Abstract
Hormone peptide tyrosine–tyrosine (PYY) is secreted into circulation from the gut L-endocrine cells in response to food intake, thus inducing satiation during interaction with its preferred receptor, Y2R. Clinical applications of systemically administered PYY for the purpose of reducing body weight were compromised as a result of the common side effect of visceral sickness. We describe here a novel approach of elevating PYY in saliva in mice, which, although reliably inducing strong anorexic responses, does not cause aversive reactions. The augmentation of salivary PYY activated forebrain areas known to mediate feeding, hunger, and satiation while minimally affecting brainstem chemoreceptor zones triggering nausea. By comparing neuronal pathways activated by systemic versus salivary PYY, we identified a metabolic circuit associated with Y2R-positive cells in the oral cavity and extending through brainstem nuclei into hypothalamic satiety centers. The discovery of this alternative circuit that regulates ingestive behavior without inducing taste aversion may open the possibility of a therapeutic application of PYY for the treatment of obesity via direct oral application.
Introduction
Two potent mechanisms that inhibit ingestive behavior are the induction of satiation and the negative modulation of palatability caused by taste aversion (TA) (Yamamoto, 2008). Several gastrointestinal peptides, acting as endocrine modulators of ingestive behavior, have been shown inducing both effects. The list of such peptides includes glucagon-like peptide-1 (GLP-1; Thiele et al., 1997), Exendin-4 (Kolterman et al., 2003), cholecystokinin (Deutsch and Hardy, 1977), and peptide tyrosine–tyrosine (PYY) (Halatchev and Cone, 2005).
PYY, a molecular mediator of satiation, is released predominantly from enteroendocrine L-cells of the distal intestine in response to food intake (FI). PYY exists in two forms, PYY1–36 and PYY3–36. The former is active on all Y receptors and is converted to PYY3–36 during cleavage by dipeptidase IV. PYY3–36 retains high affinity only for the Y2 receptor (Y2R) (Blomqvist and Herzog, 1997) and acts by reducing FI and adiposity. Although PYY3–36 systemic administration inhibits FI in rodents, monkeys, and humans (Batterham et al., 2002; Chelikani et al., 2005; Degen et al., 2005; Moran et al., 2005; Talsania et al., 2005), the exact mechanism by which it controls ingestive behavior has not been fully elucidated.
Evidence suggest that circulating PYY3–36 crosses the blood–brain barrier (Nonaka et al., 2003) and interacts with Y2Rs expressed in specific brain areas (Fetissov et al., 2004; Stanić et al., 2006). Conversely, there is evidence demonstrating that circulating PYY3–36 also inhibits FI through interaction with Y2Rs expressed in abdominal sensory branches of the vagus nerve (Koda et al., 2005). However, its role is not entirely clear because vagal denervation only blocks the anorexic response to peripheral administration of PYY3–36 in rats (Koda et al., 2005) but not in mice (Halatchev and Cone, 2005).
Adding more complexity to the physiological role of PYY3–36, we documented recently that PYY3–36 is also present in saliva (Acosta et al., 2011). Although its innate physiological functions have yet to be determined, we have shown that salivary PYY3–36 can modulate FI and body weight (BW) accumulation. The anorexigenic effect is likely mediated through the activation of Y2R-positive (Y2R+) cells in the oral mucosa. These data led us to hypothesize that a putative neuronal circuit, initiated in the oral cavity, extends through the brainstem to hypothalamic areas, via cranial nerve afferents. If such a pathway exists, it would have to relay the information through the brainstem. Incidentally, neurons in the area postrema (AP) of the brainstem are known to partially mediate TA in response to the PYY3–36 administered peripherally (Halatchev and Cone, 2005) or by intranasal spray (Gantz et al., 2007).
The purpose of the current investigation was to investigate the mechanism by which salivary PYY3–36 inhibits ingestive behavior. First, we tested whether the reduction in FI after salivary PYY3–36 augmentation was related to aversive behavioral responses. Moreover, by comparing neuronal pathways engaged by systemic versus salivary PYY3–36, we aimed to identify neurons in the AP, nucleus of the solitary tract (NST), parabrachial nucleus (PBN), and hypothalamic areas that are activated in response to the orally applied PYY3–36.
Materials and Methods
Animals
Male wild-type C57BL/6 and PYY knock-out (KO) mice (PYY−/−) (Boey et al., 2006) weighing 20–25 g were housed individually in hanging wire-mesh cages in a room with 12 h light/dark cycle. Animals were provided ad libitum access to regular mice chow and water except as otherwise noted below (see Experimental procedures). The Institutional Animal Care and Use Committee of the University of Florida approved all experimental procedures.
Test substances
The TA experiment was performed using PYY3–36 from Bachem and LiCl from Alfa Aesar. For the intraperitoneal injections, the solution was injected into the peritoneal cavity at a dose of 6 μg/100 g BW. For the oral spay treatment, PYY3–36 was diluted at the concentrations of 0.075, 0.15, or 0.225 mg/ml. The solutions were administered into the oral cavity at the doses of 6, 12, or 18 μg/100 g BW. LiCl was dissolved in sterile distilled water to a final concentration of 0.15 m; mice were injected intraperitoneally with a volume equivalent to 2% of their BW. For the conditioned TA (CTA) experiment with liquid, flavored solutions were made of diluted Kool-Aid (either 0.15% saccharine with 0.05% cherry Kool-Aid or 0.15% saccharine with 0.05% grape Kool-Aid). For the CTA with solid food, we used flavored apple and orange Crunchies (BetterPets).
Experimental procedures
Treatment with orally administered substances
With the exception of 125I-PYY, the oral treatment described herein refers to the substances administered by an oral spray (OS) targeting receptors in the oral cavity. The solutions were administrated into the oral cavity in a single puff, as described previously (Acosta et al., 2011), using a sterile -inch dram (8 × 58 mm) glass sampler bottle (each puff delivers ∼30 μl of solution to the oral cavity in a harmless manner).
In vivo treatment of mice with 125I-PYY1–36
PYY KO mice were deeply anesthetized, and 125I-labeled human PYY1–36 (Phoenix Pharmaceuticals) was administered into the oral cavity by micropipette at the dose of 7 μCi/100 g BW (equivalent to 18 μg/100 g BW) in a total volume of 155 μl, or intraperitoneally injected BIIE0246 N-[(1S)-4-[(Aminoiminomethyl)amino]-1-[[[2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]]amino]carbonyl]butyl]-1-[2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl]-cyclopentaneacetamide antagonist was applied at 50 m excess as described previously (Acosta et al., 2011). Five minutes after oral administration, mice were killed; tongue tissues were harvested and extensively washed in several changes of PBS until no above-the-background radioactivity was detected by Geiger counter. After systemic administration, the animal was killed 15 min after injection, and the tongue was treated as described above. Sagittal sections of the fresh-frozen tongue tissues were exposed to a one-sided x-ray film (Kodak BioMax MR) for 3 d at −20°C. After exposure, sections were stained with hematoxylin (Merck) and eosin (Sigma) (H&E). To investigate histological localization of 125I-labeled human PYY1–36 within the tongue epithelium, the slides were examined by polarized light microscopy.
Conditioning procedure preceding immunohistochemistry
To test whether salivary PYY augmentation activates neurons in the brainstem and hypothalamic nuclei, groups of mice were conditioned for repeated cycles of fasting for 24 h, followed by refeeding. A treatment combination of intraperitoneal injections and OS administration was incorporated into the conditioning protocol so that all mice in all control and experimental groups were subjected to the same combination of OS/intraperitoneal injections. Mice in control fasted groups were fasted over the duration of the experiment. All mice were killed at 1 h after the treatment. Brains were harvested and neuronal activity was evaluated by probing the induction of c-fos, phosphorylated extracellular signal-regulated kinase (p-ERK) expression, or other neuronal markers as described below.
c-Fos immunohistochemistry
All mice were subjected to the conditioning procedure described above. LiCl was used as a positive control treatment. PYY3–36 injected intraperitoneally was used as another positive control characterized previously to induce CTA (Halatchev and Cone, 2005; Chelikani et al., 2006). Five groups of mice (n = 4 each) were fasted for 24 h: (1) negative control (see Fig. 3, group “Fast”); (2) positive control, fed for 1 h and killed 1 h later (see Fig. 3, group “Fed”); (3) positive CTA group, injected with LiCl and killed 1 h later; (4) positive CTA group, administered PYY3–36 intraperitoneally (6 μg/100 g BW; see Fig. 3, group “PYY i.p.”) and killed 1 h after the treatment; and (5) experimental group, administered PYY3–36 OS (6 μg/100 g BW; see Fig. 3, group “PYY OS”) and killed 1 h after the treatment.
Figure 3.
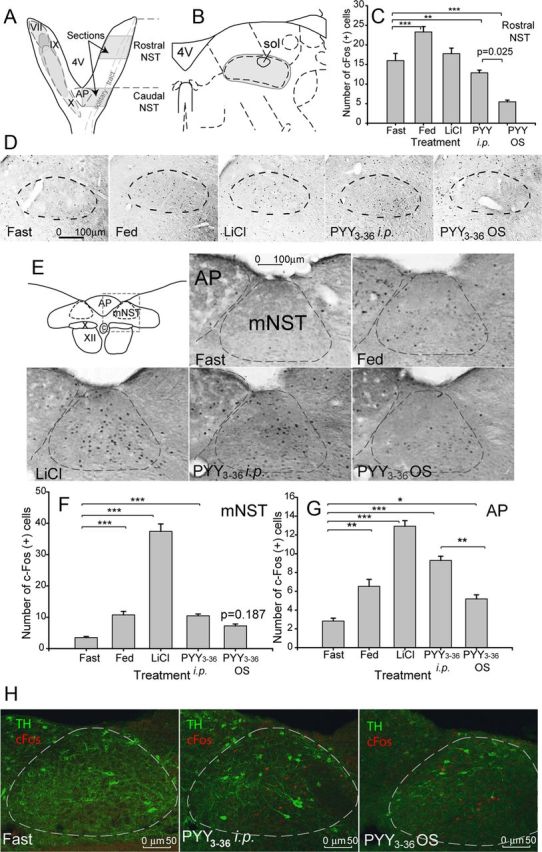
Effect of PYY3–36 OS on c-fos expression in the brainstem. A, Diagram of the horizontal representation of the NST (nucleus of solitary track) in the mouse. Filled ovals indicate the overlapping termination patterns of the facial nerve (VII), the linguotonsilar branch of the glossopharyngeal nerve (IX), and the superior laryngeal branch of the vagus nerve (X). Shaded areas on the right aspect indicate the sectioned areas in the rNST and AP; sections were collected bilaterally. 4V, Fourth ventricle. B, Diagram of the coronal representation of the medial rostral area of the solitary tract. sol, Solitary tract. Filled oval indicates tabulated area. C, Tabulated values expressed as average number of c-Fos+ cells per section (n = 4). D, Shown are representative photomicrographs of the c-fos activity in the rostral part of the solitary tract in mice treated as indicated on the respective panels. E, Diagram of the coronal representation of the intermediate area of the solitary tract and representative photomicrographs of the c-fos activity. ST, Solitary tract; C, central canal; X, dorsal motor nucleus of the vagus; XII, hypoglossal nucleus. Dashed rectangle designates the areas shown as photomicrographs; dashed ovals designate areas included in the c-Fos staining count. F, Tabulated values expressed as average number of c-Fos+ cells per section in the mNST (n = 4). G, Tabulated values expressed as average number of c-Fos+ cells per section in the AP. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. H, Representative photomicrographs of the c-fos activity in mNST catecholaminergic (TH) neurons.
To collect brains, a previously described protocol was followed (Gorbatyuk et al., 2001). Briefly, mice were deeply anesthetized with sodium pentobarbital and perfused sequentially through the ascending aorta with the following: (1) 20 ml of heparinized saline; and (2) 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 (PB). The brains were postfixed in the same fixative for 4 h and then immersed in 30% sucrose in 0.1 m PB at 4°C. A series of 40-μm-thick coronal sections were cut through the rostrocaudal extent on a cryostat (Leica CM3050 S) and collected in anti-freezing storage solution.
For the bright-field photomicrographs, sections were preincubated first with 0.5% H2O2–10% methanol for 15 min and then with 5% normal goat serum for 1 h. Sections were incubated for 36 h at 4°C with anti-c-Fos primary antibody (1:2000; Santa Cruz Biotechnology). Incubation with secondary goat anti-rabbit biotinylated antibody (1:400, for 4 h) was followed by incubation with avidin–biotin–peroxidase complex (ABC; Vector Laboratories). Reactions were visualized using 3,3-diaminobenzidine as a chromagen. c-Fos+ cells on the representative microphotographs were counted by operators blind to identity of the group.
p-ERK and vasopressin double immunofluorescence
Sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were double stained with anti-phospho-p44/42 MAP kinase (p-ERK) (Cell Signaling Technology) and vasopressin [anti-arginine–vasopressin (AVP) antibody; Millipore Bioscience Research Reagents]. Brains were collected and postfixed as described above. Series of 25-μm-thick coronal sections were cut through the rostrocaudal extent and mounted onto Fisher Superfrost Plus slides. p-ERK immunolocalization was conducted using the tyramide signaling amplification (TSA) kit (PerkinElmer Life and Analytical Sciences), whereas vasopressin was detected through the standard protocol. Tissues were blocked in 0.3% H2O2 in Tris-buffered saline for 30 min at room temperature (RT) to eliminate endogenous peroxidase activity, followed by blocking with 10% donkey serum in TNT (0.1 m Tris HCl, pH 7.5, 0.15 m NaCl, and 0.05% Tween 20) for 60 min at RT to reduce nonspecific antibody binding. Sections were then incubated simultaneously with the two primary antibodies: rabbit anti-p-ERK (1:2000) and guinea pig anti-vasopressin (1:1000) in TNT for 72 h at 4°C. Tissues were subsequently incubated with secondary goat anti-rabbit IgG (Fab′)2 conjugated to horseradish peroxidase (1:1000 for 60 min at RT; Abcam), followed by incubation with fluorescein provided in the TSA kit (1:300, for 7 min at RT; PerkinElmer Life and Analytical Sciences) to detect p-ERK. To detect vasopressin, the slides were subsequently incubated with Cy5 donkey anti-guinea pig IgG (1:100 in TNT for 60 min at RT; Jackson ImmunoResearch).
p-ERK and α-melanocyte-stimulating hormone double-immunofluorescence
Sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were double stained with anti-p-ERK and anti-α-melanocyte-stimulating hormone (α-MSH) (Millipore Bioscience Research Reagents). The protocol was identical to the one described above; anti-α-MSH was used at a 1:2000 concentration. To detect α-MSH, the slides were incubated with Alexa Fluor 555 donkey anti-sheep IgG (1:1000; Invitrogen).
c-Fos and tyrosine hydroxylase double immunohistochemistry
Sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were double stained with anti-c-Fos (1:1000; Millipore) and anti-tyrosine hydroxylase (TH) antibody (1:500; Cell Signaling Technology).
c-Fos and GLP-1 double immunohistochemistry
Sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were double stained with anti-c-Fos (1:1000; Santa Cruz Biotechnology) and anti-GLP-1 antibody (1:1000; Peninsula Lab).
c-Fos and oxytocin double immunohistochemistry
Sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were double stained with anti-c-Fos (1:1000; Santa Cruz Technology) and anti-oxytocin (OXT; 1:1000; Abcam).
CTA behavioral studies
Both oral (OS) and systemic (intraperitoneally) PYY3–36 were administered at identical matching doses (6 μg/100 g BW) because it had been shown to reliably and reproducibly inhibit FI by either OS (Acosta et al., 2011) or intraperitoneally (Halatchev and Cone, 2005). Two complementing paradigms were used to study the induction of CTA by PYY3–36: (1) one with liquid and (2) one with solid food. Both protocols were performed as described previously (Halatchev and Cone, 2005; Chelikani et al., 2006). Mice were habituated to individual housing 2 weeks before the experiments. All subsequent procedures were conducted in the animal's home cages from 7:00 P.M. and 9:00 P.M. (dark period from 7:00 P.M. to 7:00 A.M.). Animals had ad libitum access to regular rodent chow at all times. Water was withdrawn 23 h before the start of the first day of training. Mice had access to water or the flavored solutions every day for 1 h.
Liquid paradigm
Habituation procedure.
To habituate to timing of liquid presentation, mice were conditioned to consume water during a 5 d training period (Table 1, open cells). After 23 h of liquid deprivation, water was offered for 1 h (7:00 P.M. to 8:00 P.M.) in two different bottles that were situated equidistant from the food hopper. The amount consumed was determined by weighing the bottles before and after each training session. To habituate mice to intraperitoneal injections and the OS, after each training session, animals were injected intraperitoneally with a volume of sterile isotonic saline solution (SS) equal to 2% of BW, and, at the same time, water was administered to the oral cavity via an OS.
Table 1.
Schematic timeline of the CTA trials with liquid (roman font) or solid food (bold)
| Days | Habituation 1–5 | Conditioning |
Trials 18 | |||
|---|---|---|---|---|---|---|
| 6, 10, 14 | 7, 11, 15 | 8, 12, 16 | 9, 13, 17 | |||
| Bottle content | Water | Flavor 1 in both bottles | Water | Flavor 2 in both bottles | Water | Flavors 1 or 2 in separate bottles |
| Regimen | Water OS + SS, i.p. | One of 4 treatment regimens | Water OS + SS, i.p. | Water OS + SS, i.p. | Water OS + SS, i.p. | None |
| Rack content | Regular chow | Flavor 1 in both trays | Regular chow | Flavor 2 in both trays | Regular chow | Flavors 1 or 2 in each tray |
| Regimen | Water OS + SS, i.p. | One of 4 treatment regimens | Water OS + SS, i.p. | Water OS + SS, i.p. | Water OS + SS, i.p. | None |
Conditioning procedure.
Immediately after training, animals were subjected to a 12 d conditioning procedure consisting of the following three 4 d sequences (Table 1). The animals were assigned at random to either of two flavor conditions and were subjected to a regimen of OS and intraperitoneal injections. During the same 1 h period (7:00 P.M. to 8:00 P.M.) on day 1, mice were offered a novel flavored liquid in both bottles (that could be either grape or cherry Kool-Aid prepared as described previously; Chelikani et al., 2005), followed by one of the following treatment regimens: (1) PYY3–36 OS at doses indicated, accompanied by an intraperitoneal injection of SS; (2) water OS and PYY3–36 intraperitoneally; (3) water OS and LiCl intraperitoneally; or (4) water OS and SS solution intraperitoneally. On day 2, all the mice received water for 1 h to allow for recovery from the treatment regimen. On day 3, each mouse received the alternative novel flavor (i.e., if an animal received grape during the first day, it received cherry in the third and vice versa), and after 1 h, all mice received an SS injection intraperitoneally and water OS. On day 4, they again received water during the 1 h period. This 4 d sequence was repeated three times for a total of three conditioning trials over 12 consecutive days.
Preference trials.
On 2 consecutive days immediately after the conditioning period, each mouse was given simultaneous access to the two flavored solutions, and the amount consumed of each stimulus was measured. For each mouse, the left–right position of the bottles containing the two flavored solutions was reversed during the second day. Water was presented as flavored solution in two separate bottles equidistant from the food. Treatment consisted of one of the following regimens: (1) PYY3–36 OS at different doses and SS intraperitoneally; (2) water OS and PYY3–36 intraperitoneally; (3) water OS and LiCl intraperitoneally; or (4) water OS and SS intraperitoneally.
Solid food paradigm (Table 1, shaded cells)
For the CTA experiment with solid food, procedures were the same as those described above, but flavored Crunchies were used instead flavored solutions. Mice were fasted for 23 h instead of being water deprived for 23 h.
Regular chow or flavored Crunchies were presented in two separate trays equidistant from water. Treatment consisted of one of the following regimens: (1) PYY3–36 OS at different doses and SS intraperitoneally; (2) water OS and PYY3–36 intraperitoneally; (3) water OS and LiCl intraperitoneally; or (4) water OS and SS intraperitoneally.
Statistics
Statistical analyses were performed using IBM SPSS Statistics version 17 software. Data are expressed as group means ± SEM. For the TA experiments, significance across individual treatments was determined using one-way ANOVA with Dunnett's post hoc test when three or more groups were compared. Unpaired Student's (two-tailed) t test was used to determine the significance when two groups were compared. For c-Fos activation experiments, one-way ANOVA with Dunnett's test post hoc was used for comparing different treatments with the fasting control group, followed by one-way ANOVA with both Fisher's least significant difference and Tukey's post hoc tests to determine significance across individual treatments. Tukey's test was used to control type I error rate resulting from multiple pairwise comparisons. The statistical rejection criterion was set at p ≤ 0.05.
Results
Salivary PYY3–36 does not induce CTA
PYY3–36 administered systemically had been shown to reduce FI (Batterham et al., 2002) while at the same time inducing CTA (Halatchev and Cone, 2005). The latter manifestation is apparently related to the activation of neurons in the AP brainstem area mediating, in part, aversive reactions (Halatchev and Cone, 2005). To test whether anorexic doses of salivary PYY3–36 induce CTA in a similar manner, we conducted the following behavioral study.
Inducing CTA with flavored liquid
PYY3–36 OS at doses that reliably and reproducibly inhibit FI (6 μg/100 g BW) (Acosta et al., 2011) did not produce TA in mice, whereas PYY3–36 administered intraperitoneally at the same dose did (Fig. 1A). Negative controls that received saline intraperitoneally and water OS paired to both flavors did not show any preference for either of the flavors and drank equal amounts in response to both stimuli. Conversely, positive controls that were treated with LiCl showed a significant reduction of treatment–paired flavor, with the difference of consumption between the two flavors reaching 75% (t(14) = −6.6, p < 0.001). Mice that received PYY3–36 intraperitoneally (6 μg/100 g BW) drank 65% less of the PYY3–36 intraperitoneally paired flavor versus the saline paired flavor (t(14) = −5.1, p < 0.001). PYY3–36 OS-treated mice drank equal amounts from the two flavors (t(14) = 0.0, p = 0.984).
Figure 1.
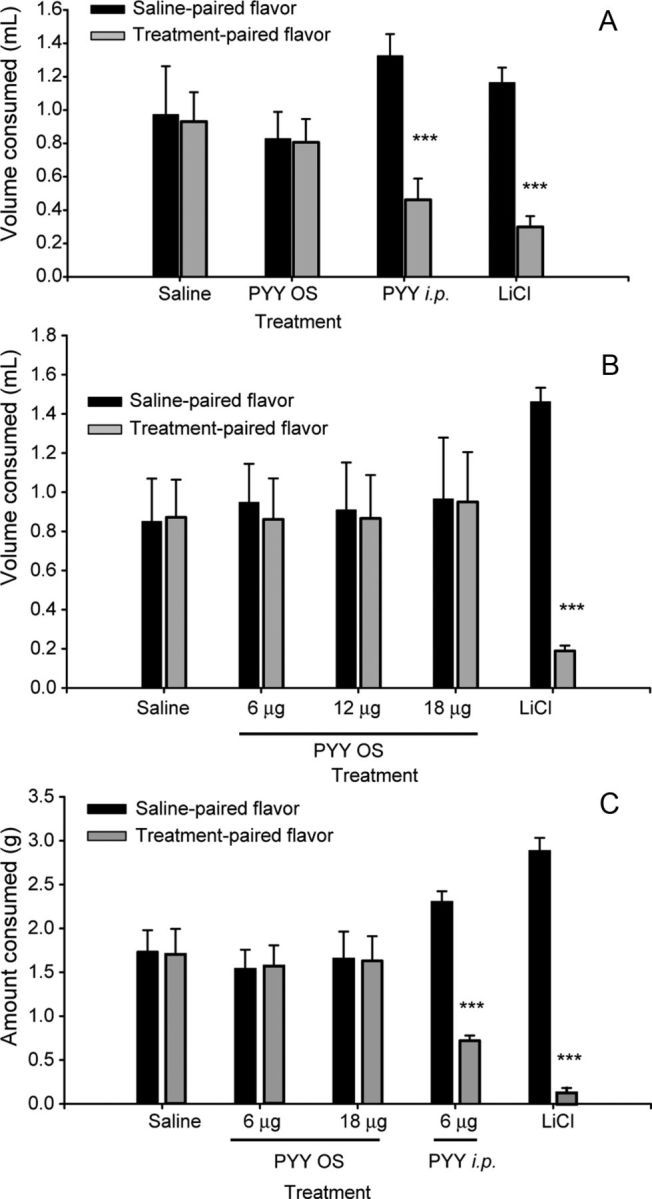
Effect of PYY3–36 treatment on aversive response. Individual flavor consumption: saline paired flavor versus treatment paired flavor. A, B, Liquid paradigm (n = 8 each group). C, Solid food paradigm (n = 8 each group). For details, see Results. *p ≤ 0.05 **p ≤ 0.01, ***p ≤ 0.001.
Previously, we showed that higher doses of PYY3–36 applied orally for 21 consecutive days results in a significant reduction of BW accumulation in mice (Acosta et al., 2011). Therefore, to exclude the possibility of mounting CTA at higher doses, the above experiment was repeated using PYY3–36 OS at 12 and 18 μg/100 g BW. Likewise, neither of these doses resulted in preference or aversion for any of the flavors (Fig. 1B), with the LiCl control group consistently showing a reduction of the paired flavor consumption (t(14) = −16.5, p < 0.001).
Inducing CTA with flavored solid food
To corroborate these data and to model a potential therapeutic application scenario, we repeated the behavioral experiment using flavored solid food. Importantly, using PYY3–36 OS at 6 or 18 μg/100 g BW treatment doses, we observed similar results to the one with the liquid paradigm (Fig. 1C). PYY3–36 OS paired flavors had no effect on the amount of food consumed, although there was a significant difference apparent for the PYY3–36 intraperitoneally treated group (t(14) = −1.4, p < 0.001) and even more so for the LiCl control group (t(14) = −16.6, p < 0.001). Thus, the augmentation of salivary PYY3–36 at a dose that reliably inhibits FI, or even at a threefold higher dose, does not induce CTA.
Salivary PYY3–36 binds to lingual Y2R receptors
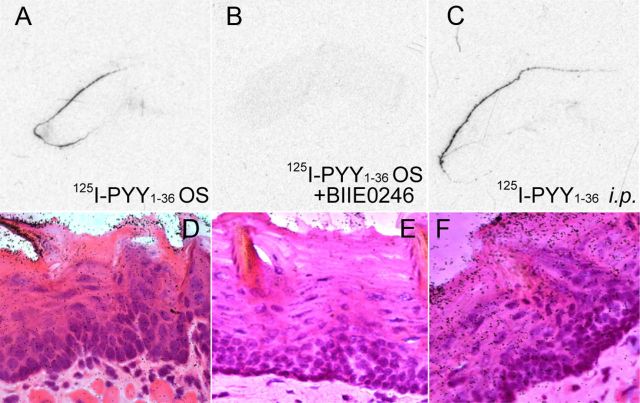
We have demonstrated that orally applied PYY3–36 does not enter into circulation, whereas circulating PYY3–36 efficiently transudates from plasma into saliva (Acosta et al., 2011). Thus, the observed anorexic effect of salivary PYY3–36 has to be related to the effector interacting locally with the cognate receptors expressed in the oral mucosa (Acosta et al., 2011; Hurtado et al., 2012). To determine whether salivary PYY binds to Y2R expressed in lingual epithelia cells, we used 125I-labeled PYY1–36 administered into the oral cavity of PYY KO mice. These mice were used to exclude a possible competition of endogenous salivary PYY with the radiolabeled ligand. Five minutes after treatment, radiolabeled PYY was found to be bound to both dorsal and ventral tongue surface epithelia (Fig. 2A,D). When labeled PYY was mixed with the Y2R-specific antagonist BIIE0246 (Doods et al., 1999), binding was abolished, thus not only providing additional support for the specificity of the interaction but also identifying the Y2R as the major interaction site (Fig. 2B,E). Moreover, when radiolabeled PYY was administered intraperitoneally, the lingual epithelial binding of 125I-PYY was robust as soon as 15 min after injection (Fig. 2C,F), confirming our recent finding that systemic PYY is efficiently transported into saliva (Acosta et al., 2011). H&E staining of the sections (Fig. 2D,F) showed no preference of the peptide distribution whereupon silver granules could be seen localizing in all layers, including superficial dorsal keratinocytes as well as in basal epithelial progenitor cells known to express Y2R (Hurtado et al., 2012).
Figure 2.
Salivary PYY binds to Y2Rs in the tongue epithelia. A, D, Representative images of a sagittal section of the murine tongue subjected to 125I-PYY binding applied orally in vivo. B, E, Images of the tongue from a mouse in which 125I-PYY was coadministered with the Y2R-specific antagonist BIIE0246. C, F, Images of the tongue from a mouse in which 125I-PYY was injected intraperitoneally. D–F, Micrographs of histological sections of the tongue epithelium with H&E staining; silver granules (black dots) colocalize with 125I-PYY.
Effect of salivary PYY3–36 on brainstem neurons
To investigate whether the postulated interaction of salivary PYY3–36 and its cognate receptors induces afferent neuronal pathways, we studied the patterns of c-fos activation in the NST, AP, and PBN.
The data of the current report have to be interpreted with the following notions in mind. On one hand, we showed previously that PYY3–36 administered peripherally is transported into the oral cavity from the bloodstream (Acosta et al., 2011), a fact now supported by the direct experiment with 125I-PYY (Fig. 2C). Thus, in the PYY intraperitoneally injected positive controls used in this study, PYY3–36 would activate hypothalamic neurons as characterized previously (Batterham et al., 2002; Halatchev and Cone, 2005), and, during diffusion into the oral cavity, it will also affect a putative pathway that originates in the oral mucosa. Conversely, PYY3–36, applied by OS, does not leak retrogradely into the bloodstream (Acosta et al., 2011). As a result, it would not affect neurons targeted by circulating PYY, while, nonetheless, activating oral Y2R cells and putative afferent pathways.
The NST has two major functional divisions: (1) an anterior–lateral oral–gustatory half [hereafter referred to as “rostral” NST (rNST)]; and (2) a posterior–medial visceral afferent half [“caudal” NST (cNST)] (Hamilton and Norgren, 1984; Travers and Norgren, 1995). In the AP, known to mediate aversive response, the different activation patterns induced by salivary versus systemic PYY would also help to explain the lack of CTA after OS PYY application. Therefore, three areas were analyzed separately: (1) the rNST; (2) the cNST; and (3) the AP (Fig. 3A, shaded areas unilaterally shown on the right aspect of the solitary tract). For this experiment, both the OS and intraperitoneal groups were treated with the identical doses of PYY3–36 (6 μg/100 g BW) that were identified previously to reliably reduce FI (Batterham et al., 2002; Acosta et al., 2011). An additional group of control mice were injected intraperitoneally with LiCl to induce visceral malaise.
rNST
In the rostral subdivision, all subnuclei (medial, rostrocentral, rostrolateral, and ventral; Corson et al., 2012) showed similar response trends; therefore, we combined c-Fos+ neurons in these areas during tabulation (Fig. 3B, the respective shaded area, D, dashed oval in the brain sections). Neuronal activation differed significantly across the five groups (F(4,121) = 60.9, p < 0.001). Tukey's post hoc comparisons of these five groups indicate that PYY OS-treated groups showed a significant reduction in the numbers of c-Fos+ neurons compared with both fasting (p = 0.001) and PYY intraperitoneally (p = 0.025; Fig. 3C) groups. Animals in the fed group responded by activating neurons, whereas there was no significant effect in the rNST neurons in the LiCl-treated group (p = 0.865).
cNST
The cNST is known to relay satiation signals from the alimentary tract to the hypothalamus (Hamilton and Norgren, 1984). In the caudal aspect, we studied the NST at the level of the AP (Fig. 3A, shaded area). Within this region, we tabulated c-Fos+ neurons within the medial NST (mNST; Fig. 3E, outlined area) in which we found only few c-Fos+ cells in fasted animals. Neuronal activation differed significantly across groups (F(4,251) = 129, p < 0.001). However, Tukey's post hoc comparison of these groups showed that, unlike the rostral part, the cNST responded to LiCl treatment in a very robust manner compared with fasted mice (p < 0.01). In addition, both fed and PYY3–36 intraperitoneal control groups showed significant increases in c-fos expression compared with fasted mice as well (p < 0.01 in both cases), whereas there was no response in the OS group (p = 0.187; Fig. 3F).
AP
In the AP, all four groups showed a significant activation of c-Fos in neurons when pairwise compared with the fasted group (Fig. 3E,G; F(4,192) = 36.7, p < 0.001). Similar to the cNST area, the PYY3–36 OS-treated group showed the least neuronal activation (p = 0.026), and this was significantly less than in the PYY3–36 intraperitoneally treated group (p = 0.003) but not different from the fed group (p = 0.501).
Overall, neurons in both the rostral and caudal brainstem clearly responded in distinctive ways to the PYY treatment depending on the administration route. For example, rostral neurons in the OS group showed a significantly lower degree of activation compared with the intraperitoneal group. At the same time, caudal mNST neurons were either not activated in the OS group or showed a significantly lower degree of activation in the AP. Such a differential pattern could reflect the distinctive mechanisms of PYY3–36 action: humoral via circumventricular organs when administered systemically versus neuronal if applied by OS.
PBN
The PBN is a major relay for gustatory and visceral information and mediates anorexia associated with malaise induced by intraperitoneal injection of LiCl (Rinaman and Dzmura, 2007). Lesions of the PBN prevent the acquisition of CTA and conditioned taste preferences (Reilly and Trifunovic, 2000; Trifunovic and Reilly, 2002). Direct injections of cannabinoid or μ-opioid receptor agonists into the PBN stimulate feeding (DiPatrizio and Simansky, 2008). Thus, the PBN can process both aversive and appetitive ascending visceral information to modulate feeding behavior.
The PBN consists of nine cytoarchitectonically and functionally different subnuclei (Fulwiler and Saper, 1984; Hashimoto et al., 2009). For the purpose of quantifying the effect of the treatment, we combined several subnuclei into medial (mPBN) and lateral (lPBN) subdivisions. This was attributable to the fact that the PBN nuclei are physically divided into medial and lateral subdivisions by fibers of the superior cerebellar peduncle, and, in some cases, it was difficult to delineate the cytoarchitectonic boundaries for these particular subdivisions of subnuclei. The segregation of taste and visceral projections to the PBN is not as clear as in the NST (Hermann et al., 1983). Nevertheless, visceral afferent projections arising from the cNST terminate primarily in nuclei of the lPBN, whereas taste-responsive neurons are found predominantly in the mPBN (Karimnamazi and Travers, 1998).
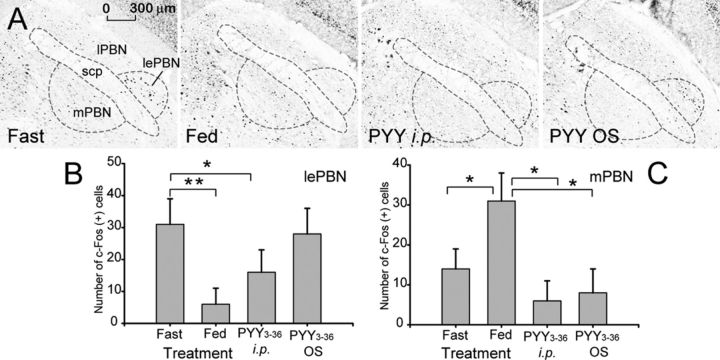
lPBN
During fasting, a well defined group of c-Fos+ neurons can be identified in the area (Fig. 4A) corresponding to the lateral external PBN (lePBN). It is established that neurons in these subnuclei receive projections from AP (Cunningham et al., 1994) and send projections to the lateral hypothalamic area (LHA) and the amygdala (Amy) (Tokita et al., 2010). In fed mice, as well as PYY intraperitoneally, the number of c-Fos+ neurons in lePBN was significantly reduced (p = 0.006 and p = 0.025, respectively), whereas in PYY OS group, there was no significant change (Fig. 4A,B; p = 0.884).
Figure 4.
Effect of PYY3–36 OS on c-fos expression in the PBN of the brainstem. A, Shown are representative photomicrographs of the c-fos activity in the PBN in mice treated as indicated on the respective panels. scp, Superior cerebellar peduncle. B, Tabulated values expressed as average number of c-Fos+ cells per section in the lePBN (n = 4). C, Tabulated values expressed as average number of c-Fos+ cells per section in the mPBN. *p ≤ 0.05, **p ≤ 0.01.
mPBN
During fasting, there were few c-Fos+ neurons in the mPBN, but their number increased approximately twofold after feeding (p = 0.009). Treatment with PYY, both intraperitoneally and OS, reduced the number of c-Fos-activated neurons compared with both fed and fasted mice, albeit not significantly so when compared with the latter group (Fig. 4A,C; p = 0.086 and p = 0.114, respectively).
Effects of systemic and salivary PYY3–36 on the expression of c-Fos in catecholaminergic neurons in the NST and AP
To characterize the chemical nature of the PYY-activated neurons, we used TH as a catecholaminergic neuronal marker. In the AP, as well as in the mNST, there were few if any TH-immunoreactive (IR) neurons that were c-Fos+, and neither intraperitoneal nor OS PYY3–36 treatment had any effect (Fig. 3H). However, both types of PYY treatment induced c-Fos in a subpopulation of TH-IR large neurons localized at the mNST ventral–lateral edge at the level of and rostral to the AP level (7.56–7.2 mm caudal of bregma; Paxinos and Franklin, 2001; Fig. 5F,I). NST TH+ neurons contribute into a major ascending pathway through which vagal sensory information reaches the hypothalamus. These neurons play a key role in the integration of visceral satiety signals and may be involved in mediating brainstem satiation circuits to the hypothalamus. However, the majority of the activated c-Fos+ neurons were shown to be noncatecholaminergic, a finding consistent with previously published observations (Blevins et al., 2008).
Figure 5.
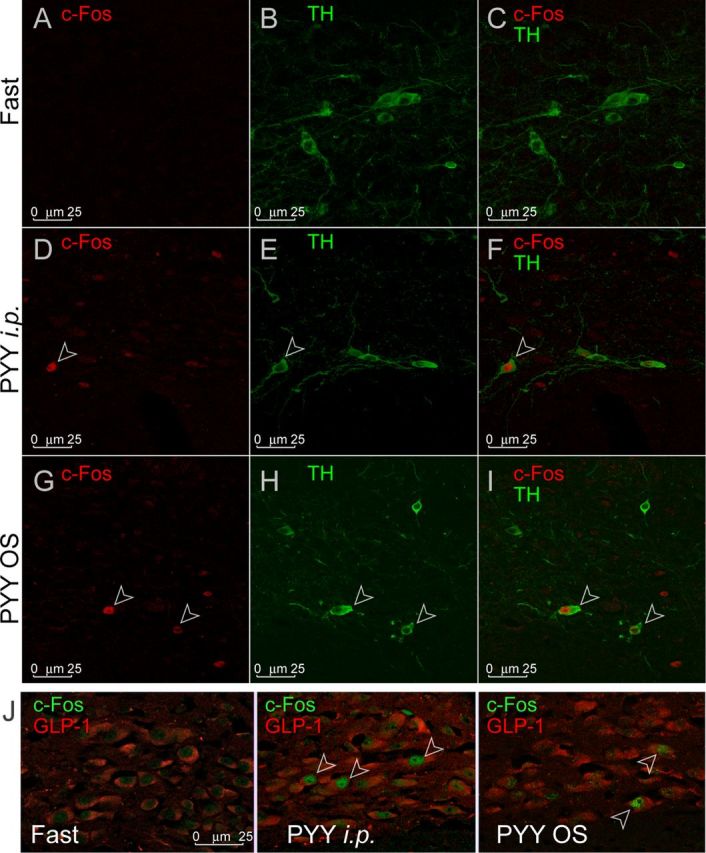
c-Fos activity in NST catecholaminergic (TH) and GLPergic neurons. A, D, and G show individual channels for c-Fos. B, E, and H show individual channels for TH. C, F, and I show combined channels. Open arrowheads indicate neurons positive for both c-Fos and TH. J shows c-Fos expression (green) in the GLP-1-IR neurons (red) in the mNST under treatment conditions indicated in each panel. Open arrowheads indicate neurons positive for both c-Fos and GLP-1.
Effect of PYY3–36 treatment on the expression of c-Fos in GLP-1 neurons in the mNST
GLP-1 neurons process pre-pro-glucagon into GLP-1, GLP-2, oxyntomodulin, and glicentin. These neurons are subject to leptin signaling (Vrang et al., 2008) and project mainly to the hypothalamic nuclei involved in appetite regulation: paraventricular nucleus (PVN), dorsomedial hypothalamus (Vrang et al., 2007), and arcuate nucleus (Arc; Llewellyn-Smith et al., 2011). To establish the possible involvement of GLP-1-signaling in response to PYY3–36 treatment, we conducted double immunohistochemistry for GLP-1 and c-Fos in mNST neurons localized ventrally of the caudal aspect of the AP. Both intraperitoneal and OS PYY3–36 treatment induced c-Fos in a subpopulation of GLP-1-IR neurons (Fig. 5J).
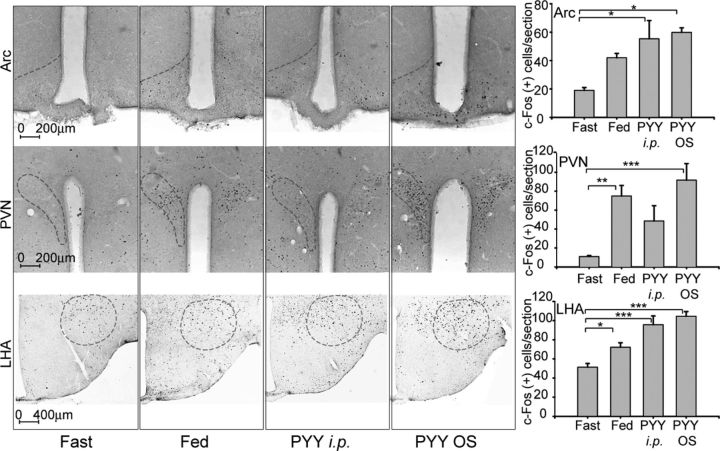
Salivary PYY3–36 activates hypothalamic c-Fos
The mechanism of the anorexigenic action of peripherally applied PYY3–36 could be related to its action on hypothalamic neurons (Batterham et al., 2002). Alternatively, peripheral PYY3–36 may inhibit FI by signaling through Y2R expressed in the vagus nerve (Koda et al., 2005). Both pathways have been shown to activate neurons in the hypothalamus. To test whether salivary PYY augmentation activates hypothalamic centers, four groups of mice were conditioned as described in Materials and Methods. There were few c-Fos+ cells in fasted animals in Arc, PVN, and LHA and their numbers were markedly induced in all areas after feeding (Fig. 6). All these activated areas are known to mediate both satiety and hunger. Data in this report are consistent with previously published findings describing activation of neurons in hypothalamic areas in anticipatory response to feeding in habituated animals (Johnstone et al., 2006). Similar to the mice from the fed control group, orally treated and intraperitoneally injected PYY3–36 groups showed activation of neurons in Arc (Fig. 6, top row; F(3,20) = 33.8, p = 0.02), PVN (Fig. 6, middle row; F(3,12) = 10.1, p = 0.001), and LHA (Fig. 6, bottom row; F(3,21) = 25.6, p < 0.001). Although PYY3–36 intraperitoneally injected mice displayed an increase in a number of c-Fos+ PVN neurons, this did not reach statistical significance after running Tukey's post hoc analysis (p = 0.13).
Figure 6.
Effect of PYY3–36 OS on c-fos expression in the Arc (top row), PVN (middle row), and LHA (bottom row). Dash ovals indicate areas included in the tabulations. Panels in the rightmost column show tabulated values expressed as average number of c-Fos+ cells per section (n = 4 mice per group). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Salivary PYY3–36 activates hypothalamic p-ERK1/2
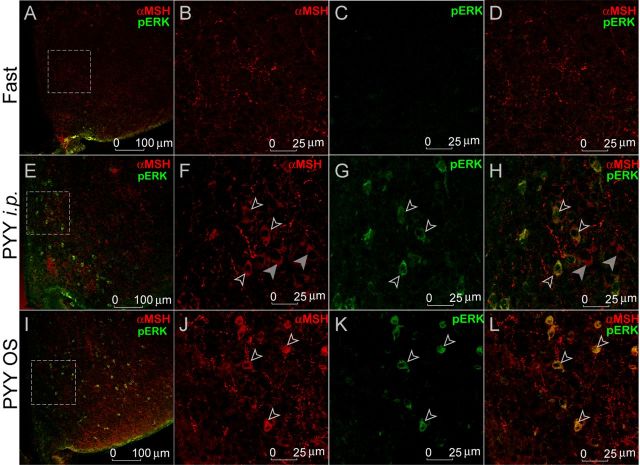
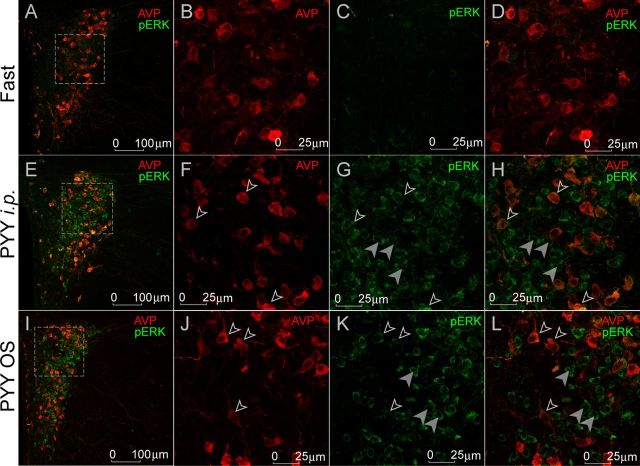
Another morphological marker of the neuronal activation is ERK1/2, also known as p44/42 mitogen-activated protein kinase (Xu et al., 2002; Belgardt and Brüning, 2010). After phosphorylation, activated p-ERK1/2 can initiate additional downstream signaling, in particular, an increase in c-Fos biosynthesis, including the upregulation of its gene transcription and protein phosphorylation-mediated stabilization (Gorbatyuk et al., 2001; Glauser and Schlegel, 2007; Aoyagi et al., 2009). There is also an association of p-ERK1/2 signaling and the expression of pro-opiomelanocortin (POMC), the precursor of α-MSH (Jenks, 2009; Kuribara et al., 2011), a strong anorexic central mediator of FI and increased metabolic activity (Morton et al., 2006). To validate c-Fos-related data and to establish the morphological identity of the activated neurons, we conducted a double immunostaining with p-ERK1/2 and α-MSH in the Arc or AVP in the PVN.
For this experiment, brain sections from fasted, PYY3–36 intraperitoneally, and PYY3–36 OS-treated mice were analyzed. The pattern of p-ERK1/2 activation followed the same trend as the pattern of c-Fos expression in the Arc and PVN: p-ERK expression level was higher in PYY3–36 intraperitoneally and PYY3–36 OS-treated mice compared with fasted mice (Fig. 7C,G,K in the Arc; Fig. 8C,G,K in the PVN). A majority of α-MSH+ cells were also p-ERK1/2+ (Fig. 7H,L, open arrowheads), indicative of their involvement in anorexigenic pathways. In the PVN, both intraperitoneal and OS PYY3–36 treatments activated AVP+ neurons (Fig. 8H,L, open arrowheads).
Figure 7.
Effect of PYY3–36 treatment on p-ERK1/2 activation in the Arc. A, E, and I show low-magnification images of p-ERK1/2 and α-MSH colocalization. B, F, J and C, G, K are magnified images (individual channels) of the areas outlined by a dotted rectangles in the leftmost column. D, H, and L are images from the combined channels. Open arrowheads indicate neurons expressing both p-ERK1/2 and α-MSH; filled arrowheads designate neurons expressing α-MSH only.
Figure 8.
Effect of PYY3–36 treatment on p-ERK1/2 activation in the PVN. A, E, and I show low-magnification images of p-ERK1/2 and AVP (shown in pseudocolored red hue for better viewing) colocalization. B, F, J and C, G, K are magnified images (individual channels) of the areas outlined by the dotted rectangles in the leftmost column. D, H, and L are images from the combined channels. Open arrowheads indicate neurons positive for both p-ERK1/2 and AVP; filled arrowheads designate neurons positive for p-ERK1/2 only.
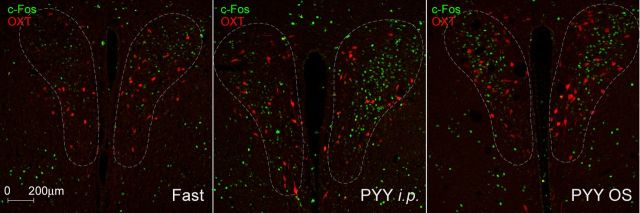
Salivary PYY3–36 does not activate OXT neurons in the PVN
To further characterize the identity of the activated neurons, we costained the PVN sections with OXT and c-Fos antibodies (Fig. 9). Although OXT+ neurons were abundantly observed in the area of c-Fos activation, there was very little if any colocalization. Considering the distinct profile of OXT and AVP expression in the parvocellular PVN, this almost certainly indicates that the area of c-Fos/ERK activation was that of the medial parvocellular division of the PVN almost exclusively containing corticotropin-releasing factor hormone (CRF) neurons (Gorbatyuk et al., 2001).
Figure 9.
Effect of PYY3–36 treatment on c-fos expression in the OXT neurons in the PVN. There is no overlap in the c-Fos expression (green) and OXT-IR neurons (red) in the PVN under the treatment conditions indicated in each panel.
Discussion
Anorexic peptide PYY3–36, administered at supraphysiological doses induces CTA in nonemetic species. In humans, this aversive reaction is associated with visceral illness and nausea (Degen et al., 2005). The current study describes an alternative route of PYY3–36 administration that reliably induces an anorectic response without inducing CTA. Furthermore, our data support the existence of the previously postulated putative anorexic pathway activated by salivary PYY3–36 (Acosta et al., 2011) and acting through the cognate YRs expressed in oral mucosa (Hurtado et al., 2012).
Because systemic PYY3–36 had been implicated in mounting CTA by activating AP neurons (Halatchev and Cone, 2005; Chelikani et al., 2006), it was of interest to test whether orally administered PYY3–36 induced aversive responses as well. Although PYY3–36 intraperitoneally injected mice indeed developed CTA to an associated flavor, no such response was documented in mice treated with OS PYY3–36, even at the highest dose of 18 μg/100 g BW.
Neuronal pathways in the brainstem
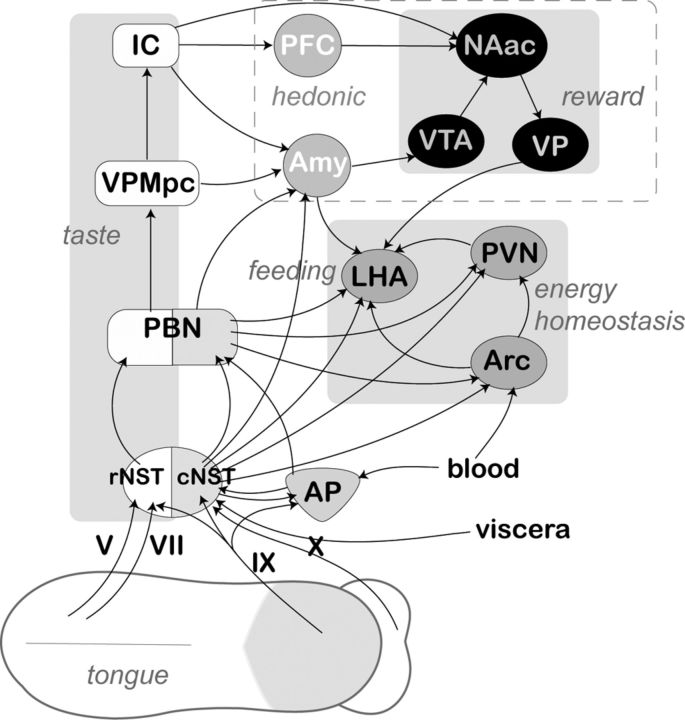
The NST consists of two major divisions, rostral and caudal, mediating and integrating gustatory and visceral information, respectively. rNST mediates gustatory perception via terminal fields of the V, VII cranial nerves from anterior 2/3 of the tongue and the palate (Contreras et al., 1982) and the projections of the lingual-tonsilar branch of the IX (Hamilton and Norgren, 1984). Conversely, cNST and AP receive projections of vagal afferent fibers that innervate different visceral organs (Kalia and Sullivan, 1982; Katz and Karten, 1983; Belecky and Smith, 1990; Travers and Nicklas, 1990). This partition is further manifested in distinctive afferent projections to the higher brains areas (Fig. 10).
Figure 10.
Basic diagram displaying main putative anorexigenic pathway originating in the tongue epithelia and/or taste cells innervated with afferent projections of neurons from trigeminal nerve V, cranial nerve VII (chorda tympani branch), glossopharyngeal nerve IX, or superior laryngeal branch of the cranial nerve X. For clarity, only ascending projection are shown, although the majority of these pathways include reciprocal descending fibers. The rostral (gustatory) and caudal (visceral) subdivisions of the NST are shown by white and shaded areas, respectively. The distinctive shading of the PBN is used to show the existence of functionally segregated subnuclei. Anatomically and functionally related nuclei of the forebrain areas are designated by similar shaped and shaded ovals, and their functional roles are displayed in italics. VPMpc, Parvicellular part of the posteromedial ventral thalamic nucleus; IC, insular cortex; PFC, prefrontal complex; VTA, ventral tegmental area; NAac, nucleus accumbens; VP, ventral pallidum.
The PYY3–36 intraperitoneal group showed significant reduction of c-Fos+ neurons in the rNST compared with fasting and feeding conditions. In the OS-treated group, the neuronal inhibition was even more pronounced. These data are consistent with the recent report showing that the inhibition of the opioid/GABAergic neurons in the rNST could suppress ingestive behavior (Kinzeler and Travers, 2011). The reduction in the numbers of activated neurons in the rNST could result from inhibiting descending projections from the gustatory cortex (Smith and Li, 2000), the lateral hypothalamus (Cho et al., 2002), and the Amy (Lundy and Norgren, 2001), all modulating gustatory activity within the NST and/or PBN. In general, descending influences from the forebrain are excitatory, although there is some evidence for inhibitory effects as well (Hayama et al., 1985; Di Lorenzo and Monroe, 1995; Smith and Li, 2000; Lundy and Norgren, 2001). Recent evidence has shown that, in rats, a major descending projection from the Amy to the NST is GABAergic (Saha et al., 2000). Together, this evidence suggests that the responses of gustatory neurons in the brainstem are subject to descending forebrain modulation, most likely reflecting the animal's physiological state or previous experience.
The neurons in the AP and cNST showed similar responses to the systemic and oral PYY administration. Both cNST and AP groups responded by significantly increasing the numbers of c-Fos-IR cells compared with the fasted but not the fed group. Both nuclei responded to LiCl in a very dramatic way, consistent with the view that the AP projects into the cNST (Date et al., 2006) and that it is a chemoreceptor trigger zone mediating nausea (Bernstein et al., 1992). However, the OS treatment induced significantly fewer neurons in the AP compared with systemic treatment, consistent with the absence of the CTA response.
It is conceivable that these c-Fos-IR neurons are pre-pro-glucagon neurons that are known to be localized in the cNST. A significant body of evidence supports a role of the NST pre-pro-glucagon neurons as mediators of the anorexia elicited by visceral malaise. Rinaman (1999a) has shown that three potent interoceptive stressors (CCK, LiCl, and LPS) induce c-Fos expression in a large proportion of GLP-1-IR neurons in the NST, and LiCl-induced suppression of FI can be partially blocked by previous central administration of GLP-1 receptor antagonists (Rinaman, 1999b; Seeley et al., 2000), pointing to a functional role of central GLP-1 neurons in LiCl-induced anorexia. Moderate gastric distension can activate hindbrain GLP-1/2-containing neurons, consistent with a role of central pre-pro-glucagon-derived peptides as FI inhibitors. Therefore, these data support the view that the medullary GLP system is involved in appetite control and is activated by stimuli within the behavioral continuum ranging from satiety to nausea (Vrang et al., 2003).
Notably, the changes in the numbers of c-Fos-IR neurons in mPBN reflected changes in rNST: compared with fasted mice, their numbers increased after feeding and were reduced by PYY3–36 treatment. This effect could be explained by the fact that the afferent projections from taste-responsive neurons arising from the rNST terminate primarily in the mPBN (Karimnamazi and Travers, 1998) and that the activation/suppression of rNST neurons directly affect mPBN neurons.
Neuronal pathways in the hypothalamus
Administration of an anorexigenic dose of PYY3–36, whether it is intraperitoneally or by an OS, increased the number of c-Fos+ neurons in the forebrain Arc, PVN, and LHA nuclei and increased p-ERK in the Arc and PVN nuclei. Therefore, the inevitable conclusion is that supraphysiological salivary PYY3–36 can modulate satiety/feeding centers without reaching plasma. Phenotypic identification neurons in the PVN shows that both AVP+ and AVP-negative but not OXT neurons were activated, suggesting that PYY induced satiation by stimulating AVP secretion. AVP is involved in appetite suppression by opposing neuropeptide Y (NPY)-induced orexigenic effects (Olson et al., 1991a,b; Verbalis et al., 1993; Aoyagi et al., 2009).
Both intraperitoneal and OS PYY also induced p-ERK in a significant population within the parvocellular subnuclei of the PVN that are known to express CRF. CRF has been shown to act on the CNS to inhibit FI in several models of hyperphagia (Currie et al., 2001; Fekete et al., 2007).
In the Arc, PYY activated α-MSH-expressing neurons known to reduce FI and increase energy expenditure (Fan et al., 1997). It is worth noting that GLP-1R is colocalized with POMC (anorexigenic) but not with NPY (orexigenic) neurons within the Arc (Sandoval et al., 2008). It is conceivable that the activation of the α-MSH-IR neurons we documented in fed and PYY-treated mice was mediated by the activation of GLP-1 neurons in the NST projecting into Arc. In this case, the increase of c-Fos-IR neurons in the LHA (Fig. 6) in PYY OS mice is probably relayed by direct Arc-to-LHA projections known to encode information related to taste hedonics and feeding (Nishijo et al., 2000).
α-MSH containing neurons in the Arc also activate downstream secondary neuronal populations at distant sites, such as the PBN, Amy, and NST (Belgardt and Brüning, 2010). In the PBN, for example, melanocortin 4 receptor-IR neurons, activated by LiCl treatment, are located in lePBN nuclei known to be critical for the acquisition of CTA (Paues et al., 2006).
Overall, these results strongly support the assumption that salivary PYY interferes directly or indirectly with neuronal networks dedicated to feeding and energy balance (Fig. 10) and that this could partially explain the OS PYY-induced hypophagia.
The PYY3–36-preferred receptor Y2R is expressed in the basal cell epithelia of the tongue (Acosta et al., 2011), as well as in taste cells of the circumvallate papillae (Hurtado et al., 2012). These PYY3–36-responsive cells could be candidates to transduce the information from salivary PYY3–36. In fact, we have shown that the Y2R+ basal epithelial layer of the tongue responded robustly to PYY3–36 (Hurtado et al., 2012). Therefore, although peripheral PYY3–36 may exert its unique effects through the vagal nerve, salivary PYY3–36 could affect the trigeminal, facial, and/or glossopharyngeal nerves, which carry afferent gustatory and somatosensory signals. At least one ascending GLPergic pathway links the NST to the Arc and PVN (Vrang et al., 2007; Llewellyn-Smith et al., 2011), and there are descending Arc–NST, PVN–NST, and LHA–NST projections that are involved in appetite control (van der Kooy et al., 1984; Belgardt and Brüning, 2010; Blevins and Baskin, 2010). Together, these data provide support for the existence of specific pathways connecting oral mucosa and hypothalamic brainstem circuits controlling ingestive behavior.
In summary, we have identified a novel putative alternative pathway that originates in sensory nerves of the oral cavity and projects, via the facial, glossopharyngeal, trigeminal nerves, and/or vagus branch to the NST in the brainstem (Fig. 10). From the NST, the signal could be relayed to the Arc and PVN. The precise phenotype(s) of the neurons and connections involved remain to be identified. However, from the activation patterns of the NST, we can infer that PYY3–36 could be inducing an anorectic effect through the regulation of the palatability of food. To the best of our knowledge, this is the first report demonstrating that PYY3–36 administered into the oral cavity does not induce the adverse effect that is observed when PYY3–36 is administered systemically. Degen et al. (2005) demonstrated that exogenously administered PYY3–36 can suppress FI in humans only when used at the supraphysiological doses. Importantly, inhibition of feeding induced with such doses was accompanied by subjective dose-dependent side effects related to gastrointestinal malaise (associated with the CTA reported in animal models). As a result, the potential of PYY to emerge as a powerful antiobesity drug was challenged by its narrow therapeutic index. The discovery of an alternative pathway mediated by salivary PYY3–36 and its receptors in the oral cavity that regulates ingestive behavior without inducing TA reveals the existence of a novel pathway for the NPY system that may serve as an important therapeutic target.
Footnotes
This work was supported in part by National Institutes of Health Grants 5P30DC010763-02, 1R01DK62302-01, and 1R01DC012819-01A1.
The authors declare no competing financial interests.
References
- Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, Baum BJ, Zolotukhin S. Salivary PYY: a putative bypass to satiety. PLoS One. 2011;6:e26137. doi: 10.1371/journal.pone.0026137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T, Kusakawa S, Sanbe A, Hiroyama M, Fujiwara Y, Yamauchi J, Tanoue A. Enhanced effect of neuropeptide Y on food intake caused by blockade of the V(1A) vasopressin receptor. Eur J Pharmacol. 2009;622:32–36. doi: 10.1016/j.ejphar.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Belecky TL, Smith DV. Postnatal development of palatal and laryngeal taste buds in the hamster. J Comp Neurol. 1990;293:646–654. doi: 10.1002/cne.902930409. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Chavez M, Allen D, Taylor EM. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res. 1992;575:132–137. doi: 10.1016/0006-8993(92)90432-9. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG. Hypothalamic-brainstem circuits controlling eating. Forum Nutr. 2010;63:133–140. doi: 10.1159/000264401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Chelikani PK, Haver AC, Reidelberger RD. PYY(3–36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides. 2008;29:112–119. doi: 10.1016/j.peptides.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes–how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/S0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–1370. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005;146:879–888. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides. 2006;27:3193–3201. doi: 10.1016/j.peptides.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. Taste responses of neurons of the hamster solitary nucleus are enhanced by lateral hypothalamic stimulation. J Neurophysiol. 2002;87:1981–1992. doi: 10.1152/jn.00765.2001. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982;6:303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Corson J, Aldridge A, Wilmoth K, Erisir A. A survey of oral cavity afferents to the rat nucleus tractus solitarii. J Comp Neurol. 2012;520:495–527. doi: 10.1002/cne.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 2001;916:222–228. doi: 10.1016/S0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3–36 on food intake in humans. Gastroenterology. 2005;129:1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Hardy WT. Cholecystokinin produces bait shyness in rats. Nature. 1977;266:196. doi: 10.1038/266196a0. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. J Neurophysiol. 1995;74:258–272. doi: 10.1152/jn.1995.74.1.258. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–R5. doi: 10.1016/S0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hökfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol. 2004;470:256–265. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gantz I, Erondu N, Mallick M, Musser B, Krishna R, Tanaka WK, Snyder K, Stevens C, Stroh MA, Zhu H, Wagner JA, Macneil DJ, Heymsfield SB, Amatruda JM. Efficacy and safety of intranasal peptide YY3–36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92:1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Schlegel W. Sequential actions of ERK1/2 on the AP-1 transcription factor allow temporal integration of metabolic signals in pancreatic beta cells. FASEB J. 2007;21:3240–3249. doi: 10.1096/fj.06-7798com. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk OS, Milner TA, Wang G, Regunathan S, Reis DJ. Localization of agmatine in vasopressin and oxytocin neurons of the rat hypothalamic paraventricular and supraoptic nuclei. Exp Neurol. 2001;171:235–245. doi: 10.1006/exnr.2001.7746. [DOI] [PubMed] [Google Scholar]
- Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab. 2005;1:159–168. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Obata K, Ogawa H. Characterization of parabrachial subnuclei in mice with regard to salt tastants: possible independence of taste relay from visceral processing. Chem Senses. 2009;34:253–267. doi: 10.1093/chemse/bjn085. [DOI] [PubMed] [Google Scholar]
- Hayama T, Ito S, Ogawa H. Responses of solitary tract nucleus neurons to taste and mechanical stimulations of the oral cavity in decerebrate rats. Exp Brain Res. 1985;60:235–242. doi: 10.1007/BF00235918. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Kohlerman NJ, Rogers RC. Hepatic-vagal and gustatory afferent interactions in the brainstem of the rat. J Auton Nerv Syst. 1983;9:477–495. doi: 10.1016/0165-1838(83)90008-5. [DOI] [PubMed] [Google Scholar]
- Hurtado MD, Acosta A, Riveros PP, Baum BJ, Ukhanov K, Brown AR, Dotson CD, Herzog H, Zolotukhin S. Distribution of Y-receptors in murine lingual epithelia. PLoS One. 2012;7:e46358. doi: 10.1371/journal.pone.0046358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci. 2009;1163:17–30. doi: 10.1111/j.1749-6632.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res. 1998;813:283–302. doi: 10.1016/S0006-8993(98)00951-2. [DOI] [PubMed] [Google Scholar]
- Katz DM, Karten HJ. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol. 1983;218:42–73. doi: 10.1002/cne.902180104. [DOI] [PubMed] [Google Scholar]
- Kinzeler NR, Travers SP. mu-Opioid modulation in the rostral solitary nucleus and reticular formation alters taste reactivity: evidence for a suppressive effect on consummatory behavior. Am J Physiol Regul Integr Comp Physiol. 2011;301:R690–R700. doi: 10.1152/ajpregu.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- Kuribara M, Kidane AH, Vos GA, de Gouw D, Roubos EW, Scheenen WJ, Jenks BG. Extracellular-signal regulated kinase regulates production of pro-opiomelanocortin in pituitary melanotroph cells. J Neuroendocrinol. 2011;23:261–268. doi: 10.1111/j.1365-2826.2010.02103.x. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol. 2001;85:770–783. doi: 10.1152/jn.2001.85.2.770. [DOI] [PubMed] [Google Scholar]
- Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R384–R388. doi: 10.1152/ajpregu.00535.2004. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Uwano T, Kondoh T, Torii K. Hypothalamic and amygdalar neuronal responses to various tastant solutions during ingestive behavior in rats. J Nutr. 2000;130(Suppl 4S):954S–959S. doi: 10.1093/jn/130.4.954S. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3–36 in the mouse. J Pharmacol Exp Ther. 2003;306:948–953. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991a;129:785–791. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol. 1991b;260:R448–R452. doi: 10.1152/ajpregu.1991.260.2.R448. [DOI] [PubMed] [Google Scholar]
- Paues J, Mackerlova L, Blomqvist A. Expression of melanocortin-4 receptor by rat parabrachial neurons responsive to immune and aversive stimuli. Neuroscience. 2006;141:287–297. doi: 10.1016/j.neuroscience.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: aversive and appetitive gustatory conditioning. Brain Res Bull. 2000;52:269–278. doi: 10.1016/S0361-9230(00)00263-X. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999a;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999b;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/S0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Li CS. GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Res. 2000;858:408–415. doi: 10.1016/S0006-8993(99)02484-1. [DOI] [PubMed] [Google Scholar]
- Stanić D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hökfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience. 2010;171:351–365. doi: 10.1016/j.neuroscience.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec. 1990;227:373–379. doi: 10.1002/ar.1092270313. [DOI] [PubMed] [Google Scholar]
- Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol. 1995;73:2144–2162. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- Trifunovic R, Reilly S. Medial versus lateral parabrachial nucleus lesions in the rat: effects on mercaptoacetate-induced feeding and conditioned taste aversion. Brain Res Bull. 2002;58:107–113. doi: 10.1016/S0361-9230(02)00766-9. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Blackburn RE, Olson BR, Stricker EM. Central oxytocin inhibition of food and salt ingestion: a mechanism for intake regulation of solute homeostasis. Regul Pept. 1993;45:149–154. doi: 10.1016/0167-0115(93)90198-H. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Jensen PB, Lykkegaard K, Artmann A, Larsen LK, Tang-Christensen M. Upregulation of the brainstem preproglucagon system in the obese Zucker rat. Brain Res. 2008;1187:116–124. doi: 10.1016/j.brainres.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang BR, Ding YQ, Kuang F, Wang X, Duan XL, Jiao XY, Ju G. Extracellular signal-regulated kinases1/2 in neurons of the dorsal motor nucleus of the vagus nerve and nucleus of the solitary tract are activated by noxious visceral stimulus in mice. Neurosci Lett. 2002;334:103–106. doi: 10.1016/S0304-3940(02)01113-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Central mechanisms of roles of taste in reward and eating. Acta Physiol Hung. 2008;95:165–186. doi: 10.1556/APhysiol.95.2008.2.2. [DOI] [PubMed] [Google Scholar]