of pancreatobiliary malignancies [18]. Generally it is not used as a prognostic or a diagnostic tool in clinical practice. Whether CA 19-9 might be an indicator of prognosis of bile duct cancer is debatable. Some retrospective studies reported that high serum levels of CA 19-9 have a close relationship with unresectability of the tumor [5]. However, in our study, serum CA 19-9 levels higher than 35 U/mL were associated with a significant survival disadvantage.
In univariate analysis, preoperative biliary drainage (P = 0.014) and tumor size larger than 2 cm (P = 0.018) had a negative effect on the survival. There are three ways to drain bile juice out of biliary tract: ERBD, ENBD, and PTBD. Subgroup analysis revealed that it is not necessary to distinguish the method used (ERBD and ENBD, P = 0.615; ERBD and PTBD, P = 0.100; ENBD and PTBD, P = 0.059). For that reason, we unified patients who had ERBD, ENBD and PTBD before the operation in the same group and compared them to another group in which drainage was not done. Although they did not have significant meaning in multivariate analysis, biliary drainage and larger tumor size might imply advanced tumor staging.
Lymph node status is a significant prognostic factor for distal bile duct cancer [19]. A study reported survival rate 65% without lymph node metastasis [20]. Also, our data showed that the survival rate 60% without lymph node metastasis and 17% with lymph node metastasis, which represents the survival significance of lymph node status of distal bile duct cancer. A Japanese study revealed that distal bile duct cancer patients with up to two positive lymph nodes had favorable prognosis [12]. Another Japanese group described that lymph node involvement is related to a high risk of liver metastasis [21].
Resection margin has been reported to be a significant prognostic factor, as it is the only factor that surgeons can control. Presently, resection margin displayed the highest hazard ratio (5.078). Therefore, it is necessary to obtain negative resection margin intraoperatively to improve survival.
This study had several limitations. As it was conducted in a single center and used a retrospective approach, selection bias may potentially have affected the results. However, the number of cases is large enough to calculate statistical values. Concerning node status, we did not analyze the number of metastasized nodes, which in any case does affect the surgical strategy.
In conclusion, as expected, surgical resection margin proved to be a significant indicator of worsened prognosis. Therefore, intraoperative frozen section should be utilized very aggressively to achieve R0 resection. For the distal bile duct cancer with elevated preoperative CA 19-9 level or advanced stage, additional study on postoperative adjuvant treatment may be warranted.
Footnotes
This original article was announced as "Best oral presentation" on July 3, 2012 at Palais des Congres, Paris/France where Tenth World Congress of the International Hepato-Pancreato-Biliary Association was exhibited.
No potential conflict of interest relevant to this article was reported.
References
- 1.The Korea Central Cancer Registry NCC. Annual report of cancer statistics in Korea in 2009. Seoul: Ministry of Health and Wellfare; 2011. [Google Scholar]
- 2.Sako K, Seitzinger GL, Garside E. Carcinoma of the extrahepatic bile ducts; review of the literature and report of six cases. Surgery. 1957;41:416–437. [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 4.Tompkins RK, Thomas D, Wile A, Longmire WP., Jr Prognostic factors in bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447–457. doi: 10.1097/00000658-198110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Ruiz M, Guerra-Vales JM, Colina-Ruizdelgado F. Comorbidity negatively influences prognosis in patients with extrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5279–5286. doi: 10.3748/wjg.15.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen PJ, Reiner AS, Gonen M, Klimstra DK, Blumgart LH, Brennan MF, et al. Extrahepatic cholangiocarcinoma: a comparison of patients with resected proximal and distal lesions. HPB (Oxford) 2008;10:341–346. doi: 10.1080/13651820802276630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahra M, Jacob D, Langrehr JM, Neumann UP, Neuhaus P. Carcinoma of the distal and middle bile duct: surgical results, prognostic factors, and long-term follow-up. J Hepatobiliary Pancreat Surg. 2008;15:501–507. doi: 10.1007/s00534-007-1308-4. [DOI] [PubMed] [Google Scholar]
- 8.Choi HS. Assessment of the definition of early extrahepatic bile duct cancer through the prognosis analysis of patients who had received curative resection. Korean J Gastroenterol. 2007;50:136–139. [PubMed] [Google Scholar]
- 9.Forsmo HM, Horn A, Viste A, Hoem D, Ovrebo K. Survival and an overview of decision-making in patients with cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:412–417. [PubMed] [Google Scholar]
- 10.Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YM, Cho EH, Lee KY, Ahn SI, Choi SK, Kim SJ, et al. Effect of preoperative biliary drainage on surgical results after pancreaticoduodenectomy in patients with distal common bile duct cancer: focused on the rate of decrease in serum bilirubin. World J Gastroenterol. 2008;14:1102–1107. doi: 10.3748/wjg.14.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. doi: 10.1001/archsurg.137.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Cho JY, Han HS, Yoon YS, Hwang DW, Jung K, Kim JH, et al. Preoperative cholangitis and metastatic lymph node have a negative impact on survival after resection of extrahepatic bile duct cancer. World J Surg. 2012;36:1842–1847. doi: 10.1007/s00268-012-1594-0. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Longmire WP., Jr Tumors of the extrahepatic biliary radicals. Curr Probl Cancer. 1976;1:1–545. doi: 10.1016/s0147-0272(76)80006-2. [DOI] [PubMed] [Google Scholar]
- 16.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: Advances and current limitations. World J Clin Oncol. 2011;2:94–107. doi: 10.5306/wjco.v2.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Enjoji M, Nakayama F. Cancer of the extrahepatic bile duct: a clinicopathologic study of immunohistochemistry for CEA, CA19-9, and p21. World J Surg. 1988;12:11–17. doi: 10.1007/BF01658480. [DOI] [PubMed] [Google Scholar]
- 19.Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–1715. doi: 10.1002/bjs.1800831217. [DOI] [PubMed] [Google Scholar]
- 20.Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Tajima H, Miwa K. Role of nodal involvement and the periductal soft-tissue margin in middle and distal bile duct cancer. Ann Surg. 1999;229:76–83. doi: 10.1097/00000658-199901000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki R, Takahashi M, Funato O, Nitta H, Murakami M, Kawamura H, et al. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery. 2001;129:677–683. doi: 10.1067/msy.2001.114555. [DOI] [PubMed] [Google Scholar]