Abstract
RhoA is a small GTPase multifunctional protein that regulates cell proliferation and cytoskeletal reorganization. Regulation of its protein stability plays an important role in its biological functions. We have shown that a Skp1-Cul1-F-box (SCF) FBXL19 E3 ubiquitin ligase targets Rac1, a related member of the Rho family for ubiquitination and degradation. Here, SCFFBXL19 mediates RhoA ubiquitination and proteasomal degradation in lung epithelial cells. Ectopically expressed FBXL19 decreased RhoA wild type, active, and inactive forms. Cellular depletion of FBXL19 increased RhoA protein levels and extended its half-life. FBXL19 bound the small GTPase in the cytoplasm leading to RhoA ubiquitination at Lys135. A RhoAK135R mutant protein was resistant to SCFFBXL19-mediated ubiquitination and degradation and exhibited a longer lifespan. Protein kinase Erk2-mediated phosphorylation of RhoA was both sufficient and required for SCFFBXL19-mediated RhoA ubiquitination and degradation. Thus, SCFFBXL19 targets RhoA for its disposal, a process regulated by Erk2. Ectopically expressed FBXL19 reduced phosphorylation of p27 and cell proliferation, a process mediated by RhoA. Further, FBXL19 cellular expression diminished lysophosphatidic acid (LPA)-induced phosphorylation of myosin light chain (MLC) and stress fiber formation. Hence, SCFFBXL19 functions as a RhoA antagonist during cell proliferation and cytoskeleton rearrangement. These results provide the first evidence of an F-box protein targeting RhoA thereby modulating its cellular lifespan that impacts cell proliferation and cytoskeleton rearrangement.
Keywords: small GTPase protein, protein stability, ubiquitin-proteasome system, phosphorylation, cell proliferation, stress fiber
1.1. Introduction
Small GTPase proteins regulate numerous cellular functions including cell cycle progression, cytoskeletal rearrangement, and regulation of transcriptional factor activity [1, 2]. Their activation is dependent on interaction with guanine nucleotides. The small GTPases are activated in a GTP bound state but are inactivated when bound to GDP [3]. RhoA is a member of the RhoGTPases that control cell proliferation and cytoskeletal stress fiber formation [4-6]. Similar to other small GTPases, RhoA activity is activated by guanine-nucleotide exchange factors (GEFs) and is inactivated by guanine-nucleotide dissociation inhibitors (GDIs), which regulate cycling between a GTP- and a GDP-bound state [7]. Recent studies suggest that regulatory control of RhoA protein stability plays a critical role in RhoA-mediated cellular signaling and biological functions [8, 9].
Protein ubiquitination is a major post-translational modification that regulates protein stability [10-14]. Ubiquitin is a small protein consisting of 76 amino acids, which covalently attaches to proteins, thereby directing proteins to the proteasome or lysosome for degradation. Protein ubiquitination requires a sequential series of steps: activation of ubiquitin by E1 ubiquitin-activating enzyme; transferring ubiquitin from an E1 enzyme to a ubiquitin-conjugating enzyme E2; and attachment of a mono- or poly-ubiquitin chain to substrates via an E3 ligase [14]. The Skp1-Cul1-F-box protein (SCF) ligase complex is one of the largest E3 ubiquitin ligase families [15, 16]. The F-box protein in the E3 ligase complex specifically interacts with substrates. It is common for one F-box protein to target more than one substrate. For example, β-Trcp1 (also termed FBXW1a) targets both phosphorylated-I-κB [17] as well as cortactin [18] for their ubiquitination and degradation. We have demonstrated that FBXL19, a new member of F-box protein family, targets the IL-33 cognate receptor, ST2L, for its ubiquitination and proteasomal degradation [19]. In addition to ST2L, we recently found that Rac1 is another target for FBXL19. Over-expression of FBXL19 reduced both active and inactive forms of Rac1 protein [20].
Several groups have attempted to identify which E3 ligase apparatus catalyzes RhoA ubiquitination for its ultimate degradation in cells [8, 9, 21]. Smurf1 appears to target activated RhoA [9], while Cullin-3/BACURD targets only GDP-bound RhoA for its ubiquitination and degradation [8]. Here, we show that SCFFBXL19 uniquely targets both the active and inactive forms of RhoA for ubiquitination and degradation, a process facilitated by extracellular signal-regulated kinase 2 (Erk2) that phosphorylates the GTPase. Further, we demonstrated that ectopically expressed FBXL19 reduces RhoA-mediated cell proliferation and stress fiber formation. These data suggest that in addition to ST2L and Rac1, RhoA is a new identified substrate for SCFFBXL19.
2. Materials and Methods
2.1. Cell culture and reagents
Murine lung epithelial cells (MLE12) were from ATCC (Manassas, VA, USA). Cells were cultured with HITES medium containing 10 % fetal bovine serum (FBS) and antibiotics in a 5% CO2 incubator at 37°C. V5 antibody, mammalian expression plasmid pcDNA3.1D/His-V5-TOPO, and Escherichia coli Top10 competent cells were from Invitrogen (Carlsbad, CA, USA). HA tag (29F4), and ubiquitin (P4D1) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Cycloheximide, leupeptin, β-actin ntibody, individual FBXL19 shRNAs, and scrambled shRNA were from Sigma-Aldrich (St. Louis, MO, USA). MG-132 and PD98059 were from Calbiochem. RhoA, Erk2, phospho-MLC, and phospho-p27 antibodies, immunobilized protein A/G beads, and control IgG were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). FBXL19 antibody was from Abgent (San Deigo, CA, USA). All materials in the highest grades used in the experiments are commercially available.
2.2. Construction of FBXL19 and RhoA plasmids
The FBXL19 cDNA was inserted into a pcDNA3.1D/V5-His vector (Invitrogen, CA, USA) [19]. Site directed mutagenesis was performed to generate RhoA lysine or serine mutants according to the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA, USA).
2.3. Immunoblotting and immunoprecipitation
Cells were washed with cold PBS and collected in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM β-glycerophosphate, 1 mM MgCl2, 1 % Triton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. Equal amounts of cell lysates (20 μg) were subjected to SDS-PAGE, electrotransferred to membranes and immunoblotted with indicated antibodies. For immunoprecipitation, equal amounts of cell lysates (1 mg) were incubated with specific primary antibodies overnight at 4 °C followed by the addition of 40 μl of protein A/G-agarose for 2 h at 4°C. The immunoprecipitated complex was washed three times with 1% Tritonx-100 in ice-cold phosphate-buffered saline and analyzed by immunoblotting with indicated antibodies.
2.4. Immunostaining
Cells grown on 35-mm glass-bottom culture dishes were fixed in 3.7% of formaldehyde for 20 min, followed by permeabilization with 0.1% of TritonX-100 for 2 min. Cells were incubated with a 1:200 dilution of antibodies to RhoA or V5 tag, followed by a 1:200 dilution of fluorescence-conjugated secondary antibody sequentially for immunostaining. The actin cytoskeleton was stained with fluorescence-conjugated phalloidin. Immunofluorescent cell imaging was performed using a Nikon confocal microscope.
2.5. Plasmid transfection by electroporation
MLE cells were suspended in 120 μl of nucleofection buffer and mixed well with 3 μg of plasmid DNA in an electroporation cuvette. Electroporation was performed in the Nucleofection™ II System (Lonza, Gaithersburg, MD, USA), and the cells were cultured in 2 ml of complete HITES medium for 48 h. ShRNA plasmids were also delivered into cells by using nucleofection with the same protocol. Cells were cultured for 72 h [19].
2.6. In vitro translation of cDNA of RhoA and FBXL19
In vitro transcription and translation (TnT) was performed using an in vitro translation system from Promega, Inc according to the manufacturer’s instructions [19]. Translated RhoA and FBXL19 were confirmed by immunoblotting.
2.7. In vitro ubiquitin conjugation assay
The ubiquitination of RhoA by FBXL19 was performed in a reaction mixture containing synthesized substrates, 50 mM Tris (pH 7.6), 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, 1.5 ng/μl E1, 10 ng/μl Ubc5, 10 ng/μl Ubc7, 1 μg/μl ubiquitin, 1 μM ubiquitin aldehyde, and His-purified recombinant Cullin 1, Skp1, Rbx1 (Boston Biochem, Cambridge, MA, USA), and synthesized F-box proteins from TnT system. The mixtures were subjected to immunoblotting.
2.8. Cell proliferation assay
MLE 12 cells were plated in a 96-well microplate at a density of 5000 cells per well in serum-free (blank) medium or 2% FBS medium. After culturing for 48 h, cell proliferation was measured by a CyQUANT NF Cell Proliferation Assay Kit (Invitrogen). Briefly, 100 μl of 1 × dye binding solution was dispensed into each microplate well using a multichannel pipettor. The microplate was incubated at 37 °C for 30 minutes. The fluorescence intensity of each sample was then measured using a fluorescence microplate reader with excitation at ~485 nm and emission detection at ~530 nm.
2.9. Statistics
All results were subjected to statistical analysis using two-way analysis of variance, and, wherever appropriate, analyzed by Student–Newman-Keuls test. Data are expressed as mean ± S.D. of triplicate samples from at least three independent experiments and values that were p<0.05 were considered statistically significant.
3. Results
3.1. RhoA degradation is through the proteasome system
To investigate if lung epithelial cells contain a functional apparatus to degrade RhoA, we first examined the half-life of RhoA, both the wild type, active form, and the inactive form in MLE12 cells. V5 tagged RhoA wild type, active form (RhoAV14), or an inactive form (RhoAN19) over-expressing MLE12 cells were treated with a protein synthesis inhibitor (cycloheximide, CHX); the half-life of RhoA wild type, RhoAV14, or RhoAN19 was then determined by immunoblotting. As shown in Fig. 1A, in the presence of CHX, all the wild type, active, and inactive forms of RhoA degraded to ~50 % around 2 h. To investigate which degradation pathway is involved in RhoA degradation, RhoAV14 or RhoAN19 over-expressing cells were incubated with proteasome inhibitor (MG-132) or lysosome inhibitor (leupeptin) prior to CHX treatment. CHX-mediated RhoAV14 or RhoAN19 degradation was attenuated by pretreatment with MG-132, but not leupeptin (Fig. 1B). Consistent with findings of others, these results suggest that RhoA is degraded by the proteasome system [22, 23].
Figure 1. RhoA degrades in the proteasome system in lung epithelial cells.

A. MLE12 cells were transfected with V5 tagged RhoA wild type (RhoA-V5), RhoA active form (RhoAV14-V5), or RhoA inactive form (RhoAN19-V5) plasmids for 48 h. Cells were treated with cycloheximide (CHX, 20 μg/ml) for indicated times and then cell lysates were analyzed for over-expressed RhoA and β-actin by immunoblotting with V5 tag and β-actin antibodies. B. RhoAV14-V5 over-expressed MLE12 cells were treated with 20 μg/ml of CHX with or without MG-132 (20 μM) or leupeptin (100 μM) for 2 h. Cell lysates were analyzed for RhoAV14-V5 and β-actin by immunoblotting with V5 tag and β-actin antibodies. C. RhoAN19-V5 over-expressed MLE12 cells were treated with 20 μg/ml of CHX with or without MG-132 (20 μM) or leupeptin (100 μM) for 2 h. Cell lysates were analyzed for RhoAN19-V5 and β-actin by immunoblotting with V5 tag and β-actin antibodies. Shown are representative blots from three independent experiments.
3.2. SCFFBXL19 triggers RhoA degradation
We have shown that a subunit of the SCF E3 ligase family (termed FBXL19) targets Rac1 for its ubiquitination and degradation [20]. To investigate if FBXL19 also regulates RhoA stability, first we examined endogenous RhoA levels in FBXL19 knock down cells. MLE12 cells were transfected with three distinct individual FBXL19 shRNA for 72 h. As shown in Fig. 2A, FBXL19 shRNAs transfection increased RhoA levels with significant knock down efficiency. To investigate if FBXL19-induced RhoA degradation is associated with RhoA activity, we co-expressed V5 tagged FBXL19 (FBXL19-V5) plasmid with either RhoA wild type, RhoAV14, or RhoAN19 in MLE12 cells. All of the RhoA forms were degraded by FBXL19-V5 (Fig. 2B). Thus, we used RhoAN19 plasmid as an over-expression system to study the mechanisms of RhoA degradation in the rest of the experiments. Next, the degradation rate of RhoAN19-V5 in FBXL19 depleted cells was compared to control shRNA transfected cells. FBXL19 shRNA transfection attenuated RhoAN19-V5 degradation, an effect that was partially reversed by over-expression of FBXL19-V5 in FBXL19 shRNA transfected cells (Fig. 2C). Further, MLE12 cells were also co-transfected with RhoAN19-V5 and HA tagged FBXL19. Co-immunoprecipitation (co-IP) with an antibody to the V5 tag revealed that FBXL19-HA associates with RhoAN19-V5 (Fig. 3A). Further, RhoA was detected in the FBXL19 immunoprecipitation complex (Fig. 3B). The association between over-expressed FBXL19 and endogenous RhoA were also confirmed by immunostaining (Fig. 3C). RhoA and GFP tagged FBXL19 co-localize in the cytoplasm, however over-expressed FBXL19 was also detected in the nuclei (Fig. 3C). These results indicate that SCFFBXL19 targets RhoA for degradation.
Figure 2. FBXL19 regulates RhoA stability.

A. MLE12 cells were transfected with three distinct FBXL19 shRNA plasmids (#1 - #3) for 72 h. RhoA, FBXL19, and β-actin expression were analyzed by immunoblotting. B. MLE12 cells were co-transfected with FBXL19-V5 plasmid and RhoA-V5 or RhoAV14-V5, or RhoAN19-V5 plasmids. Cell lysates were analyzed for RhoA-V5 or RhoAV14-V5, RhoAN19-V5, FBXL19-V5, and β-actin by immunoblotting with V5 tag and β-actin antibodies. C. RhoAN19-V5 over-expressed MLE12 cells were co-transfected with vector alone, shFBXL19, or shFBXL19 + FBXL19-V5 plasmids and then cells were treated with CHX (20 μg/ml) for 0 - 4 h. Cell lysates were analyzed for RhoAN19-V5, FBXL19-V5, FBXL19, and β-actin by immunoblotting with V5 tag, FBXL19, and β-actin antibodies. Shown are representative blots from three independent experiments.
Figure 3. FBXL19 interacts with RhoA.

A. MLE12 cells were co-transfected with RhoAN19-V5 and FBXL19-HA plasmids. Cell lysates were subjected to immunoprecipitation with HA tag, followed by V5 tag immunoblotting. Input lysates were analyzed by immunoblotting with V5 tag and HA tag antibodies. Shown are representative blots from three independent experiments. B. MLE12 cell lysates were subjected to immunoprecipitation with FBXL19 antibody, followed by RhoA immunoblotting. Input lysates were analyzed by immunoblotting with RhoA and β-actin antibodies. Shown are representative blots from three independent experiments. C. MLE12 cells grown on glass-bottom dishes were transfected with FBXL19-GFP plasmid (2 μg) for 48 h followed by MG-132 (20 μM, 18 h) treatment. Localization of FBXL19-GFP (green), RhoA (red), and nuclei (blue) were examined by immunofluorescence staining. Arrows show co-localization of FBXL19 and RhoA. Shown are representative images from three independent experiments. Scale bars represent 10 μm.
3.3. RhoA lysine135 is the ubiquitin acceptor site for FBXL19
Ubiquitin covalently attaches to lysine (K) residue(s) within a target protein [14]. To identify the putative ubiquitin acceptor site within RhoA for the SCFFBXL19 complex, we substituted several candidate K residues of RhoA with Arg (R). Of several mutants tested, only RhoAN19K135R was resistant to FBXL19-mediated degradation (Fig. 4A). RhoAN19K135R displayed greater protein stability than another mutant, RhoAN19K140R, in response to CHX treatment (Fig. 4B). In vitro ubiquitination assays showed that SCFFBXL19 induced–polyubiquitination was reduced using RhoAN19K135R as a substrate versus RhoAN19 (Fig. 4C). The results suggest that FBXL19 mediates site-specific RhoA polyubiquitination.
Figure 4. Lysine 135 within RhoA is the ubiquitin acceptor site for FBXL19.

A. MLE12 cells were co-transfected with FBXL19-V5 and RhoAN19-V5 or lysine mutant plasmids. Cell lysates were analyzed for RhoAN19-V5, mutants, FBXL19-V5, and β-actin by immunoblotting with antibodies to V5 tag and β-actin. B. MLE12 cells were transfected with RhoAN19K135R-V5 or RhoAN19K140R-V5 plasmid and then cells were treated with CHX for 0, 2, and 4 h. Cell lysates were analyzed for V5 tagged RhoAN19 mutants and β-actin by immunoblotting with V5 tag and β-actin antibodies. C. FBXL19-HA, RhoAN19-V5, and RhoAN19K135R–V5 were synthesized by a TnT system, and in vitro ubiquitinations were measured by incubation with E1, E2, ubiquitin, Cullin1, Skp1, and ATP, followed by V5 tag immunoblotting. Input lysates were analyzed for RhoAN19-V5, mutant, and FBXL19-HA by immunoblotting. Shown are representative blots from three independent experiments.
3.4. Erk2 regulates RhoA phosphorylation and degradation
Substrate phosphorylation is often an essential step for F-box proteins to recognize their substrates during proteolysis [24, 25]. We have shown that Erk2 is a key kinase in the regulation of cortactin phosphorylation and subsequent degradation by SCFβ-Trcp in MLE12 cells [18]. To investigate if Erk2 is also involved with FBXL19-mediated RhoA degradation, we first examined the effect of Erk2 on RhoA phosphorylation. Immunoprecipitation with a RhoA antibody followed by immunoblotting with a specific antibody to phospho-serine shows that over-expressed V5 tagged Erk2 increased RhoA serine phosphorylation (Fig. 5A), while depletion of Erk2 by transfected cells with Erk2 shRNA reduced the phosphorylation of RhoA (Fig. 5B). To investigate if Erk2-mediated phosphorylation of RhoA is associated with RhoA degradation, RhoAN19-V5 over-expressed cells were pretreated with an inhibitor of the Erk pathway (PD98059) prior to CHX treatment. As shown in Fig. 6A, PD98059 attenuated CHX-mediated RhoAN19-V5 degradation. Over-expression of Erk2 reduced protein levels of either over-expressed RhoAN19-V5 or endogenous RhoA (Fig. 6B and 6C). Further, PD98059 attenuated FBXL19-mediated RhoA degradation (Fig. 6D), as well as FBXL19-mediated polyubiquitination of RhoA (Fig. 6E), suggesting that Erk2 promotes SCFFBXL19-mediated RhoA degradation.
Figure 5. Erk2 regulates RhoA phosphorylation.
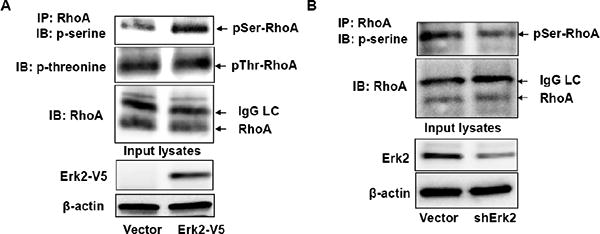
A. MLE12 cells were transfected with Erk2-V5 plasmid for 48 h. Cell lysates were subjected to immunoprecipitation with a RhoA antibody. The precipitates were probed with phospho-serine, phospho-threonine, and RhoA antibodies. Input cell lysates were subjected to V5 tag and β-actin immunoblotting. B. MLE12 cells were transfected with shCont or shErk2 plasmid for 72 h. Cell lysates were subjected to immunoprecipitation with a RhoA antibody. The precipitates were probed with phospho-serine and RhoA antibodies. Input cell lysates were subjected to Erk2 and β-actin immunoblotting. Shown are representative blots from three independent experiments.
Figure 6. Erk2 regulates RhoA stability.
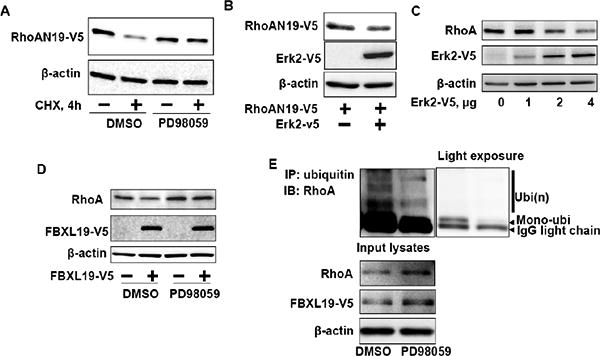
A. RhoAN19-V5 over-expressing MLE12 cells were pretreated with DMSO or PD98059 (10 μM, 1 h) prior to CHX treatment (20 μg/ml, 4 h) and then cell lysates were analyzed for RhoAN19-V5 and β-actin by immunoblotting with V5 tag and β-actin antibodies. B. MLE12 cells were co-transfected with RhoAN19-V5 and Erk2-V5 plasmids and then cell lysates were analyzed for RhoAN19-V5, Erk2-V5, and β-actin by immunoblotting with V5 tag and β-actin antibodies. C. MLE12 cells were transfected with Erk2-V5 plasmids (0 – 4 μg) and then cell lysates were analyzed for RhoA, Erk2-V5, and β-actin by immunoblotting with RhoA, V5 tag, and β-actin antibodies. Shown are representative blots from three independent experiments. D. MLE12 cells were transfected with FBXL19-V5 plasmid and then cells were treated with DMSO or PD98059 (5 μM, 16 h). Cell lysates were analyzed for RhoA, FBXL19-V5, and β-actin by immunoblotting with RhoA, V5 tag, and β-actin antibodies. Shown are representative blots from three independent experiments. E. FBXL19-V5 over-expressing MLE12 cells were pretreated with DMSO or PD98059 (5 μM, 16 h) prior to immunoprecipitation with ubiquitin antibody. The immunoprecipitated complex was analyzed for RhoA by immunoblotting. Input lystes were analyzed for RhoA, FBXL19-V5, and β-actin by immunoblotting with RhoA, V5 tag, and β-actin antibodies. Right panel is a light exposure image.
3.5. FBXL19 regulates cell proliferation and stress fiber formation
RhoA plays a critical role in regulating cell proliferation and cytoskeleton rearrangement [26, 27]. Our findings show that FBXL19 plays a direct role in the polyubiquitination and degradation of RhoA suggesting that FBXL19 may inhibit RhoA-mediated cell proliferation and cytoskeleton rearrangement. First, we found that down-regulation of FBXL19 by FBXL19 shRNA transfection increased cell proliferation (Fig. 7A), suggesting that FBXL19 plays a role in regulation of cell proliferation. Phosphorylation of p27Kip1 at serine10 is known to promote cell cycle progression [28, 29]; FBS induced this effect was attenuated in FBXL19 over-expressed cells (Fig. 7B). As expected, over-expression of RhoA increased cell growth and the effect was lessened in the FBXL19 over-expressed cells (Fig. 7C). Further, the inhibitory effect of RhoA down-regulation on cell proliferation was restored by over-expression of RhoA wild type or RhoAK135R mutant (Fig. 7D). To investigate if SCFFBXL19-mediated RhoA degradation regulates stress fiber formation, an empty vector or FBXL19-V5 plasmid transfected MLE12 cells were treated with LPA; phosphorylation of myosin light chain (MLC) was then examined by immunoblotting. LPA induced the phosphorylation of MLC in empty vector transfected cells, but not in FBXL19-V5 over-expressed cells (Fig. 8A). Immunostaining shows that LPA treatment induced stress fiber formation and the effect was attenuated by over-expression of FBXL19 (Fig. 8B). LPA and FBS treatment had no effect of RhoA expression (data not shown). These results suggest that SCFFBXL19 targets RhoA degradation and functions as an antagonist of RhoA in the regulation of the cell proliferation and cytoskeletal rearrangement.
Figure 7. FBXL19 regulates cell proliferation.

A. MLE12 cells were transfected with shCont and shFBXL19 plasmid for 72 h. Cell proliferation were measured. *p<0.01, compared to shCont. B. MLE12 cells were transfected with FBXL19-V5 plasmid and then cells were treated with 2 % of FBS for 30 min. Cell lysates were analyzed for FBXL19-V5, phospho-p27, and β-actin by immunoblotting with V5 tag, p-p27, and β-actin antibodies. C. MLE12 cells were co-transfected with RhoA-V5 and FBXL19-HA. Cell numbers were accounted for 0 – 3 days. D. MLE12 cells were transfected with shCont, shRhoA, RhoA-V5, or RhoAK135R plasmids as indicted. Cells were treated with or without 2% of FBS for 48 h and cell proliferation were measured. Cell proliferation was normalized to the cells without FBS (veh). *p<0.01, compared to untransfected cells; **p<0.05, compared to shRhoA transfected cells.
Figure 8. FBXL19 regulates stress fiber formation.

A. MLE12 cells were transfected with FBXL19-V5 plasmid and then cells were treated with LPA (5 μM) for 5 min. Cell lysates were analyzed for phospho-MLC, FBXL19-V5, and β-actin by immunoblotting with p-MLC, V5 tag, and β-actin antibodies. B. MLE12 cells were transfected with empty vector (a and b) or FBXL19-V5 plasmid (c-h) and then cells were treated with LPA (5 μM, 10 min) (b, f-h). Cells were fixed and actin filaments were stained with phalloidin (red), FBXL19-V5 was stained with a V5 tag antibody (green), and nuclei were stained with DAPI (blue). Shown are representative images from three independent experiments. Scale bar represents 2 μm.
4. Discussion
Post-translational modifications, including ubiquitination and phosphorylation, regulate the function of key signaling biomolecules by modulating their activity, localization, and protein stability. Ubiquitination of small GTPases controls their behavior in cells, including migratory ability and cell cycle progression [20, 29, 30]. Here, RhoA lifespan is regulated by the SCFFBXL19 E3 ligase complex by mediating RhoA ubiquitination. While the ability of FBXL19 on protein ubiquitination is mediated by protein kinases, such as glycogen synthase kinase 3β (GSK3β) and AKT, RhoA ubiquitination and degradation by the F-box protein was facilitated by an Erk2-mediated phosphorylation dependent mechanism. FBXL19 targets RhoA for ubiquitination and degradation, thus regulating cell growth and stress fiber formation suggesting that this F-box protein might serve as an endogenous feedback inhibitor of RhoA. The biologic relevance of F-box proteins in modulating cellular responses after various stimuli such as LPA will be more apparent by using more sophisticated in vivo models such as FBXL19 knockout mice. Nevertheless, the data presented here provide evidence that this SCF subunit modulates processes that may ultimately relate to lung repair, injury, and possibly tumorogenesis.
Ubiquitination is a necessary step for protein degradation in the proteasome system, which is catalyzed by a series critical reactions [14, 31]. F-box proteins, the major component of the SCF E3 ligase machinery, directly targets substrates for their ubiquitination, thereby playing a key role in fundamental processes such as cell replication [32, 33], transcriptional activities [17, 34, 35], and cell motility [20, 36]. We have shown that FBXL19 targets the IL-33 receptor ST2L and Rac1 for their polyubiquitination and turnover within the proteasome system [19]. Here we uncover that RhoA is a new substrate for FBXL19. While the other two known E3 ligases, Smurf1 and Cullin-3/BACURD, have been known to target only active or inactive RhoA for degradation [8, 9], over-expression of FBXL19 reduced both active and inactive RhoA for disposal. This finding is similar to what we have recently demonstrated that SCFFBXL19 induces Rac1 ubiquitination and degradation in a guanine nucleotide binding independent mechanism [20]. Hence, this study reveals a new substrate for SCFFBXL19. It is common for F-box proteins to target multiple substrates. For example, SCFFBXW7 induces polyubiquitination and degradation of Notch [35], cyclin E [37], c-Myc [38, 39] and c-Jun [35]. Lysines6,7 within RhoA have been identified as ubiquitin acceptor sites by Smurf1 [22], while ubiquitin acceptor site(s) by Cullin-3/BACURD have not been demonstrated. Here, we identified that lysine135 is the ubiquitin acceptor site by SCFFBXL19, since the corresponding RhoAK135R mutant has less ubiquitination, and it is resistant to SCFFBXL19-mediated degradation. Unlike lysines 6 and 7 that are in close proximity to first guanine nucleotide binding site (G1), lysine135 is distant from the GTP/GDP binding sites and resides in the linker region within α-helices 3 and 4, therefore allowing ubiquitin linking to RhoA in both GTP- and GDP-bound states by SCFFBXL19. Cullin-3 is a member of the SCF E3 ligase complex. Chen et al. demonstrated that BACURD regulates Cullin-3-mediated RhoA ubiquitination [8]. The involvement of FBXL19 in Cullin-3-mediated RhoA ubiquitination and FBXL19 docking site within RhoA will be determined in the future.
Phosphorylation of substrates is often an essential step, functioning as a recognition signal for F-box protein interaction with substrates [24, 25]. SCFβ-Trcp-mediated ubiquitination of I-κB is dependent on I-κB phosphorylation by IKKs [40]. We have shown that GSK3β [19] and AKT [20] facilitate FBXL19-mediated ubiquitination and degradation of substrates, such as ST2L and Rac1. Phosphorylation of RhoA by PKA at serine188 has been known to protect RhoA from Smurf1-mediated ubiquitination and degradation in vascular smooth muscle cells [9]. The role of phosphorylation of RhoA in promoting its degradation has not been investigated. Erk2 is a serine/threonine kinase that regulates a variety of cellular responses including cellular growth, differentiation, gene expression, and apoptosis by phosphorylating numerous substrates. Several studies have demonstrated that Erk2 promotes protein ubiquitination and degradation of various targets, such as dual-specificity phosphatase 1 [41], GATA3 [42], and cortactin [18]; however, the role of Erk2 in regulation of RhoA stability and activity has not been well studied. Hamadmad et al. reported that inhibition of Erk attenuated erythropoietin-induced RhoA activation [43]. Here, RhoA phosphorylation by Erk2 promotes FBXL19-mediated RhoA degradation, since inhibition of the Erk pathway attenuates RhoA ubiquitination, while over-expression of Erk2 reduces RhoA stability. Future studies will need to further clarify the phosphodegron-like molecular signature within RhoA that is important in recruiting SCFFBXL19 in the context of Erk phosphorylation.
The current study unveils a new molecular mechanism of RhoA degradation. RhoA plays a critical role in regulation of cell growth and cytoskeleton rearrangement. Thus, it is likely that SCFFBXL19 functions as an antagonist of RhoA and exerts divergent actions on several fundamental processes that regulate cell growth and cell motlity. Future studies will also examine the role of FBXL19 in RhoA-mediated immune responses and will focus on high throughput small molecule screening of novel FBXL19 peptide mimics to manipulate cell motility.
Research Highlights.
SCFFBXL19 mediates RhoA ubiquitination and degradation.
RhoA lys135 is an ubiquitin acceptor site by SCFFBXL19.
Erk2 promotes SCFFBXL19-induced RhoA ubiquitination and degradation.
SCFFBXL19 regulates cell proliferation and stress fiber formation.
Acknowledgments
This study was supported by the US National Institutes of Health RO1 HL01916 and HL112791 (to Y.Z.), HL096376, HL097376 and HL098174 (to R.K.M.), American Heart Association awards 12SDG9050005 (J.Z.), and a Merit Review Award from the US Department of Veterans Affairs (to R.K.M.). All authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall A. Rho GTPases and the actin cytoskeleton. Science (New York, NY. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 3.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature reviews. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 4.Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar WH. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. Journal of cell science. 1997;110(pt 19):2417–2427. doi: 10.1242/jcs.110.19.2417. [DOI] [PubMed] [Google Scholar]
- 5.Sauzeau V, Le Mellionnec E, Bertoglio J, Scalbert E, Pacaud P, Loirand G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circulation research. 2001;88:1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 6.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. The Journal of cell biology. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein- coupled receptor signaling. Molecular pharmacology. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Molecular cell. 2009;35:841–855. doi: 10.1016/j.molcel.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang HR, Wrana JL. Smurf1: a link between cell polarity and ubiquitination. Cell cycle (Georgetown, Tex. 2004;3:391–392. doi: 10.4161/cc.3.4.772. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. The Journal of biological chemistry. 2002;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 11.Gronroos E, Hellman U, Heldin CH, Ericsson J. Control of Smad7 stability by competition between acetylation and ubiquitination. Molecular cell. 2002;10:483–493. doi: 10.1016/s1097-2765(02)00639-1. [DOI] [PubMed] [Google Scholar]
- 12.Brooks CL, Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Current opinion in cell biology. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 13.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 14.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochimica et biophysica acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Willems AR, Goh T, Taylor L, Chernushevich I, Shevchenko A, Tyers M. SCF ubiquitin protein ligases and phosphorylation-dependent proteolysis. Philosophical transactions of the Royal Society of London. 1999;354:1533–1550. doi: 10.1098/rstb.1999.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Ghosh S. beta-TrCP mediates the signal-induced ubiquitination of IkappaBbeta. The Journal of biological chemistry. 1999;274:29591–29594. doi: 10.1074/jbc.274.42.29591. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Wei J, Mialki R, Zou C, Mallampalli RK, Zhao Y. Extracellular signal-regulated kinase (ERK) regulates cortactin ubiquitination and degradation in lung epithelial cells. The Journal of biological chemistry. 2012;287:19105–19114. doi: 10.1074/jbc.M112.339507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Wei J, Mialki RK, Mallampalli DF, Chen BB, Coon T, Zou C, Mallampalli RK, Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nature immunology. 2012;13:651–658. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Mialki RK, Wei J, Coon TA, Zou C, Chen BB, Mallampalli RK, Zhao Y. SCF E3 ligase F-box protein complex SCFFBXL19 regulates cell migration by mediating Rac1 ubiquitination and degradation. Faseb J. 2013 doi: 10.1096/fj.12-223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science (New York, NY. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 22.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science (New York, NY. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 23.Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circulation research. 2005;96:1152–1160. doi: 10.1161/01.RES.0000170084.88780.ea. [DOI] [PubMed] [Google Scholar]
- 24.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 25.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science (New York NY. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 26.Faried A, Faried LS, Kimura H, Nakajima M, Sohda M, Miyazaki T, Kato H, Usman N, Kuwano H. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42:1455–1465. doi: 10.1016/j.ejca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Guasch RM, Tomas M, Minambres R, Valles S, Renau-Piqueras J, Guerri C. RhoA and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. Journal of neuroscience research. 2003;72:487–502. doi: 10.1002/jnr.10594. [DOI] [PubMed] [Google Scholar]
- 28.Chappell J, Leitner JW, Solomon S, Golovchenko I, Goalstone ML, Draznin B. Effect of insulin on cell cycle progression in MCF-7 breast cancer cells. Direct and potentiating influence. The Journal of biological chemistry. 2001;276:38023–38028. doi: 10.1074/jbc.M104416200. [DOI] [PubMed] [Google Scholar]
- 29.Zheng D, Sun Y, Gu S, Ji C, Zhao W, Xie Y, Mao Y. LNX (Ligand of Numb-protein X) interacts with RhoC, both of which regulate AP-1-mediated transcriptional activation. Molecular biology reports. 2010;37:2431–2437. doi: 10.1007/s11033-009-9754-5. [DOI] [PubMed] [Google Scholar]
- 30.Visvikis O, Lores P, Boyer L, Chardin P, Lemichez E, Gacon G. Activated Rac1, but not the tumorigenic variant Rac1b, is ubiquitinated on Lys 147 through a JNK-regulated process. The FEBS journal. 2008;275:386–396. doi: 10.1111/j.1742-4658.2007.06209.x. [DOI] [PubMed] [Google Scholar]
- 31.Barbier O, Chryssagi AM, Hugon S, Rombouts JJ, Thonnard JL. Prospective functional analysis of trapeziectomy combined with intermetacarpal tendon stabilisation in trapeziometacarpal arthritis. Acta orthopaedica Belgica. 2004;70:410–416. [PubMed] [Google Scholar]
- 32.Craig KL, Tyers M. The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Progress in biophysics and molecular biology. 1999;72:299–328. doi: 10.1016/s0079-6107(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 33.Vodermaier HC. APC/C and SCF: controlling each other and the cell cycle. Curr Biol. 2004;14:R787–796. doi: 10.1016/j.cub.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie. 2001;83:351–356. doi: 10.1016/s0300-9084(01)01237-8. [DOI] [PubMed] [Google Scholar]
- 35.Hoeck JD, Jandke A, Blake SM, Nye E, Spencer-Dene B, Brandner S, Behrens A. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nature neuroscience. 2010;13:1365–1372. doi: 10.1038/nn.2644. [DOI] [PubMed] [Google Scholar]
- 36.Lander R, Nordin K, LaBonne C. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. The Journal of cell biology. 2011;194:17–25. doi: 10.1083/jcb.201012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klotz K, Cepeda D, Tan Y, Sun D, Sangfelt O, Spruck C. SCF(Fbxw7/hCdc4) targets cyclin E2 for ubiquitin-dependent proteolysis. Experimental cell research. 2009;315:1832–1839. doi: 10.1016/j.yexcr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Welcker M, Orian A, Grim JE, Eisenman RN, Clurman BE. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol. 2004;14:1852–1857. doi: 10.1016/j.cub.2004.09.083. [DOI] [PubMed] [Google Scholar]
- 39.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes & development. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvisi DF, Pinna F, Meloni F, Ladu S, Pellegrino R, Sini M, Daino L, Simile MM, De Miglio MR, Virdis P, Frau M, Tomasi ML, Seddaiu MA, Muroni MR, Feo F, Pascale RM. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer research. 2008;68:4192–4200. doi: 10.1158/0008-5472.CAN-07-6157. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. The Journal of biological chemistry. 2005;280:29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 43.Hamadmad SN, Hohl RJ. Erythropoietin stimulates cancer cell migration and activates RhoA protein through a mitogen-activated protein kinase/extracellular signal-regulated kinase-dependent mechanism. The Journal of pharmacology and experimental therapeutics. 2008;324:1227–1233. doi: 10.1124/jpet.107.129643. [DOI] [PubMed] [Google Scholar]


